Abstract
Planar cell polarization entails establishment of cellular asymmetries within the tissue plane. An evolutionarily conserved Planar Cell Polarity (PCP) signaling system employs intra- and intercellular feedback interactions between its core components, including Frizzled, Van Gogh, Flamingo, Prickle and Dishevelled, to establish their characteristic asymmetric intracellular distributions and coordinate planar polarity of cell populations. By translating global patterning information into asymmetries of cell membranes and intracellular organelles, PCP signaling coordinates morphogenetic behaviors of individual cells and cell populations with the embryonic polarity. In vertebrates, by polarizing cilia in the node/Kupffer’s vesicle, PCP signaling links the anteroposterior to left-right embryonic polarity.
Introduction
The generation of asymmetries is one of the hallmarks of embryonic development. Individual cells, such as an egg or a migrating macrophage, can be asymmetric. Moreover, one of the first tasks an embryo has to accomplish is determination of its anteroposterior (AP), dorsoventral (DV) and left-right (LR) axes. Polarization of cell populations or tissues is most evident in the case of epithelia, in which cells develop two types of polarity, apico-basal and planar (Figure 1A)(Adler, 2002; Nejsum and Nelson, 2009). In the first, cells elaborate distinct apical, lateral and basal membranes, thus affording specialized epithelial surfaces (Nejsum and Nelson, 2009). An evolutionarily conserved set of proteins establishes the apical-basal epithelial polarity, which does not appear to be coordinated with the AP, DV or LR body axes. The second, or planar, polarity entails cell polarization along the axis orthogonal to the epithelial cell sheet (Adler, 2002), and as accumulating evidence indicates, it coordinates polarity and behaviors of individual cells and tissues with the embryonic axes.
Figure 1. Fz/PCP pathway in epithelial and mesenchymal cell populations.

(A) Asymmetric localization of core PCP components in Drosophila epithelial cells. (B) PCP protein localization in polarized and vertebrate mesenchymal cells during C&E cell movements, showing asymmetric localization of Dvl and Pk.
Initially characterized in insects, the process of planar polarization ensures that epithelial cells are polarized during wound healing, that all bristles in the wing or abdomen point posteriorly, that ommatidia in the compound eye exhibit crystalline-like organization, or that cells intercalate in a polarized fashion to elongate the germ band during Drosophila melanogaster gastrulation (Gubb and Garcia-Bellido, 1982; Nubler-Jung et al., 1987; Zallen and Wieschaus, 2004). Discoveries in the recent decade also implicate planar polarization in diverse processes in vertebrates, including gastrulation movements of convergence and extension (C&E)(Heisenberg et al., 2000; Wallingford et al., 2000), ear morphogenesis (Wang et al., 2006b), hair and cilia polarization (Guo et al., 2004; Mitchell et al., 2009) and tangential neuronal migration (Jessen et al., 2002). Indeed, planar polarization can be considered as an ever-present property of all, or nearly all, epithelial sheets that is manifest to various degrees (Lawrence et al., 2004). It is also now apparent that mesenchymal cell populations can acquire planar polarity (Figure 1B).
In terms of the underlying mechanisms, planar polarization can be mediated by different molecular pathways (Zallen, 2007). Here we focus on the planar polarity elaborated by the Frizzled/Planar Cell Polarity (PCP) signaling system. We start by reviewing our current understanding of the molecular mechanisms of PCP signaling in Drosophila. Next, we compare the Drosophila and vertebrates in terms of the mechanisms of PCP mediated cell polarization and the morphogenetic processes regulated by this signaling system. We highlight differences in the molecular components of the Drosophila and vertebrate pathway and the recently suggested relationships between the vertebrate PCP pathway and primary cilia. We also review recent advances in our understanding of the upstream regulators of the PCP signaling system. Finally, we discuss the problem of coordination between the embryonic, tissue and cellular polarities and an emerging model whereby the PCP signaling relays the embryonic AP patterning to the level of individual cells to polarize their behaviors, and thus coordinate morphogenesis with embryonic polarity.
Mechanisms of planar Fz/PCP in Drosophila
The Drosophila model has provided key mechanistic insights into PCP signaling. The stereotyped arrangement of sensory bristles and cellular hairs (trichomes) on the wing, abdomen and thorax (notum) or ommatidia in the eye all serve as models of planar polarity in Drosophila, and mutations in, or misexpression of, the core PCP genes results in the loss of polarity in these tissues, albeit to differing extents (Adler, 2002; Lawrence et al., 2004; Strutt, 2003). First, at the organismal and tissue levels, morphogen gradients instruct embryonic axes, coordinate morphogenetic movements, and orient cell divisions and growth of tissues and organs. At the cellular level, cell-cell communication mediated by the so-called “core PCP components” allows cells to coordinate their polarity in a uniform direction, as instructed directly or indirectly by both embryonic polarity and extracellular signals. The core PCP proteins identified in Drosophila include: Van Gogh/Strabismus (Vang, Vangl1/2 in vertebrates), a four-pass transmembrane protein (Taylor et al., 1998; Wolff and Rubin, 1998), Frizzled (Fz), a seven-pass transmembrane protein (Adler et al., 1997; Vinson et al., 1989), and Flamingo (Fmi, or Starry Night, Clsr in vertebrates), a seven-pass transmembrane atypical cadherin (Chae et al., 1999; Usui et al., 1999); and cytoplasmic proteins: Prickle (Pk) (Gubb et al., 1999), Dishevelled (Dsh/Dvl) (Theisen et al., 1994) and Diego (Dgo) (Feiguin et al., 2001)(Table 1; Figure 1A). At the sub-cellular level, core PCP proteins are initially recruited uniformly to the apical cell membrane, then assume asymmetric distributions in polarized epithelia. For instance, in the Drosophila wing cells, Vang and Pk accumulate at the proximal (Tree et al., 2002) and Fz, Dsh and Dgo at the distal apical cell membranes (Axelrod, 2001; Strutt, 2001). Whereas, Fmi localizes at both proximal and distal cell edges (Chen et al., 2008; Usui et al., 1999) (Figure 1A). Fmi homodimers are proposed to have a central role in promoting these protein asymmetries in an Fz activity-dependent manner (Chen et al., 2008; Lawrence et al., 2004; Usui et al., 1999).
Table 1.
Fz/PCP pathway components
| drosophila | vertebrates | protein type | reference | |
|---|---|---|---|---|
| PCP core components | frizzled (fz) | frizzled 2 (fz2) | seven pass transmembrane receptor, extracellular cysteine-rich domain | Adler et al., 1997; Vinson et al., 1989, Dann et al., 2001; Quesada-Hernandez et al., 2010. |
| frizzled 3 (fz3) | ||||
| frizzled 6 (fz6) | ||||
| frizzled 7 (fz7) | ||||
|
| ||||
| van gogh/strabismus (Vang/stbm) | vangogh like 2 (Vangl2) | four pass transmembrane protein, PDZ binding motif | Taylor et al., 1998; Wolff and Rubin, 1998; Illescu et al., 2010; Jessen et al., 2002; Jessen and Solnica-Krezel 2004. | |
| vangogh like 1 (Vangl1) | ||||
|
| ||||
| flamingo/starry night (fmi/stan) | celsr 1 | seven pass transmembrane protein, extracellular cadherin repeats | Chae et al., 1999; Usui et al., 1999, Strutt 2008; Nakayama et al. 1998. | |
| celsr 2 | ||||
| celsr 3 | ||||
|
| ||||
| dishevelled (dsh) | dishevelled 1 (dvl1) | DEP, DIX, PDZ, Cytoplasmic domains | Theisen et al., 1994; Wong et al., 2000; Wallingford and Habas 2005; Park et al., 2008; Ehebauer and Arias 2009; Yu et al., 2010. | |
| dishevelled 2(dvl2) | ||||
| dishevelled3 (dvl3) | ||||
|
| ||||
| prickle (pk) | prickle 1 (pk1) | Cytoplasmic, triple LIM domains, PET domain | Gubb et al., 1999. | |
| prickle 2 (pk2) | ||||
|
| ||||
| diego (dgo) | inversin (inv) | Cytoplasmic, ankyrin repeats | Feiguin et al., 2001. | |
|
| ||||
| PCP effectors | fuzzy (fy) | fuzzy (fy/fuz) | predicted N-terminal transmembrane domain and C-terminal longin-like fold domain | Collier and Gubb 2007; Gray R.S. et al., 2009. |
|
| ||||
| inturned (in) | inturned (in) | predicted transmembrane domains | Park et al., 1996; Park TJ. Et al., 2006; Zeng et al., 2010 | |
|
| ||||
| fritz (frtz) | fritz (frtz) | coiled-coil WD40 repeat domains | Collier et al., 2005; Kim et al., 2011 | |
|
| ||||
| Vertebrate ligands and co-factors for PCP | wnt5 | secreted | Kilian et al., 2003; Qian et al., 2007 | |
|
| ||||
| wna1 | secreted | Heisenberg et al., 2000; Tada et al., 2000 | ||
|
| ||||
| knypek/glypican (kny/gpc4) | membrane-associated Heparan sulfate proteoglycan | Topczewski et al., 2001; Ohkawara et al., 2003 | ||
|
| ||||
| Protein tyrosine kinase 7 (PTK7) | transmembrane protein, tyrosine kinase homology domain | Lu X et al., 2007 | ||
|
| ||||
| receptor for activated protein kinase C1 (rack1) | adaptor/scaffolding protein, WD40 repeats | Wehner et al., 2011; Ren et al., 2011 | ||
|
| ||||
| Cthrc1 | secreted glycoprotein | Yamamoto et al., 2008. | ||
|
| ||||
| ror2 | Tyrosine-protein kinase receptor, type I transmembrane protein | Masiakowski and Carroll 1992; Gao et al 2011 | ||
|
| ||||
| Parallel signaling molecules for planar polarity | fat (ft) | fat 1 | transmembrane protein, atypical cadherin, extracellular cadherin repeats, EGF domain, laminin domain | Mahoney PA. et al., 1991; Rock et al., 2005; Tanoue and Takeichi., 2005; Saburi et al., 2008 |
| fat 2 | ||||
| fat 3 | ||||
| fat 4 | ||||
|
| ||||
| dachsous (ds) | dachsous 1 (ds1/dsch 1) | transmembrane protein, atypical cadherin, extracellular cadherin repeats | Clark et al., 1995; Mao et al., 2011 | |
| dachsous 2 (ds2/dsch 2) | ||||
|
| ||||
| four-jointed (fj) | four-jointed (fjx) | type II transmembrane protein, potentially secreted after cleavage | Rock et al., 2005; Probst et al., 2007 | |
|
| ||||
| chascon (chas) | ND | ND | Olguin et al., 2011. | |
Fmi-Fz complexes are endocytosed and trafficked distally, along apical proximo-distal microtubule arrays, to generate sub-cellular asymmetric localization of core PCP components (Shimada et al., 2006; Strutt and Strutt, 2008). In addition, cell-cell communication plays an essential role in establishing these intracellular asymmetries and in the propagation of planar polarity within the plane of the epithelium. Notably, cell clones that harbor mutations in specific PCP core components (Vang, Fz) disrupt and modify the planar polarity of the surrounding wild-type cells, several cell diameters from the clone and in a directional manner, a phenomenon known as domineering non-autonomy (Vinson and Adler, 1987). Specifically, loss-of-function (LOF) fz mutant clones reorient the outgrowth of wild-type neighbor cells towards the clone, while gain-of-function (GOF) of fz or vang LOF mutant cells instruct distally positioned wild-type neighbors to orient their hairs away from the clone (Lawrence et al., 2004). These observations suggest that regional defects in PCP signaling act as local symmetry breaking events, and that planar polarity is spread within the plane of the epithelium along a gradient of Fz activity. Protein-protein interactions between Fmi and Fz, as well as Fz and Vang, suggest a plausible molecular mechanism that involves the extracellular interactions between Fz-Fmi and Vang-Fmi complexes in relaying planar polarity signals between cell neighbors (Chen et al., 2008; Strutt and Strutt, 2008; Wu and Mlodzik, 2008) (Figure 1 and 2). Furthermore, genetic, molecular and computational analyses have led some to propose that an embryonic symmetry breaking event, or a ‘global directional cue’, is translated into graded Fz activity in individual cells and is amplified by the action of the core PCP components at cell boundaries via a feed-back loop type of mechanism (Amonlirdviman et al., 2005; Chen et al., 2008; Jenny et al., 2005; Tree et al., 2002). As discussed below, the exact nature of the embryonic symmetry-breaking events that drive planar polarity during morphogenesis and how its activity is coordinated with embryo patterning remain unclear.
Figure 2. Model for Wnt/β-catenin and Fz/PCP pathway in vertebrates.
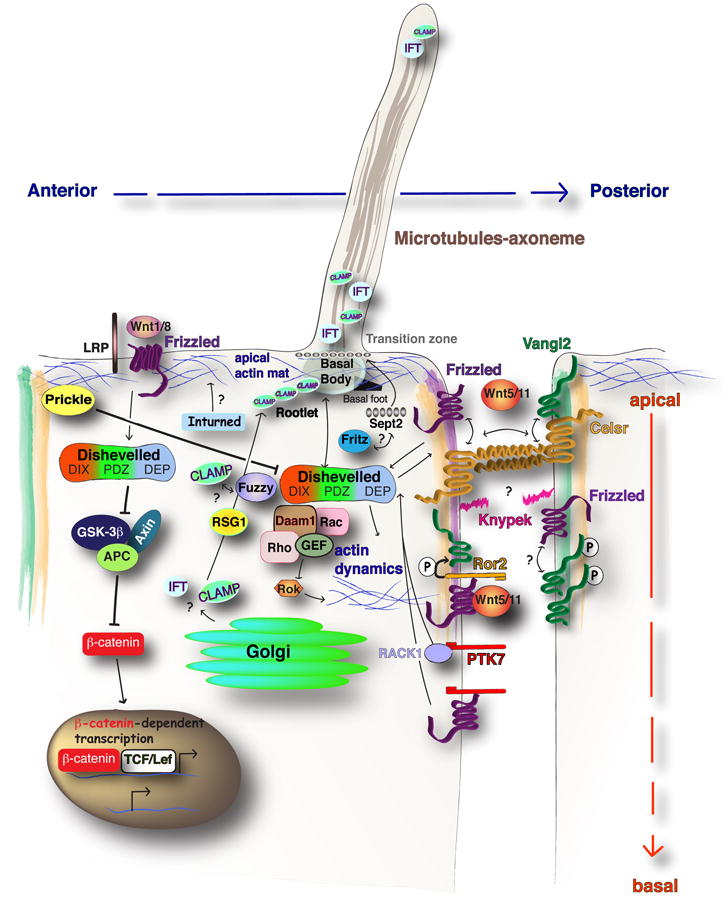
The divergence of canonical Wnt/β-catenin signaling or non-canonical Fz/PCP signaling relies on Dvl protein. The canonical arm of Dvl activation is downstream of Wnt and may involve Frizzled (Fz) co-receptors such as LRP. This activation serves to inhibit the β-catenin destruction complex of GSK-3β, Axin and APC, leading to β-catenin dependent transcription in the nucleus. Fz/PCP signaling is activated due to the interaction of Wnt/Fz/Dvl complex at the membrane. This can result in activation of effectors of the Actin cytoskeleton. Interactions between apically localized Celsr/Vang and Celsr/Fz heterodimers are proposed to propagate planar polarity between neighboring cells. The PCP co-factors, Glypican4/kynpek, Ptk7 and Ror2 provide additional regulation of PCP signals in vertebrates. A Wnt5a dependent Ror2/Fz interaction promotes phosphorylation of Vang protein, which may promote anterior Vangl accumulation. Parallel mechanisms of Ptk7 can promote membrane recruitment of Dvl through RACK1, or independent of RACK1 via interaction with Fz. Anterior localized Pk inhibits Dvl accumulation at the anterior cell edge. PCP signaling is necessary for posterior localization of the MTOC or basal body structures. The PCP effector genes, Fuz, Intu, and Fritz, support distinct aspects of ciliogenesis.
Ever-increasing involvement of Fz/PCP pathway in vertebrate morphogenesis
Since the discovery that the equivalent of the Fz/PCP signaling system regulates C&E movements in the frog, Xenopus laevis and the zebrafish, Danio rerio, a decade ago (Heisenberg et al., 2000; Tada and Smith, 2000; Topczewski et al., 2001; Wallingford et al., 2000), ongoing explorations continue to implicate this pathway in a multitude of morphogenetic processes in vertebrates. Gastrulation entails C&E movements, which narrow the nascent germ layers along the mediolateral (ML) axis and elongate them anteroposteriorly, and employs many well characterized polarized cell behaviors. One such behavior, known as ML cell intercalation, proceeds by polarized planar (in the same cell layer) intercalation of ML elongated mesenchymal cells between their anterior and posterior neighbors, resulting in extension along the AP axis and a concomitant ML narrowing of tissues (Figure 3A) (Keller et al., 2000). In addition, directed cell migration and polarized radial intercalations, whereby cells intercalate preferentially between anterior and posterior neighbors in the more shallow or deeper cell layers, also contribute to C&E (Jessen et al., 2002; Yin et al., 2008) (Figure 3A). Indeed, C&E defects, much like wing hair defects in Drosophila, are considered a hallmark of impaired vertebrate PCP. PCP dependent C&E defects result in widening of the neural plate, and consequent neural tube malformations in frogs and fish (Ciruna et al., 2006; Tawk et al., 2007; Wallingford and Harland, 2002). Likewise, mouse mutants for core PCP components present craniorachischisis, or an open neural tube, from hindbrain to tail (Table 2). Importantly, mutations in VANGL1 and VANGL2 are associated with neural tube closure defects in humans (Kibar et al., 2007; Lei et al., 2010).
Figure 3. Coordination of cell behaviors with embryonic polarity during vertebrate development.

Representative illustrations of embryos and PCP dependent planar polarized cell behaviors observed during embryonic development. (A) The early segmentation stage zebrafish embryo displays several AP polarized cell behaviors (blue box), including (i) mediolateral intercalation (Jessen et al., 2002), (ii) radial intercalation (Yin et al., 2008), (iii) polarized cell division (Gong et al., 2004), and (iv) anterior directed cell migrations (yellow box) (Yin et al., 2008). (B) The late segmentation (15-somite stage) zebrafish embryo and Kupffer’s vesicle (KV), the LR patterning organ. The basal body (green cylinders) in the KV cells, is positioned near the posterior cell edge (Borovina et al., 2010). (C) The E16.5 mouse embryo displays (i) AP polarity of hair follicles (Guo et al., 2004), (ii) ML polarity of the kinocilium (green circle) and adjacent stereocilia Actin bundles (red circles) (Wang et al., 2006), and (iii) a gradient of Wnt5a orients the proximal-distal (initially oriented along the AP axis) polarity of phosphorylated Vangl2 protein (green shading) and primary cilia/basal body (red circle) in chondrocytes of the mouse limb (Gao et al., 2011).
Table 2.
Processes mediated by Fz/PCP pathway
| Protein Family | Gene manipulation | Process/ Tissue Dysmorphogenesis | Reference |
|---|---|---|---|
| WNT | pipetail/Wnt5 | Convergent-extension; Axial Elongation | Kilian et al., 2003. |
| - | Wnt5a | Convergent-extension; Axial Elongation | Wallingford et al., 2001. |
| - | silberblick/Wnt11 homozyogus mutant zebrafish | Convergent-extension; Axial Elongation | Heisenberg et al., 2000. |
|
| |||
| Celsr | Spin and Crash/Celsr1 mutant mice | Craniorachischisis; Polarity of hair follicles | Curtin JA et al., 2003; Davenport and Fuchs 2008. |
| Celsr 2; Celsr2/3 mutant mice | polarity of ependymal basal body | Tissir et al., 2010. | |
| - | membrane targeted Fmi injection | Axial extension defect | Carreira-Barbosa et al., 2009. |
|
| |||
| VANGL | Stbm/(vangl2) loss of function | Gastrulation/ convergent extension defects | Goto et al., 2002. |
| - | Looptail/Vangl2 double mutant mice; electroporation of GFP-VanglLP | Craniorachischisis; translation polarity of the basal body | Kibar et al., 2001; Guirao et al., 2010. |
| - | trilobite/vangl2 | Convergent-extension/ Axial Elongation; Translation polairty of cilia in Kupffer’s vesicle. | Jessen JR et al., 2002; Borovina et al., 2010. |
|
| |||
| Prickle | Pk1 gain of function; loss of function | Axial extension defect | Carreira-Barbosa et al., 2003.;Cao et al., 2010. |
| - | Pk2 gain of function/mutations | Convergent extension in zebrafish | Tao H et al., 2011. |
|
| |||
| Frizzled | Fz 8 | Convergent-extension; Axial Elongation | Wallingford et al., 2001. |
| - | Fz3, 6 double homozygous | Craniorachischisis | Wang Y. et al., 2006 a. |
| - | Fz6 homozygous | Coat hair polarity defect; polarity of hair follicles | Wang Y. et al., 2006 b.; Davenport and Fuchs 2008. |
| - | Fz7 maternal-zygotic | convergent-extension; defects in the orientation of cell division | Quesada-Hernandez et al., 2010. |
|
| |||
| Dishevelled | Dvl gain of function; loss of function | Convergent extension of neural plate and lateral plate mesoderm | Wallingford et al., 2000; 2001. |
| - | Dvl1, 2 double homozygous mutants | Craniorachischisis | Hamblet et al., 2002. |
| - | Dvl2, 3 double homozygous mutants | Craniorachischisis | Etheridge SL et al., 2008. |
|
| |||
| Diego/Inversin | Inversin loss of function | Axial extension defect, convergent extension | Simons et al., 2005. |
In addition, PCP mutants are characterized by other types of tissue dysmorphogenesis, including defective orientation of mouse coat hair shafts and follicles (Devenport and Fuchs, 2008; Guo et al., 2004); disruption of stereocilia polarity in the inner ear (Wang et al., 2006b) (Figure 3C); and irregularities in the posterior placement of the microtubule organizing center (MTOC) and of the nodal cilia and consequently LR axis determination (Antic et al., 2010; Borovina et al., 2010; Hashimoto et al., 2010; Sepich et al., 2011) (Table 2; Figure 3A). Finally, PCP components are also implicated in orienting cell divisions in the zebrafish gastrula (Gong et al., 2004; Quesada-Hernandez et al., 2010) and guiding axonal projections in the mouse (Fenstermaker et al., 2011; Goodrich, 2008); however, these cell behaviors will not be discussed further due to space constraints.
Conserved function of core PCP components in vertebrates
The expanded number of vertebrate core PCP homologues presents a challenge to deciphering vertebrate PCP signaling. For example, in the mouse, there are 10 annotated Frizzled (Fzd1-10), two Vangl (Vangl1-2) and three Dvl homologues (Dvl1-3), compared to four Fz (fz1-4) and the single Vang and Dvl genes in the fly (Table 1). Despite potential genetic redundancies, single and compound mutants in some core PCP genes manifest C&E defects in frogs and fish and/or craniorachischisis in the mouse (Table 2). For instance, the mouse Fzd6 mutants exhibit disrupted polarity of the coat hair (Guo et al., 2004). Expression of a mouse homologue of the dsh1 point mutation, a PCP specific mutant in Drosophila, fails to rescue the polarity of the stereocilia in the cochlea (Wang et al., 2006a). Another parallel with Drosophila is that both loss- and gain-of-function of core PCP components in vertebrates impair PCP pathway dependent morphogenetic processes, such as C&E (Goto and Keller, 2002; Jessen et al., 2002; Wallingford and Harland, 2001).
It is more challenging to document the asymmetric localization of the core components in dynamic tissues during chordate morphogenesis, as so clearly observed in the fly wing disc epithelium. Regardless, in a chordate, Ciona savigyni, during a phase of notochord morphogenesis analogous to C&E, MYC-Pk localized diffusely mostly to the anterior, while FLAG-Vang was detected at both anterior and posterior cell edges. In contrast, FLAG-Dvl was diffuse throughout the cytoplasm, with some concentration at the medial cell edges. In the fully elongated notochord cells, both MYC-Pk and FLAG-Vangl become tightly localized as anterior puncta. Furthermore, membrane recruitment of FLAG-Dvl was abolished in the aimless/Pk mutant, as observed in core component mutants in Drosophila (Jiang et al., 2005). In Xenopus dorsal marginal zone explants that undergo C&E movements, Venus-tagged Xenopus Dvl (Venus-XDvl) accumulates around the medial tips of ML elongated cells (Kinoshita et al., 2003). In contrast, in less elongated cells of such marginal zone explants, GFP-Dvl was symmetrically associated with the membrane (Wallingford et al., 2000). Similarly, no asymmetry of Dvl-GFP fusion protein was observed in mouse neuroepithelium (Wang et al., 2006a). By contrast, in zebrafish gastrulae, mosaically expressed GFP-tagged Drosophila Pk was observed as puncta accumulating near anterior cell edges in mesodermal and neuroectodermal cells (Ciruna et al., 2006; Yin et al., 2008). Whereas, enrichment of Xenopus GFP-Dvl at the posterior cell membranes was observed in ML-elongated mesodermal cells engaged in C&E (Yin et al., 2008) (Figure 1B). Importantly, the asymmetric localization of Pk and Dvl fusion proteins was lost in tri/vangl2 mutants (Ciruna et al., 2006; Yin et al., 2008). Likewise, in the mouse node, the LR asymmetry organ, Pk2 and Vangl1, are localized to anterior cell edges (Antic et al., 2010), whereas GFP-Dvl2 and Dvl3 localize to the posterior cell edges (Hashimoto et al., 2010). In mouse epidermis Celsr1/Flamingo, Vangl2 and Fz6 proteins were detected along the anterior and posterior cell edges, although mosaic analyses are needed to accurately determine whether any anterior versus posterior intracellular asymmetries exist. Moreover, in Celsr1 (Crash) and Vangl2 (Looptail) mutant epidermis, the membrane localization of Fz6 protein was disrupted (Devenport and Fuchs, 2008).
In addition, the phenomenon of domineering non-autonomy has also been observed for the vertebrate PCP signaling. In zebrafish, wild-type cells transplanted into tril/vangl2 mutant gastrulae fail to mediolaterally elongate (Jessen et al., 2002). In Xenopus epidermis, the cilia rootlet orientation is reversed in cells anterior to tissues over-expressing Vangl2 (Mitchell et al., 2009). Furthermore, Fz-PCP control of the mouse hair follicle planar polarity is propagated through adjacent epidermis, as wild-type hair follicle explants remained unpolarized when flanked by homozygous Vangl2/Lp mutant epidermis. Finally, membrane recruitment of Vangl2 or Fzd6 is dependent on the intercellular interactions of Celsr1 homodimers (Devenport and Fuchs, 2008), analogous to the requirement of Fmi for PCP signaling in Drosophila (Bastock et al., 2003; Chen et al., 2008; Lawrence et al., 2004).
Altogether, many observations support the notion that core PCP components interact to polarize epithelial and mesenchymal cells by evolutionarily conserved mechanisms. Indeed, the localization of Pk and Dvl fusion proteins to the anterior and posterior cell edges of zebrafish gastrula cells (Yin et al., 2007) parallels localization of these proteins at the proximal and distal edges, respectively, of cells in the Drosophila wing disc. However, other reports of PCP core component intracellular localization challenge the notion of functional conservation in some vertebrate tissues. For example, the polarity of stereocilia and core PCP proteins aligns with the ML axis of the organ of Corti (Figure 3C). Wherein, Dvl accumulates at the lateral cell edges (Wang et al., 2005), however Fzd3 and Fzd6 were reported to accumulate with Vangl2 along the medial cell edges (Montcouquiol et al., 2006; Wang et al., 2006b). Conversely, mosaic labeling revealed a lateral localization of fluorescently tagged Fz6 while antibody labeling a medial localization of Pk2 (Deans et al., 2007). These studies suggest a conservation of PCP components’ localization in the inner ear as seen the fly wing epithelium. They also underscore the importance of mosaic analysis for the determination of PCP protein components’ distribution in various tissues.
Additional regulators of planar polarity in vertebrates
In contrast to Drosophila, studies of vertebrate models implicated several Wnts as ligands for Fz receptors during such diverse planar polarity processes as C&E (Heisenberg et al., 2000; Rauch et al., 1997), the establishment of kidney tubules diameter (Karner et al., 2009) and cartilage extension in the mouse limb bud (Gao et al., 2011). Moreover, zebrafish knypek (kny) mutants display C&E and cartilage morphogenesis defects due to inactivation of Glypican 4, which appears to promote Wnt/PCP activity (Topczewski et al., 2001).
In addition, multiple other proteins, informally called PCP co-receptors, appear to function in vertebrate PCP signaling. Commonly, mutations in genes encoding these and core PCP proteins interact genetically, exacerbating known PCP defects (Table 1). For instance, while Ror2-/- mutants or Vangl2Lp/+ mutants rarely display severe PCP defects, the double Ror2-/-;Vangl2Lp/+ mutant mice display craniorachischisis, and polarity defects of coat hairs and stereocilia in the inner ear (Gao et al., 2011). Mechanistically, Ror2 enhances the asymmetric localization of Vangl2, in part through Wnt5a dependent phosphorylation of Vangl2 in chondrocytes of the developing limb bud (Figure 2, 3C). Mouse Protein Tyrosine Kinase 7 (PTK7) mutants display craniorachischisis (Lu et al., 2004; Paudyal et al., 2010), defective ML cell polarization and consequently gastrulation cell movements defects (Yen et al., 2009). PTK7 regulates membrane recruitment of Dvl through divergent mechanisms; one dependent on the downstream effector molecule, Receptor of Activated Protein Kinase C1 (RACK1), and another dependent on the interaction with Fz7 (Wehner et al., 2011) (Figure 2). It will be important to separate the role of these proteins as inputs into vertebrate PCP signaling from their potential PCP-independent functions.
Functional interactions between Fz/PCP and Fat/Dachsous systems
Whereas the PCP signaling system mediates polarity of cell populations and tissues, a question remains as to how it interprets global embryonic and/or organ polarity. Molecules expressed in gradients along embryonic axes are considered to play an important role in establishing a global directional cue, which could align planar cell polarity with embryonic/organ polarity (Lawrence et al., 2004; Ma et al., 2003). Whereas Wnt ligands have not been shown to play a direct role in the PCP pathway in Drosophila, they feature prominently in vertebrate PCP signaling (Gao et al., 2011; Heisenberg et al., 2000; Kilian et al., 2003; Marlow et al., 2002; Qian et al., 2007; Rauch et al., 1997; Tada and Smith, 2000; Yamamoto et al., 2008) and could serve an instructive role, linking both cellular and organ polarity (Gao et al., 2011).
The Fat/Dachsous (Fat/Ds) pathway is considered to provide this global directional polarity cue in some Drosophila epithelia (Adler et al., 1998; Casal et al., 2002; Ma et al., 2003; Yang et al., 2002). Fat and Ds are very large transmembrane cadherin domain-containing proteins, which promote adhesion through heterophilic interactions (Matakatsu and Blair, 2004). Four-Jointed (Fj), a type II membrane glycoprotein, Golgi-localized kinase, phosphorylates the cadherin domains of Fat and Ds (Brodsky and Steller, 1996; Ishikawa et al., 2008), to modulate their binding affinity (Brittle et al., 2010; Simon et al., 2010). Ds and Fj transcripts and proteins are expressed in opposing gradients (Casal et al., 2002; Yang et al., 2002). These gradients are influenced by the early action of primary morphogens responsible for overall organ patterning, like Wg and Decapentaplegic in the developing imaginal discs (Brodsky and Steller, 1996; Villano and Katz, 1995), and Wg and Hedgehog signaling in the abdomen (Casal et al., 2006; Casal et al., 2002). These observations support a model in which the early morphogen gradients regulate tissue growth and planar polarity along a particular axis. In the wing, the proximal expression of Ds and distal expression of Fj are proposed to orient PCP signaling along the proximal-distal axis (Zeidler et al., 2000). In the Drosophila eye, it is proposed that a Fat gradient promotes Fz activity, placing the Fat/Ds system directly upstream of the core PCP proteins (Ma et al., 2003; Yang et al., 2002). In addition, Fj expression in the eye has been shown to be under the control of Atrophin, a transcriptional co-repressor that binds the cytoplasmic domain of Fat and regulates PCP (Fanto et al., 2003). Furthermore, Fat/Ds signaling functions in the Drosophila wing epidermis to establish the apically located and proximo-distally oriented set of acentrosomal microtubules (MTs) (Harumoto et al., 2010), which are important for distal transport of Fz-vesicles (Shimada et al., 2006). Whereas these data suggest the importance of Fat/Ds signaling for PCP in the fly, other studies in the Drosophila abdomen support an alternative model in which the Fat/Ds and core PCP components act in parallel pathways to influence cell polarity (Casal et al., 2006; Lawrence et al., 2007).
Like core PCP components, the vertebrate Fat/Ds system is complicated by the existence of multiple genes. The mouse genome contains four Fat homologues (Fat1-4), two Ds homologues (Dchs1-2) and one orthologue of Four-jointed (Fjx1). Fat1 mutant mice present partly penetrant holoprosencephaly and anophthalmia, without any defects in Hh signaling (Ciani et al., 2003). It is tempting to speculate that these abnormalities could be due to morphogenetic defects in partitioning of the eye field akin to defects observed with the zebrafish PCP mutants, tri/vangl2, kyp/gpc4 and wnt11/slb (Heisenberg and Nusslein-Volhard, 1997; Marlow et al., 1998). However, planar polarity phenotypes have not been reported for mice with inactivated Fjx1 gene (Probst et al., 2007). Whereas, neither Fat4 mutants nor Dchs1; Fat4 double mutant mice display defects in neural tube closure or in coat hair orientation, Fat4 mutant mice do manifest cystic kidneys due to misorientation of cell divisions (Mao et al., 2011; Saburi et al., 2008). Moreover, Fjx1;Fat4 double mutants display an enhanced cystic kidney phenotype (Saburi et al., 2008). Strikingly, Fat4; Dchs1 double mutants phenocopy loss of Fat4, without any increase in the severity of the cystic kidney defect, and only a mild decreases in the cochlear duct length and a mild increase in the neural tube width (Mao et al., 2011). The precise relationship between the Fat/Ds and PCP systems in planar polarity establishment will remain important avenues of exploration.
The role of mechanical cues in planar polarization
Accumulating evidence in the PCP field lends support to the idea that polarization of cells relative to the embryonic axes is tightly linked to cell movements and tissue constraints. A recent study in Drosophila showed that morphogenetic movements and epithelial tissue constraints during growth of the wing imaginal disc play key roles in the regulation of planar cell polarity (Aigouy et al., 2010). In particular, the planar polarization of Drosophila wing epithelium evidenced by Fz/Stbm intracellular asymmetries is present already in the wing imaginal disc at prepupal stages. However, at these early stages, the planar polarization is aligned with the wing margin (the site of wg/Wnt expression in the wing imaginal disc) rather than proximo-distal, as in the adult wing. Interestingly, the re-establishment of the core component proximo-distal asymmetries is dependent on the mechanical tension produced by the contraction of hinge tissue during pupal stages (Aigouy et al., 2010).
In agreement, planar polarity of epithelial hairs in the Drosophila notum also depends on the proper regulation of mechanical tension, in this case, transduced from the underlying indirect flight muscles via tendon cells (Olguin et al., 2011). At the molecular level, mechanotransduction by tendon cells is controlled by chascon, as well as Filamen and MyosinII. Moreover, the role of mechanotransduction from tendon cell to the notum epithelium is parallel to PCP signaling and independent of Fat/Ds signaling. Altogether, these recent studies in Drosophila support a model whereby PCP is additionally regulated by extrinsic mechanical signals during morphogenesis.
Ciliogenesis and PCP
One intriguing mechanism for PCP regulation has been suggested by the discovery of the critical role the primary cilia play in vertebrates in reception of extracellular signals, such as Hedgehog (Huangfu et al., 2003). Indeed, most cells in vertebrates generally contain at least one non-motile primary cilium (Figure 2), which is now regarded as a distinct organelle compartment, functioning in normal organismal physiology through a diverse range of sensory stimuli, including bio- and photochemical and mechanical (Nachury et al., 2009). A simplified view of a cilium will diagram an extracellular extended membrane compartment covering an array of microtubules, called the axoneme. The axoneme extends from an apically docked basal body, a structure analogous to the microtubule-organizing center (MTOC), that in many cases is associated with an apical array of filamentous Actin (Figure 2). The basal foot and ciliary rootlet extend in a planar polarized fashion from the basal body into the intracellular space, while a Septin-based diffusion barrier is important for the signaling abilities of the ciliary compartment (Hu et al., 2011). Protein synthesis does not occur in cilia, and entry of proteins into the cilium is highly regulated and involves specialized vesicle trafficking machinery (Nachury et al., 2009). The establishment and maintenance of the cilia or ‘ciliogenesis’ is accomplished, in part, through intraflagellar transport proteins (IFT) (Baldari and Rosenbaum, 2010) and a class of vesicle coat proteins, implicated in Bardet-Biedl syndrome (BBS), a complex ciliopathic disease (Zaghloul and Katsanis, 2009).
Intriguingly, the core PCP components Vangl2, Dvl and Inversin, are found within the cilia or the base of the basal body (Park et al., 2008; Ross et al., 2005; Simons et al., 2005). Moreover, a class of Drosophila “PCP effector” genes, inturned, fuzzy, and fritz previously shown to impact wing hair polarity without effecting the asymmetric distribution of the core components, also seem to function in ciliogenesis in frogs, and where studied, in the mouse (Gray et al., 2009; Heydeck et al., 2009; Park et al., 2006; Zeng et al., 2010). Furthermore, mutations in, or functional interference with, IFT or BBS genes enhance C&E and hair cell orientation defects in mice and zebrafish harboring mutations in core PCP genes (Cao et al., 2010; Ross et al., 2005). These observations lend credence to the hypothesis that cilia serve as functional antennae, receiving and possibly transmitting a cue to enhance PCP signaling. In support, the homozygous null Polaris/Ift88 mutant mouse is prenatal lethal, completely lacks all node cilia and displays neural tube defects (Murcia et al., 2000). Moreover, a conditional Ift88 mouse mutant, specifically ablating Ift88 expression in the cochlea, disrupts the formation of the kinocilium and exhibits defects in the polarity of the stereociliary bundles, analogous to other PCP core component mutants (Jones and Chen, 2008). However, these mice do not display defects in membrane recruitment of GFP-Vangl2 or endogenous Fzd3 in the cells of the organ of Corti (Montcouquiol et al., 2006; Wang et al., 2006b). Likewise, zebrafish mutants lacking both maternal and zygotic ift88 function, and consequently all ciliary axonemes, display no C&E defects (Huang and Schier, 2009). Finally, the “PCP effector” gene, Fuzzy, is crucial for ciliogenesis in the mouse, yet these mutants do not exhibit craniorachischisis (Gray et al., 2009; Heydeck et al., 2009) or defects in polarity of hair follicles (Dai et al., 2011). It follows that elaboration of an extended ciliary axoneme is dispensable for robust PCP signaling in many tissues.
What then is the connection between the cilium, PCP core and ‘effector’ proteins? During ciliogenesis, the basal body localizes to the apical cell membrane, serving as a nucleation center for targeted vesicle trafficking along microtubule tracks, crucial for the axoneme extension. As discussed, the polarized transport of GFP-Fz containing vesicles along planar polarized microtubules is required to achieve asymmetric localization of Fz in the Drosophila wing (Shimada et al., 2006). In addition to IFT and BBS proteins, targeted vesicle transport of cilia proteins depends on protein components of the evolutionary conserved exocyst complex, Sec 6, 8 and 10 (Zuo et al., 2009), as well as the Sec4p homologue, Rab8 (Nachury et al., 2007). Interestingly, core PCP mutants exhibit defects in polarized trafficking of Cadherin-containing exocyst vesicles in the Drosophila wing (Classen et al., 2005). Finally, Dvl appears to control recruitment of Sec8 protein to basal bodies (Park et al., 2008), Fuzzy mediates the inclusion of specific cargo into the ciliary axoneme (Gray et al., 2009) and Fritz interacts with the Septin cytoskeleton forming a diffusion barrier at the basal body (Kim et al., 2010) (Figure 2). Perhaps the intersection of PCP and ciliogenesis in vertebrates resides in a common requirement for proteins that function in targeted vesicle trafficking of specific vesicle cargos. A comprehensive recent review of the links between Wnt, PCP and cilia can be found in (Wallingford and Mitchell, 2011).
PCP regulates MTOC and basal body positioning
Whereas definitive evidence for regulation of PCP signaling by cilium is still missing, multiple observations support the notion that PCP signaling influences cilium. Recent studies in zebrafish have shown that PCP signaling regulates MTOC localization within cells engaged in C&E. In particular, a random intracellular distribution of MTOCs during early gastrulation transitions to a posterior-medial or posterior-lateral localization in ML-elongated cells undergoing PCP-dependent C&E movements (Sepich et al., 2011). Moreover, this biased intracellular MTOC position is dependent on PCP components Dvl and Kny/Gpc4 (Figure 1B). Likewise, maternal-zygotic (MZ) tri/vangl2 zebrafish mutants fail to position posteriorly the basal bodies in the floor plate and in Kupffer’s vesicle cells (Borovina et al., 2010). The Kupffer’s vesicle and the gastrocoel roof plate are analogous embryonic structures to the mouse node, the LR asymmetry organ. Indeed, antisense morpholino oligonucleotide mediated knockdown of Vangl2 disrupts the posterior position of cilia in cells of the gastroceol roof plate of Xenopus embryos (Antic et al., 2010). In agreement, Vangl1;Vangl2 compound mutant mice exhibit LR patterning defects and fail to establish posterior placement of the basal bodies in the node cells (Song et al., 2010). Finally, loss of Dvl function disrupts rotational polarity of the basal body and subsequently the polarity of cilia beating (Hirota et al., 2010; Mitchell et al., 2009; Park et al., 2008).
PCP signaling has also been implicated in apical docking of the basal body in some ciliated tissues. The mouse Celsr2 and Celsr3 double mutant exhibits defects in apical positioning and polarity of the basal foot of multi-ciliated ependymal cells (Tissir et al., 2010). Moreover, interference with Dvl function generates defects in apical docking of basal bodies in the multiciliated epidermis of frogs and planaria (Almuedo-Castillo et al., 2011; Park et al., 2008). However, such defects have not been reported by others using similar methods to disrupt Dvl function in the node or ependymal cells of the mouse brain (Hashimoto et al., 2010; Hirota et al., 2010). Whereas it is clear that PCP serves to establish and maintain the polarity of the cilium in a diverse range of phyla and cell types, it remains possible that core PCP and “effector” proteins may function during ciliogenesis through biochemical pathways that are distinct from PCP signaling in vertebrates.
Links between PCP signaling and embryo patterning
Normal embryogenesis requires that the processes of embryonic axes determination and morphogenesis are precisely coordinated. Therefore, it is tempting to hypothesize that the morphogenetic behaviors of individual cells and of cell populations are synchronized with embryo patterning. The PCP signaling system emerged as a major and evolutionarily conserved regulator of morphogenetic processes including gastrulation and neurulation, tangential neuronal migration, extension of kidney and ear ducts, and limb/wing morphogenesis (Table 2). Whereas the precise molecular mechanisms upstream of PCP signaling remain to be elucidated, accumulating evidence supports the notion that, in vertebrates, the PCP signaling system activity relays global AP and DV patterning information to individual cells, thus instructing and coordinating their morphogenetic behaviors with embryonic polarity (Figure 3)(Hashimoto et al., 2010; Yin et al., 2008). Moreover, by regulating intracellular polarity and cilia position in the node/Kupffer’s vesicle, the PCP signaling system links AP embryo polarity to LR axis specification (Hashimoto et al., 2010).
The links between AP and DV patterning and PCP pathway activity are most striking during the C&E gastrulation movements. Indeed, C&E movements are driven by cells that become elongated mediolaterally (dorsoventrally), and thus perpendicular to the AP embryonic axis, in a variety of gastrulae (Jessen et al., 2002; Keller, 2002; Shih and Keller, 1992; Topczewski et al., 2001; Wallingford et al., 2000). A common feature of the polarized planar and radial intercalations driving C&E is that they promote AP axis extension as cells preferentially separate their anterior and posterior neighbors (Figure 3A)(Shih and Keller, 1992; Yin et al., 2008). Several lines of evidence indicate that Wnt/PCP signaling plays an instructive role in coordinating these polarized cell behaviors with the AP/DV embryonic axes. First, manipulation of Wnt/PCP signaling, either by elevated or diminished function of pathway components, inhibits ML-cell elongation (Goto and Keller, 2002; Jessen et al., 2002; Keller, 2002; Marlow et al., 2002; Shih and Keller, 1992; Topczewski et al., 2001; Wallingford et al., 2000; Yen et al., 2009), and impairs the AP bias of the planar and radial cell intercalations (Lin et al., 2005; Wallingford et al., 2000; Yen et al., 2009; Yin et al., 2008). Importantly, the AP/ML polarized cell morphology during C&E is correlated with the accumulation at the anterior cell membrane of Pk-GFP fusion proteins in such diverse gastrulae as tunicate Ciona (Jiang et al., 2005), zebrafish (Ciruna et al., 2006; Yin et al., 2008) and mouse (Gao et al., 2011). The enrichment of Dvl-GFP at the posterior membranes and the posterior bias in the intracellular MTOC positioning in the cells of zebrafish gastrulae further highlight the crosstalk between PCP signaling and AP embryonic patterning (Sepich et al., 2011; Yin et al., 2008).
This remarkable Wnt/PCP dependent coordination of morphogenetic cell behaviors with the embryonic axes is also observed in later developmental processes. LR axis determination entails unidirectional fluid flow in the laterality organ, dependent on the posterior tilt of cilia, which are anchored by the basal body in the posterior cell region (Nonaka et al., 2005). As in zebrafish gastrula cells, Dvl is enriched at the posterior cell membranes, while Pk and Vangl1 accumulate at the anterior membranes of epithelial node/KV cells (Antic et al., 2010). In zebrafish and mouse, the asymmetric localization of PCP components, the posterior placement of the basal body, the posterior tilt of cilia, the asymmetric fluid flow and LR asymmetry determination all depend on normal activity of the PCP signaling system (Antic et al., 2010; Borovina et al., 2010; Hashimoto et al., 2010). Later, in the developing hindbrain of zebrafish and mouse, a set of facial motoneurons migrates posteriorly from rhombomere 4 to rhombomere 6. This posterior tangential migration is dependent on the normal function of several PCP components, including Tri/Vangl2 (Jessen et al., 2002), Fz, and Fmi/Clsr (Wada et al., 2006), although no asymmetric distribution of PCP proteins in the hindbrain has been reported. Recent studies also implicate several PCP components, including Fz3, Cels3 and Vangl2, in guidance and organization of the axonal projections of monoaminergic neurons along the AP axis in the brainstem of mouse embryos (Fenstermaker et al., 2011). Finally, new elegant genetic studies in the mouse demonstrate that a Wnt5a signaling gradient mediates limb elongation by establishing planar polarity in chondrocytes along the proximal-distal axis via regulation of Vangl2 phosphorylation by the Ror2 receptor. Hyperphosphorylated Vang2 becomes enriched at the proximal cell membranes of the polarized chondrocytes, and thus at the lower end of the distal to proximal gradient of Wnt5a (Gao et al., 2011) (Figure 2, 3). As the proximodistal limb axis is aligned with the AP embryonic axis, it is intriguing that this intriguing regulatory mechanism of Vangl2, constitutes another example of coordination between the embryonic and cellular polarity during morphogenesis by PCP signaling.
What molecular mechanisms coordinate embryo patterning with Wnt/PCP pathway activity, and in turn morphogenetic cell behaviors? Whereas we are far from having a complete understanding of this complex problem, the first clues emerge from studies of vertebrate gastrulation. An intrinsic AP polarity of axial mesoderm that involves graded Activin-like activity is necessary for C&E to occur during Xenopus gastrulation (Ninomiya et al., 2004). However, the identity of the Activin-like signal in vivo, whether it directly or indirectly regulates Wnt/PCP signaling, or whether it acts in parallel to regulate C&E, remains to be determined. Genetic studies in zebrafish revealed that the ventral to dorsal gradient of BMP activity is an important player in the process of coordinating embryo patterning and morphogenesis during gastrulation. BMP is an evolutionarily conserved morphogen that promotes ventral and posterior, and limits dorsoanterior cell fates in all germ layers during vertebrate gastrulation (De Robertis et al., 2000). However, the BMP activity gradient also regulates C&E movements in the zebrafish gastrula (Myers et al., 2002). In particular, C&E movements do not occur and cells do not exhibit ML polarization in the ventral region of zebrafish gastrula where BMP activity levels are high. In the lateral regions of the gastrula, C&E movements of increasing speeds are observed as cells become ML elongated migrating down the BMP activity gradient. Dorsally, at low BMP activity, C&E movements occur via polarized intercalation of ML-elongated cells. Likewise, in Xenopus, high levels of BMP activity inhibit C&E of tissue explants (Graff et al., 1994). One mechanism via which the ventral to dorsal BMP activity gradient limits C&E in the ventrolateral gastrula regions involves negative regulation of expression of wnt11/slb and wnt5/ppt genes (Myers et al., 2002), which are required for polarized cell behaviors underlying C&E movements (Figure. 3A) (Heisenberg et al., 2000; Kilian et al., 2003). In agreement, during Xenopus gastrulation, BMP signaling negatively regulates expression of Glypican 4(Ohkawara et al., 2003), the orthologue of the zebrafish kny/gpc4 gene, essential for C&E movements (Topczewski et al., 2001). These studies provide a model of a morphogen gradient coordinating embryo patterning with morphogenetic cell behaviors via regulation of Wnt/PCP signaling.
Conclusion
In this review, we discussed several inputs proposed to regulate Wn/Fz/PCP pathway activity in Drosophila and vertebrates, including the Fat/Ds system, Wnt, BMP, and Activin-like ligands, and mechanical signals. Wnt5a in the limb bud, as well as Wnt11 and Wnt5 during gastrulation, are expressed in a graded fashion from posterior to anterior (distal to proximal in the limb), and evidence exists for a gradient of Wnt5a signaling to regulate Ror2/Vangl planar polarity signaling in the limb (Gao et al., 2011). Moreover, Nodal morphogenic activity (Activin-like signals) has been proposed to pattern the AP axis in zebrafish (Thisse et al., 2000). It will be important to determine which of these inputs integrate PCP signaling with embryonic patterning. Another key area of inquiry is to delineate the mechanisms via which the exquisite polarization of individual cells by the PCP pathway (proximo-distal or anteroposterior) is established and subsequently translated into morphogenetic cell behaviors, such as polarized planar and radial intercalations or directed cell migration. Additional decisive experiments are needed to dissect the relationship between the Fat/Ds and PCP signaling systems, in particular whether the Fat/Ds system acts upstream and/or in parallel to the PCP signaling system in regulating cell polarity and morphogenesis. The relationships discussed here between the PCP signaling system and primary cilia warrant further experiments to determine the molecular and cellular mechanisms via which PCP signaling system impacts on primary cilia formation and function, and whether primary cilia influence PCP signaling system directly or whether the two share common molecular components.
Acknowledgments
We would like to thank Drs. Jarema Malicki, Florence Marlow, Diane Sepich, John Wallingford, Yingzi Yang and Xin Li for critical comments and/or discussion, and Linda Lobos for text editing. We apologize to all authors whose relevant work could not be cited due to space constraints. This work in the Solnica-Krezel laboratory is supported in part by an R01 GM55101 grant from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Almuedo-Castillo M, Salo E, Adell T. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc Natl Acad Sci U S A. 2011;108:2813–2818. doi: 10.1073/pnas.1012090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky MH, Steller H. Positional information along the dorsal-ventral axis of the Drosophila eye: graded expression of the four-jointed gene. Dev Biol. 1996;173:428–446. doi: 10.1006/dbio.1996.0038. [DOI] [PubMed] [Google Scholar]
- Cao Y, Park A, Sun Z. Intraflagellar transport proteins are essential for cilia formation and for planar cell polarity. J Am Soc Nephrol. 2010;21:1326–1333. doi: 10.1681/ASN.2009091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Struhl G, Lawrence PA. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Dai D, Zhu H, Wlodarczyk B, Zhang L, Li L, Li AG, Finnell RH, Roop DR, Chen J. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–310. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci. 2007;27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2011;30:16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Goodrich LV. The plane facts of PCP in the CNS. Neuron. 2008;60:9–16. doi: 10.1016/j.neuron.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP Receptor Suggest That Ventral Mesoderm-Inducing Signals Override Dorsal Signal In Vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, Collier S, Roote J. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn. 2009;238:3035–3042. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS, Inoue M, Ito T, Kato H, Sakaguchi M, et al. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–3046. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2011;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. doi: 10.1016/S0070-2153(08)00808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei YP, Zhang T, Li H, Wu BL, Jin L, Wang HY. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;362:2232–2235. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, Hamm H. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, Basson MA, Francis-West P, Irvine KD. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, Driever W, Solnica-Krezel L. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev Biol. 1998;203:382–399. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2009;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum LN, Nelson WJ. Epithelial cell surface polarity: the early steps. Front Biosci. 2009;14:1088–1098. doi: 10.2741/3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubler-Jung K, Bonitz R, Sonnenschein M. Cell polarity during wound healing in an insect epidermis. Development. 1987;100:163–170. doi: 10.1242/dev.100.1.163. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- Olguin P, Glavic A, Mlodzik M. Intertissue mechanical stress affects Frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr Biol. 2011;21:236–242. doi: 10.1016/j.cub.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, Wells S, Lu X, Norris DP, Dean CH, et al. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst B, Rock R, Gessler M, Vortkamp A, Puschel AW. The rodent Four-jointed ortholog Fjx1 regulates dendrite extension. Dev Biol. 2007;312:461–470. doi: 10.1016/j.ydbio.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Hernandez E, Caneparo L, Schneider S, Winkler S, Liebling M, Fraser SE, Heisenberg CP. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol. 2010;20:1966–1972. doi: 10.1016/j.cub.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–234. [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–552. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tawk M, Araya C, Lyons DA, Reugels AM, Girdler GC, Bayley PR, Hyde DR, Tada M, Clarke JD. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature. 2000;403:425–428. doi: 10.1038/35000200. [DOI] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Villano JL, Katz FN. four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development. 1995;121:2767–2777. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wada H, Tanaka H, Nakayama S, Iwasaki M, Okamoto H. Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development. 2006;133:4749–4759. doi: 10.1242/dev.02665. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006a;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006b;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner P, Shnitsar I, Urlaub H, Borchers A. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development. 2011;138:1321–1327. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]


