Abstract
The synthesis and ΔF508-CFTR corrector activity of a 148-member methylbithiazole-based library is reported. Synthetic routes were devised and optimized to generate methylbithiazole analogs in four steps. Corrector potency and efficacy was assayed using epithelial cells expressing human ΔF508-CFTR. These structure-activity data establish that the bithiazole substructure plays a critical function; eight novel methylbithiazole correctors were identified with low micromolar potencies.
Keywords: ΔF508-CFTR correctors, bithiazole, structure-activity, small molecule library
Cystic fibrosis (CF), a lethal genetic disease afflicting ~0.04% of white individuals,1 results in chronic lung infections because mutant cystic fibrosis transmembrane conductance regulator (CFTR) protein fails to confer chloride permeability to epithelial cells in lung and other tissues.2 ΔF508-CFTR, the most common CF mutation (present in at least 1 allele in ~90% of CF patients),1 contains a single amino acid deletion of phenylalanine 508, which causes the nascent protein to be retained in the endoplasmic reticulum and rapidly degraded. 3 When allowed to reach the cell plasma membrane by low-temperature (27° C) rescue, ΔF508-CFTR can function as a cAMP-activated chloride channel, but with significantly decreased activity compared with WT-CFTR.4 While programs aimed at the discovery of small-molecule effectors of defective ΔF508-CFTR folding and cellular processing (e.g., correctors) and channel gating (e.g., potentiators) have identified a number of ΔF508-CFTR potentiators,5 the discovery of effective correctors is a substantially greater challenge as protein folding and trafficking are complex processes involving multiple cellular targets, some of which may be cell type-specific. As previously reported, we identified several small-molecule ΔF508-CFTR correctors [aminoarylthiazoles (e.g., 1 and 2), quinazolinylaminopyrimidinones (e.g., 3), and 4′-methyl-4,5′-bithiazole (e.g., 4); Figure 1] by screening a structurally diverse set of 150,000 compounds. 6 Functional and biochemical analyses established methylbithiazoles as particularly promising for further development based on their efficacy in human ΔF508-CFTR airway epithelial cells and their CFTR-specificity. Herein, we report the preparation and screening of 148 new methylbithiazole analogs aimed at establishing initial SAR data for this lead class of correctors.
Figure 1.
Correctors of defective human ΔF508-CFTR.
To verify our initial screening result as well as to develop an effective route to methylbithiazoles, we began with a re-synthesis of corrector 4. This work commenced by treating 3-chloropentane-2,4-dione with thiourea (Scheme 1) under reflux in absolute ethanol; 5-acetyl-2-amino-4-methylthiazole (5) was obtained in 90% yield. Surprisingly, attempts to N-acylate the C2-amino moiety in 5 with benzoyl chloride under various base, solvent and temperature conditions failed or delivered the targeted N-acyl aminothizole 6 in low yield. While perhaps surmountable in any particular case, the malfunction of 5 → 6 is a consequence of the 5-acetyl moiety reducing the nucleophilicity of the C2-amino group of 5. Since the plan was to ultimately diversify with a spectrum of acid chlorides, this problematic reaction caused us to evaluate the inverse of these two reactions – i.e., use benzoyl isothiocyanate as the starting material in place of thiourea (Scheme 2). Passing ammonia gas through a dichloromethane solution of benzoyl isothiocyanate delivered N- carbamothioylbenzamide (7) which then reacted with 3-chloropentane-2,4-dione to afford 6 in good yield. α-Bromination of the acetyl group of 6 proved to be quite challenging with the recovery of unreacted 6 being the principle issue [Br3 on Amberlite A-26 resin, CHCl3, rt, 24h/no reaction; Br2 in AcOH, rt, 24h/>50% recovered 6; NBS, CHCl3, reflux, 24h/>50% recovered 6]. Pyridinium tribromide in a 30% HBr in acetic acid solution resolved this problem, presumably because this strongly acidic medium promotes enolization. Finally, bromoacetyl thiazole 8 (obtained from 6) was treated with thiourea 9, in turn prepared by reaction of 5-chloro-2-methoxyaniline with ammonium thiocyanate, in refluxing ethanol to deliver corrector 4 in excellent yield. Importantly, no chromatic purifications were required throughout the four-step process outlined in Scheme 2.
Scheme 1.
Aminothiazole N-acylation problem. Conditions used: 1. Et3N (1.2eq), C6H5COCl, DCM, 0 °C to rt, overnight; 5 is recovered. 2. ibid 1, except 5 was pre-washed with 10% aq. NaOH; results in complex mixture. 3. Et3N (1.2eq), C6H5COCl, toluene, reflux, overnight; 5 is recovered + trace of 6. 4. i-Pr2NEt (15eq), C6H5COCl (10eq), DCM, rt, overnight; 5 is consumed; mono-and bis-benzoylation products.
Scheme 2.
Synthesis of corrector 4.
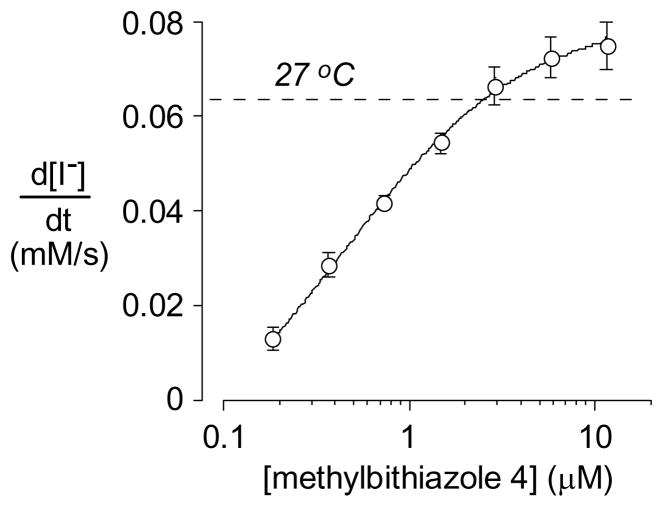
The concentration-activity data for resythesized methylbithiazole 4 is shown in Figure 2 where the dashed line indicates the level of activity with low-temperature (27° C) rescue which is used as a positive control and reference. Activation of ΔF508-CFTR was confirmed for each of the compounds by showing no activity on non-transfected FRT cells and near-complete inhibition of the increased iodide influx by the thiazolidinone CFTRinh-172 at 10 μM (data not shown).7
Figure 2.
Concentration-activity analysis of methylbithiazole 4 (mean ± S.E., n = 4).
To establish SAR information, it was decided to begin by making a small collection of analogs of 4 in which the N-(4′-methyl-4,5′-bithiazol-2′-yl)benzamide moiety was held constant while the aniline moiety was varied. As outlined in Scheme 3, each of these derivatives was available in one step by condensation of the appropriate arylthiourea with 8.
Scheme 3.
Aniline derivatives of the methylbithiazole corrector.
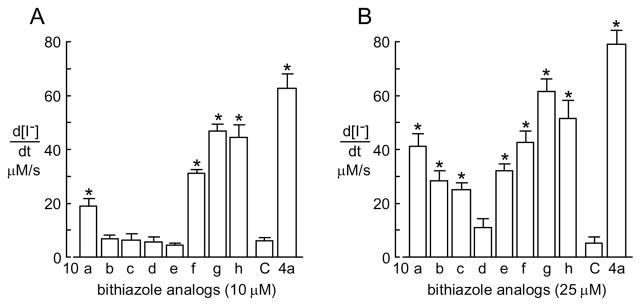
The corrector efficacies of methybithiazoles 10a-h are summarized in Figure 3. Although all eight of these methylbithiazoles were less effective correctors of ΔF508-CFTR than initial hit compound 4, we were encouraged to find that three (10f, 10g, and 10h) were moderately effective. Indeed, compounds 10g and 10h showed comparable corrector activity to 4 at both 10 and 25 μM concentrations. Additionally, this small set of compounds established that placing an electron withdrawing flourine group on the aniline moiety (e.g., 10c and 10d) caused a significant decrease in activity and this decrease was independent of where the fluoride was located (10c and 10d). This small initial set of compounds established that peripheral modification of the methylbithiazole core modulates corrector activity and, as such, a broader study of the amide and aniline substructures of methybithiazole 4 was undertaken.
Figure 3.
Maximal iodide influx in ΔF508-CFTR-expressing FRT cells incubated at 37 °C for aniline-based methylbithiazole correctors 10a-h (see scheme 3) compared with 4a (C, negative DMSO vehicle control) (mean ± S.E., n = 4, * P < 0.05 tested by Student’s test comparing with C).
With these data for bithiazoles 10a-h in hand, a 40-member second library set (11Aa-11Dj; Figure 4) was prepared by reagent-based modification of the protocol outlined in Scheme 2 (e.g., the isothiocyanate employed in step 1 incorporate aryl moieties A-D and the N-aryl thioureas used is step 4 incorporated aryl moieties a-j). Based on the results with 10c and 10d, fluorine was precluded from the R2 aryls.
Figure 4.
Library set two bithiazoles.
While library set two was under preparation, a third library set was targeted in which R1 diversity (Scheme 2) was expanded to include non-aryl substituents. Scheme 2 chemistry afforded high yielding reactions and required no chromatographic purifications, but R1 diversity in the bithiazoles accessible by this chemistry was limited in that few isothiocyanates are commercially available. Since previous attempts at benzoylation 5 → 6 (Scheme 1) were problematic, it was decided to construct library set three by assembly of the bithiazole first with subsquent N-acylation (Scheme 4). It was postulated that the C2′-amino group in the planned 4′-methyl-N-aryl-4,5′-bithiazole-2,2′-diamine intermediate (e.g., 14a-j) would be considerably more nucleophilic than the corresponding amine in aminothiazole 5 with its C5 acyl moiety. In addition, adding the R1 diversity input in the last step was expected to provide considerable time- and labor-saving benefits, particularly with regards to product purification.
Scheme 4.
Synthesis of library set three bithiazoles.
Bromination of 5 using pyridinium tribromide plus 30% HBr in acetic acid afforded 12 in 50% yield. While the isolated product yield of this reaction was significantly lower than the nearly quantitative bromination 6 → 8, the cause of this modest yield was the water solubility of the ammonium salt of 12. Aromatic thioureas 13a-j were prepared by bubbling ammonia gas through the commercially available aromatic isothiocyantates in dichloromethane. After simply evaporating the dichloromethane, pure aromatic thioureas 13a-j were obtained in high yield. Bromothiazole 12 was subsequently refluxed in ethanol with thioureas 13a-j to afford C2′-aminobithiazoles 14a-j in 60–80% yields. These compounds were contaminated by modest quantities of impurities which proved difficult to remove by flash column chromatography as product and impurity Rf values were similar. Consequently, the crude C2′-aminobithiazoles were used in the next step without purification. As hoped, acylation of the C2′-amino moiety of 14a-j with various acid chlorides in the presence of triethylamine in chloroform afforded library set three bithiazoles 15Ea-15Nj (Scheme 4). LC-MS analysis of this last reaction generally showed 40–60% reaction completion. Additional reaction time or the addition of more acid chloride made product purification more difficult due to a corresponding increase in side products. Upon work-up, the products were purified by prep-HPLC and again analyzed by LC-MS. All showed 90% or better purity as well as the correct molecular ion peak.
The Kd/Vmax data for the eight most active methyl-bithiazoles from the 11Aa-11Dj and 15Ea-15Nj series are shown in Figure 5. It is interesting to note that six out of the eight best methylbithiazole share either a 4-chlorobenzamide (11Ca, 11Cd, 11Ci) or a pivalamide (15Jf, 15Jh, 15Jj) at the C2′-position and five out of these eight share either o-methoxy or o-ethoxy aromatic amines (11Ci, 15Jf, 15Jh, 15Jj, 15Ni) at the C2-position.
Figure 5.
Kd/Vmax data for the most active bithiazoles in library sets two and three.
When there is a para substituted aromatic amide at the C2′-position (11Ca, 11Cd, 11Ci, 11Dd), p-substituted aromatic amines at the C2-position have elevated activity (11Ca, 11Cd, 11Dd). It is also interesting that bithiazole 15Ni, which presents a relatively long alkyl amide chain, expressed comparable corrector activity. None of the methylbithiazoles incorporating a 2,4-dichloroaniline substituent showed ΔF508-CFTR corrector activity. Nevertheless, among the bithiazoles screened (including the original hit 4), new bithiazole 15Jf is the most effective corrector as judged by both Vmax and Kd data.
ΔF508-CFTR corrector activity measurements were made in this study as described previously6 using FRT epithelial cells stably co-expressing human ΔF508-CFTR and the sensitive halide-sensing green fluorescent protein YFP-H148Q/I152L.8 Cells were grown at 37°C (90% humidity; 5% CO2) for 24 hours and then incubated for 20 hours with 50 μl of medium containing test compounds. At the time of the assay, cells were washed with PBS and then incubated with PBS containing forskolin (20 μM) and genistein (50 μM) for 20 minutes. Measurements were carried out using FLUOstar fluorescence plate readers (Optima; BMG LABTECH Gmbh), each equipped with 500 ± 10 nm excitation and 535 ± 15 nm emission filters (Chroma Technology Corp.). Each well was assayed individually for iodide influx by recording fluorescence continuously (200 ms per point) for 2 seconds (baseline) and then for 12 seconds after rapid (<1 second) addition of 165 μl PBS in which 137 mM chloride was replaced by iodide. The iodide influx rate was computed by fitting the final 11.5 seconds of the data to an exponential for extrapolation of initial slope and normalized for background-subtracted initial fluorescence. All experiments contained negative controls (DMSO vehicle) and positive controls (methybithiazole 4).
In conclusion, we have developed two versatile synthetic routes for the reliable preparation of bithiazole derivatives. Screening data for the 148 bithiazoles provides valuable structural activity information. In particular, the information gathered from bithiazoles 11Ca, 11Cd, 11Ci, 11Dd, 15Jf, 15Jh, 15Jj, and 15Ni is guiding our ongoing efforts to further refine and improve the ΔF508-CFTR corrector activity of bithiazoles.
Acknowledgments
The authors thank the National Institutes of Health (DK072517 and GM076151) and the National Science Foundation [CHE-0614756 as well as CHE-0443516 and CHE-9808183 (NMR spectrometers)] for their generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations-correlation with incidence data and application to screening. Human Mutation. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol CheM. 2000;275:3729–3732. doi: 10.1074/jbc.275.6.3729. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.Benharouga M, Haardt M, Kartner N, Lukacs GL. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J Cell Biol. 2001;153:957–970. doi: 10.1083/jcb.153.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Al-Nakkash L, Hwang TC. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pfluegers Archiv. 1999;437:553–561. doi: 10.1007/s004240050817. [DOI] [PubMed] [Google Scholar]; (b) Drumm Mitchell L, Wilkinson Daniel J, Smit Lisa S, Worrell Roger T, Strong Theresa V, Frizzell Raymond A, Dawson David C, Collins Francis S. Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science (Washington, DC, United States) 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]; (c) Hwang TC, Sheppard DN. Molecular pharmacology of the CFTR Cl- channel. Trends in Pharmacological Sciences. 1999;20:448–453. doi: 10.1016/s0165-6147(99)01386-3. [DOI] [PubMed] [Google Scholar]; (d) Hwang TC, Wang F, Yang ICH, Reenstra WW. Genistein potentiates wild-type and DF508-CFTR channel activity. Am J Physiology. 1997;273:C988–C998. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]; (e) Pedemonte Nicoletta, Sonawane ND, Taddei Alessandro, Hu Jie, Zegarra-Moran Olga, Suen Yat Fan, Robins Lori I, Dicus Christopher W, Willenbring Dan, Nantz Michael H, Kurth Mark J, Galietta Luis JV, Verkman AS. Phenylglycine and sulfonamide correctors of defective ΔF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Molecular Pharmacology. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]; (f) Yang Hong, Shelat Anang A, Guy R Kiplin, Gopinath Vadiraj S, Ma Tonghui, Du Kai, Lukacs Gergely L, Taddei Alessandro, Folli Chiara, Pedemonte Nicoletta, Galietta Luis JV, Verkman AS. Nanomolar Affinity Small Molecule Correctors of Defective ΔF508-CFTR Chloride Channel Gating. Journal of Biological Chemistry. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 6.Pedemonte Nicoletta, Lukacs Gergely L, Du Kai, Caci Emanuela, Zegarra-Moran Olga, Galietta Luis JV, Verkman AS. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. Journal of Clinical Investigation. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman A. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera-toxin induced intestinal fluid secretion. J Clin Investig. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]