Abstract
Bioprocess forces such as shear stress experienced during routine cell culture are considered to be harmful to cells. However, the impact of physical forces on cell behavior is an area of growing interest within the tissue engineering community, and it is widely acknowledged that mechanical stimulation including shear stress can enhance osteogenic differentiation. This paper considers the effects of bioprocess shear stress on cell responses such as survival and proliferation in several contexts, including suspension-adapted cells used for recombinant protein and monoclonal antibody manufacture, adherent cells for therapy in suspension, and adherent cells attached to their growth substrates. The enhanced osteogenic differentiation that fluid flow shear stress is widely found to induce is discussed, along with the tissue engineering of mineralized tissue using perfusion bioreactors. Recent evidence that bioprocess forces produced during capillary transfer or pipetting of cell suspensions can enhance osteogenic responses is also discussed.
1. Introduction
One of the most recent and exciting advances in modern medicine is the emerging field of regenerative medicine, where live cell-based therapies are used to restore function to ailing tissues and organs. The key challenge still faced for successful commercialisation of cell therapies is their production on a large scale [1, 2]. Consequently, the bioprocessing steps involved in the manufacture of cell therapies have to be scalable and capable of producing cellular products of a reproducibly high quality [2]. It is important that the cells maintain their integrity throughout the whole bioprocess and that the resulting cell product has the appropriate functional identity that is required for therapy. In order to achieve this, it is essential to have a detailed understanding of how the bioprocessing environment impacts on the cells at both the laboratory and industrial manufacture scales.
Cell behavior is influenced and affected by the microenvironment in which they reside. In vivo, cells are exposed to a combination of both biochemical and physical cues that regulate their function. Biochemical stimuli such as growth factors and chemokines stimulate a wide range of responses by binding to their appropriate receptors and initiating intracellular signaling pathways. Physical cues can be provided by other cells or matrix components and be passive in nature or the result of applied force.
Bioprocessing of cells for therapy involves ex vivo expansion and differentiation in an artificial microenvironment [1]. Therefore, careful consideration has to be given to the impact that this artificial environment will have on cells that will be transplanted into a patient. It is important that nutrients and growth factors are delivered in suitably formulated growth medium, providing the cells with the appropriate nutritional support. It is also important to monitor and control the physicochemical environment, as subtle changes in temperature, O2, CO2, and pH will all potentially alter cell behaviour. A further consideration that is often overlooked in basic science research is the impact of bioprocess forces on those cells. Bioprocess forces encountered during cell culture include hydrodynamic shear caused by shaking of the flasks to aid their detachment following trypsinisation, forces produced during centrifugation prior to resuspending and shear stresses resulting from transfer through capillaries or by pipetting to resuspend cell pellets [3]. To date, few studies have been conducted to assess the impact of bioprocess forces on human stem-cell populations, but by understanding their impact on suspension-adapted mammalian cells, combined with evidence of the impact of shear stress on stem cell populations, we can begin to develop an understanding of the impact that bioprocess forces will have on stem-cell survival, proliferation, and differentiation capacity in vitro. Furthermore, we review evidence that shear stress can enhance cellular responses in the context of bone regeneration strategies.
2. The Impact of Bioprocess Forces on Recovery, Viability, and Proliferation of Mammalian Cells
2.1. Suspension-Adapted Cell Lines
Suspension adapted mammalian cells are commonly used in the biopharmaceutical industry for the production of monoclonal antibodies and recombinant proteins. Stirred tank and air-lift bioreactors are extensively used in the culturing of these cells (Figure 1(a)) and whilst increasing agitation improves mixing and nutrient transport, which is necessary to achieve high purity and yield of therapeutic product, they can also cause harm to the cells. Stirring and sparging of the suspension culture results in the formation of spatial differences in the pattern of liquid flow, leading to cell damage caused by hydrodynamic shear stress [4–6]. Undesired cell lysis can affect the stability of therapeutic protein products due to the large amounts of proteases that are released from the dying cells, making purification of the target protein difficult [7, 8].
Figure 1.
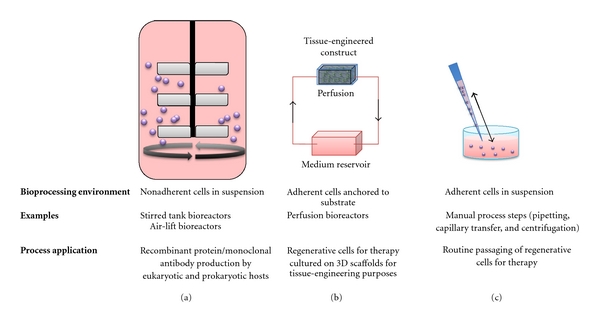
Different circumstances in which mammalian cells become exposed to shear stress that can impact on their behavior. (a) During the manufacture of biopharmaceuticals such as recombinant proteins or monoclonal antibodies, shear stress encountered by suspension-adapted cells in stirred tank bioreactors is often considered harmful. (b) Adherent cells can experience shear stress as a mechanical stimulus that promotes osteogenic differentiation and this can be exploited in perfusion bioreactors, where active transport of oxygen and nutrients throughout 3D scaffolds exposes cells to shear stress. (c) Bioprocess forces produced during manual processing of multipotent cell populations can also enhance osteogenic differentiation potential.
Many studies have been carried out to investigate the sensitivity of suspension-adapted mammalian cells to shear forces, and there is an overwhelming consensus that shear forces are damaging to these cells [9–11]. Turbulent shear stress is generally thought to be more damaging to cells than laminar shear stress of the same magnitude [12–14]. In terms of the critical level of shear stress that cells can withstand, there is great variation in opinion as to what magnitude of shear stress is harmful. One notable study reported that cell loss at high shear stress (100 Nm−2) and low shear stress (1 Nm−2) is greater than that at intermediate shear stress (10 Nm−2) for several animal cell lines in stirred-bioreactors [15]. Cell lysis at high shear is caused by the applied shear force exceeding the cell bursting force, whereas the damage caused at low shear stress is mainly due to the papillated state of the cell, something that occurs when the turgor pressure pushes the cytoplasm out through transient pores formed in the cell [15]. At high levels of hydrodynamic shear stress (induced by high intensity agitation of 1500 rpm in a turbine impeller baffled fermenter), the viability of murine hybridoma cells is affected [14].
Studies conducted on suspended human cells such as primary T-cells suggested that no immediate effects were observed on the metabolism or the extent of apoptosis as a result of hydrodynamic forces arising from agitation or sparging, up to agitation rates of 180 rpm [16]. Even so, the overall expansion potential of the population was reduced due to rapid downregulation of the interleukin-2 receptor. However, in the same study, transformed T cells were extremely sensitive, with reduced growth rates recorded even at agitation rates as low as 30 rpm [16].
In 1992, Born and colleagues proposed a cell-damage model [9]. The model proposed that cell loss is independent of duration of exposure to shear stress as a cell exposed to laminar shear stress is either disrupted or unaffected [9]. However, this model may apply specifically to suspension-adapted cells, because for adherent cells in suspension, increased shear stress exposure time results in an increase in cell damage [11, 17–19].
The large variation in susceptibility of cells in suspension to shear damage is dependent not just on the specific cell type but also on inherent variation within a single population of cells. For instance, the susceptibility of cells to hydrodynamic damage can vary as they move through the cell cycle, with larger cells in S and G2 phase being more prone to damage than G1 cells, presumably due to their size [14].
Many studies have been conducted on haematopoietic cells, as they have an important role in the development of new medicines and therapeutics. Furthermore, they are often in suspension in vivo and, hence, experience hydrodynamic shear stress, something that cells are likely to encounter during cell bioprocessing, for example during capillary transfer [1, 4, 9]. The use of erythrocytes as a cellular standard for comparative analysis of the damage caused by hydrodynamic shear stress has been suggested by Zhang et al. [20]. The main advantage of using erythrocytes is that they do not multiply in vitro so the effects of cell damage can be seen clearly, without apoptosis events in some cells being obscured by the impact of proliferation in others. Studies of erythrocytes from different mammals revealed that the critical shear stress cells can experience without losing viability increases significantly as the cell volume decreases, further demonstrating the relationship between cell size and susceptibility to shear stress [9]. Another type of circulating haematopoietic cells that experience hydrodynamic shear stress are leukocytes. The requirement for these cells to withstand shear stress in vivo results from their adhesion to vascular endothelium when migrating to sites of tissue damage or infection. The continual flow of blood past these cells exposes them to shear stress and detachment of leukocytes adhering to vascular endothelium occurs when shear stresses are in the range of 26.5–106.0 Nm−2 [21]. Additionally, shear stress of 60 Nm−2 applied to leukocytes in vitro for duration of 10 minutes will lyse around 25% of the population [12], and this demonstrates that shear stress levels that can detach leukocytes from vascular endothelium also have debilitating effects on those cells when applied in vitro.
2.2. Adherent Cells in Suspension
Experience gained from suspension-adapted mammalian cells used for production of vaccines, recombinant proteins and antibodies provides valuable insights that can be applied to the scalable manufacturing of stem cell-based therapies, but because the cells themselves are the therapeutic product, bioprocess forces might, either favourably or unfavourably, alter the phenotype of those cells [1, 3, 17–19, 22]. This is one of the factors that make cell-based therapeutics more challenging to produce at scale than protein-based therapeutics [23]. However, advancement of the regenerative medicine field relies on the ability to expand and differentiate stem cells into suitable candidates for therapy ex vivo, and consequently, bioprocess forces are unavoidable (Figures 1 and 2(c)).
Figure 2.
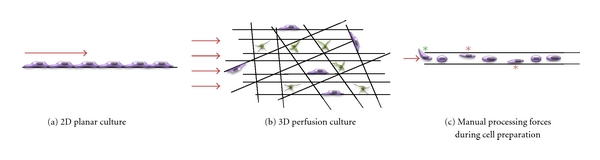
The application method and magnitude of shear stress can both impact on cell survival, proliferation and osteogenic differentiation. (a) Cells cultured in 2D and subjected to fluid flow shear stress, for example using parallel plate perfusion systems, are exposed to shear stress in one plane at the exposed surface of the cells and relatively high shear stress drives osteogenic differentiation. (b) In 3D perfusion culture, typically used when seeding cells throughout biomaterial scaffolds, cell dynamics are different: some cells are flattened and adhered firmly to the scaffold surface. These cells (shown in purple) experience shear stress at their exposed surface similar to those cultured in 2D culture. Other cells bridge between scaffold components and these cells (shown in green) experience shear stress as a 3D stimulus. Consequently, lower levels of shear stress than those required in 2D culture can drive osteogenic differentiation. (c) Bioprocess forces experienced during pipetting and capillary transfer of adherent cells in suspension provide non-uniform shear stress stimuli in the form of wall shear stress as the cells randomly hit the capillary walls during transport (red stars). Furthermore, shear stress and extensional forces upon entry to the capillary may provide further positive mechanical stimulus (green star). Red arrows indicate initial direction of fluid flow.
Bioprocess forces experienced during the isolation of primary cells from tissues for ex vivo expansion have to be considered carefully, as they may introduce a source of poorly characterized stimuli to the cells that can alter their phenotype even before the onset of expansion and/or differentiation. Harvesting of bone marrow requires physical removal from the marrow cavity, introducing low-level shear stress (due to the large diameter of the 11G biopsy needle ~3 mm) during capillary transfer. However, when obtaining adipose tissue as a source of adipose-derived stem cells, procedures often involve mechanically abrasive and high shear stress during suction of often-fibrous adipose tissue. For other tissue types, such as retrieval of dermal fibroblasts for the production of induced pluripotent cells, the forces encountered during retrieval of primary tissue are minimal, as tissue biopsy needles have a large diameter (~6 mm), and hence, the majority of cells do not experience mechanical force. However, when liberating these cells from the collagenous matrix, shear stresses are unavoidably encountered during mechanical digestion of ECM components.
Once isolated, routine passaging of cells subjects them to shear stress caused by pipetting, capillary transfer, and centrifugation [3]. The limited work conducted on adherent cells in suspension to date suggests that cells from different sources have differing capacities to withstand shear stress [3, 17–19, 22] and numerous physical properties are likely to impact such as cell size, density, membrane strength, and flexibility and surface characteristics such as cohesion [22]. Even functionally distinct subsets of a cell population from a single tissue can tolerate shear stress to different degrees [18].
In order to mimic the effect of manual pipetting under constant, reproducible flow rates, Veraitch et al. [3] used a capillary fluid flow device to determine the impact of shear stress on two murine embryonic stem cell lines. Repeated passes through the capillary at a flowrate of 0.80 mLs−1 did not cause a significant drop in viability, but a small decline in total cell number recovered was noted with increasing number of capillary passes [3]. This loss was attributed to surface adhesion to the syringe barrel and overall demonstrates the robustness of these cells.
However, the robustness of murine embryonic stem cells is not universally noted in other sources of adherent mammalian cells. Studies indicate that shear stress can be debilitating to adult primary cells including bone marrow-derived mesenchymal stem cells (MSCs) [24], muscle-derived multipotent precursor cells [18, 19], and vascular smooth muscle cells [17, 22]. Cultured rat aortic smooth muscle cells repeatedly exposed to (lamina) wall shear stress between 2–120 Nm−2 by passing back and forth through a narrow-gauge capillary underwent a decline in cell number with increasing number of capillary passes, indicating that cell damage took place during each capillary pass [17]. A large proportion of the cell population survived repeated exposure to shear stress, and this was postulated to be either due to the cells travelling along the centre of the capillary and hence not experiencing damaging levels of shear stress; or heterogeneity in membrane strength (burst tension) throughout the cell population. The optimum wall shear stress for cell survival was found to be 10–50 Nm−2, which is similar to that reported by others for low adherence leukaemic cells [15]. Subsequent studies also demonstrated cell loss at low shear stress (0.5, 1.0 Nm−2) during capillary transfer but this was not a result of cell disruption, rather cell attachment to the capillary wall at low flow rates [22]. This phenomenon mirrors that in vivo, where circulating leukocytes attach to vascular endothelium [25, 26].
The debilitating effects of flow-induced shear stress were also observed when murine MSCs were passed through needles of various bore sizes [24]. A direct relationship between the bore diameter of the needle, flow rate and cell trauma was evident. When needle bore diameter was decreased or flow rate was increased, cell viability, spreading, and proliferation were all seen to decrease [24]. Increased production of caspase-3 protein, an early indicator of apoptosis, was also reported. Subsequent studies of murine muscle-derived precursor cells exposed to high wall shear stress using the same capillary fluid flow system as Zoro et al. [17, 22] indicate that the effect of shear stresses may not be immediately observed, as viable cell recovery immediately following exposure to high capillary wall shear stress was not significantly affected [19]. However, after 24 hours, a significant reduction in cell viability was evident, similar to the reports of apoptosis reported for murine MSCs [24]. Interestingly, there seems to be differential ability of functionally distinct cell subsets isolated from a single tissue to withstand the effects of shear stress, based on our recent observation that rapidly adherent cells isolated from murine muscle are more susceptible to damage caused by capillary fluid flow-induced shear stress than late adherent cells from the same population [18].
Manual processing can introduce several sources of bioprocess forces (e.g., when detaching trypsinised cells from the culture dish and resuspending cell pellets) that are highly variable between operators and the impact of different forces on cells being prepared for therapy can potentially be of detriment to those cells. Automated approaches may overcome the variability and enable the bioprocess forces experienced by cells to be controlled to a greater degree, but still, these forces will remain. However, bioprocess forces do not necessarily have to be viewed as debilitating to cells and there is an emerging body of evidence suggesting that bioprocess forces that cells experience during capillary transfer can actually be beneficial in some contexts. They can, for example, be used to enhance osteogenic differentiation and synthetic activity in muscle-derived multipotent precursor cells [18, 19]. It has been observed using in vitro models that even short-term exposure of these multipotent precursor cells to fluid flow shear stress during capillary transfer upon passaging can enhance osteogenic differentiation beyond that induced by defined osteogenic medium alone and that functionally distinct subsets, isolated on the basis of differential adhesion capacity, have different intrinsic osteogenic potential [18].
2.3. Adherent Cells Attached to Surfaces
Understanding the impact of flow-induced shear stress on adherent cell responses provides useful information for production of cell-based therapies and tissue-engineered constructs. Seeding cells throughout 3D porous scaffolds using perfusion methods for even distribution exposes those cells to shear stress. Even low levels of shear stress (0.25–0.60 N·m−2) can interfere with attachment of anchorage-dependent mammalian cells to surfaces [27]. Exposing baby hamster kidney cells to shear stress using a flow chamber demonstrated that critical shear stress of 0.75–1.0 Nm−2 applied for 24 h resulted in decreased cell viability [28], and hence, careful control of perfusion rate is necessary.
Expanding adherent cells in perfusion bioreactors or on microcarriers in stirred bioreactors may potentially be advantageous for producing large quantities of cells in controlled environment, where the critical culture parameters can be monitored and controlled [29]. Hematopoietic stem cells (HSCs) were one of the first potential therapeutic stem-cell populations to be studied in such bioreactors and expansion is often carried out in stirred or suspension culture [30, 31]. MSCs, on the other hand, that are often explored for their potential use in tissue engineering for skeletal regeneration are mostly cultured on 3D scaffolds in perfusion bioreactors [29]. The main advantage of using continuous perfusion with fresh or recycled medium is that oxygen transport to the cells within the scaffold is increased [32]. A number of studies have confirmed that perfusion bioreactor culture for 3D tissue production facilitates uniform distribution of cells with MSC proliferation being achievable without loss of multilineage differentiation potential [33–37]. This is very encouraging, because being able to direct the expansion of cells as well as their differentiation within 3D scaffolds in vitro is crucial for successful tissue engineering. Furthermore, osteogenic induction of MSCs expanded and differentiated in 3-D scaffolds under perfusion flow conditions is enhanced compared to static culture [38–40] and this is, therefore, of benefit for production of mineralized tissue.
3. Fluid Flow Shear Stress Is a Bioprocess Force that Potentiates Intracellular Signaling and Osteogenic Responses
In vivo, bone tissue is continually exposed to mechanical stresses. The hard mineralized matrix protects cells against the full impact of load bearing. However, it is hypothesized that these macroscale forces lead to pressure differences on fluid contained within the uncalcified matrix immediately surrounding the mature bone cells (osteocytes) throughout the bone matrix [41, 42]. The pressure differences result in flow of interstitial fluid through the pores and channels in the bone [42], essentially converting the macroscale forces to microscale shear stresses that mechanically stimulate the osteocytes. It has been predicted that in vivo, a fluid shear stress of between 8–30 dyn/cm2 is experienced by bone cells as a result of interstitial fluid flow [43]. Communication networks that span from the marrow to the periosteum ensure coordinated activity of osteoblasts, osteoclasts, immature osteoprogenitors and bone marrow stromal cells to maintain the continual bone remodeling that occurs [41]. In vitro studies indicate that mature osteocytes are more sensitive to mechanical forces such as fluid flow shear stress than osteoblasts are, determined by more rapid and sustained production of prostaglandin E2 in response to mechanical stimulation [44, 45], and it has been hypothesized that in vivo, the osteocytes, therefore, are the main mechanosensitive cells, responding to fluid flow shear stresses by producing factors that regulate bone metabolism [44].
However, osteoblasts also respond to fluid flow shear stress in vitro and temporary exposure to fluid shear stress of 6 dyn/cm2 was shown to stimulate sustained release of nitric oxide, an osteoblast mitogen for up at least 12 hours following exposure [46]. Osteoblasts exposed to fluid flow shear stress experience enhanced phosphorylation of mitogen activated protein kinase (MAPK) intracellular signal transduction proteins, especially those in the extracellular signal-related kinase (ERK) pathway, which is also activated by mitogenic growth factors such as epithelial growth factor, insulin-like growth factor, and basic fibroblast growth factor [41, 47]. This physical mitogenic stimulus drives proliferation of osteoblasts in an ERK-dependent fashion [48–51]. Knockdown of either ERK-1 or ERK-2 resulted in complete abolition of shear stress-induced proliferation [49]. Fluid flow shear stress also induces proliferation via the PI3K/AKT signaling pathway via a focal adhesion kinase (FAK)/Shc-dependent mechanism upon activation of integrin receptors [52].
MAPK signaling also stimulates the expression of osteoblast-specific genes including early growth response-1, c-fos proto-oncogene, cyclooxygenase-2, type I collagen and osteopontin [53–58] downstream of integrin activation due to fluid shear stress. αvβ3- and β1-containing integrins are specifically reported to mediate mechanotransduction in osteoblasts exposed to shear stress [52, 55, 59] and their mechanotransduction activity is more readily sustained on fibronectin than on type I collagen or laminin [55]. Integrin-mediated mechanotransduction and gene expression in response to shear stress can be further augmented by additional signals, such as estrogen [60]. In addition to enhanced proliferation and osteogenic gene expression by osteoblasts in response to shear stress, their functional differentiation towards a mineralizing phenotype is also enhanced, as determined by increased or sustained alkaline phosphatase production [50], secretion of osteogenic proteins: osteopontin, osteocalcin, decorin, and type I collagen [61], and increased calcified matrix deposition [61]. The enhanced cellular responses of osteoblasts to shear stress indicate that this form of mechanical stimulation can provide appropriate cues for bone induction pathways and fluid flow shear stress is a much more potent cue for enhancing osteoblast regenerative responses than other forms of mechanical stimulation [62, 63].
In multipotent MSCs, the impact of shear stress on osteogenic differentiation has also been studied in some detail. It is an appropriate stimulus for the recruitment of MSCs from the bone marrow to sites of injury and enhances their osteogenic differentiation [64–66]. This information is very useful for tissue engineering, because it suggests that the kind of stresses created when dynamic culture methods necessary for 3D scaffolds are used can be beneficial, rather than inhibitory for osseoinduction.
Whilst the stimulatory effect of shear stress on osteoblast proliferation is well known [48–51], its impact on undifferentiated MSCs remains controversial. Conflicting reports have been made when studying shear stress in 2D culture with descriptions of either enhanced proliferation [64, 67], reduced proliferation and cell number decline [68, 69], or no net difference in overall cell number [70] in response to fluid flow shear stress compared to nonsheared controls. In the studies where increased proliferation was reported, the cell monolayer was exposed to oscillatory fluid flow of 1 Hz and a shear stress of 10 dyn/cm2 for 2 h, and proliferation was assessed after 24 h [64]. In studies where lack of cell growth was reported, shear stress was applied at constants levels of between 0.012–2.7 dyn/cm2, an order of magnitude lower [68–70]. It is difficult to determine what variables might impact on MSC proliferation as the form of application, magnitude and frequency of shear stress could have varying effects, so too could variability between the MSC populations used in different studies. For example, Kreke et al. [69] hypothesized that as MSCs are undifferentiated, cell loss could be due to apoptosis and/or detachment of an immature, nonosteogenic subset of cells that is unable to respond with appropriate mechanotransduction and osteogenic specification. This was further supported by their observation of elevated normalised alkaline phosphatase activity in the remaining cell population and after 20 days culture with exposure to fluid flow shear stress every 2nd day, very high mRNA levels for osteopontin and bone sialoprotein were detected, along with decreased mRNA levels of adipogenic marker lipoprotein lipase, suggesting that osteogenic selection had taken place [69]. In another study, decreased MSC proliferation upon exposure to uniform shear stress of 1.2 × 10−3 N/m2 (equivalent of 0.012 dyn/cm2) for 10 days was reported, but so too was significantly higher type I collagen levels and high intensity of von Kossa staining of mineralized matrix [68].
This notion of “osteogenic selection” of undifferentiated MSCs is not uncommon. In other studies characterizing the influence of titanium implant microtopography on differentiation of MSCs, a similar observation of osteogenic cell selection was made—whereas osteoblast proliferation was enhanced on microrough titanium compared to smooth polished surfaces [71], MSCs underwent a significant decline [72]. In spite of this decline, their ultimate ability to produce calcified matrix on microrough surfaces was greatly enhanced, suggesting that the physical environment selects MSCs that have osteogenic induction potential and is damaging to those that do not.
Shear stress has a pivotal role in regulating the transition from progenitor cell proliferation to osteogenic induction to maturation. It has been observed to directly stimulate and enhance expression of osteogenic genes including alkaline phosphatase, osteopontin, bone sialoprotein, and osteocalcin [69, 70, 73], and in parallel to elevated alkaline phosphatase levels, a decline in type I collagen gene expression has been reported [73] indicating that shear stress can invoke this transition away from matrix production towards calcification and mineralization. Decreased type I collagen expression was reported to be ERK-mediated, whereas the concurrent increase in alkaline phosphatase expression was dependent on p38 MAP kinase, activated in response to 12 dyn/cm2 fluid flow shear stress [73]. In an earlier study applying shear stress levels of 2.3 dyn/cm2, increased phosphorylation of p38 and ERK was evident within just 30 minutes after stimulation [74].
Mechanical stimulation of MSCs can enhance the activation of several matrix metalloproteases (MMPs) and their inhibitors, the tissue inhibitors of metalloproteases (TIMPs), which may further regulate osteogenic events. Specifically, collagenase activity associated with MMP2, MMP9, MMP13, and TIMP2 was elevated in MSCs in response to mechanical stimuli [75–77] and this was related to posttranslational activation, as mRNA expression levels were not affected by the mechanical signal [77]. MMP13 in particular is reported to be involved in osteogenic differentiation of MSCs [77]. The precise nature of osteogenesis enhancing effects that MMPs have on MSCs remains to be determined, but candidate mechanisms include exposure of cryptic binding domains by cleavage of ECM components [78, 79], release of matrix-bound bioactive molecules [78] and alterations in matrix architecture and assembly [78], which may facilitate cytoskeletal modifications that direct morphology changes during differentiation.
Certainly in 2D culture, it seems that shear stress has an overall net effect of directing differentiation of MSCs, preventing their proliferation and selecting cell subsets that have osteogenic potential from those that do not. Subsequent upregulation of osteogenic genes following MAP kinase-dependent mechanotransduction then results in enhanced calcified matrix production. However, tissue-engineering approaches for bone regeneration require cell seeding onto 3D scaffolds, and so, it is necessary to take caution when inferring observations from 2D fluid flow assays to scaffold-based approaches.
4. Activation of Mechanotransduction Pathways by Shear Stress
Integrin-mediated mechanotransduction is one of several mechanotransduction pathways that are widely known to promote osteogenic differentiation in response to mechanical stimuli such as fluid flow shear stress. Different ECM molecules are capable of supporting osteogenic differentiation of MSCs including type I collagen, fibronectin, laminin, and vitronectin [55, 80, 81], and cells attach to these ECM molecules via integrins. Fibronectin has the most potent ability to promote osteogenic differentiation [55], likely due to it is ability to activate α5β1 integrins, which are important for the osteogenic process [82]. Osteogenic responses are promoted not just by α5β1, but also via activation of αvβ3 and possibly other β1-containing integrins [52, 55, 59]. These integrins mediate cellular attachment to several different matrix components including fibronectin, type I collagen, laminin, and vitronectin. However, the reason that osteogenesis occurs most readily on fibronectin rather than on type I collagen or laminin under fluid shear stress [55] probably reflects the rapid and sustained activation of α5β1 and αvβ3 receptors by fibronectin. Shear stress upregulates α5β1 expression in MSCs used for production of vascular grafts [83] and β1 subunit is itself upregulated in osteoblasts by shear stress [50]. It is thought that this is an important event for differentiation and ultimately matrix mineralization not just in osteoblasts but also in MSCs, where α5β1 activation by fibronectin is an important regulator for osteogenic differentiation of MSCs both in 2D and 3D culture [82]. Furthermore, fibronectin fragments that specifically activated α5β1, rather than αvβ3, yielded greater osteogenic responses [82].
Focal adhesions at sites of high integrin clustering and activation mediate mechanotransduction pathways downstream of extracellular outside-in signals. FAK is a nonreceptor tyrosine kinase that is essential for mechanotransduction from the focal adhesion and if FAK signal transduction in osteoblasts is blocked, osteogenesis mediated by fluid flow shear stress is attenuated [84]. FAK is also essential for ECM-directed osteogenic differentiation of MSCs [85], indicating the general importance of mechanical activation of integrins and FAK during osteogenic events.
Osteogenic differentiation and maturation in response to mechanical stimulation by fluid shear stress is also dependent on downstream activation of ERK1 and ERK2 proteins. Several different convergent signal transduction mechanisms result in phosphorylation of ERK proteins upon mechanical stimulation, and integrin signaling represents one such mechanism. Shear stress-induced ERK activation can result from upstream coupling of β1 integrin subunit with the Shc adaptor protein [50, 55] and ERK phosphorylation subsequently leads to transcriptional activation of osteogenic genes including the potent osteogenic transcription factor RUNX2 [81, 85].
A second key pathway that promotes ERK-mediated osteogenic differentiation in response to fluid flow shear stress is Ca2+ signaling [86]. Ca2+ signaling enhances proliferation of MSCs in response to fluid flow [67], leading to the activation of AP-1 transcription factors and consequently expression of important osteogenic genes encoding type I collagen, RUNX2 and osteocalcin [87–89], ultimately promoting mineralized matrix formation [87]. Activation of ERK downstream of Ca2+ signaling is mediated by calmodulin-dependent protein kinase [87] and protein kinase C [89]. Nitric oxide is also produced in response to Ca2+-dependent upregulation of nitric oxide synthase activity in MSCs [90]. Therefore, the reported increase in nitric oxide when cells are exposed to shear stress [46] and during osteogenic differentiation [91] is likely a downstream event of cellular influx of Ca2+. Nitric oxide itself can then participate in osteogenic mechanotransduction by enhancing the phosphorylation of ERK proteins [92].
The intracellular responses of MSCs to shear stress also includes structural changes to the cytoskeleton [93, 94] important for osteogenic differentiation to take place [95]. Recent studies indicate that Rho-mediated contraction of the actin cytoskeleton seems to be critical for osteogenic differentiation of MSCs and further enhances RUNX2 expression [96, 97]. Overexpression studies showed that RhoA signaling via its effector protein Rho-associated kinase (ROCKII) significantly upregulated osteogenic differentiation in response to oscillatory fluid flow whilst suppressing chondrogenic and adipogenic induction [96]. However, in wild-type controls oscillatory fluid flow was found to concurrently and indiscriminately enhance expression of trilineage differentiation markers Runx2, Sox9, and PPARγ, markers of osteogenic, chondrogenic, and adipogenic differentiation, respectively [96].
5. Bioreactors for Bone Tissue Engineering: Impact of Shear Stress
Bone tissue engineering is an area of considerable interest, as it would enable scalable production of autologous bone material for regeneration within critical-sized skeletal defects that do not regenerate well using current strategies such as external fixing devices or implantable permanent materials [98]. It would also eliminate the need to obtain sizeable bone grafts from the patient, as MSCs isolated from the bone marrow or even from more easily accessible sources of multipotent cells such as adipose tissue could be expanded to the required cell number and differentiated into appropriate osteoblast cells. Researchers in the field are already capable of producing tissue-engineered bone in appropriate clinical quantities [99, 100] and desired anatomical shape to human bones [101]. One major challenge, however, is that producing 3D tissues such as bone is not achievable using conventional cell culture methods. This is because during static culture of cell-seeded scaffolds, nutrient gradients develop due to a lack of active nutrient transport to the core of the tissue. Therefore, cells at the surface of the scaffold receive adequate nutritional support, but this is at the growing expense of cells located progressively closer the center of the scaffold. Oxygen in the culture medium that is delivered via passive diffusion can only support the 150–200 μm thick outermost layer of cells in a scaffold [102, 103]. Consequently, when nutrient and oxygen levels decline below a minimum threshold necessary for cell survival, the cells die [32].
Development of bioreactors that facilitate even distribution of nutrients and oxygen to cells throughout a 3D scaffold using active transport methods have taken considerable steps to addressing this fundamental challenge. Several types of bioreactor have been extensively tested for potential use in bone tissue engineering: spinner flasks, rotating wall, and perfusion systems (for an excellent detailed review, see [104]). These dynamic bioreactors, particularly perfusion bioreactors, have successfully been used to enhance cell-seeding efficiency and uniformity [36, 105–109] and facilitate proliferation throughout the entire 3D structure [34–36, 109–113]. Importantly, for bone tissue engineering strategies, flow perfusion bioreactors have been successfully demonstrated to promote osteogenic differentiation and mineralization on scaffolds [38, 40, 109, 112–116] even in the absence of dexamethasone [117]. Flow perfusion systems also seem to be superior to spinner flasks and rotating wall bioreactors, as they result in more homogeneous mineralized matrix formation throughout the scaffold when compared to spinner flasks [106, 114] and spinner flasks themselves induce greater osteogenic marker expression than rotating wall bioreactors [111, 118]. These observations of enhanced osteogenic responses in 3D perfusion bioreactors are consistent when using stem cells derived from adipose tissue [119] as well as bone marrow [120].
The responses of cells in 3D scaffolds to fluid flow shear stress are dose-dependent [33, 38, 105, 112, 115, 121–123] and it seems that a critical shear stress level exists, after which point cell detachment and death occur [105, 122]. It is particularly interesting to note that in two separate studies, application of a low medium perfusion rate was stimulatory to proliferation compared to higher perfusion rate, whereas expression of osteogenic differentiation markers was elevated at the higher perfusion rate relative to low perfusion [33, 123]. This indicates that a mechanical “switch” likely exists, whereby mechanotransduction pathways due to mild fluid flow shear stress promote proliferation and increasing levels of stress beyond a given threshold promotes differentiation at the expense of proliferation and self-renewal. This is an important consideration, because ensuring that the appropriate cell number for functional bone formation is achieved before initiating the program of osteogenic differentiation is essential for creating the gold standard in tissue engineered bone. According to studies by Zhao et al. [33] and Cartmell et al. [123], maintaining shear stress at a magnitude that is stimulatory to proliferation will achieve the required cell number and subsequently raising shear stress above the threshold that leads to differentiation will drive the osteogenic response. With this in mind, an even greater understanding of the biophysical environment in which MSCs reside in vivo and the subsequent biophysical changes that take place during the different stages of bone healing could provide useful indications of the change in mechanical forces such as shear stress that required for enhanceing the proliferation and subsequent differentiation and maturation phases of osteoblast development.
A final important consideration for osteogenic induction on 3D scaffolds in perfusion bioreactors is that mathematical models of the effects of shear stress on cell behavior are mostly based on 2D culture conditions using cells in monolayer, where the effective parameters and outcomes can be easily drawn. However, cells adhered to the surface of 3D scaffolds are variable in their adhesion status (Figure 2(b)), such as in the form of bridging (occupying a position in 3D between several scaffold components) or flattened (firmly adhered to a single section of scaffold in 2D) [124]. Under flow perfusion culture these two different cell arrangements may differentially sense the fluid flow shear force. Cells bridging the scaffolds sense shear force with greater sensitivity than those occupying a flattened 2D morphology as the flow is more perpendicular to the cytoskeleton of the former cells [125]. Therefore, the mechanical force generated on the cells and the shear stress that is required for directing osteogenic cellular processes is lower in the 3D cultured cells than in 2D culture. For example, studies focused on osteogenesis in 3D culture have employed fluid shear stress in the range of 0.01~0.05 Pa [33, 94, 115, 126], which is far lower than the stress ranges (0.1~1 Pa) that are effective for osteoinduction in 2D culture [69, 96, 127]. It is also presumed that the flattened subset of cells in 3D perfusion culture corresponds to those under 2D laminar flow. As a result, during flow perfusion culture, adhesion, proliferation and differentiation of cells present in 3D scaffolds are considered to be more variable and complicated than those in 2D culture under laminar flow.
6. Conclusions
Regenerative medicine strategies for treatment of difficult-to-heal critical sized bone defects are a realistic goal and tissue engineering approaches that incorporate regenerative cell populations and appropriate biocompatible scaffold materials are an exciting prospect for successful widespread treatment of patients in the very near future. Bioreactors that utilize perfusion culture systems are necessary to overcome the limitations of standard cell-culture practice by producing viable and structurally suitable tissues that have uniform distribution of essential oxygen and nutrients throughout the full depth of the tissue. Many studies have shown that shear stresses inherent in perfusion culture are extremely beneficial for enhancing osteogenic differentiation and improving resulting matrix mineralization by activating appropriate mechanotransduction pathways that may regulate osteogenesis in vivo. It is also evident that even when adult multipotent cells in suspension are exposed to fluid flow shear stresses similar to those that cells experience during routine bioprocessing, osteogenic induction in those cells is significantly enhanced. Therefore, shear stress can be a powerful tool for enhancing cell differentiation that will benefit many patients requiring cell therapy or tissue engineering for bone regeneration.
Acknowledgments
This work was supported by WCU (World Class University) Program (Grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science, and Technology, Republic of Korea.
References
- 1.Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3(4):369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Mason C, Hoare M. Regenerative medicine bioprocessing: building a conceptual framework based on early studies. Tissue Engineering. 2007;13(2):301–311. doi: 10.1089/ten.2006.0177. [DOI] [PubMed] [Google Scholar]
- 3.Veraitch FS, Scott R, Wong JW, Lye GJ, Mason C. The impact of manual processing on the expansion and directed differentiation of embryonic stem cells. Biotechnology and Bioengineering. 2008;99(5):1216–1229. doi: 10.1002/bit.21673. [DOI] [PubMed] [Google Scholar]
- 4.Chisti Y. Animal-cell damage in sparged bioreactors. Trends in Biotechnology. 2000;18(10):420–432. doi: 10.1016/s0167-7799(00)01474-8. [DOI] [PubMed] [Google Scholar]
- 5.van der Pol L, Tramper J. Shear sensitivity of animal cells from a culture-medium perspective. Trends in Biotechnology. 1998;16(8):323–328. doi: 10.1016/s0167-7799(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 6.Gregoriades N, Clay J, Ma N, Koelling K, Chalmers JJ. Cell damage of microcarrier cultures as a function of local energy dissipation created by a rapid extensional flow. Biotechnology and Bioengineering. 2000;69(2):171–182. doi: 10.1002/(sici)1097-0290(20000720)69:2<171::aid-bit6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Chisti Y. Hydrodynamic damage to animal cells. Critical Reviews in Biotechnology. 2001;21(2):67–110. doi: 10.1080/20013891081692. [DOI] [PubMed] [Google Scholar]
- 8.Lütkemeyer D, Ameskamp N, Tebbe H, Wittler J, Lehmann J. Estimation of cell damage in bench- and pilot-scale affinity expanded- bed chromatography for the purification of monoclonal antibodies. Biotechnology and Bioengineering. 1999;65(1):114–119. doi: 10.1002/(sici)1097-0290(19991005)65:1<114::aid-bit14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Born C, Zhang Z, Al-Rubeai M, Thomas CR. Estimation of disruption of animal cells by laminar shear stress. Biotechnology and Bioengineering. 1992;40(9):1004–1010. doi: 10.1002/bit.260400903. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Briones MA, Chalmers JJ. Flow parameters associated with hydrodynamic cell injury. Biotechnology and Bioengineering. 1994;44(9):1089–1098. doi: 10.1002/bit.260440910. [DOI] [PubMed] [Google Scholar]
- 11.Kretzmer G, Schugerl K. Response of mammalian cells to shear stress. Applied Microbiology and Biotechnology. 1991;34(5):613–616. doi: 10.1007/BF00167909. [DOI] [PubMed] [Google Scholar]
- 12.Prokop A, Bajpai RK. The sensitivity of biocatalysts to hydrodynamic shear stress. Advances in Applied Microbiology. 1992;37:165–232. [Google Scholar]
- 13.Al-Rubeai M, Singh RP, Goldman MH, Emery AN. Death mechanisms of animal cells in conditions of intensive agitation. Biotechnology and Bioengineering. 1995;45(6):463–472. doi: 10.1002/bit.260450602. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rubeai M, Singh RP, Emery AN, Zhang Z. Cell cycle and cell size dependence of susceptibility of hydrodynamic forces. Biotechnology and Bioengineering. 1995;46(1):88–92. doi: 10.1002/bit.260460112. [DOI] [PubMed] [Google Scholar]
- 15.Mardikar SH, Niranjan K. Observations on the shear damage to different animal cells in a concentric cylinder viscometer. Biotechnology and Bioengineering. 2000;68(6):697–704. doi: 10.1002/(sici)1097-0290(20000620)68:6<697::aid-bit14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Carswell KS, Papoutsakis ET. Culture of human T cells in stirred bioreactors for cellular immunotherapy applications: shear, proliferation, and the IL-2 receptor. Biotechnology and Bioengineering. 2000;68(3):328–338. doi: 10.1002/(sici)1097-0290(20000505)68:3<328::aid-bit11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Zoro BJ, Owen S, Drake RAL, Hoare M. The impact of process stress on suspended anchorage-dependent mammalian cells as an indicator of likely challenges for regenerative medicines. Biotechnology and Bioengineering. 2008;99(2):468–474. doi: 10.1002/bit.21544. [DOI] [PubMed] [Google Scholar]
- 18.Patel M, et al. Muscle-derived precursor cells isolated on the basis of differential adhesion properties respond differently to capillary flow. Biotechnology Letters. 2011;33(7):1481–1486. doi: 10.1007/s10529-011-0570-3. [DOI] [PubMed] [Google Scholar]
- 19.Mulhall H, et al. Effect of capillaryshear stress on recovery and osteogenic differentiation of muscle-derived precursor cell populations. Journal of Tissue Engineering and Regenerative Medicine. 2011;5(8):629–635. doi: 10.1002/term.355. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Chisti Y, Moo-young M. Effects of the hydrodynamic environment and shear protectants on survival of erythrocytes in suspension. Journal of Biotechnology. 1995;43(1):33–40. doi: 10.1016/0168-1656(95)00111-8. [DOI] [PubMed] [Google Scholar]
- 21.Schid-Schonbien GW. Rheology of leukocytes. In: Skalak R, Chien S, editors. Handbook of Bioengineering. New York, NY, USA: McGraw-Hill; 1987. pp. 13.1–13.25. [Google Scholar]
- 22.Zoro BJ, Owen S, Drake RAL, Mason C, Hoare M. Regenerative medicine bioprocessing: concentration and behavior of adherent cell suspensions and pastes. Biotechnology and Bioengineering. 2009;103(6):1236–1247. doi: 10.1002/bit.22356. [DOI] [PubMed] [Google Scholar]
- 23.Mason C, Dunnill P. Translational regenerative medicine research: essential to discovery and outcome. Regenerative Medicine. 2007;2(3):227–229. doi: 10.2217/17460751.2.3.227. [DOI] [PubMed] [Google Scholar]
- 24.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regenerative Medicine. 2009;4(1):49–64. doi: 10.2217/17460751.4.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 26.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Molecular Immunology. 2005;42(7):799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 27.Olivier LA, Truskey GA. A numerical analysis of forces exerted by laminar flow on spreading cells in a parallel plate flow chamber assay. Biotechnology and Bioengineering. 1993;42(8):963–973. doi: 10.1002/bit.260420807. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig A, Kretzmer G, Schugerl K. Determination of a “critical shear stress level“ applied to adherent mammalian cells. Enzyme and Microbial Technology. 1992;14(3):209–213. doi: 10.1016/0141-0229(92)90068-y. [DOI] [PubMed] [Google Scholar]
- 29.King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Current Opinion in Chemical Biology. 2007;11(4):394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Liu Q, Cai H, Tan WS. A comparative gene-expression analysis of CD34+ hematopoietic stem and progenitor cells grown in static and stirred culture systems. Cellular and Molecular Biology Letters. 2006;11(4):475–487. doi: 10.2478/s11658-006-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Liu T, Fan X, Ma X, Cui Z. Ex vivo expansion of hematopoietic stem cells derived from umbilical cord blood in rotating wall vessel. Journal of Biotechnology. 2006;124(3):592–601. doi: 10.1016/j.jbiotec.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Volkmer E, Drosse I, Otto S, et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Engineering Part A. 2008;14(8):1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 33.Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnology and Bioengineering. 2007;96(3):584–595. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y, Hardouin P, Zhu Z, Tang T, Dai K, Lu J. Three-dimensional flow perfusion culture system for stem cell proliferation inside the critical-size β-tricalcium phosphate scaffold. Tissue Engineering. 2006;12(12):3535–3543. doi: 10.1089/ten.2006.12.3535. [DOI] [PubMed] [Google Scholar]
- 35.Zhao F, Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnology and Bioengineering. 2005;91(4):482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T. Effects of oxygen transport on 3-D human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnology Progress. 2005;21(4):1269–1280. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Xu H, Wan C, McCaigue M, Li G. Bioreactor expansion of human adult bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(9):2052–2059. doi: 10.1634/stemcells.2005-0591. [DOI] [PubMed] [Google Scholar]
- 38.Bancroft GN, Sikavitsas VI, Van Den Dolder J, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braccini A, Wendt D, Jaquiery C, et al. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23(8):1066–1072. doi: 10.1634/stemcells.2005-0002. [DOI] [PubMed] [Google Scholar]
- 40.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. Journal of Biomedical Materials Research—Part A. 2003;67(1):87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 41.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and Bone Mechanotransduction. Biomaterials. 2001;22(19):2581–2593. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 42.Burger EH, Klein-Nulend J. Mechanotransduction in bone—role of the lacunocanalicular network. FASEB Journal. 1999;13(8):S101–S112. [PubMed] [Google Scholar]
- 43.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of Biomechanics. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 44.Klein-Nulend J, Van der Plas A, Semeins CM, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB Journal. 1995;9(5):441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 45.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton-dependent process. Biochemical and Biophysical Research Communications. 1996;225(1):62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 46.Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. American Journal of Physiology. 1996;271(1, part 1):E205–E208. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- 47.Ogata T. Fluid flow-induced tyrosine phosphorylation and participation of growth factor signaling pathway in osteoblast-like cells. Journal of Cellular Biochemistry. 2000;76(4):529–538. [PubMed] [Google Scholar]
- 48.Kapur S, Mohan S, Baylink DJ, Lau KHW. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. Journal of Biological Chemistry. 2005;280(20):20163–20170. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- 49.Kapur S, Chen ST, Baylink DJ, Lau KHW. Extracellular signal-regulated kinase-1 and -2 are both essential for the shear stress-induced human osteoblast proliferation. Bone. 2004;35(2):525–534. doi: 10.1016/j.bone.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32(3):241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- 51.Jiang GL, White CR, Stevens HY, Frangos JA. Temporal gradients in shear stimulate osteoblastic proliferation via ERK1/2 and retinoblastoma protein. American Journal of Physiology—Endocrinology and Metabolism. 2002;283(2):E383–E389. doi: 10.1152/ajpendo.00547.2001. [DOI] [PubMed] [Google Scholar]
- 52.Lee DY, Li YSJ, Chang SF, et al. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by αvβ3 and β1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. Journal of Biological Chemistry. 2010;285(1):30–42. doi: 10.1074/jbc.M109.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H, Wu C, Dai K, Chang J, Tang T. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials. 2006;27(33):5651–5657. doi: 10.1016/j.biomaterials.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 54.You J, Reilly GC, Zhen X, et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. Journal of Biological Chemistry. 2001;276(16):13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 55.Lee DY, Yeh C-R, Chang S-F, et al. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. Journal of Bone and Mineral Research. 2008;23(7):1140–1149. doi: 10.1359/jbmr.080302. [DOI] [PubMed] [Google Scholar]
- 56.González O, Fong KD, Trindade MCD, Warren SM, Longaker MT, Smith RL. Fluid shear stress magnitude, duration, and total applied load regulate gene expression and nitric oxide production in primary calvarial osteoblast cultures. Plastic and Reconstructive Surgery. 2008;122(2):419–428. doi: 10.1097/PRS.0b013e31817d5ff1. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka SM, Sun HB, Roeder RK, Burr DB, Turner CH, Yokota H. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcified Tissue International. 2005;76(4):261–271. doi: 10.1007/s00223-004-0238-2. [DOI] [PubMed] [Google Scholar]
- 58.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. Journal of Biomechanical Engineering. 2000;122(4):387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 59.Weyts FA, Li YS, Van Leeuwen J, Weinans H, Chien S. ERK activation and α v β 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. Journal of Cellular Biochemistry. 2002;87(1):85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- 60.Yeh CR, Chiu J-J, Lee C-I, et al. Estrogen augments shear stress-induced signaling and gene expression in osteoblast-like cells via estrogen receptor-mediated expression of β1-integrin. Journal of Bone and Mineral Research. 2010;25(3):627–639. doi: 10.1359/jbmr.091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fassina L, Visai L, Asti L, et al. Calcified matrix production by SAOS-2 cells inside a polyurethane porous scaffold, using a perfusion bioreactor. Tissue Engineering. 2005;11(5-6):685–700. doi: 10.1089/ten.2005.11.685. [DOI] [PubMed] [Google Scholar]
- 62.Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. American Journal of Physiology—Endocrinology and Metabolism. 1997;273(4):E751–E758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- 63.Owan I, Burr DB, Turner CH, et al. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. American Journal of Physiology—Cell Physiology. 1997;273(3):C810–C815. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- 64.Li YJ, Batra NN, You L, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. Journal of Orthopaedic Research. 2004;22(6):1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomechanics and Modeling in Mechanobiology. 2010;9(6):659–670. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 66.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Annals of Biomedical Engineering. 2008;36(12):1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 67.Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. American Journal of Physiology—Cell Physiology. 2006;290(3):C776–C784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- 68.Scaglione S, Wendt D, Miggino S, et al. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2D model system. Journal of Biomedical Materials Research—Part A. 2008;86(2):411–419. doi: 10.1002/jbm.a.31607. [DOI] [PubMed] [Google Scholar]
- 69.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Kreke MR, Goldstein AS. Hydrodynamic shear stimulates osteocalcin expression but not proliferation of bone marrow stromal cells. Tissue Engineering. 2004;10(5-6):780–788. doi: 10.1089/1076327041348455. [DOI] [PubMed] [Google Scholar]
- 71.Brett PM, Harle J, Salih V, et al. Roughness response genes in osteoblasts. Bone. 2004;35(1):124–133. doi: 10.1016/j.bone.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Wall I, Donos N, Carlqvist K, Jones F, Brett P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009;45(1):17–26. doi: 10.1016/j.bone.2009.03.662. [DOI] [PubMed] [Google Scholar]
- 73.Grellier M, Bareille R, Bourget C, Amédée J. Responsiveness of human bone marrow stromal cells to shear stress. Journal of Tissue Engineering and Regenerative Medicine. 2009;3(4):302–309. doi: 10.1002/term.166. [DOI] [PubMed] [Google Scholar]
- 74.Kreke MR, Sharp LA, Woo Lee Y, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Engineering—Part A. 2008;14(4):529–537. doi: 10.1089/tea.2007.0068. [DOI] [PubMed] [Google Scholar]
- 75.Charoonpatrapong-Panyayong K, Shah R, Yang J, et al. Nmp4/CIZ contributes to fluid shear stress induced MMP-13 gene induction in osteoblasts. Journal of Cellular Biochemistry. 2007;102(5):1202–1213. doi: 10.1002/jcb.21349. [DOI] [PubMed] [Google Scholar]
- 76.Chen YJ, Huang CH, Lee IC, Lee YT, Chen MH, Young TH. Effects of cyclic mechanical stretching on the mRNA expression of tendon/ ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connective Tissue Research. 2008;49(1):7–14. doi: 10.1080/03008200701818561. [DOI] [PubMed] [Google Scholar]
- 77.Kasper G, Glaeser JD, Geissler S, et al. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells. 2007;25(8):1985–1994. doi: 10.1634/stemcells.2006-0676. [DOI] [PubMed] [Google Scholar]
- 78.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current Opinion in Cell Biology. 2004;16(5):558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parikka V, Väänänen A, Risteli J, et al. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biology. 2005;24(6):438–447. doi: 10.1016/j.matbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Klees RF, Salasznyk RM, Vandenberg S, Bennett K, Plopper GE. Laminin-5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biology. 2007;26(2):106–114. doi: 10.1016/j.matbio.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Communication and Adhesion. 2004;11(5-6):137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 82.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30(6):1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McIlhenny SE, Hager ES, Grabo DJ, et al. Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by upregulation of the α5β 1 integrin. Tissue Engineering—Part A. 2010;16(1):245–255. doi: 10.1089/ten.tea.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young SRL, Gerard-O'Riley R, Kim J-B, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. Journal of Bone and Mineral Research. 2009;24(3):411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Experimental Cell Research. 2007;313(1):22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu D, Genetos DC, Shao Y, et al. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42(4):644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin MK, Kim MK, Bae YS, et al. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cellular Signalling. 2008;20(4):613–624. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Haasper C, Jagodzinski M, Drescher M, et al. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Experimental and Toxicologic Pathology. 2008;59(6):355–363. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochemical and Biophysical Research Communications. 2005;328(3):751–755. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 90.Foreman MA, Gu Y, Howl JD, Jones S, Publicover SJ. Group III metabotropic glutamate receptor activation inhibits Ca2+ influx and nitric oxide synthase activity in bone marrow stromal cells. Journal of Cellular Physiology. 2005;204(2):704–713. doi: 10.1002/jcp.20353. [DOI] [PubMed] [Google Scholar]
- 91.Orciani M, Trubiani O, Vignini A, Mattioli-Belmonte M, Di Primio R, Salvolini E. Nitric oxide production during the osteogenic differentiation of human periodontal ligament mesenchymal stem cells. Acta Histochemica. 2009;111(1):15–24. doi: 10.1016/j.acthis.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Rangaswami H, Marathe N, Zhuang S, et al. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. Journal of Biological Chemistry. 2009;284(22):14796–14808. doi: 10.1074/jbc.M806486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu Q, Wu C, Shen Y, Zheng S, Chen R. Effect of LIMK2 RNAi on reorganization of the actin cytoskeleton in osteoblasts induced by fluid shear stress. Journal of Biomechanics. 2008;41(15):3225–3228. doi: 10.1016/j.jbiomech.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Jaasma MJ, O’Brien FJ. Mechanical stimulation of osteoblasts using steady and dynamic fluid flow. Tissue Engineering—Part A. 2008;14(7):1213–1223. doi: 10.1089/ten.tea.2007.0321. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez JP, González M, Ríos S, Cambiazo V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. Journal of Cellular Biochemistry. 2004;93(4):721–731. doi: 10.1002/jcb.20234. [DOI] [PubMed] [Google Scholar]
- 96.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of Cell Science. 2009;122, part 4:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circulation Research. 2008;103(3):234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Advances in Biochemical Engineering/Biotechnology. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 99.Janssen FW, Oostra J, Van Oorschot A, Van Blitterswijk CA. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials. 2006;27(3):315–323. doi: 10.1016/j.biomaterials.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 100.Janssen FW, Van Dijkhuizen-Radersma R, Van Oorschot A, Oostra J, De Bruijn JD, Van Blitterswijk CA. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. Journal of Tissue Engineering and Regenerative Medicine. 2010;4(1):12–24. doi: 10.1002/term.197. [DOI] [PubMed] [Google Scholar]
- 101.Grayson WL, Fröhlich M, Yeager K, et al. Engineering anatomically shaped human bone grafts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Folkman JH, Hochberg M. Self regulation of growth in three dimensions. Journal of Experimental Medicine. 1973;138(4):745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colton CK. Implantable biohybrid artificial organs. Cell Transplantation. 1995;4(4):415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 104.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2010;4(2):171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 105.Alvarez-Barreto JF, Linehan SM, Shambaugh RL, Sikavitsas VI. Flow perfusion improves seeding of tissue engineering scaffolds with different architectures. Annals of Biomedical Engineering. 2007;35(3):429–442. doi: 10.1007/s10439-006-9244-z. [DOI] [PubMed] [Google Scholar]
- 106.Wendt D, Marsano A, Jakob M, Heberer M, Martin I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnology and Bioengineering. 2003;84(2):205–214. doi: 10.1002/bit.10759. [DOI] [PubMed] [Google Scholar]
- 107.Alvarez-Barreto JF, Sikavitsas VI. Improved mesenchymal stem cell seeding on RGD-modified poly(L-lactic acid) scaffolds using flow perfusion. Macromolecular Bioscience. 2007;7(5):579–588. doi: 10.1002/mabi.200600280. [DOI] [PubMed] [Google Scholar]
- 108.Wendt D, Stroebel S, Jakob M, John GT, Martin I. Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions. Biorheology. 2006;43(3-4):481–488. [PubMed] [Google Scholar]
- 109.Yang J, Cao C, Wang W, et al. Proliferation and osteogenesis of immortalized bone marrow-derived mesenchymal stem cells in porous polylactic glycolic acid scaffolds under perfusion culture. Journal of Biomedical Materials Research—Part A. 2010;92(3):817–829. doi: 10.1002/jbm.a.32378. [DOI] [PubMed] [Google Scholar]
- 110.Stiehler M, Bünger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. Journal of Biomedical Materials Research—Part A. 2009;89(1):96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- 111.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. Journal of Biomedical Materials Research. 2002;62(1):136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 112.Grayson WL, Bhumiratana S, Cannizzaro C, et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Engineering—Part A. 2008;14(11):1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MAK, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Annals of Biomedical Engineering. 2005;33(1):63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 114.Meinel L, Karageorgiou V, Fajardo R, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Annals of Biomedical Engineering. 2004;32(1):112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 115.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11203–11208. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. Journal of Biomedical Materials Research—Part A. 2005;72(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 118.Wang TW, Wu HC, Wang HY, Lin FH, Sun JS. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. Journal of Biomedical Materials Research—Part A. 2009;88(4):935–946. doi: 10.1002/jbm.a.31914. [DOI] [PubMed] [Google Scholar]
- 119.Oh CH, Hong SJ, Jeong I, Yu HS, Jegal SH, Kim HW. Development of robotic dispensed bioactive scaffolds and human adipose-derived stem cell culturing for bone tissue engineering. Tissue Engineering—Part C: Methods. 2010;16(4):561–571. doi: 10.1089/ten.TEC.2009.0274. [DOI] [PubMed] [Google Scholar]
- 120.Fröhlich M, Grayson WL, Marolt D, Gimble JM, Kregar-Velikonja N, Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Engineering—Part A. 2010;16(1):179–189. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li D, Tang T, Lu J, Dai K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Engineering—Part A. 2009;15(10):2773–2783. doi: 10.1089/ten.TEA.2008.0540. [DOI] [PubMed] [Google Scholar]
- 122.Ichinohe N, Takamoto T, Tabata Y. Proliferation, osteogenic differentiation, and distribution of rat bone marrow stromal cells in nonwoven fabrics by different culture methods. Tissue Engineering—Part A. 2008;14(1):107–116. doi: 10.1089/ten.a.2007.0021. [DOI] [PubMed] [Google Scholar]
- 123.Cartmell SH, Porter BD, García AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Engineering. 2003;9(6):1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 124.Annaz B, Hing KA, Kayser M, Buckland T, Di Silvio L. Porosity variation in hydroxyapatite and osteoblast morphology: a scanning electron microscopy study. Journal of Microscopy. 2004;215, part 1:100–110. doi: 10.1111/j.0022-2720.2004.01354.x. [DOI] [PubMed] [Google Scholar]
- 125.McCoy RJ, O’Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Engineering—Part B: Reviews. 2010;16(6):587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- 126.Plunkett NA, Partap S, O’Brien FJ. Osteoblast response to rest periods during bioreactor culture of collagen-glycosaminoglycan scaffolds. Tissue Engineering—Part A. 2010;16(3):943–951. doi: 10.1089/ten.TEA.2009.0345. [DOI] [PubMed] [Google Scholar]
- 127.Sharp LA, Lee YW, Goldstein AS. Effect of low-frequency pulsatile flow on expression of osteoblastic genes by bone marrow stromal cells. Annals of Biomedical Engineering. 2009;37(3):445–453. doi: 10.1007/s10439-008-9632-7. [DOI] [PubMed] [Google Scholar]


