Abstract
Objective
To examine the reciprocal associations between depressive symptoms and clinical definitions of the metabolic syndrome in childhood and adulthood.
Design
Population-based prospective cohort study of 921 participants (538 women and 383 men) in Finland. The components of the metabolic syndrome were measured in childhood (mean age 12 years) and again in adulthood (mean age 33 years). A revised version of the Beck Depression Inventory was used to assess depressive symptoms at the mean ages of 24 and 33.
Main Outcome Measures
Metabolic syndrome defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP), the European Group for the Study of Insulin Resistance, and the International Diabetes Federation criteria.
Results
In women, depressive symptoms were associated with increased risk of the metabolic syndrome in adulthood (odds ratio for NCEP metabolic syndrome per 1 SD increase in depressive symptoms 1.40, 95% confidence interval 1.05-1.85). The metabolic syndrome in childhood, in turn, predicted higher levels of depressive symptoms in adulthood (p= 0.03). In men, no associations were found between depressive symptoms and the clinical definitions of the metabolic syndrome.
Conclusion
The process linking depressive symptoms with the metabolic syndrome may go into both directions and may begin early in life.
Keywords: metabolic syndrome, depressive symptoms, obesity, cardiovascular disease, childhood
The association of depression with cardiovascular disease is well-established (Musselman, Evans, & Nemeroff, 1998; Wulsin & Singal, 2003). However, the mechanisms that account for this association remain unclear (Nicholson, Kuper, & Hemingway, 2006). It has been suggested that depression may be linked to cardiovascular disease through metabolic disturbances that constitute the metabolic syndrome (Kinder, Kamarck, Baum, & Orchard, 2002; Nicholson et al., 2006). The metabolic syndrome is a risk factor for cardiovascular disease and mortality (Lakka et al., 2002; Ninomiya et al., 2003), consisting of the clustering of several cardiovascular risk factors, such as central obesity, glucose intolerance, insulin resistance, hypertension and dyslipidemia, within one individual (Grundy et al., 2005; Reaven, 1988).
Studies on the relationship between depression and the metabolic syndrome are mostly cross-sectional or limited to restricted groups, such as middle-aged or clinical populations. Prospective evidence in young and healthy populations not selected to be at high cardiovascular risk is scarce. In middle-aged women it has been found that high levels of depression predicted the risk for developing the metabolic syndrome over 7.4 years (Räikkönen, Matthews, & Kuller, 2002). In the Third National Health and Nutrition Examination Survey, young adult women with a history of at least one major depressive episode were twice as likely to have the metabolic syndrome as those with no history of depression, whereas no such relationship existed in men (Kinder, Carnethon, Palaniappan, King, & Fortmann, 2004). In the Northern Finland 1966 Birth Cohort Study, no cross-sectional relation was found between depression and the metabolic syndrome, although depression was directly related with a large waist circumference in the same study (Herva et al., 2006).
The above-mentioned studies have used different definitions of the metabolic syndrome which makes it difficult to compare the findings with each other. Some studies have used the National Cholesterol Education Program Adult Treatment Panel III criteria (Herva et al., 2006; Kinder et al., 2004; Räikkönen et al., 2002), while others have used various measures of reduced insulin sensitivity (Everson-Rose, 2004; Suarez, 2006; Timonen, 2005; Lawlor, 2003). The only study so far that has compared several clinically valid definitions of the metabolic syndrome is that by Räikkönen, Matthews, and Kuller (2007) who found that depressive symptoms predicted the development of the metabolic syndrome in middle-aged women, regardless of whether the metabolic syndrome definition. The present study examines whether depressive symptoms are similarly associated with various clinical definitions of the metabolic syndrome in a nationally representative sample of young Finnish adults of both genders.
Adverse health behaviors are one of the mechanisms through which depressive symptoms may contribute to the metabolic syndrome. Depressive individuals are known to report poorer lifestyle habits such as sedentary lifestyles, high intake of saturated fats, smoking, and heavy alcohol consumption (Carney, Freedland, Miller, & Jaffe, 2002; Franko et al., 2005). These behaviors, in turn, significantly increase the risk for developing the metabolic syndrome (Eckel, Grundy, & Zimmet, 2005; Grundy et al., 2005). For this reason, we examined whether the association between depressive symptoms and the metabolic syndrome would be explained by the aforementioned health behaviors. The immune system's functioning is another candidate that recently has been suggested to play a role in the link between depressive symptoms and the metabolic syndrome. C-reactive protein is a marker of systemic inflammation that is involved both in the development of depressive symptoms (Elovainio et al., 2006) and the metabolic syndrome (Haffner, 2006). It has been suggested that depression promotes and maintains inflammatory responses by down-regulating glucocorticoid receptor expression, resulting in the immune system's inability to benefit from cortisol's anti-inflammatory actions (Carney et al., 2002). C-reactive protein was also examined in the present study as a potential confounder in the association between depressive symptoms and the metabolic syndrome.
Finally, most studies have concentrated on the hypothesis that the metabolic syndrome is a consequence of depression. The possibility of reverse causality, i.e., that depression may be a consequence of earlier metabolic syndrome or its correlates, has been almost completely ignored in the literature. The only longitudinal study so far is that by Räikkönen and others (2002) who showed that the metabolic syndrome predicted increasing anger and anxiety, but not depression, in middle-aged women over 7.4 years. Whether a bi-directional relationship between depressive symptoms and the metabolic syndrome would be found in other populations, or using a different time-scale, remains unclear. The present study sought to examine this in young adult men and women by following them from childhood into their adulthood.
In this population-based study of young adults, we examined : a) whether high levels of depressive symptoms in adulthood are predictive of the metabolic syndrome or its components 9 years later, and b) whether having the metabolic syndrome in childhood and during the life-course (childhood and/or adulthood) is related to depressive symptoms in adulthood (the reverse causality hypothesis). We used 3 recently published guidelines to diagnose the metabolic syndrome: the International Diabetes Federation guideline (IDF; Alberti, Zimmet, & Shaw, 2005), the National Institute of Health Adult Treatment Panel III guideline (NCEP; Grundy et al., 2005) and the European Group for the Study of Insulin Resistance guideline (EGIR; Balkau & Charles, 1999). Women and men were examined separately, because previous research suggests that depressive symptoms are more prevalent in women and that health-related risks related to depression may be different in women than in men (Kinder et al., 2004; Räikkönen et al., 2002; Suarez, 2006).
Methods
Participants
The participants were from the Cardiovascular Risk in Young Finns Study which is a population-based, geographically representative sample of Finnish children and adolescents that has been followed up during 21 years, starting in 1980 (N at baseline=3,596). The study plan was accepted by the ethics committees of all participating universities, and the study protocol of each study phase corresponded to the proposal by the World Health Organization. All subjects gave written informed consent and the study was conducted in accordance with the Helsinki declaration.
The present study consisted of participants who were accessible at the measurement of adulthood metabolic syndrome in 2001 (N=2283). Participants who had missing data on any of the study variables (N=1279), who were pregnant (N=62) or who had a diagnosis of type 1 diabetes (N=20) or coronary heart disease (N=1) were excluded, resulting in 921 participants (538 women and 383 men) who were examined at three study phases: in 1980 for childhood metabolic syndrome (mean age 12 years), in 1992 for depressive symptoms (mean age 24 years), and in 2001 for depressive symptoms, the metabolic syndrome, and adulthood risk factors (mean age 33 years). Compared to those who had dropped out during the follow-up period between 1980 and 2001, the participants in the present study were more often women (58% of the present sample vs. 48% of the drop-outs), had a slightly lower body mass index (17.8 kg/m2 vs. 18.1 kg/m2), lower systolic blood pressure (112 mm Hg vs. 114 mm Hg), lower serum insulin (9.3 mU/L vs. 10.3 mU/L), and a came from families with a higher, income.
Measurement of the Metabolic Syndrome
Adulthood metabolic syndrome was measured at the mean age of 33 years including waist circumference, blood pressure, serum glucose, serum triglycerides, high-density lipoprotein (HDL) cholesterol, and serum insulin. A detailed description of the procedures has been described previously (Juonala et al., 2004). In order to differentiate individuals with clinical metabolic syndrome from those without, we used the following recently established definitions.
The NCEP (Grundy et al., 2005) is diagnosed as 3 or more of the following conditions: waist ≥102 cm in men and ≥88 cm in women, serum triglycerides ≥1.695 mmol/l (150 mg/dl), HDL cholesterol <1.036 mmol/l (40 mg/dl) in men and <1.295 mmol/l (50 mg/dl) in women, blood pressure ≥130 or ≥85 mmHg or treated, and plasma glucose ≥5.6 mmol/l (100 mg/dl).
The EGIR (Balkau & Charles, 1999) criteria were the presence of hyperinsulinemia (defined as non-diabetic subjects having fasting insulin level in the highest quartile, the cut-off point of our study was 9 mU/l), and at least two of the following conditions: fasting blood glucose ≥6.1 mmol/l, blood pressure ≥140/≥90 mmHg or current use of antihypertensive medication, serum triglyceride level >2.0 mmol/l or HDL level <1.0 mmol/l, and waist at least 94 cm in men and 80 cm in women.
The IDF (Alberti et al., 2005) is diagnosed as abdominal obesity and ≥ 2 of the following criteria: waist ≥94 cm in men and ≥80 cm in women, fasting plasma glucose ≥5.6 mmol/l, hypertriglyceridemia ≥1.695 mmol/L and HDL-cholesterol levels <1.036 mmol/L in men and <1.295 in women and blood pressure ≥130/≥85 mmHg or treatment.
Childhood metabolic syndrome was measured in 1980 at the mean age of 12 years. In order to form a comparable metabolic syndrome status rating for each participant in childhood than was done in adulthood (having versus not having the metabolic syndrome), we used a dichotomized metabolic syndrome variable for children. By following previous studies in children and adolescents, we adopted the definition of childhood metabolic syndrome from the Bogalusa Heart Study (Chen et al., 2000; Räikkönen, Matthews, & Salomon, 2003). It comprised body mass index (kg/m2), HDL-cholesterol, serum triglycerides, systolic and diastolic blood pressure, and serum insulin (Åkerblom et al., 1985). Glucose levels were not available in childhood. Children having values exceeding the 75th percentiles (except in HDL-cholesterol where < 25th percentile was used as the cut-off point) in 3 or more components were classified as having the metabolic syndrome, resulting in 126 children (13.7%) with and 795 children (86.3%) without the metabolic syndrome. To sum up, we used dichotomized metabolic syndrome variables (having versus not having) for childhood and adulthood metabolic syndrome throughout the statistical analyses, whereas continuous variables were used for the individual components of the metabolic syndrome.
Measurement of Depressive Symptoms
Depressive symptoms were self-rated in 1992 and in 2001 using a revised version of the Beck Depression Inventory (BDI; Beck & Steer, 1987; Katainen, Räikkönen, & Keltikangas-Järvinen, 1999). The original BDI is a 21-item questionnaire that offers 4 alternative statements for each item. In the present study, the BDI was modified so that each statement represents the second mildest level of depression in the original BDI. The participants were asked to choose between 1 (totally disagree) and 5 (totally agree). The individual items of the modified scale are shown in the Appendix (http://…please, insert hyperlink here).
We used this modified BDI because it was thought to more accurately bring out inter-individual variance in depressive symptoms in a healthy and young population in which rates of clinical depression are known to be low (Salmela-Aro & Nurmi, 1996). The theoretical model of the revised questionnaire has been tested by confirmatory factor analyses in the Cardiovascular Risk in Young Finns data which indicate that this model is empirically identifiable (Katainen et al., 1999). The modified BDI has previously been associated with psychological characteristics that are known to be associated with depression, such as child's difficult temperament (negative emotionality and low sociability), adulthood sentimentality and fatigability, and low social support (Elovainio et al., 2004; Heponiemi et al., 2006; Katainen et al., 1999). Moreover, our measure has been associated with cardiovascular risk factors such as C-reactive protein (Elovainio et al., 2006) and pre-clinical atherosclerosis (Elovainio et al., 2005) in the Cardiovascular Risk in Young Finns Study. Cronbach's α for the revised BDI in 1992 and 2001 was .0.89 and 0.92. The Pearson correlation between depressive tendencies in 1992 and 2001 was r=0.58 (p<0.0001).
Potential explanatory variables
Adult health behaviors were acquired by questionnaires in 2001 and included the frequency of heavy alcohol use (more than 6 units per session, ranging from 1 (less than 2 times a year) to 6 (at least twice a week), dietary fat usage (main source of fat: butter vs. vegetable-based fats), physical activity (a sum index of five questions assessing the intensity, duration, and frequency of sports by Telama, Yang, Laakso, & Viikari, 1997), and smoking: regular smoking (non-smoker/less than daily/daily smoker), number of cigarettes per day, and total years of smoking during lifetime.
Participants' educational level in 2001 and their parents' educational level in 1980 was classified as low (elementary school), intermediate (high school or vocational school), and high (college/university). Parental occupational status in 1980 was categorized as manual, lower non-manual, and upper non-manual. Family income in childhood was calculated as gross annual income, ranging from 1 (corresponding to <USD 3,000) to 8 (corresponding to >USD 22,000). These criteria correspond to the median income per household in Finland which was 12 920 USD in 1983 (Statistics Finland, 1983).
C-reactive protein was assessed from venous blood samples using an automated analyzer (Olympus AU400, Olympus, USA) and a highly sensitive turbidimetric immunoassay kit (“CRP-UL”-assay, Wako Chemicals, Neuss, Germany) (Raitakari, Mansikkaniemi, Marniemi, Viikari, & Raitakari, 2005). Owing to a skewed distribution (skewness=6.83), C-reactive protein was log-transformed before the analyses.
Statistical Analyses
We used logistic regression analysis to calculate odds ratios for having the metabolic syndrome per 1-standard deviation increase in standardized depressive symptoms score. In addition to age, we controlled for the potential explanatory variables by entering them in separate blocks. The first block included childhood metabolic syndrome components entered as continuous variables (body mass index, HDL cholesterol, triglycerides, systolic and diastolic blood pressure, and serum insulin), the second block included three indicators of childhood socioeconomic status (parental educational level, parental occupational status, family income), the third block included adulthood health behaviors (smoking, alcohol use, physical activity, type of dietary fat used), the fourth block included adulthood socioeconomic status (educational level), and the fifth block included C-reactive protein, and finally we controlled simultaneously for all of the above-mentioned risk factors. All statistical analyses were conducted separately for women and men. The association between depressive symptoms and the individual components of the metabolic syndrome was examined by regression analysis using the components included into the NCEP criteria as continuous variables. The NCEP is widely used in clinical settings and there is strong evidence for its predictive power with regards cardiovascular disease and mortality (Grundy et al., 2005; Sundström et al., 2006).
The reverse causality hypothesis (whether metabolic syndrome predicts depressive symptoms) was examined by testing whether the mean levels of adulthood depressive symptoms in 1992 or 2001 were different in participants having versus not having the metabolic syndrome in childhood (one-way analysis of variance). To test the possibility that life-course exposure to the metabolic syndrome is associated with depressive symptoms, we formed 4 groups based on whether the participant had metabolic syndrome (1) neither in childhood nor in adulthood, (2) only in childhood, (3) only in adulthood, and (4) both in childhood and in adulthood. Means of depressive symptoms in these four groups were compared by one-way analysis of variance. Finally, we used regression analysis to examine whether childhood or adulthood metabolic syndrome components were more important in predicting adulthood depressive symptoms. The continuous metabolic syndrome components were entered as predictor variables in three separate models including childhood components, adulthood components, and finally both childhood and adulthood components. We compared changes in R2 between the models to examine the contribution of each set of predictors to depressive symptoms.
Results
Risk Factor Levels
Men had worse levels than women in all of the metabolic syndrome components in adulthood (waist circumference, blood pressure, serum glucose, serum triglycerides, HDL cholesterol, and serum insulin). Compared to women, men also had a higher prevalence of adulthood metabolic syndrome as defined by the NCEP (16% vs. 10%), the EGIR (12% vs. 8%) and the IDF criteria (19% vs. 12%). Women had higher levels in depressive symptoms than men at both examinations (means 2.0 vs. 2.2 in 1992 and means 1.9 and 2.1 in 2001).
Depressive Symptoms as Predictors of the Metabolic Syndrome
In women, the odds ratio for having the metabolic syndrome was approximately 1.4-fold per each 1-standard deviation increase in the level of depressive symptoms both in 1992 and 2001 (table 1, age-adjusted models). This was true across all 3 definitions of the metabolic syndrome, except one association of only borderline significance for the EGIR. The age-adjusted models in Table 1 explained 2%-5% and the fully adjusted models explained 30%-42% of the variance in the metabolic syndrome in women (Nagelkerke R2). In men, depressive symptoms were not associated with any of the metabolic syndrome definitions (table 1). As shown in Table 1, no single covariate was able to attenuate the associations in women, but the associations became non-significant after adjustment for all of the childhood and adulthood risk factors simultaneously (Table 1).
Table 1. Risk for having the metabolic syndrome in adulthood (in 2001) defined by the NCEP, the EGIR, and the IDF criteria according to levels of depressive symptoms. OR=odds ratio; CI=confidence interval.
| Women (N=538) | Men (N=383) | |||||
|---|---|---|---|---|---|---|
| NCEP | EGIR | IDF | NCEP | EGIR | IDF | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Depressive symptoms in 1992a | ||||||
| Adjustment in addition to age: | ||||||
| None | 1.43 (1.00-1.15)** | 1.33 (0.99-1.79) | 1.43 (1.11-1.84)** | 1.06 (0.81-1.38) | 0.93 (0.68-1.27) | 1.18 (0.92-1.51) |
| Childhood metabolic componentsb | 1.39 (1.05-1.86)* | 1.29 (0.94-1.76) | 1.39 (1.06-1.81)* | 1.08 (0.82-1.43) | 0.87 (0.63-1.22) | 1.12 (0.86-1.46) |
| Childhood socioeconomic statusc | 1.43 (1.09-1.88)* | 1.32 (0.98-1.77) | 1.43 (1.11-1.83)** | 1.03 (0.78-1.36) | 0.90 (0.65-1.25) | 1.16 (0.90-1.49) |
| Adulthood health behaviorsd | 1.36 (1.02-1.80)* | 1.29 (0.95-1.76) | 1.38 (1.06-1.79)* | 1.03 (0.82-1.41) | 0.92 (0.66-1.29) | 1.19 (0.92-1.53) |
| Adulthood socioeconomic statuse | 1.40 (1.06-1.84)* | 1.30 (0.97-1.76) | 1.41 (1.09-1.82)** | 1.05 (0.80-1.37) | 0.91 (0.66-1.25) | 1.16 (0.90-1.49) |
| Adulthood C-reactive protein | 1.89 (1.02-1.88)* | 1.22 (0.88-1.67) | 1.37 (1.04-1.82)* | 1.06 (0.80-1.39) | 0.91 (0.65-1.26) | 1.18 (0.91-1.54) |
| All of the above | 1.37 (0.96-1.94) | 1.20 (0.84-1.73) | 1.34 (0.99-1-82) | 1.05 (0.76-1.45) | 0.85 (0.57-1.29) | 1.12 (0.84-1.51) |
| Depressive symptoms in 2001a | ||||||
| Adjustment in addition to age: | ||||||
| None | 1.50 (1.15-1.97)** | 1.42 (1.06-1.90)* | 1.48 (1.15-1.90)** | 1.14 (0.87-1.48) | 1.02 (0.75-1.39) | 1.09 (0.85-1.40) |
| Childhood metabolic componentsb | 1.39 (1.05-1.84)* | 1.32 (0.98-1.79) | 1.36 (1.04-1.76)* | 1.13 (0.85-1.02) | 0.92 (0.66-1.28) | 1.02 (0.78-1.33) |
| Childhood socioeconomic status c | 1.50 (1.15-1.97)** | 1.39 (1.04-1.87)* | 1.48 (1.15-1.90)** | 1.18 (0.90-1.55) | 1.04 (0.76-1.42) | 1.12 (0.86-1.45) |
| Adulthood health behaviorsd | 1.35 (1.01-1.79)* | 1.33 (0.97-1.81) | 1-36 (1.05-1.78)* | 1.08 (0.82-1.42) | 0.91 (0.66-1.25) | 1.05 (0.81-1.37) |
| Adulthood socioeconomic statuse | 1.49 (1.14-1.95)** | 1.41 (1.05-1.88)* | 1.47 (1.15-1.89)* | 1.16 (0.89-1.52) | 1.04 (0.76-1.43) | 1.12 (0.86-1.44) |
| Adulthood C-reactive protein | 1.51 (1.12-2.04)** | 1.33 (0.98-1.82) | 1.45 (1.10-1.90)** | 1.09 (0.82-1.44) | 0.95 (0.68-1.32) | 1.03 (0.79-1.12) |
| All of the above | 1.34 (0.94-1.90) | 1.21 (0.86-1.72) | 1.28 (0.94-1.75) | 1.11 (0.81-1.52) | 0.83 (0.56-1.23) | 1.00 (0.74-1.35) |
Scores were standardized; odds ratios indicate risk for having the metabolic syndrome per each 1-standard deviation change in depressive symptoms.
Body-mass index, HDL-cholesterol, triglycerides, systolic and diastolic blood pressure, and serum insulin entered as continuous variables.
The following indicators of SES were entered separately into the model: parental educational level (low, intermediate, high), parental occupational status (manual, lower non-manual, upper non-manual), family annual income (an 8-point scale).
Smoking (regular vs. irregular, number of cigarettes per day, and total years of smoking during lifetime), main source of dietary fat, heavy alcohol consumption, physical activity.
Participants' educational level (low, intermediate, high).
p < .05,
p < .01
p < .001
Examination of the individual metabolic syndrome components (as continuous variables) in table 2 showed that higher scores in depressive symptoms in 1992 and in 2001 were associated with a greater waist circumference both in women and in men, whereas depressive symptoms did not have consistent associations with the other components.
Table 2. Standardized beta coefficients of depressive symptoms in 1992 and in 2001 in predicting the individual components of the metabolic syndrome. WC=waist circumference, SBP=systolic blood pressure, DBP=diastolic blood pressure, TG=triglycerides.
| Depressive symptoms | Metabolic syndrome component | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WC | SBP | DBP | HDL-chol | TG | Glucose | |||||||
| β | R2a | β | R2a | β | R2a | β | R2a | β | R2a | β | R2a | |
| Women | ||||||||||||
| In 1992 | ||||||||||||
| Age-adjusted | 0.12** | 0.03 | 0.07 | 0.02 | 0.06 | 0.02 | -0.08 | 0.01 | 0.08 | 0.01 | 0.04 | 0.01 |
| Fully adjustedb | 0.04 | 0.43 | 0.04 | 0.20 | 0.04 | 0.18 | -0.06 | 0.39 | 0.06 | 0.25 | 0.01 | 0.07 |
| In 2001 | ||||||||||||
| Age-adjusted | 0.23*** | 0.06 | 0.09* | 0.02 | 0.06 | 0.02 | -0.05 | 0.00 | 0.10* | 0.01 | 0.09 | 0.01 |
| Fully adjustedb | 0.13*** | 0.44 | 0.04 | 0.20 | 0.01 | 0.18 | -0.03 | 0.39 | 0.06 | 0.25 | 0.07 | 0.08 |
| Men | ||||||||||||
| In 1992 | ||||||||||||
| Age-adjusted | 0.13** | 0.04 | 0.02 | 0.01 | -0.02 | 0.05 | -0.03 | 0.01 | 0.06 | 0.02 | -0.02 | 0.01 |
| Fully adjustedb | 0.08* | 0.46 | 0.04 | 0.18 | -0.01 | 0.21 | -0.04 | 0.38 | 0.04 | 0.21 | -0.03 | 0.08 |
| In 2001 | ||||||||||||
| Age-adjusted | 0.14** | 0.05 | 0.07 | 0.02 | 0.04 | 0.05 | -0.04 | 0.01 | 0.09 | 0.02 | 0.01 | 0.01 |
| Fully adjustedb | 0.06 | 0.46 | 0.11* | 0.18 | 0.05 | 0.21 | -0.04 | 0.38 | 0.02 | 0.21 | -0.01 | 0.08 |
Refers to the model including depressive symptoms and covariates.
Childhood covariates were body-mass index, HDL-cholesterol, triglycerides, systolic and diastolic blood pressure, serum insulin, parental occupational status, parental educational level, and family income.
Adulthood covariates were: participant's educational level, smoking, main source of dietary fat, heavy alcohol consumption, physical activity, C-reactive protein.
p < .05,
p < .01
p < .001
Childhood Metabolic Syndrome as a Predictor of Depressive Symptoms in Adulthood (the reverse causality hypothesis)
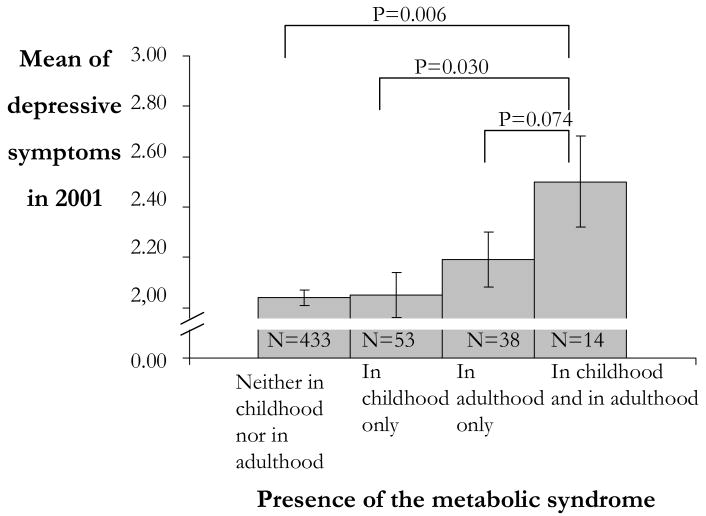
Women having the metabolic syndrome in childhood had slightly higher levels of depressive symptoms in adulthood (in 2001) than women free of childhood metabolic syndrome (F=4.70, p= .031; means of depressive symptoms were 2.25 vs. 2.07). In men, childhood metabolic syndrome did not predict any of the measurements of depressive symptoms (ps ranged between 0.159 and 0.448). As depicted in Figure 1, the level of women's depressive symptoms increased according to lifetime exposure to the metabolic syndrome (F=4.64, p = 0.003). Tukey's pairwise comparisons showed that women with persistent metabolic syndrome (diagnosed as having the metabolic syndrome both in childhood and in adulthood) had higher levels of depressive symptoms in adulthood than women who were free of the metabolic syndrome or who had it only in childhood or in adulthood.
Figure 1.
Adulthood depressive symptoms according to presence of the metabolic syndrome over the life-course in women.
We further examined the reverse causality hypothesis for the individual components of the metabolic syndrome by entering 1) childhood, 2) adulthood, and 3) both childhood and adulthood components simultaneously as predictors of depressive symptoms. As shown in table 3, entering the childhood components showed that in men, body-mass index in childhood (B=0.15, p= .047 in Model 1) predicted adulthood depressive symptoms whereas no significant associations were found in women. Entering the adulthood components showed that high waist circumference was the strongest predictor of depressive symptoms in both women and in men (Bs=0.22 and 0.13, ps = < .001 and .032 in Model 2). When both childhood and adulthood components were entered at the same time, high waist circumference in adult women remained a robust predictor of depressive symptoms (B=0.22, p = < .001), while higher systolic and diastolic blood pressure in childhood predicted lower future depressive symptoms in men (Bs=-0.14 and -0.11, ps=.041 and .047 in Model 3). Childhood components explained 1.6% of depressive symptoms in women, while the respective figure was 4.5% for men (shown in the R2 statistics section in table 3). Adulthood components explained 5.4% of women's and 2.5% of men's depressive symptoms. Altering the entry of childhood and adulthood metabolic syndrome components showed that adding childhood components after adulthood components improved the model only marginally in women (R2 increased by 0.5%) but considerably more in men (R2 increased by 3.5%). This suggests that childhood risk factor levels are important for men's future depressive symptoms, while current risk factor levels are more important for women's depressive symptoms.
Table 3. Age-adjusted regression coefficients (B) of childhood metabolic syndrome components (Model1), adulthood metabolic syndrome components (Model 2), and both childhood and adulthood metabolic syndrome components (Model 3) as predictors of depressive symptoms in 2001.
| Metabolic syndrome component | Women (n=538) | Men (n=383) | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| In childhood (1980)a | ||||||
| Body-mass index | 0.08 | -0.04 | 0.15* | 0.11 | ||
| Systolic blood pressure | 0.02 | 0.02 | -0.12 | -0.14* | ||
| Diastolic blood pressure | 0.04 | 0.03 | -0.10 | -0.11* | ||
| HDL-cholesterol | 0.00 | 0.08 | 0.11 | |||
| Triglycerides | 0.08 | 0.05 | 0.07 | 0.07 | ||
| Insulin | 0.03 | 0.03 | 0.07 | 0.06 | ||
| In adulthood (2001)a | ||||||
| Waist circumference | 0.22*** | 0.22*** | 0.13* | 0.06 | ||
| Systolic blood pressure | 0.01 | 0.01 | 0.04 | 0.09 | ||
| Diastolic blood pressure | -0.02 | 0.02* | -0.04 | -0.02 | ||
| HDL-cholesterol | 0.02 | 0.02 | 0.00 | -0.06 | ||
| Triglycerides | 0.04 | 0.04 | 0.04 | 0.02 | ||
| Glucose | 0.04 | 0.04 | -0.02 | -0.01 | ||
| R2 statistics: | ||||||
| R2 of Model 1 (%) | 1.6 | 4.5 | ||||
| R2 of Model 2 (%) | 5.4 | 2.5 | ||||
| R2 of Model 3 (%) | 5.9 | 6.0 | ||||
| ΔR2 between Model 1 and 3 (%) | 4.3 | 1.5 | ||||
| ΔR2 between Model 2 and 3 (%) | 0.5 | 3.5 | ||||
All components were entered simultaneously into the model.
p < .05,
p < .01
p < .001
Discussion
This study is to our knowledge the first to show an association of depressive symptoms in young adult women with three clinically valid definitions of the metabolic syndrome, namely those established by the Adult Treatment Panel-III, the European Group for the study of insulin resistance, and the International Diabetes Federation. Our findings also extend prior observations by showing a potentially lifelong reciprocal nature of the associations: higher level of depressive symptoms predicted the metabolic syndrome in women but also having the metabolic syndrome in childhood predicted later depressive symptoms. It is worth noting, however, that the magnitude of the effects was rather small, ranging between 1% and 6%. Nevertheless, it is noteworthy that associations of even this magnitude were found in this young and healthy population not selected to be at high risk for cardiovascular disease. It may be concluded that the process linking depressive symptoms with the metabolic syndrome may go into both directions and it may begin early in life, most likely already in childhood. The cumulative exposure to the metabolic syndrome over the life-course seems to be especially important in predicting future risk for depressive symptoms.
The association between depressive symptoms and the metabolic syndrome in women did not hold against rigorous controls for childhood and adulthood risk factors such as education, physical activity, smoking, and alcohol consumption, which suggests a mediating effect by these variables. However, no single risk factor alone (health behavior or biological) was responsible for the attenuations. Perhaps single risk behaviors alone do not have enough power to act as mediators, but they must occur in combination to have a mediating effect. The association between depressive symptoms and the metabolic syndrome may also be explained by unmeasured factors. Psychosocial factors, such as lack of social integration, have been suggested to co-occur with depressive symptoms and accentuate their effect on cardiovascular outcomes (Horsten, Mittleman, Wamala, Schenck-Gustafsson, & Orth-Gomér, 2000). Another candidate is shared pathophysiological liability that accounts for the co-occurrence of depressive symptoms and the metabolic syndrome (Bornstein, Schuppenies, Wong, & Licinio, 2006). Genes that regulate dysregulation of the hypothalamic-pituitary-adrenocortical axis activity have been suggested to partially explain the link between depression and the metabolic syndrome (Bornstein et al., 2006; Brown, Varghese, & Mc Ewen, 2004).
A large part of the associations between depressive symptoms and the metabolic syndrome was attributable to central obesity. The proposed mechanism whereby depression leads to obesity is, in addition to a positive energy balance, through dysregulation of the autonomic nervous system and the hypothalamic-pituitary-adrenocortical axis (Björntorp, Holm, & Rosmond, 1999; Bornstein et al., 2006). Stress hormones, including epinephrine, norepinephrine, and cortisol, contribute to increased central adiposity and increased sympathetic tone which, in turn, has been suggested to be a central mechanism in the development of insulin resistance and cardiovascular diseases (Goldbacher, Matthews, & Salomon, 2005; Schneiderman & Skyler, 1996). We also found evidence for the reverse causality hypothesis regarding the individual components of the metabolic syndrome predicting depressive symptoms. Notably, childhood components were important for adulthood depressive symptoms in men, while current components, especially obesity, played a more important role for women's depressive symptoms. The possibility that the timing of exposure to the metabolic syndrome may have different consequences for men and women is a topic to be investigated in future studies.
The lack of an association between depressive symptoms and the metabolic syndrome in men is in line with previous research suggesting that health-related risks related to depression may be more critical for women than for men (Kinder et al., 2004; Räikkönen et al., 2002; Suarez, 2006). In the Third National Health and Nutrition Examination Survey depression was cross-sectionally associated with a 2-fold risk of having the metabolic syndrome in young adult women but not in men (Kinder et al., 2004). Likewise, Suarez (2006) found that depressive symptoms were associated with insulin resistance in women, whereas no association was found in men. This suggests that depressive symptoms may produce different cardiovascular outcomes in men and women. Sex-specific relationships between endogenous sex hormones and psychological states may partly explain the observed differences between women and men, but evidence on this is only beginning to accumulate (Suarez, 2006).
This study had limitations regarding the measurement instruments and the sample. First of all, the scale of depressive symptoms did not qualify as a measure of clinical depression and therefore our findings should be replicated using a standard measure of depression with clinical cut-off points. However, our measure of mild depression can be justified in the present sample because it may better bring out subtle variations in depressive symptoms in a population of healthy young adults among whom rates of clinical depression are rather low. Even mild, sub-clinical, levels of depression may be important as they have been suggested to have cardiotoxic effects if they persist over time (Kubzansky, Davidson, & Rozanski, 2005; Suarez, 2006). Secondly, regarding childhood metabolic syndrome, we did not have serum glucose levels available in childhood which weakens the definition of the metabolic syndrome. We did, however, have childhood insulin available which is considered as an important feature of childhood metabolic syndrome (Brambilla et al., 2007). Thirdly, even though the original study sample was representative of all Finns, it did not remain representative in the follow-up examinations. Attrition analyses showed that the present sample was biased towards better health and wealth compared to the original participants. The large loss of participants was due to stringent inclusion requirements: the participant had to be present at three study phases (in 1980, in 1992, and in 2001) and he had to have complete data for all of the variables. The generalizability to other populations is also limited because our sample was ethnically homogeneous, consisting of Caucasian men and women.
To conclude, these findings suggest an association between depressive symptoms and the metabolic syndrome which may be bi-directional. High depressive symptoms in adulthood were found to predict increased risk for the metabolic syndrome in women and the metabolic syndrome in childhood, in turn, predicted depressive symptoms. If these associations were causal, interventions designed to reduce both the metabolic syndrome and the development of depressive symptoms should be targeted at an early stage, because the roots of their association seem to lie in childhood.
Acknowledgments
This study was financially supported by the Academy of Finland (project number 123621 [L.P.-R.], project numbers 209514, 209518, and 111056 [L.K.-J.], project numbers 77841 and 210283 [O.T.R], project no 117604 [M.K.], Research Funds of the University of Helsinki (project number 2106012 [L.P.-R.], the Social insurance institution of Finland, the Finnish Foundation of Cardiovascular Research, and the Yrjö Jahnsson Foundation.
Appendix. Individual items of the modified Beck depression inventory
I am sad all the time and I can't snap out of it.
I feel discouraged about the future.
I feel I have failed more than the average person.
I don't enjoy things the way I used to.
I feel guilty a good part of the time.
I feel I may be punished.
I am disappointed in myself.
I am critical of myself for my weaknesses or mistakes.
I have thoughts of killing myself, but I would not carry them out.
I cry more now than I used to.
I am slightly more irritated now than usual.
I am less interested in other people than I used to be.
I put off making decisions more than I used to.
I am worried that I am looking old or unattractive.
It takes an extra effort to get started at doing something.
I don't sleep as well as I used to.
I get tired more easily than I used to.
My appetite is not as good as it used to be.
I have lost more than five pounds.
I am worried about physical problems such as aches and pains, or upset stomach, or constipation.
I am less interested in sex than I used to be.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
Contributor Information
Laura Pulkki-Råback, University of Helsinki.
Marko Elovainio, University of Helsinki.
Mika Kivimäki, University College London.
Noora Mattsson, University of Turku.
Olli T. Raitakari, University of Turku
Sampsa Puttonen, Finnish Institute of Occupational Health.
Jukka Marniemi, National Public Health Institute.
Jorma S.A. Viikari, University of Turku
Liisa Keltikangas-Järvinen, University of Helsinki.
References
- Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Balkau B, Charles MA. Comment of the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabetic Medicine. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. San Antonio:TX: Psychological Corporation; 1987. [Google Scholar]
- Björntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabetic Medicine. 1999;16:373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: The stress axis as the locus of gene-environment interactions. Molecular Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Lissau I, Flodmark CE, Moreno LA, Widhalm K, Wabitsch M, et al. Metabolic risk factor clustering in children: to draw a line across pediatric metabolic syndrome. International Journal of Obesity. 2007;31:591–600. doi: 10.1038/sj.ijo.0803581. [DOI] [PubMed] [Google Scholar]
- Brown ES, Varghese FP, Mc Ewen B. Association of depression with medical illness: does cortisol play a role? Biological Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. Journal of Psychosomatic Research. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovasculart risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects. The Bogalusa Heart Study. Diabetes. 2000;49:1042–1048. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome 2005 [Google Scholar]
- Elovainio M, Keltikangas-Järvinen L, Kivimäki M, Pulkki L, Puttonen S, Heponiemi T, et al. Depressive symptoms and carotid artery intima-media thickness in young adults: the Cardiovascular Risk in Young Finns Study. Psychosomatic Medicine. 2005;67:561–567. doi: 10.1097/01.psy.0000170340.74035.23. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Keltikangas-Järvinen L, Pulkki-Råback L, Kivimäki M, Puttonen S, Viikari L, et al. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychological Medicine. 2006;36:797–805. doi: 10.1017/S0033291706007574. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Kivimäki M, Puttonen S, Heponiemi T, Pulkki L, Keltikangas-Järvinen L. Temperament and depressive symptoms: a population-based longitudinal study on Cloninger's psychobiological temperament model. Journal of Affective Disorders. 2004;83:227–232. doi: 10.1016/j.jad.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Franko DL, Striegel-Moore RH, Bean J, Tamer R, Kraemer HC, Dohm FA, et al. Psychosocial and health consequences of adolescent depression in black and white young adult women. Health Psychology. 2005;24:586–593. doi: 10.1037/0278-6133.24.6.586. [DOI] [PubMed] [Google Scholar]
- Goldbacher EM, Matthews KA, Salomon K. Central adiposity is associated with cardiovascular reactivity to stress in adolescents. Health Psychology. 2005;24:375–384. doi: 10.1037/0278-6133.24.4.375. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. American Journal of Cardiology. 2006;97(suppl):3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Kivimäki M, Pulkki L, Puttonen S, Keltikangas-Järvinen L. The longitudinal effects of social support and hostility on depressive tendencies. Social Science & Medicine. 2006;63:1374–1382. doi: 10.1016/j.socscimed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Herva A, Räsänen P, Miettunen J, Timonen M, Läksy K, Veijola J, et al. Co-occurence of metabolic syndrome with depression and anxiety in young adults: The Northern Finland 1966 Birth Cohort Study. Psychosomatic Medicine. 2006;68:213–216. doi: 10.1097/01.psy.0000203172.02305.ea. [DOI] [PubMed] [Google Scholar]
- Horsten M, Mittleman MA, Wamala S, Schenck-Gustafsson K, Orth-Gomér K. Depressive symptoms and lack of social integration in relation to prognisis of CHD in middle-aged women. European Heart Journal. 2000;21:1072–1080. doi: 10.1053/euhj.1999.2012. [DOI] [PubMed] [Google Scholar]
- Juonala M, Viikari J, Hutri-Kahonen N, Pietikainen M, Jokinen E, Taittonen L, et al. The 21-year follow-up of the Cardiovascular Risk in Young Finns Study: risk factor levels, secular trends and east-west difference. Journal of Internal Medicine. 2004;255:457–468. doi: 10.1111/j.1365-2796.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- Katainen S, Räikkönen K, Keltikangas-Järvinen L. Adolescent temperament, perceived social support, and depressive tendencies as predictors of depressive tendencies in young adulthod. European Journal of Personality. 1999;13:183–207. [Google Scholar]
- Kinder LS, Carnethon MR, Palaniappan LP, King AC, Fortmann S. Depression and the metabolic syndrome in young adults: Findings from the Third National Health and Nutrition Examination Survey. Psychosomatic Medicine. 2004;66:316–322. doi: 10.1097/01.psy.0000124755.91880.f4. [DOI] [PubMed] [Google Scholar]
- Kinder LS, Kamarck TW, Baum AB, Orchard TJ. Depressive symptamology and coronary heart disease in type I diabetes mellitus: A study of possible mechanisms. Health Psychology. 2002;21:542–552. doi: 10.1037//0278-6133.21.6.542. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosom Med. 2005;67(suppl. 1):S10–S14. doi: 10.1097/01.psy.0000164012.88829.41. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Journal of the American Medical Association. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatement. Archives of General Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational countries. European Heart Journal. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2003;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- Raitakari M, Mansikkaniemi K, Marniemi J, Viikari J, Raitakari OT. Distribution and determinants of serum high-sensitive C-reactive protein in a population of young adults. The Cardiovascular Risk in Young Finns Study. Journal of Internal Medicine. 2005;258:428–434. doi: 10.1111/j.1365-2796.2005.01563.x. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988: Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews K, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: Antecedent or consequence? Metabolism: Clinical and Experimental. 2002;51:1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews KA, Salomon K. Hostility predicts metabolic syndrome risk factors in children and adolescents. Health Psychology. 2003;22:279–286. doi: 10.1037/0278-6133.22.3.279. [DOI] [PubMed] [Google Scholar]
- Salmela-Aro K, Nurmi JE. Depressive symptoms and personal project appraisals: A cross-lagged longitudinal study. Personality and Individual Differences. 1996;21:373–381. [Google Scholar]
- Schneiderman N, Skyler JS. Insulin metabolism, sympathetic nervous system regulation, and coronary heart disease prevention. In: Orth-Gomér K, Schneiderman N, editors. Behavioral medicine approaches to cardiovascular disease prevention. New Jersey: Lawrence Erlbaum; 1996. [Google Scholar]
- Statistics Finland. Distribution of household income in Finland 1983 [Google Scholar]
- Suarez E. Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults. Health Psychology. 2006;25:484–492. doi: 10.1037/0278-6133.25.4.484. [DOI] [PubMed] [Google Scholar]
- Sundström J, Risérus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: Prospective, population-based cohort study. British Medical Journal. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telama R, Yang X, Laakso L, Viikari J. Physical activity in childhood and adolescence as predictor of physical activity in young adulthood. American Journal of Preventive Medicine. 1997;13:317–323. [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? a systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K, et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross-sectional study of 1980, and an account of the children's and families' state of health. Acta Paediatrica Scandinavica Supplement. 1985;318(suppl):S49–S63. doi: 10.1111/j.1651-2227.1985.tb10082.x. [DOI] [PubMed] [Google Scholar]