Abstract
The kappa-opioid receptor (KOR) antagonist norbinaltorphimine (nor-BNI) attenuates behavioral antinociception produced by spinal administration of the cannabinoid receptor agonist delta-9-tetrahydorcannabinol (THC). The present study examined the ability of nor-BNI to prevent cannabinoid-induced inhibition of medullary dorsal horn (MDH) nociceptive neurons and antinociception produced by the cannabinoid agonist WIN 55,212-2 (WIN-2). Extracellular, single unit recordings of lamina I and lamina V MDH neurons was performed in urethane anesthetized rats. Heat-evoked activity was measured before and after local brainstem application of nor-BNI or vehicle followed by WIN-2. In both lamina I and lamina V neurons, prior application of nor-BNI prevented the inhibition of heat-evoked activity by WIN-2. In separate experiments, the contribution of KOR to cannabinoid-induced increases in heat-evoked head withdrawal latencies was assessed in lightly urethane-anesthetized rats. Antinociception produced by intrathecal administration of WIN-2 and THC was attenuated by prior administration of nor-BNI. In contrast, antinociception produced by the cannabinoid CP55940 remained unaffected by prior administration of nor-BNI. These results indicate that cannabinoid inhibition of nociceptive reflexes produced by WIN-2 and THC may result from inhibition of dorsal horn neurons through a KOR-dependent mechanism.
1. Introduction
Cannabinoids and opioids act on common elements of the circuitry in the brain and spinal cord that produces analgesia. Administered spinally or microinjected into brain regions involved in the descending modulation of pain, cannabinoids and opioids reduce nociceptive signals and produce analgesia in behavioral tests (Fields et al., 1988; Fields et al., 2005; Walker and Hohmann, 2005). At the level of the spinal cord, studies indicate an interaction between cannabinoids and opioids in producing analgesia. Spinal administration of the cannabinoid agonist delta-9-tetrahydrocannabinol (THC) produces antinociception that is antagonized by the kappa opioid receptor (KOR) antagonist, norbinaltorphimine (nor-BNI), and the administration of antisense oligonucleotides to the KOR blocks intrathecal THC-induced antinociception (Mason et al., 1999; Pugh et al., 1995; Pugh et al., 1997; Welch, 1993).
It has been hypothesized that intrathecal administration of cannabinoids produces antinociception by stimulating the release of endogenous opioid peptides. As evidence, spinal administration of cannabinoids induces the release of dynorphin, an endogenous opioid peptide with high affinity for the KOR, as measured by microdialysis (Mason et al., 1999). Additionally, intrathecal administration of antibodies to dynorphin attenuates intrathecal cannabinoid-induced antinociception (Pugh et al., 1997). Therefore, a KOR antagonist should block the suppression of dorsal horn nociceptive neurons produced by cannabinoid receptor agonists.
Several studies have shown inhibition of spinal and medullary dorsal horn (MDH) neurons following administration of cannabinoid receptor agonists (Akerman et al., 2007; Drew et al., 2000; Hohmann et al., 1995; Hohmann et al., 1998; Hohmann et al., 1999; Johanek and Simone, 2005; Kelly and Chapman, 2003; Ogawa and Meng, 2009; Papanastassiou et al., 2004). It remains unknown, however, whether this inhibition involves the endogenous release of a KOR agonist. Recently, we have demonstrated inhibition of noxious thermal stimulation evoked activity of MDH neurons located in both superficial and deep laminae following local brainstem application of the CB1/CB2 receptor agonist WIN 55,212-2 (WIN-2) (Ogawa and Meng, 2009). Identical thermal stimuli were also used to demonstrate the ability of WIN-2 to inhibit the head withdrawal reflex. The present study sought to determine whether cannabinoid-induced inhibition of the head withdrawal reflex and inhibition of heat evoked activity from superficial or deep MDH neurons could be attenuated by prior application of the KOR antagonist, nor-BNI.
2. Results
General properties
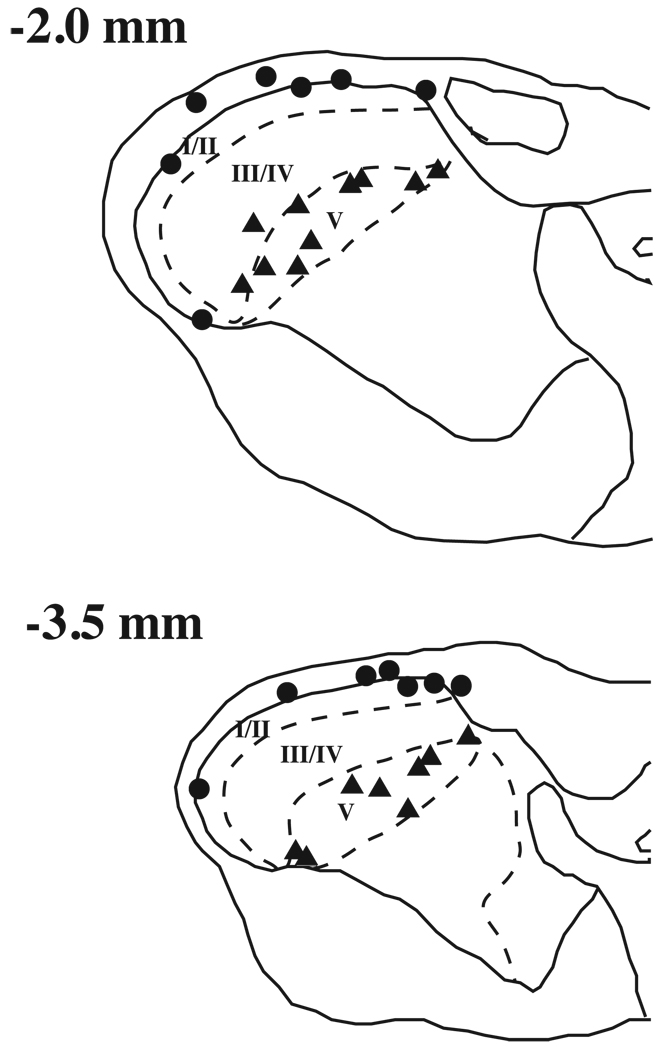
Single unit activity was recorded from 19 lamina I and 19 lamina V MDH neurons located between 2.0 and 3.5 mm caudal to obex. Fourteen recording sites were confirmed in lamina I and 18 sites were identified in lamina V based on electrolytic lesions (Fig1). The location of the remaining neurons into lamina I or lamina V treatment groups were based on microdrive readings of recording depths. Based on microdrive readings, the recording depth of lamina I neurons ranged from 0 to 295 µ with a median of 25 µ. Recording depths of lamina V neurons ranged from 550 to 1614 µ with a median of 696 µ. All lamina I neurons could be classified as either NS (n=10) or WDR (n=8). In lamina V, 5 neurons were classified as NS and 16 as WDR. In order to compare the effect of drug treatments on fast and slow heat-evoked responses in lamina I and lamina V neurons, data were converted to percent of control. The baseline heat-evoked activity (spikes/s) for all groups was not significantly different (Table 1, 2-way ANOVA, p > 0.05).
Figure 1.
A) Histological reconstruction of electrolytic lesion sites from lamina I and lamina V medullary dorsal horn (MDH) neurons. Numbers to the left represent approximate distance from obex (mm). Circles represent lamina I neurons and triangles represent lamina V neurons.
Table 1.
Average evoked activity (spikes/s) for pre-drug baseline control stimulation trials for each experimental group. The number of cells in each treatment group is indicated in parentheses.
| Lamina I | Lamina V | |||
|---|---|---|---|---|
| Fast heat | Slow heat | Fast heat | Slow heat | |
| Saline + WIN-2 | 16.2 ± 3.2 | 18.3 ± 3.3 (6) | 22.3 ± 5.0 | 15.9 ± 3.0 (7) |
| Nor-BNI + WIN-2 | 6.1 ± 1.6 | 7.5 ± 1.4 (7) | 23.1 ± 6.1 | 18.3 ± 7.0 (7) |
| Nor-BNI + Vehicle | 16.7 ± 4.1 | 10.1 ± 2.9 (6) | 20.0 ± 2.6 | 9.6 ± 2.4 (6) |
Effect of nor-BNI on cannabinoid-induced inhibition of lamina I and lamina V neurons
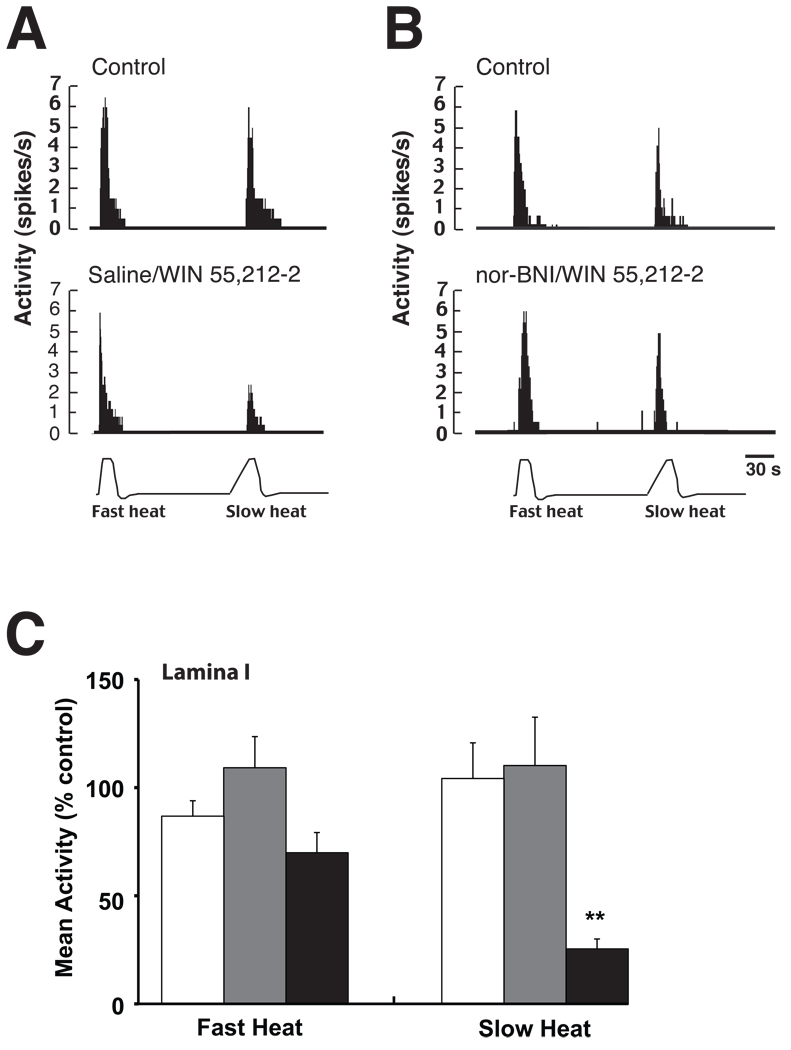
In a previous study, we demonstrated WIN-2 differentially inhibits MDH neurons based on their laminar location (Ogawa and Meng, 2009). In lamina I neurons, WIN-2 inhibited activity evoked by slow but not fast heat ramps. In contrast, WIN-2 inhibited both slow and fast heat evoked responses in lamina V neurons. Consistent with these earlier findings, bath application of WIN-2 in the present study inhibited activity evoked by the slow but not fast heat ramp in lamina I neurons (Fig 2 A & C), and inhibited both slow and fast heat-evoked responses in lamina V neurons (Fig 3 A & C). The effect of nor-BNI pretreatment on inhibition of lamina I and V neurons produced by WIN-2 was also examined. Pre-treatment with nor-BNI blocked the WIN-2 induced inhibition of heat evoked activity from both lamina I (Fig 2 B & C, one-way ANOVA, p < 0.01) and lamina V neurons (Fig 3 B & C, one-way ANOVA, p < 0.01). Treatment with nor-BNI followed by the vehicle to WIN-2 had no effect on either fast or slow heat-evoked activity of neurons recorded in both lamina I and lamina V (Figs 2C & 3C). Finally, drug treatments did not affect ongoing baseline activity (data not shown).
Figure 2.
The effect of nor-BNI pretreatment on WIN-2 inhibition of lamina I MDH neurons. A) A lamina I neuron exhibiting fast and slow heat evoked activity before (top panel) and after (bottom panel) bath application of WIN-2. WIN-2 preferentially inhibited activity evoked by the slow heat ramp. B) Fast and slow heat evoked activity of a lamina I neuron remained constant before and after bath application of nor-BNI followed by WIN-2. C) Grouped data from all lamina I neurons indicate that prior administration of nor-BNI blocked WIN-2 inhibition of slow heat-evoked responses in lamina I neurons. White bars, nor-BNI + vehicle; gray bars, nor-BNI + WIN-2; black bars, saline + WIN-2. n = 6–7 neurons/treatment group. Data represents mean ± SEM. ** p < 0.01 versus nor-BNI/vehicle and nor-BNI/WIN-2 treatment groups.
Figure 3.
The effect of nor-BNI pretreatment on WIN-2 inhibition of lamina V MDH neurons. A) Example of WIN-2 inhibition of both fast and slow heat evoked activity in a lamina V neuron. B) Heat evoked activity of a lamina V neuron remained constant following application of nor-BNI prior to WIN-2. C) Data averaged from all lamina V neurons demonstrate the prevention of WIN-2 inhibition with prior administration of nor-BNI. White bars, nor-BNI + vehicle; gray bars, nor-BNI + WIN-2; black bars, saline + 25 WIN-2. N = 6–7 neurons per treatment group. Data represents mean ± SEM. ** p < 0.01 versus nor-BNI/vehicle and nor-BNI/WIN-2 treatment groups.
Effect of nor-BNI on cannabinoid-induced increases in head withdrawal latencies
In parallel experiments, the ability of nor-BNI to prevent antinociception produced by the cannabinoid agonists WIN-2, THC, and CP55940 was tested in lightly anesthetized animals. Head withdrawal latencies were analyzed as % MPE because of the differences in baseline withdrawal latencies between the fast and slow heat ramps (Table 2). Behavioral withdrawal latencies were measured following fast and slow heat ramps applied to the whisker pad region. In agreement with our previous results (Ogawa and Meng, 2009), intrathecal administration of WIN-2 to the C1 region increased head withdrawal latencies only to the slow heat ramp (Fig 4A & B). The antinociception produced by WIN-2 was prevented by prior administration of either the CB1 antagonist SR141716 or the kappa opioid receptor antagonist nor-BNI (Fig 4A). Furthermore, nor-BNI treatment alone did not affect head withdrawal latencies to either the fast or slow heat ramps (Fig 4A & B).
Table 2.
Average baseline head withdrawal latencies in response to fast and slow heat ramps. Vehicle for WIN-2 is 10% emulphor solution in 0.9% saline, and vehicle for THC, CP55940, and U69593 is 98% ethanol.
| Fast heat | Slow heat | |
|---|---|---|
| Saline + WIN-2 | 8.4 ± 0.4 | 18.7 ± 0.3 |
| Nor-BNI + WIN-2 | 7.6 ± 0.5 | 17.5 ± 0.5 |
| SR141716 + WIN-2 | 7.3 ± 0.4 | 18.1 ± 0.5 |
| Saline + Vehicle | 7.4 ± 0.3 | 17.9 ± 0.4 |
| Nor-BNI + Vehicle | 7.2 ± 0.3 | 17.8 ± 0.9 |
| Saline + THC | 6.8 ± 0.3 | 17.9 ± 0.4 |
| Nor-BNI + THC | 6.2 ± 0.4 | 17.4 ± 0.2 |
| SR141716 + THC | 6.4 ± 0.3 | 17.5 ± 0.6 |
| Saline + CP55940 | 6.3 ± 0.4 | 17.0 ± 0.4 |
| Nor-BNI + CP55940 | 6.6 ± 0.3 | 17.9 ± 0.4 |
| SR141716 + CP55940 | 7.3 ± 0.5 | 17.7 ± 0.2 |
| Saline + U69593 | 6.6 ± 0.2 | 17.4 ± 0.3 |
| Nor-BNI + U69593 | 8.2 ± 0.9 | 18.2 ± 0.6 |
| Saline + 98% Ethanol | 6.2 ± 0.2 | 17.1 ± 0.4 |
Figure 4.
A) Intrathecal injections of WIN-2 to the Vc/C1 region increased head withdrawal latencies evoked by the slow heat ramp applied to the face. The WIN-2 induced antinociception was blocked by pre-treatment with either nor-BNI or the CB1 receptor antagonist SR141716. B) Withdrawal latencies evoked by the fast heat ramp remained unchanged after WIN-2 injections. ** p < 0.01 versus all other drug treatment groups. Data are expressed as group means ± SEM of the percent maximal potential analgesic effect (% MPE). N = 7–8 animals per treatment group.
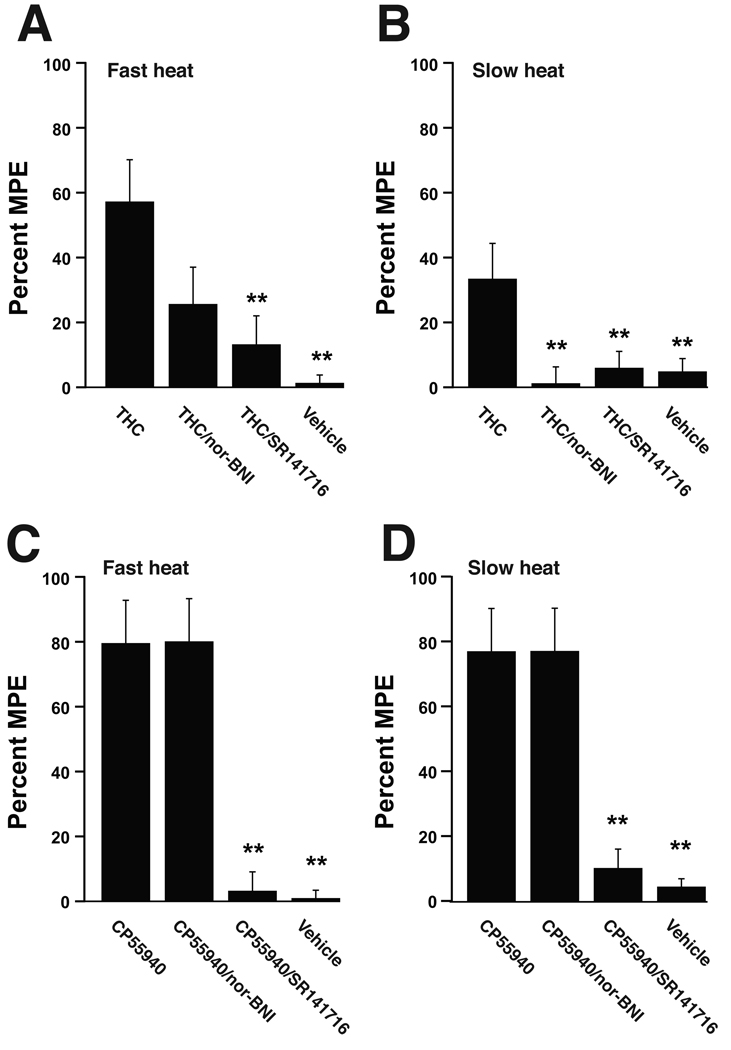
The CB1 agonists THC and CP55940 also increased head withdrawal latencies, however in the case of these compounds latencies to both fast and slow heat ramps were increased (Fig 5 A–D). As with WIN-2, antinociception produced by THC was blocked by prior administration of either SR141716 or nor-BNI (Fig 5 A & B). In contrast, antinociception produced by CP55540 was attenuated by SR141716 but remained unaffected by prior administration of nor-BNI (Fig 5 B, C). Intrathecal administration of the kappa opioid receptor agonist U69593 (20 µg) produced only a mild antinociception to the fast heat ramp (21.4±3.6% MPE) that was blocked by prior administration of nor-BNI (−9.7±5.0% MPE, p < 0.01). U69593 did not significantly affect withdrawal latencies to the slow heat ramp (7.2±3.5% MPE).
Figure 5.
Intrathecal administration of the CB1/CB2 receptor agonist THC produced antinociception to head withdrawal latencies evoked by both the slow (A) and fast (B) heat ramps. Administration of nor-BNI attenuated the antinociception produced by THC. In contrast, CP55940 produced antinociception to both the slow (C) and fast (D) heat ramps that was not affected by pre-treatment with nor-BNI. ** p < 0.01 versus THC treatment (panels A & B) and versus CP55940 and CP55940/nor-BNI treatment groups (panels C & D). Data are expressed as group means ± SEM of the percent maximal potential analgesic effect (% MPE). N = 7–8 animals per treatment group.
3. Discussion
Previous studies have revealed KOR involvement in behavioral antinociception produced by the cannabinoid receptor agonists THC and CP55,940 (Mason et al., 1999; Pugh et al., 1995; Pugh et al., 1997; Welch, 1993). Although these studies employed intrathecal injections to administer drugs, they did not examine direct effects on dorsal horn nociceptive neurons, leaving open the possibility that cannabinoids may interact with kappa opioids in the ventral horn of the spinal cord. Such a scenario could produce changes in motor reflexes without altering the activity of dorsal horn sensory neurons. In the present study, both noxious heat-evoked reflexes and activity of MDH neurons were measured to more directly determine the involvement of KOR activation in cannabinoid-induced suppression of sensory neurons and antinociception. Using this approach, it was possible to determine whether cannabinoid and KOR interactions occurred within the dorsal horn. One caveat to making direct comparisons between the behavioral and electrophysiology findings is the difference in anesthesic agents and depth of anesthesia in these two paradigms.
In confirmation of our previous findings, WIN-2 inhibited activity in lamina I neurons evoked by the slow, but not fast, heat ramp (Ogawa and Meng, 2009). In contrast, WIN-2 inhibited activity evoked by both the fast and slow heat ramps in lamina V neurons. Furthermore, WIN-2 increased head withdrawal latencies only to the slow heat ramp, which is also consistent to our previous findings (Ogawa and Meng, 2009). The present study employed fast and slow heat ramps to determine the involvement of KORs in cannabinoid induced inhibition of A-delta and C-fiber evoked activity; slow heat ramps have been demonstrated to primarily activate C-fiber primary afferent neurons, whereas fast heat ramps preferentially activate A-delta afferent neurons (Lu et al., 1997; Lu et al., 2004; McMullan et al., 2004; McMullan and Lumb, 2006a; McMullan and Lumb, 2006b; Yeomans and Proudfit, 1996a; Yeomans and Proudfit, 1996b; Zachariou et al., 1997). The KOR antagonist nor-BNI was equally effective in attenuating the inhibition of fast and slow heat evoked activity produced by WIN-2 administration. In addition, no differences were found in the ability of nor-BNI to block WIN-2 inhibition of lamina I or lamina V neurons. These results indicate that the involvement of kappa opioids in cannabinoid induced suppression of MDH activity is not restricted to a particular subset of MDH neurons, as defined by laminar location or primary afferent fiber activation.
At the level of the spinal cord, kappa but not mu or delta opioid receptors appear to be involved in cannabinoid analgesia (Welch and Stevens, 1992; Welch, 1993; Welch, 1994). While THC tolerant mice demonstrate cross-tolerance to intrathecal KOR agonists, there is no cross-tolerance to mu or delta opioid agonists (Smith et al., 1994; Welch, 1997). Antinociception produced by intrathecal administration of THC is also not affected by mu or delta receptor antagonists, whereas nor-BNI blocks cannabinoid-induced analgesia in the mouse and rat (Welch and Stevens, 1992; Welch, 1993; Welch, 1994). In addition, the more non-selective opioid receptor antagonist naloxone benzoylhydrazone attenuates cannabinoid-induced analgesia, demonstrating that these effects are not limited to the use of nor-BNI (Welch, 1994). Finally, as further evidence of KOR involvement in cannabinoid analgesia, THC-induced antinociception is attenuated by intrathecal administration of antisense oligonucleotides to the KOR (Pugh et al., 1995).
Some species differences have been reported in the ability of nor-BNI to attenuate spinal cannabinoid analgesia. Although nor-BNI blocks analgesia produced by both intrathecal THC and CP55,940 in the mouse, results from studies in rats indicate that nor-BNI attenuates antinociception produced by intrathecal THC but not CP55,940 (Mason et al., 1999; Pugh et al., 1997). The results in the rat are consistent with observations from the present study in which nor-BNI prevented analgesia produced by THC and WIN-2 but was ineffective in blocking CP55,940 induced analgesia. It should be noted, however, that only one dose of CP55,940 was used, and this dose produced greater antinociception than either THC or WIN-2. A complete dose response for CP55,940 or use of a dose closer to its ED50 would more properly compare the effect of nor-BNI on antinociception produced by the different cannabinoid receptor agonists.
The reported discrepancies between rats and mice have been traced to differences in the ability of THC and CP55,940 to evoke the release of dynorphin A in the spinal cord (Houser et al., 2000). Dynorphin A-(1-17) and its metabolite dynorphin A-(1-8) are KOR agonists derived from prodynorphin, and intrathecal administration of dynorphin A produces a KOR mediated antinociception (Nakazawa et al., 1991; Xue et al., 1995). In the rat, intrathecal CP55,940 increases dynorphin B immunoreactivity, whereas THC increases dynorphin A immunoreactivity (Mason et al., 1999; Pugh et al., 1997). Consequently, pretreatment with antisera to dynorphin A blocks THC, but not CP55,940 induced antinociception and THC shows cross tolerance with dynorphin A but not with dynorphin B (Pugh et al., 1996; Pugh et al., 1997). The capacity of THC and CP 55,940 to facilitate the release of different dynorphin peptides may also explain differences in their ability to potentiate morphine antinociception (Welch et al., 1995).
The effect of nor-BNI on WIN-2 antinociception had not been previously examined. In the present study, both nor-BNI and the cannabinoid receptor antagonist, SR141716, attenuated WIN-2 induced antinociception. Additionally, nor-BNI blocked antinociception produced by the KOR agonist U69593, confirming KOR mediated actions of nor-BNI. If similar mechanisms are involved with kappa opioids in the production of THC and WIN-2 antinociception, then WIN-2 administration should increase the spinal release of dynorphin A. Although the effect of acute WIN-2 administration on dynorphin A levels is not known, a previous study has reported an increase in dynorphin A levels after five days of treatment with WIN-2 (Gardell et al., 2002b).
The importance of dynorphin release to cannabiniod induced antinociception is not entirely clear, with some studies producing contradictory results. One study found no effect of nor-BNI on the spinal WIN-2 or THC antinociception in mice (Gardell et al., 2002a). In this same study, spinal WIN-2 and THC produced equivalent antinociception in prodynorphin knockout mice (Gardell et al., 2002a). These results led to an alternative interpretation of the previous studies, in which nor-BNI may be antagonizing a separate, tonically active antinociceptive system. By decreasing the activity of this system, nor-BNI would in effect counteract CB1 induced antinociception. This interpretation, however, is not supported by the present study in which nor-BNI treatment alone had no effect on head withdrawal latencies or neuronal activity.
Additionally, if cannabinoid-induced antinociception requires KOR activation, then both cannabinoid receptor and KOR agonists should inhibit dorsal horn neurons. Previous studies that have examined the effect of KOR agonists on dorsal horn neurons have produced mixed results. Iontophoretic or direct spinal cord application of KOR agonists have caused either excitation, inhibition, or had no effect on the activity of dorsal horn nociceptive neurons (Dong et al., 1991; Hylden et al., 1991; Knox and Dickenson, 1987; Sullivan and Dickenson, 1991; Wang et al., 1999). These previous studies are in contrast to the relatively consistent findings of cannabinoid receptor mediated inhibition of spinal and MDH neurons, again raising questions as to the role of endogenous kappa opioids in cannabinoid-induced antinociception.
In summary, application of the KOR antagonist nor-BNI to the MDH blocked WIN-2 induced suppression of heat evoked activity in both lamina I and lamina V neurons. In parallel experiments, nor-BNI attenuated antinociception produced by intrathecal administration of the cannabinoid receptor agonists WIN-2 and THC as well as the KOR agonist U69593. While our results are consistent with previous behavioral studies indicating KOR involvement in cannabinoid analgesia, it is acknowledged that alternative explanations may account for these observations.
4. Experimental Procedure
All experimental procedures were approved by the Institutional Animal Care and Use Committee at University of New England, and were treated according to the policies and recommendations of the NIH guidelines for the handling and use of laboratory animals.
Single unit recordings
Male Sprague-Dawley rats (320–350g; Charles River) were anesthetized with 2–3 % isoflurane mixed with oxygen for surgery. The trachea was cannulated for artificial ventilation and end-tidal CO2 was maintained from 3.5 to 4.5 %. The right jugular vein and femoral artery were catheterized. At the start of recording, continuous infusion of pancuronium bromide (0.6 mg/kg/hr, i.v.) was initiated primarily to minimize movement caused by respiration. Arterial blood pressure was continuously monitored to ensure anesthesia remained at therapeutic levels during recording. Body temperature was maintained at 37 °C with a feedback controlled heating pad.
Rats were placed in a stereotaxic apparatus and access to the MDH was provided by a C1 laminectomy. A pool for bath administration of drugs was made encompassing the exposed brainstem and a PE-50 catheter was placed at the level of C1 for drug infusion. A stimulating electrode was inserted into the contralateral thalamus or parabrachial area in order to test for the antidromic activation of units. Electrophysiological recordings took place under 1.75–2.25 % isoflurane, beginning at least 45 min after completion of surgery.
Extracellular single-unit recordings from MDH neurons located in superficial and deep laminae were performed using tungsten microelectrodes (impedance = 7–9 MOhms, FHC, Bowdoinham, ME). Isolated neurons were categorized based on their receptive field properties as either wide-dynamic range (WDR) or nociceptive specific (NS) (Hu, 1990; Meng et al., 1997). WDR neurons responded to light brushing of the receptive field and increased their rate of activity in response to increasing pressure, whereas NS neurons responded only to pressure and noxious pinching of the receptive field. Electrical stimulation of the receptive field was used to determine the conduction velocities of afferent fiber input (Meng et al., 1997).
The effect of drugs on activity evoked by thermal stimulation applied to the center of the receptive field was tested using a 5 × 5 mm contact thermode (Medoc, Minneapolis, MN). Two different heat ramps were used to preferentially activate A-delta or C-fiber primary afferents, as previously described (Ogawa and Meng, 2009). Rises in temperature of < 2 °C/s activates C-fiber afferents, whereas heat ramps > 2 °C/s results in activation of A-delta primary afferents (McMullan et al., 2004; Yeomans and Proudfit, 1996a; Yeomans and Proudfit, 1996b). Fast (3.4 °C/s) and slow (1.0 °C/s) heat ramps increased from a holding temperature of 35 °C to a plateau of 52 °C. The plateau temperature was maintained for 10 and 5 s for the fast and slow heat ramps, respectively. The sequence of fast and slow heat stimulation was applied with a three-minute interval between trials to avoid sensitization of the units.
After two stable baseline trials of fast and slow heat stimulation, the bath was drained and filled with the KOR antagonist nor-BNI (1.0 µg/µl) or saline 15 min prior to WIN-2 (1.0 µg/µl) infusion. Approximately 100 µl of fluid was required to fill the bath covering the brainstem. Thermal stimulation was repeated 20 min after WIN-2 administration. Only one neuron was tested per animal.
At the end of each experiment, animals were perfused with saline followed by 10 % formalin after lesions of recording (18 µA, 20 s) and stimulation (10 µA, 10 s) sites. Frozen sections (40 µm) were cut, mounted and stained with cresyl violet for histological identification of the recording and stimulation sites.
Electrophysiology data analysis
Heat evoked responses were determined by subtracting the average spontaneous activity for 10 s prior to the stimulus onset (ongoing activity) from the average rate of activity during the peak temperature. Activity was converted to percent of control (based on the average of the two baseline control trials) and the three treatment groups (saline/WIN-2, nor-BNI/WIN-2 and nor-BNI/vehicle) were compared using a one-way ANOVA followed by Tukey’s posthoc test after it was determined that the dataset conformed to a normal distribution with equal variances. Data are expressed as means ± SEM, and P<0.05 was considered to be statistically significant.
Head withdrawal latencies
In additional experiments, the ability of nor-BNI to attenuate analgesia produced by three different cannabinoid receptor agonists (WIN-2, delta-9-tetrahydrocannabinol, and CP55953) was assessed. The KOR agonist U69593 was administered as a control for the actions of nor-BNI. Drugs were delivered at the C1 level and head withdrawal reflexes were elicited by thermal stimulation of the face in lightly anesthetized rats according to previously described methods (Ogawa and Meng, 2009). This method allowed for the application of heat ramps to the face that were similar to those used in the electrophysiology studies. Briefly, five to seven days prior to behavioral testing catheters were implanted into the intrathecal space so that the tip protruded just beyond C1 into cisterna magna, as verified by dissection around the C1 region following the experiment. At least 30 min prior to testing, rats were lightly anesthetized with urethane (1.0 g/kg). The level of anesthesia eliminated spontaneous movements but still allowed for withdrawal reflexes to light pinching of the forepaw (Ogawa and Meng, 2009; Yeomans and Proudfit, 1996a). Using a contact thermal probe applied to the center of the rat’s shaved whisker pad region, fast (3.4 °C/s) and slow (1.0 °C/s) heat ramps were applied to the face at four min intervals, and the latency to withdraw was timed with a hand-held stop watch and recorded. The plateau temperature was 52 °C, and a cut-off period of 17 s for fast heat and 33 s for slow heat was used to avoid tissue damage. After three stable baseline controls, intrathecal injections were delivered in a total volume of 10 µl followed by a 10 µl saline flush.
The following intrathecal injections were given 30 min prior to measurement of head withdrawal latencies:
-
WIN-2
Saline (0.9%) 15 min prior to WIN-2 (300 µg; n = 8)
Nor-BNI (100 µg) 15 min prior to WIN-2 (300 µg; n = 8)
SR141716 (200 µg) 15 min prior to WIN-2 (300 µg; n = 8)
Saline (0.9%) 15 min prior to 10 % emulphor solution (vehicle of WIN-2; n=7)
Nor-BNI (100 µg) 15 min prior to 10 % emulphor solution (n = 8)
-
THC
Saline (0.9%) 15 min prior to THC (260 µg; n=8)
Nor-BNI (100 µg) 15 min prior to THC (260 µg; n=8)
SR141716 (200 µg) 15 min prior to THC (260 µg; n = 8)
-
CP55940
Saline (0.9%) 15 min prior to CP55940 (37.7 µg; n=8)
Nor-BNI (100 µg) 15 min prior to CP55940 (37.7 µg; n=7)
SR141716 (200 µg) 15 min prior to CP55940 (37.7 µg; n=7)
Behavioral data analysis
Head withdrawal latencies were converted into the percent maximal possible effect (%MPE) using the formula:
Percent MPE = [(post-drug latency - pre-drug latency)/(cut-off time - pre-drug latency)] × 100.
Latencies from the final two pre-drug trials were averaged for analysis. The effect of each treatment on withdrawal latencies was determined by a one-way ANOVA on the %MPE values after testing the data set for normality and equal variances. Post hoc comparisons were made using Tukey’s posthoc test. All data are expressed as means ± SEM, and P<0.05 was considered to be statistically significant.
Drugs
WIN-2 and SR141716 were dissolved in solutions of 9:1 saline:emulphor (Alkamuls EL-620, Rhone-Poulenc, Cranbury, NJ) (Hohmann et al., 1995; Martin et al., 1996; Ogawa and Meng, 2006; Ogawa and Meng, 2009; Papanastassiou et al., 2004; Tsou et al., 1996). Nor-BNI was dissolved in 0.9% saline, and THC, CP55940 and U69593 were dissolved in 98% ethanol. WIN-2 and U69593 were purchased from Sigma-Aldrich (Saint Louis, MO); nor-BNI, THC and SR141716 were a generous gift from the National Institute on Drug Abuse. Drug doses were based on findings from our previous study that examined the effect of WIN-2 and SR141716 on the activity of MDH neurons and head withdrawal latencies (Ogawa and Meng, 2009).
Acknowledgements
This work was supported by NIDA grants K02DA018408 and R01DA014548 to IDM, and KAKENHI#20791552 to AO. The authors wish to thank Dr. Barbara Winterson for her helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerman S, Holland PR, Goadsby PJ. Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. J Pharmacol Exp Ther. 2007;320:64–71. doi: 10.1124/jpet.106.106971. [DOI] [PubMed] [Google Scholar]
- Dong X-W, Parsons CG, Headley PM. Effects of intravenous mu and kappa opioid receptor agonists on sensory responses of convergent neurones in the dorsal horn of spinalized rats. Br J Pharmacol. 1991;103:1230–1236. doi: 10.1111/j.1476-5381.1991.tb12329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Harris J, Millns PJ, Kendall DA, Chapman V. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2000;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Barbaro NM, Heinricher MM. Brain stem neuronal circuitry underlying the antinociceptive action of opiates. Prog Brain Res. 1988;77:245–257. doi: 10.1016/s0079-6123(08)62792-2. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. Vol. Edinburgh: Elsevier, Churchill Livingstone; 2005. [Google Scholar]
- Gardell L, Ossipov M, Vanderah T, Lai J, Porreca F. Dynorphinein-dependent spinal cannabinoid antinociception. Pain. 2002a;100:243–248. doi: 10.1016/S0304-3959(02)00173-2. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Burgess SE, Dogrul A, Ossipov MH, Malan TP, Lai J, Porreca F. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002b;98:79–88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sciences. 1995;56:2111–2118. doi: 10.1016/0024-3205(95)00196-d. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci Lett. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J Neurophysiol. 1999;81:575–583. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Res. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Hu JW. Response properties of nociceptive and non-nociceptive neurons in the rat's trigeminal subnucleus caudalis (medullary dorsal horn) related to cutaneous and deep craniofacial afferent stimulation and modulation by diffuse noxious inhibitory controls. Pain. 1990;41:331–345. doi: 10.1016/0304-3959(90)90010-B. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Nahin RL, Traub RJ, Dubner R. Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain. 1991;44:187–193. doi: 10.1016/0304-3959(91)90136-L. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. Cannabinoid agonist, CP 55,940, prevents capsaicin-induced sensitization of spinal cord dorsal horn neurons. J Neurophysiol. 2005;93:989–997. doi: 10.1152/jn.00673.2004. [DOI] [PubMed] [Google Scholar]
- Kelly S, Chapman V. Cannabinoid CB1 receptor inhibition of mechanically evoked responses of spinal neurones in control rats, but not in rats with hindpaw inflammation. Eur J Pharmacol. 2003;474:209–216. doi: 10.1016/s0014-2999(03)02085-5. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Dickenson AH. Effects of selective and non-selective kappa-opioid receptor agonists on cutaneous C-fiber-evoked responses of rat dorsal horn neurones. Brain Research. 1987;415:21–29. doi: 10.1016/0006-8993(87)90265-4. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Meth. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lu Y, Pirec V, Yeomans D. Differential antinociceptive effects of spinal opioids on foot-withdrawal responses evoked by C fibre or A delta nociceptor activation. Br J Pharmacol. 1997;121:1210–1216. doi: 10.1038/sj.bjp.0701239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sweitzer SM, Laurito CE, Yeomans DC. Differential opioid inhibition of C- and A delta- fiber mediated thermonociception after stimulation of the nucleus raphe magnus. Anesth Analg. 2004;98:414–419. doi: 10.1213/01.ANE.0000094334.12027.06. table of contents. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 1996;16:6601–6611. doi: 10.1523/JNEUROSCI.16-20-06601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DJJ, Lowe J, Welch SP. Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur J Pharmacol. 1999;378:237–248. doi: 10.1016/s0014-2999(99)00479-3. [DOI] [PubMed] [Google Scholar]
- McMullan S, Simpson DA, Lumb BM. A reliable method for the preferential activation of C- or A-fibre heat nociceptors. J Neurosci Methods. 2004;138:133–139. doi: 10.1016/j.jneumeth.2004.03.020. [DOI] [PubMed] [Google Scholar]
- McMullan S, Lumb BM. Spinal dorsal horn neuronal responses to myelinated versus unmyelinated heat nociceptors and their modulation by activation of the periaqueductal grey in the rat. J Physiol. 2006a;576:547–556. doi: 10.1113/jphysiol.2006.117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan S, Lumb BM. Midbrain control of spinal nociception discriminates between responses evoked by myelinated and unmyelinated heat nociceptors in the rat. Pain. 2006b;124:59–68. doi: 10.1016/j.pain.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Furuya Y, Kaneko T, Yamatsu K. Spinal kappa receptor-mediated analgesia of E-2078, a systemically active dynorphin analog, in mice. J Pharmacol Exp Ther. 1991;256:76–81. [PubMed] [Google Scholar]
- Ogawa A, Meng ID. The cannabinoid receptor agonist, WIN 55,212-2, inhibits cool-specific lamina I medullary dorsal horn neurons. Neuroscience. 2006;143:265–272. doi: 10.1016/j.neuroscience.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Meng ID. Differential effects of the cannabinoid receptor agonist, WIN 55,212-2, on lamina I and lamina V spinal trigeminal nucleus caudalis neurons. Pain. 2009;141:269–275. doi: 10.1016/j.pain.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastassiou AM, Fields HL, Meng ID. Local application of the cannabinoid receptor agonist, WIN 55,212-2, to spinal trigeminal nucleus caudalis differentially affects nociceptive and non-nociceptive neurons. Pain. 2004;107:267–275. doi: 10.1016/j.pain.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Pugh G, Jr, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–616. [PubMed] [Google Scholar]
- Pugh GJ, Abood ME, Welch SP. Antisense oligodeoxynucleotides to the k-1 receptor block delta-9-THC-induced antinociception. Brain Res. 1995;689:157–158. doi: 10.1016/0006-8993(95)00560-d. [DOI] [PubMed] [Google Scholar]
- Pugh GJ, Mason DJJ, Combs V, Welch SP. Involvement of dynorphin B in the antinociceptive effects of the cannabinoid CP55,940 in the spinal cord. J Pharm Exp Ther. 1997;281:730–737. [PubMed] [Google Scholar]
- Smith PB, Welch SP, Martin BR. Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther. 1994;268:1381–1387. [PubMed] [Google Scholar]
- Sullivan AF, Dickenson AH. Electrophysiological studies on the spinal antinociceptive action of kappa opioid agonists in the adult and 21-day-old rat. J Pharmacol Exp Ther. 1991;256:1119–1125. [PubMed] [Google Scholar]
- Tsou K, Lowitz A, Hohmann AG, Martin WJ, Hathaway CB, Bereiter DA, Walker JM. Suppression of noxious stimulus-evoked expression of Fos protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. 1996;70:791–798. doi: 10.1016/s0306-4522(96)83015-6. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhang KM, Long LO, Mokha SS. Orphanin FQ (nociceptin) modulates responses of trigeminal neurons evoked by excitatory amino acids and somatosensory stimuli, and blocks the substance P-induced facilitation of N-methyl-D-aspartate-evoked responses. Neuroscience. 1999;93:703–712. doi: 10.1016/s0306-4522(99)00188-8. [DOI] [PubMed] [Google Scholar]
- Welch SP, Stevens DL. Antinociceptive activity of intrathecally-administered cannabinoids alone, and in combination with morphine in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- Welch SP. Blockade of cannabinoid-induced antinociception by norbinaltorphimine, but not N,N-diallyl-tyrosine-aib-phenylalanine-leucine, ICI 174,864 or naloxone in mice. J Pharm Exp Ther. 1993;265:633–640. [PubMed] [Google Scholar]
- Welch SP. Blockade of cannabinoid-induced antinociception by naloxone benzoylhydrazone (NalBZH) Pharmacol Biochem Behav. 1994;49:929–934. doi: 10.1016/0091-3057(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Welch SP, Thomas C, Patrick GS. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J Pharmacol Exp Ther. 1995;272:310–321. [PubMed] [Google Scholar]
- Welch SP. Characterization of anandamide-induced tolerance: comparison to delta-9-THC-induced interactions with dynorphinergic systems. Drug Alcohol Dep. 1997;45:39–45. doi: 10.1016/s0376-8716(97)01342-2. [DOI] [PubMed] [Google Scholar]
- Xue JC, Yu YX, Han JS, Jen MF. Comparative study of the analgesic and paralytic effects induced by intrathecal dynorphin a in rats. Int J Neurosci. 1995;82:83–93. doi: 10.3109/00207459508994292. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996a;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996b;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD, Yeomans DC. Low but not high rate noxious radiant skin heating evokes a capsaicin-sensitive increase in spinal cord dorsal horn release of substance P. Brain Res. 1997;752:143–150. doi: 10.1016/s0006-8993(96)01466-7. [DOI] [PubMed] [Google Scholar]