Abstract
Simultaneous recordings from connected neuron pairs have brought important insights into synaptic communication between neurons. However, patch clamp recordings from neuron pairs have been largely restricted to brain areas in which connections among nearby neurons exist at a relatively high probability. In the case of more distant connections or in areas in which neurons are connected with low-probability, recordings from synaptically connected neuron pairs have remained scarce. Here, we present a method that allows dual recordings from remotely connected neuron pairs by scanning potential presynaptic neurons. The applicability of this new approach was tested in the inhibitory pathway from the medial nucleus of the trapezoid body (MNTB) to the lateral superior olive (LSO), a sound localization pathway in the auditory brainstem. Using a three-step approach that sequentially combines focal uncaging of glutamate, pressure application of glutamate, and loose patch recordings allowed us to reliably achieve recordings from distant, synaptically connected GABA/glycinergic MNTB-LSO neuron pairs. Our results demonstrate that single MNTB neurons evoke highly variable mono-synaptic responses in developing LSO neurons, and heterogeneous short term synaptic dynamics, suggesting local variations in the refinement of these inhibitory connections. Paired recordings, enabled by scanning of remotely connected pairs, will be highly useful to perform detailed investigations of the synaptic function and plasticity from these circuits during the period of developmental refinement. In general, this method should provide a valuable tool to find connected neurons in other brain areas in which recording from candidate pairs has a low success rate.
Keywords: caged glutamate, paired recording, GABA, glycine, auditory, sound localization
1. Introduction
Simultaneous intracellular recordings from connected neuron pairs allow detailed investigation of synaptic transmission between identified pre- and postsynaptic neurons. Patch clamp recordings of connected pairs have been obtained in neuronal culture (Bi and Poo, 1998; Tao et al., 2000) and in brain slice preparations from a variety of brain regions (e.g., Debanne et al., 1996; Markram et al., 1997; Pouzat and Hestrin, 1997; Petersen and Sakmann, 2000; Sjöström et al., 2001). Most of these paired recordings have been performed in brain areas in which nearby neurons make synaptic connections with a relatively high probability (e.g. mammalian neocortex). Recordings from connected neuron pairs that are farther apart or sparsely connected are rather difficult to obtain.
The chance of achieving recordings from connected neuron pairs can be increased by screening for pre- or postsynaptic partners. In cortical slices, screening of postsynaptic target neurons has been achieved using calcium imaging (Peterlin et al., 2000; Kozloski et al., 2001). With this method, spikes are evoked in a presynaptic neuron to reveal monosynaptic responses in postsynaptic neurons, which are detected as a rise in intracellular Ca2+ levels. This method, however, has the limitation that it is restricted to identify excitatory connections because activation of inhibitory synapses generally does not elicit a calcium signal in the postsynaptic neuron. In this study, we present a new method that can be used for finding inhibitory as well as excitatory connected pairs by scanning for presynaptic neurons at multiple spatial scales.
We applied this method to an auditory brainstem pathway in which inhibitory synaptic connections are present between nuclei that are separated by hundreds of microns. In the lateral superior olive (LSO), a binaural sound localization nucleus, neurons receive synaptic inputs from the contralateral ear via the medial nucleus of the trapezoid body (MNTB) (Boudreau and Tsuchitani, 1968; Cant and Casseday, 1986; Sanes and Rubel, 1988; Bledsoe et al., 1990; Sommer et al., 1993). The mature MNTB-LSO pathway is glycinergic, but during development MNTB neurons also release GABA and glutamate (Kotak et al., 1998; Gillespie et al., 2005). The developing MNTB-LSO pathway undergoes extensive refinement (Sanes and Friauf, 2000; Kandler et al., 2009), which involves the silencing of most initial MNTB inputs and the strengthening of maintained connections (Kim and Kandler, 2003; Kim and Kandler, 2010). Recordings with minimal stimulation techniques indicated the presence of highly variable responses by single MNTB inputs in developing LSO neurons (Kim and Kandler, 2003; Gillespie et al., 2005; Noh et al., 2010; Kim and Kandler, 2010). However, in these experiments activation of more than one axon could have contributed to this variability, and in addition some responses may have been elicited by fibers that did not originate in the MNTB. Recordings from unambiguously identified pairs with known cell body locations could help resolve these issues.
By prescreening potential presynaptic neurons in the MNTB using the sequential application of focal uncaging of glutamate, pressure application of glutamate, and loose patch recordings, we were able to establish recordings from connected MNTB-LSO pairs. Our results from neonatal rats and mice show that the synaptic inputs from a single MNTB neuron are highly variable in respect to amplitude and short-term synaptic dynamics. Our methods will enable detailed investigations of these inhibitory synaptic connections and their developmental plasticity. Because the prescreening approach can be easily applied to a variety of other brain areas, the method described here will be of general use to increase the success rate of achieving paired recordings.
2. Materials and Methods
2.1 Slice preparation
All experimental procedures were in accordance with NIH guidelines and approved by the IACUC at the University of Pittsburgh. Acute brain stem slices were prepared from Sprague Dawley rats aged postnatal day (P) 4 and 6, and CD1 mice aged P6-7. Animals were anesthetized with isoflurane before decapitation and brainstem slices were prepared as described previously (Kim and Kandler, 2003; Kullmann and Kandler, 2008). In brief, transverse slices (300 μm) were cut on a vibrating microtome (DTK-1500E, Ted Pella, Redding, CA, USA) in ice-cold ACSF (artificial cerebrospinal fluid (in mM): 124 NaCl, 26 NaHCO3, 10 Glucose, 5 KCl, 1.25 KH2PO4, 1.3 MgSO4, 2 CaCl2, 1 kynurenic acid, pH = 7.4 when infused with 95% O2 / 5% CO2). Slices that contained the MNTB and the LSO were selected and incubated at room temperature in an interface-type chamber. For recordings, slices were transferred to a recording chamber and continuously perfused with oxygenated ACSF (without kynurenic acid) at room temperature at a rate of 3-4 ml/min.
2.2. Electrophysiology and data analysis
Recordings of MNTB and LSO neurons were made using standard patch clamp techniques. Recording electrodes (2–3 MΩ) contained (in mM): 76 Cs-methanesulfonate, 56 CsCl, 10 EGTA, 1 MgCl2, 1 CaCl2, 2 ATP-Mg, 0.3 GTP-Na, 5 Na2-phosphocreatine, and 10 HEPES (pH = 7.4, 290 mOsm). With the internal and external solution, ECl was −20 mV and LSO neurons were held at −70 mV (corrected for −5 mV liquid junction potential), resulting in 50 mV driving force for Cl−. Presynaptic recordings were made with an Axoclamp 2B amplifier, and postsynaptic recordings were made with an Axopatch 1D amplifier. The signals were filtered at 2 kHz and digitized at 10 kHz using custom-written programs in the Labview environment (Kullmann and Kandler, 2001). After establishing a whole cell configuration in an LSO neuron, the objective was switched from 40x to 4x and an optical fiber for glutamate uncaging was placed over the MNTB. Switching between 40x (water immersion) and 4x objectives was done using the standard manual toggle system installed on an Olympus BX50 upright microscope, and this switching did not disturb our whole cell recordings. The extracellular solution contained caged glutamate (200-400 μM, γ-(α-carboxy-2-nitrobenzyl) ester L-glutamic acid) and 50-100 msec long UV flashes were delivered via an optical fiber (20 μm core diameter) for focal glutamate uncaging (Kim and Kandler, 2003). The MNTB was scanned with uncaging in small steps (~50 μm) to identify the location of presynaptic neurons. A successful uncaging that evoked a synaptic response defined a relatively small area (< 100 μm diameter) that contained a presynaptic neuron. To further constrain the area, a pipette filled with 1-2 mM glutamate was placed within the target region for pressure application of glutamate (Picospritzer, 10 msec pulse at 10 psi). At 40x magnification, the puffing pipette was moved in small steps to define a search area (<50 um diameter). Typically, the search area was defined as a small elongated area along the long axis of the puffing pipette. In the final step, individual MNTB neurons inside this elongated area were scanned one cell at a time using a loose-seal configuration with a patch pipette filled with ACSF. For each candidate presynaptic neuron, a gentle suction was applied to form a loose seal (~100 MΩ). Current pulses (0.5-2 nA for 4-5 msec) were applied to elicit spikes. The spikes were visible as a small peak riding on top of the voltage response to the current pulses (Fig. 1C: Petersen and Sakmann, 2000). If no response was seen in the LSO neuron, the pipette was pulled away from the current candidate MNTB neuron and re-used to test a next, nearby neuron. Although we did not systematically keep track of the number of neurons we scanned before we found a connected neuron, typically we did not have to scan more than 10 candidate neurons. Synaptic responses were evoked at 0.1 Hz. In a subset of experiments, pairs of presynaptic pulses separated by 50 msec were applied to investigate paired-pulse responses.
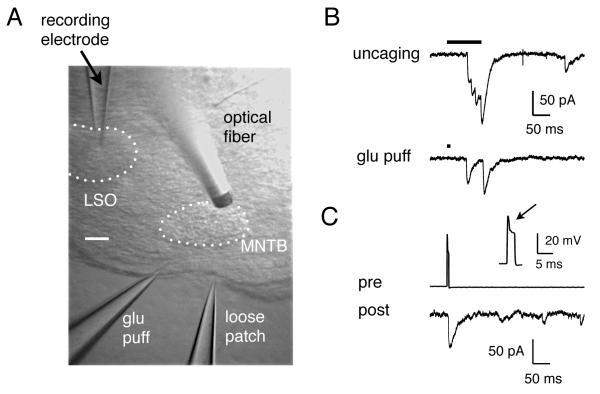
Fig. 1.
Illustration of 3-step scanning approach to find connected neuron pairs. (A) Photomicrograph of a brainstem slice (rat, P6) that contained the MNTB and the LSO. Also shown are a recording electrode in the LSO, an optical fiber, a pipette for pressure application of glutamate, and a loose patch recording in the MNTB. Scale bar = 100 μm. (B) Synaptic responses in an LSO neuron evoked by glutamate uncaging (top) or pressure application of glutamate (bottom). (C) Membrane voltage recordings of a presynaptic MNTB neuron in a loose seal mode and a postsynaptic current in an LSO neuron. The inset shows the presynaptic spike (arrow) detected in the loose seal recording.
Custom routines written in MATLAB (Mathworks) were used for data analysis. The peak amplitude of a synaptic current was measured with respect to the base line (average over 5 msec) before a presynaptic spike. Paired pulse ratio was measured as the peak amplitude of the second response divided by the first.
3. Results
To find monosynaptically connected MNTB-LSO pairs, we scanned the MNTB in three sequential steps (Fig. 1). In the first step, we focally uncaged glutamate in the MNTB using an optical fiber, a method that has been used previously to map functional MNTB-LSO connectivity (Kim and Kandler, 2003; Noh et al., 2010). The tonotopic axis runs mediolaterally in the MNTB, and it has been shown that in neonatal animals, the MNTB input area tends to span the entire dorsoventral extent (Kim and Kandler, 2003). Therefore, we scanned the MNTB along the mediolateral axis at about the dorsoventral midpoint so as to intersect the input area.
Once we identified a site at which glutamate uncaging elicited synaptic responses in the LSO neuron (Fig. 1B, top), we placed a glutamate containing pipette and pressure injected glutamate (1-2 mM) within the area defined by uncaging (Fig. 1B, bottom). This second step at a finer spatial scale further narrowed down the candidate area. In a last step, we used a patch pipette to scan individual MNTB neurons, eliciting spikes by applying brief current pulses (4-5 msec) in a loose seal configuration (Petersen and Sakmann, 2000). An example of pre- and postsynaptic traces is shown in Fig. 1C.
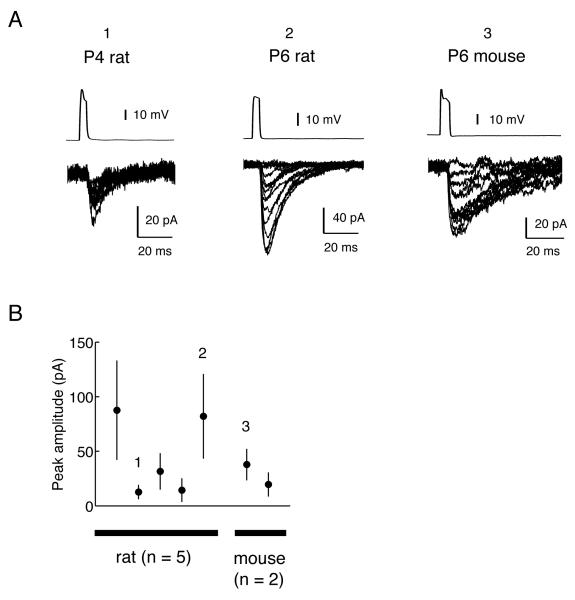
Previous recordings using minimal stimulation techniques have shown that single fiber MNTB inputs to immature LSO neurons generate responses with variable amplitudes. Because such variable amplitudes were not expected to result from the stimulation of single fibers, the possibility was raised that the minimal stimulation conditions may have activated more than one axon. To test whether variable amplitudes of synaptic responses also occur in paired recordings, we measured the synaptic responses to 40 to 50 consecutive MNTB spikes. As shown in the example traces (Fig. 2A) and the summary plot for peak amplitude (Fig. 2B), we observed significant variability in the PSC amplitude. While an example pair with smaller PSCs (Fig. 2A, left) shows a limited variability (range: 6 –31 pA), example pairs with larger PSCs (Fig. 2A, center and right) show a greater variability in the amplitudes of PSCs elicited by single MNTB spikes (center: 19-156 pA; right: 13-75 pA). The latter examples suggest that, assuming a quantal amplitude of 30 pA (Kim and Kandler, 2010), the number of release sites activated by an action potential fluctuates from one to at least three to five. In all of the 4 pairs that showed mean amplitudes greater than 30 pA, PSC amplitudes ranged from 6-18 pA to 75-196 pA, indicating that variations in the number of activated release sites is a common characteristic of immature MNTB-LSO connections. The variable amplitudes of these PSCs are reminiscent of those previously observed in LSO neurons using minimal stimulation techniques (Kim and Kandler, 2003; Noh et al., 2010; Kim and Kandler, 2010). These results indicate that the variation in PSC amplitudes observed with minimal stimulations is likely a property of single axons, lending support to the previously made estimations on single-fiber PSC amplitudes. A summary of PSC amplitudes from 7 pairs are shown in Fig. 2B. The average peak amplitude varied considerably during this development stage when some connections are in the process of elimination and others are strengthened (Kim and Kandler, 2003; Noh et al., 2010; Kim and Kandler, 2010).
Fig. 2.
Synaptic responses in MNTB-LSO pairs. (A) Pre- and postsynaptic recordings in three MNTB-LSO neuron pairs (P4 and P6 rat, and P6 mouse). Postsynaptic current amplitudes were variable as illustrated in the overlay of synaptic currents, and occasional failures were observed despite presynaptic firing (~10% on average). (B) Summary of peak amplitudes from all 7 pairs. In the plot, the mean PSC amplitudes are shown for 5 pairs in rats (P4-6) and 2 pairs in mice (P6 and 7). The numbers 1, 2, and 3 indicate the corresponding examples shown in (A). The error bars indicate standard deviations.
In three pairs from rat LSO neurons, we also examined short-term plasticity of responses evoked by a pair of presynaptic action potentials separated by 50 msec. In two pairs, we observed paired-pulse facilitation (paired-pulse ratio (PPR) of 1.5; n = 2), while in the third pair, we observed PPR of 0.97. The observation of paired-pulse facilitation was unexpected because previous studies, using the same inter-pulse interval, reported PPR values of about 0.9 in the mouse LSO (P1-12) (Kim and Kandler, 2010; Noh et al., 2010). In these studies, PPR was measured by stimulating the MNTB fiber bundle extracellularly at relatively high intensities, a condition that activates multiple presynaptic axons. Although it is highly preliminary, our data from MNTB-LSO pairs now reveal that single MNTB-LSO connections can also show paired-pulse facilitation, indicating heterogeneity in short term synaptic dynamics in the developing MNTB-LSO pathway and likely reflecting heterogeneity in the probability of release (Zucker and Regehr, 2002). Interestingly, those connections that showed paired-pulse facilitation also had smaller amplitudes (32 and 14 pA vs. 82 pA). This correlation may indicate that changes in the probability of release contribute to the strengthening and weakening of MNTB-LSO connections that occur during this developmental stage.
4. Discussion
We described a new approach that combined three scanning techniques with increasing spatial resolution to achieve paired recordings from distant, inhibitory, synaptically connected neuron pairs. We then applied this approach to the developing MNTB-LSO pairs and showed that synaptic responses elicited by single MNTB spikes had variable amplitudes with an estimated quantal content of 1 to 3 (based on mean amplitudes) consistent with previous estimates obtained by minimal stimulation (Kim and Kandler, 2010).
Our results also indicate that single MNTB-LSO connections differ in their short term plasticity as we observed both paired-pulse facilitation and paired pulse depression. Previous studies which analyzed paired-pulse plasticity using multi-fiber stimulation did not detect paired-pulse facilitation (Noh et al., 2010; Kim and Kandler, 2010). This may indicate that most fibers or fibers eliciting larger responses which dominate multi-fiber response amplitudes, show paired pulse depression. A detailed analysis of developmental changes of paired-pulse plasticity of paired responses could shed more light on the question of whether weakening and strengthening of single MNTB-LSO connections may involve changes in the probability of presynaptic release, which usually co-varies with short term plasticity.
Although the pre-screening method enabled us to find synaptically connected neuron pairs in the inhibitory MNTB-LSO pathway, several limitations of this approach should be noted. First the sequential screening is time consuming and it required about 30 min after establishing a whole cell recording from an LSO neuron to achieve paired recordings, limiting the remaining recording time. It may also prevent investigations of synaptic processes that are sensitive to the wash-out of intracellular components, such as long term potentiation. Another consideration is that presynaptic neurons are probed with loose patch techniques without breaking in. While re-using the loose patch pipette was necessary to scan multiple candidate neurons, it also prevented the formation of tight-seal whole cell recordings. Although loose seal recordings leave some uncertainty to the exact timing of firing, we were able to reliably detect presynaptic action potential as a small peak in the membrane voltage. Thus, unless probing for reciprocal connections is required, using the loose patch approach for pre-synaptic neurons should not impose a limiting downside. In some cases, the threshold current level for eliciting spikes increased during recording, making it necessary to increase stimulation strength. If the experimental questions require whole-cell recordings from the presynaptic neurons, it should be possible to introduce a new clean electrode for whole cell recording after identifying a presynaptic neuron because a loose seal can be removed without damaging the neuron.
We note that in applying our approach to various systems, it may be possible to skip the uncaging step. We believe that for scanning at a spatial scale of the whole MNTB, uncaging was superior to pressure injection because uncaging not only allows fast scanning, but also better defines the area of activation due to the highly focal release of free glutamate. Although we find it advantageous to use uncaging as an intermediate step, it may not be necessary depending on the circuitry being studied and the goals of the experiments.
Our results indicate that the unitary synaptic responses in MNTB-LSO pairs varied considerably consistent with previous results using minimal stimulation techniques. In the MNTB-LSO pair with the mean response amplitude of 82 pA (Fig. 2A, center), for instance, amplitudes ranged from 19 pA to 156 pA. Given the mean quantal amplitude of 30 pA in the neonatal LSO (P1-3), at the lowest amplitudes, it is likely that only one of the release sites was active, whereas at the highest, about 5 release sites were active. Considering that smallest PSC amplitudes observed (6-18 pA), which may represent distal inputs, were smaller than the mean quantal amplitude (30 pA), the range of the number of activated release sites may even be greater. These results indicate that at the ages examined, individual release sites are not reliably activated by each action potential, adding variability in addition to quantal variability. We hypothesize that age-related changes in the reliability of recruiting release sites contributes to the developmental strengthening and weakening of single MNTB-LSO connections during the topographic refinement of this pathway.
Another important aspect of the MNTB-LSO transmission is the developmentally regulated release of glutamate, in addition to GABA and glycine (Gillespie et al., 2005). This co-release of glutamate has been implicated in the refinement process (Noh et al., 2010). Direct demonstration of the co-release of three neurotransmitters by a single MNTB neuron will be possible with recordings from MNTB-LSO pairs.
Further investigation of synaptic transmission between MNTB-LSO pairs will be helpful in uncovering the mechanisms underlying the developmental specification of inhibitory circuits. During the refinement of the MNTB-LSO pathway, the rules by which a subset of the initial inputs is maintained while others are silenced are incompletely understood. Investigating differences in synaptic properties among the converging MNTB neurons with respect to topographic location may shed light on this question.
The method presented in this paper is applicable to other inhibitory pathways or to other brain areas with relatively distant connections. Our approach allowed us to record from connected pairs ~500 μm apart (Fig. 1A) and should be applicable to similarly or even more distant pairs as long as the connections can be preserved in slice preparations. This technique would allow investigation of unequivocally unitary inputs of known source in many neuronal connections and should bring important insights into the basic synaptic function, heterogeneity, and plasticity as recordings from nearby neuron pairs have in neocortex and cerebellum.
Acknowledgements
This work was supported by the Center for the Neural Basis of Cognition (GK) and a grant from the National Institute for Deafness and other Communication Disorders (KK) (DC 04199).
Abbreviations
- MNTB
medial nucleus of the trapezoid body
- LSO
lateral superior olive
- PSC
postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Snead CR, Helfert RH, Prasad V, Wenthold RJ, Altschuler RA. Immunocytochemical and lesion studies support the hypothesis that the projection from the medial nucleus of the trapezoid body to the lateral superior olive is glycinergic. Brain Res. 1990;517:189–94. doi: 10.1016/0006-8993(90)91025-c. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–54. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–76. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Cooperative interactions in the induction of long-term potentiation and depression of synaptic excitation between hippocampal CA3-CA1 cell pairs in vitro. Proc Natl Acad Sci USA. 1996;93:11225–30. doi: 10.1073/pnas.93.20.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–38. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–17. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Synaptic changes underlying the strengthening of GABA/glycinergic connections in the developing lateral superior olive. Neuroscience. 2010;171:924–33. doi: 10.1016/j.neuroscience.2010.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–90. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–55. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloski J, Hamzei-Sichani F, Yuste R. Stereotyped position of local synaptic targets in neocortex. Science. 2001;293:868–72. doi: 10.1126/science.293.5531.868. [DOI] [PubMed] [Google Scholar]
- Kullmann PHM, Kandler K. Dendritic Ca2+ responses in neonatal lateral superior olive neurons elicited by glycinergic/GABAergic synapses and action potentials. Neuroscience. 2008;154:338–45. doi: 10.1016/j.neuroscience.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann PH, Kandler K. Glycinergic/GABAergic synapses in the lateral superior olive are excitatory in neonatal C57Bl/6J mice. Brain Res Dev Brain Res. 2001;131:143–47. doi: 10.1016/s0165-3806(01)00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–15. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–38. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proc Natl Acad Sci USA. 2000;97:3619–24. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. J Neurosci. 2000;20:7579–86. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of basket/stellate cell-->Purkinje cell synapses in the cerebellum. J Neurosci. 1997;17:9104–12. doi: 10.1523/JNEUROSCI.17-23-09104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–64. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sommer I, Lingenhöhl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res. 1993;95:223–39. doi: 10.1007/BF00229781. [DOI] [PubMed] [Google Scholar]
- Tao H, Zhang LI, Bi G, Poo M. Selective presynaptic propagation of long-term potentiation in defined neural networks. J Neurosci. 2000;20:3233–43. doi: 10.1523/JNEUROSCI.20-09-03233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]