Abstract
Increased human population in the Western Kenya highlands has led to reclamation of natural swamps resulting in the creation of habitats suitable for the breeding of Anopheles gambiae, the major malaria vector in the region. Here we report on a study to restore the reclaimed swamp and reverse its suitability as a habitat for malaria vectors. Napier grass-shaded and non-shaded water channels in reclaimed sites in Western Kenya highlands were studied for the presence and density of mosquito larvae, mosquito species composition, and daily variation in water temperature. Shading was associated with 75.5% and 88.4% (P < 0.0001) reduction in anopheline larvae densities and 78.1% and 88% (P < 0.0001) reduction in Anopheles gambiae sensu lato (s.l.) densities in two sites, respectively. Shading was associated with a 5.7°C, 5.0°C, and 4.7°C, and 1.6°C, 3.9°C, and 2.8°C (for maximum, minimum, and average temperatures, respectively) reduction (P < 0.0001) in water temperatures in the two locations, respectively. An. gambiae s.l. was the dominant species, constituting 83.2% and 73.1%, and 44.5% and 42.3%, of anophelines in non-shaded and shaded channels, respectively, in the two sites, respectively. An. gambiae sensu stricto (s.s.) constituted the majority (97.4%) of An. gambiae s.l., while the rest (2.6%) comprised of Anopheles arabiensis. Minimum water temperature decreased with increasing grass height (P = 0.0039 and P = 0.0415 for Lunyerere and Emutete sites, respectively). The results demonstrate how simple environmental strategies can have a strong impact on vector densities.
Keywords: Anopheles gambiae s.l., mosquito breeding, Napier grass, water channels, larval density, water temperature
Introduction
Land-use changes have led to the creation of anthropogenic larval habitats suitable for malaria vectors, and thus have increased malaria transmission in the Western Kenya highlands. In recent times, increased human population pressure has led to the clearance of natural swamps, massive deforestation and the cultivation of crops in the valley bottoms (Munga et al., 2006; Mushinzimana et al., 2006). These changes can create ideal conditions for vector proliferation and increased malaria transmission (Briet et al., 2003) and also alter the local microclimate in favor of malaria transmission (Lindblade et al., 2000).
The majority of breeding habitats in the highlands are confined to the valley bottoms because the hillside gradients provide efficient drainage (Minakawa et al., 2004; Githeko et al., 2006) and appropriate management of these habitats can suppress malaria transmission (Minakawa et al., 1999).
The productivity of a given larval habitat is affected by both biotic and abiotic factors. In that regard, shading decreases photosynthesis at the level of the aquatic habitat and thus impairs the larval food chain. For example, Tuno et al. (2005) reported diminished populations of An. gambiae s.l. and other invertebrate fauna in shaded forest habitats in Western Kenya highlands
This study tests the hypothesis that intentionally shading the habitats of immature malaria vectors in wetlands will alter the densities of the anopheline larvae and their relative species composition as a result of reduced water temperature. Under natural conditions An. gambiae s.l. adults were shown to emerge only from farmland habitats, but not from forest and swamp habitats (Munga et al., 2006). The great majority of An. gambiae s.l. adults were collected within a distance of <500 m from the valley bottoms where streams, rivers and reclaimed swamps were located (Minakawa et al., 2005a; Munga et al., 2006). Studies of the spatial distribution on the spatial distribution of plasmodium parasites in humans and the associated malaria vectors in the same area suggested that transmission was focal and that targeted vector control was feasible (Minakawa et al., 2005a; Githeko et al., 2006; Zhou et al., 2004).
The rate at which a mosquito larva develops is dependent on the prevailing temperature, development of An. gambiae s.l. mosquito larvae ceases at temperatures below 16°C and below 14°C they die. Temperature affects the rate of larval development, pupation rates (Paaijmans et al., 2008; Munga et al., 2006); larval survivorship (Tuno et al., 2005); larval-to-adult survivorship and larval-to-adult development time (Afrane et al., 2005). The presence of An. gambiae s.l. and An. funestus in natural aquatic habitats in the Western Kenya highlands was inversely related to canopy cover (Minakawa et al., 2002).
An. gambiae s.l. generally avoids standing water populated with vegetation (Minakawa et al., 1999; Mutuku et al., 2006a, b), however, An. gambiae s.l. larvae can commonly be found in habitats with emergent grasses (Minakawa et al., 2004). An. gambiae s.l. females deposited significantly more eggs in rainwater than in waters from forests and wetlands, suggesting that An. gambiae s.l. prefers water with few impurities (Munga et al., 2005).
The cultivation of swamps increases water temperatures by exposing the immature habitats to direct sunlight thus reducing the larval to adult developmental time of An. gambiae s.l. in the cool highlands of Western Kenya (Minakawa et al., 2004). The amount of sunlight reaching the water and associated water temperature changes through the removal of canopy influences not only the quantity, but also the species composition of photosynthetic aquatic biomass, which may influence the larval food chain in favour of immature anophelines.
Informed larval interventions that target the more prolific habitats have been suggested to have great potential in combating malaria (Killeen et al., 2006; Gu and Novak, 2005), especially at a local, rather than nationwide scale resulting in increased cost-effectiveness of the operation.
Vector control programs with community participation have significant and lasting impacts on vector density, and are more cost-effective than vertically structured programs (Geissbuhler et al., 2009). In addition, the programs often integrate with other health or development programs, promote an enduring sense of pride in home and community, and make use of politically viable vector control strategies (Bryan et al., 1994; Hahn et al., 2009). The present study investigated the effects of intentionally shading mosquito breeding habitats using Napier grass on mosquito larval populations, species composition and water temperatures.
Materials and Methods
Study Area
This study was conducted in the Lunyerere and Emutete areas located in the Vihiga and Emuhaya districts, respectively, in the highlands of Western Kenya. Lunyerere is situated at an altitude of 1538 m above sea level, 00.10307 N and 34.72157E, while Emutete, 1545 m above sea level, is at 00.02854 N and 34.64003E. Both areas experience monthly mean temperatures ranging between 12°C and 29°C, with two rainy seasons in March to May and September to November. The other months are relatively dry. According to observations by the Kakamega meteorological station, the 1960–1999 average annual rainfall in the region was 1977 mm, mean minimum temperature was 13.8°C, and a mean maximum temperature was 28.0°C.
Topographical features in these highland areas comprise hills, valleys, and plateaus, with small rivers and streams running along the valley bottoms. Swamps are a common feature in valleys. The characteristic broad, flat valley bottoms, surrounded by undulating hills, make these areas prone to flooding, especially during the rainy season.
According to Githeko et al. (2006), malaria parasite prevalence among school-going children in an area bordering Lunyerere was approximately 68%. Following the scaling up of mosquito control interventions, in particular insecticide-treated bed-nets, the parasite prevalence levels have dropped to below 20%. Parasite prevalence among school-going children in Emutete is estimated at 44.3% (Fillinger et al., 2009).
Until around 1999, both sites were natural swamps dominated by papyrus (Cyperus papyrus). The cultivated swamps are now used for small-scale subsistence farming with maize, beans, vegetables, sweet potatoes, arrowroots, and Napier grass being grown as a livestock feed.
Study Design
The flood-prone, flat-bottomed valley requires extensive drainage to prevent crop damage, particularly during the rainy season. The design, arrangement, and network of drainage channels in the study areas have been graphically illustrated in Figure 1.
Figure 1.
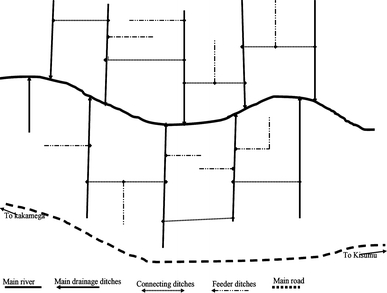
Graphic of the cultivated valley bottoms showing the design and arrangement of drainage channels.
The depth of water in drainage channels ranges between 25 and 35 cm in the two sites (Lunyerere and Emutete). The depth, width, and length of the channels varied from one plot to another, depending on the actual plot location, size, and the plot owner. The water channels in Lunyerere had an average length of 5.5 m compared to 8.5 m in Emutete. The channels had an average width of 0.5 m in the two sites.
Three factors were considered in selecting the study channels, namely, slow-flowing water, permanent channel, and plot owners consent to plant the Napier grass. Information about the persistence of water in the channels was obtained from the farmers. A total of 22 drainage channels (12 in Lunyerere and 10 in Emutete) were selected. A randomized controlled study design was used in allocating non-shaded and shaded plots. The process involved listing the selected drainage channels, assigning each an identification number, and randomly assigning individual channels intervention or non-intervention status (Fig. 2a, b).
Figure 2.

Non-shaded (open) (a) and Napier grass-shaded (b) channels as seen in the field.
Napier grass (Pennisetum purpureum) was planted on both sides of the entire length of drainage channels assigned intervention status. The non-intervention channels were left unplanted, hence open and exposed to direct sunlight. The usual farm activities, including the occasional cleaning and draining of water channels and land cultivation, went on uninterrupted throughout the study period, thus ensuring that conditions remained as natural as possible.
Larval Densities
Larval sampling was conducted weekly at each of the sampling locations (water channel) using the standard dipping method (WHO, 1975; Service, 1993). The process involved random sampling with no effort made to determine larval concentrations and their distribution within each habitat. The number of dips taken at each sampling plot was proportional to the size of channel, as sizes varied from plot to another; a constant number of dips across all larval plots would have introduced bias in the study results. The mean larval densities for each channel were calculated based on the actual number of dips. Mosquito larvae collected were differentiated in the dipper into anophelines or culicines, depending on whether they float parallel with the water surface (anopheline) or hang down from the surface (culicines) (Rozendaal, 1997). Counting and recording was performed on site before proceeding to the next sampling plot. Anopheline larvae were preserved in plastic vials containing 96% ethanol, carefully sealed, placed, and transported in cool boxes to the laboratory for further analysis.
Larval Species Composition
In the laboratory, the anopheline larvae were removed from the plastic vials and preserved in new vials containing fresh ethanol. The contents of each vial were poured into a glass Petri-dish and larvae sorted according to their respective developmental stages, i.e., early instars (1st and 2nd instars larvae); late instars (3rd and 4th instars). All larvae were identified morphologically to the species level (Gillies and Coetzee, 1987). Randomly selected samples of An. gambiae s.l. larvae were subjected to the DNA-based polymerase chain reaction (PCR) to determine the proportion of the sibling species of An. gambiae s.s. and Anopheles arabiensis Patton in the sample (Scott et al., 1993).
Water Temperature
Water temperatures in both non-shaded and shaded sites were recorded with the water-resistant temperature loggers (Optic Stowaway Temperature Logger, Onset Computer Corporation, Bourne, MA) at 1-minute intervals. Data loggers were carefully secured into the water to record temperature in the upper water layer. The data were downloaded using Box car Pro 4.3 software program once each month, re-launched, and returned to their respective locations.
Napier Grass Height
The height of Napier grass was recorded at every stage of larval sampling. Data were collected between November 2006 and August 2007, and January 2007 to August 2007, for Lunyerere and Emutete sites, respectively.
Ethical Consideration
The study was approved by the Scientific Review Committee (SSC) of the Kenya Medical Research Institute (KEMRI), Protocol KEMRI SSC# 1121. Verbal informed consent was obtained from selected plot owners before accessing their land.
Statistical Analysis
Data entry, transformation, and analysis (sums, means, and proportions) of larval reduction levels were carried out in Microsoft Excel. Regression and analysis of variance (ANOVA) were carried out using the JMP version 5.0.1 (SAS Institute, Cary, NC) program to test for differences in larval density between the shaded and non-shaded water channels. Correlation and regression analysis were used to determine the association between height of the grass and larval densities and water temperature.
Monthly larval and temperature data were log transformed to stabilize variances and then used to calculate the geometric means and 95% confidence intervals. The results were then back-transformed. All statistical analyses results were compared at a 5% level for significance.
Results
Species Composition
In Lunyerere, a total of 173 and 252 sampling visits were made to the non-shaded and shaded sites, respectively. Eighty-three percent of the selected sites contained An. gambiae s.l. larvae on at least one occasion. In non-shaded sites, a total of 627 larvae (53.3% anophelines and 46.7% culicines) and 115 pupae (47 anophelines and 68 culicines) were collected. In the shaded sites, 303 larvae (27.1% anophelines and 72.9% culicines) and 98 pupae (8 anophelines and 90 culicines) were collected (Table 1).
Table 1.
Larval Mosquito Species Composition, Density, and Reduction Levels
| Site | Species | Total collected in non-shaded channels | Total collected in shaded channels | Proportion of total collected in non-shaded channels | Proportion of total collected in shaded channels | Mean larval density (No. per dip) in non-shaded channels | Mean larval density (No. per dip) in shaded channels | Difference between shaded and non-shaded (ANOVA) P value | Observed reduction (% ) in species abundance attributable to shading |
|---|---|---|---|---|---|---|---|---|---|
| Lunyerere | An. gambiae s.l, | 278 | 60 | 83.2 | 73.2 | 1.61 ± 0.236 | 0.24 ± 0.078 | <0.0001 | 78.4 |
| An. funestus | 13 | 10 | 3.9 | 12.2 | 0.08 ± 0.02 | 0.04 ± 0.014 | 0.1293 | 23.1 | |
| An. coustani | 19 | 3 | 5.7 | 3.7 | 0.11 ± 0.024 | 0.01 ± 0.007 | <0.0001 | 84.2 | |
| An. rufipes | 2 | 2 | 0.6 | 2.4 | 0.01 ± 0.008 | 0.01 ± 0.006 | 0.7046 | 0 | |
| An. marshalli | 4 | 2 | 1.2 | 2.4 | 0.02 ± 0.011 | 0.01 ± 0.006 | 0.1932 | 50 | |
| An. maculipalpis | 5 | 0 | 1.5 | 0 | 0.03 ± 0.013 | 0 | 0.0066 | 100 | |
| An. azaniae | 9 | 4 | 2.7 | 4.9 | 0.05 ± 0.017 | 0.01 ± 0.008 | 0.0335 | 55.6 | |
| An. implexus | 4 | 1 | 1.2 | 1.2 | 0.02 ± 0.011 | 0 ± 0.004 | 0.0723 | 75 | |
| Total anophelines | 334 | 82 | 100 | 100 | 0.19 ± 0.028 | 0.03 ± 0.01 | <0.0001 | 75.4 | |
| Total culicines | 293 | 221 | 100 | 100 | 0.17 ± 0.023 | 0.09 ± 0.02 | 0.0061 | 24.6 | |
| Emutete | An. gambiae | 500 | 60 | 41.2 | 39.5 | 3.82 ± 0.34 | 0.45 ± 0.09 | <0.0001 | 88 |
| An. funestus | 5 | 9 | 0.4 | 5.9 | 0.04 ± 0.02 | 0.07 ± 0.02 | 0.2801 | −80 | |
| An. coustani | 357 | 28 | 29.4 | 18.4 | 2.72 ± 0.25 | 0.21 ± 0.05 | <0.0001 | 92.2 | |
| An. squamous | 23 | 11 | 1.9 | 7.2 | 0.18 ± 0.04 | 0.08 ± 0.03 | 0.0542 | 52.2 | |
| An. rufipes | 11 | 7 | 0.9 | 4.6 | 0.08 ± 0.03 | 0.05 ± 0.02 | 0.3414 | 36.4 | |
| An. marshalli | 5 | 3 | 0.4 | 2 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.4612 | 40 | |
| An. maculipapis | 195 | 8 | 16.1 | 5.3 | 1.49 ± 0.13 | 0.06 ± 0.02 | <0.0001 | 95.9 | |
| An. azaniae | 53 | 3 | 4.4 | 2 | 0.4 ± 0.05 | 0.002 ± 0.001 | <0.0001 | 94.3 | |
| An. pretoriensis | 10 | 0 | 0 | 6.6 | 0.08 ± 0.03 | 0 | 0.0031 | 100 | |
| An. implexus | 60 | 9 | 4.9 | 5.9 | 0.46 ± 0.05 | 0.07 ± 0.02 | <0.0001 | 85 | |
| An. flavicosta | 4 | 4 | 0.3 | 2.6 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.9913 | 0 | |
| Total anophelines | 1223 | 142 | 100 | 100 | 9.33 ± 0.83 | 1.07 ± 0.19 | <0.0001 | 88.4 | |
| Total culicines | 632 | 562 | 100 | 100 | 4.82 ± 0.60 | 4.26 ± 0.73 | 0.5492 | 11.1 |
In Lunyerere, 416 anopheline larvae were collected, comprising 334 (80.3%) and 82 (19.7%) larvae from non-shaded and shaded sites, respectively. Larvae from the non-shaded channels comprised of 45.5% early instars and 54.5% late instars larvae, whereas larvae from shaded channels comprised of 41.5% and 58.5% early and late instar larvae, respectively. A total of 55 anopheline pupae were collected, comprising 47 (85.5%) from the non-shaded channels and 8 (14.5%) from shaded channels.
In Emutete, 131 and 132 sampling visits were made in non-shaded and shaded sites, respectively. All (100%) the study plots had An. gambiae s.l. larvae on at least one occasion. A total of 1855 larvae (65.9% anophelines and 34.1% culicines) and 52 pupae (9 anophelines and 43 culicines) were collected in the non-shaded sites, while in the shaded sites, 704 larvae (20.2% anophelines and 79.8% culicines) and 38 pupae (2 anophelines and 36 culicines) were collected (Table 1).
A total of 1365 anopheline larvae comprising 89.6% and 10.4% from the non-shaded and shaded channels, respectively, were collected (Table 1). In non-shaded channels, the anopheline larvae consisted of 793 (64.8%) early and 430 (35.2%) late instars larvae, respectively, whereas larvae from shaded channels consisted of 95 (66.9%) and 47 (33.1%) early and late instar larvae, respectively.
Morphological identification (Table 1) of the Anopheles larvae sampled revealed the presence of a variety of species in varying proportions. These included An. gambiae Giles, An. funestus Giles, An. squamous, An. coustani Laveran, An. rufipes Gouph, An. marshalli Theobald, An. maculipapis Giles, An. azaniae Bailly-Choumara, An. flavicosta Edwards, An. pretoriensis Theobald, and An. implexus Theobald.
The anopheline pupae collected were not distinguished to the species level.
An. gambiae s.l. was the dominant species complex collected, irrespective of whether the channel was shaded or not.
PCR Analysis
The principal malaria vectors in the Anopheles gambiae sensu lato (s.l.) complex are Anopheles gambiae sensu stricto and Anopheles arabiensis. Polymerase chain reaction (PCR) analysis was conducted on 329 samples (187 and 142 samples from Emutete and Lunyerere sites, respectively). The An. gambiae s.l. samples from Emutete comprised of 150 and 37 samples from non-shaded and shaded channels, respectively, while An. gambiae s.l. from the Lunyerere site comprised of 74 and 68 samples from non-shaded and shaded channels, respectively. A total of 56 samples (32 from Emutete and 14 from Lunyerere) failed to amplify, thus could not be identified. This could have resulted due to misidentification of samples, for example, An. christi larvae may have been identified as An. gambiae s.l. because the larvae of the two species are nearly identical.
Results showed that An. gambiae s.l. comprised of 97.7% and 96.7% of An. gambiae s.s. in the non-shaded channels as compared to 100% and 89.5% in shaded channels (for Emutete and Lunyerere sites, respectively. The rest comprised of An. arabiensis Patton.
Larval Densities (Lunyerere)
In testing the differences in larval densities between the non-shaded and shaded channels, one-way ANOVA was used. The mean densities of anopheline larvae were significantly higher (F 1, 424 = 67.63, P < 0.0001) in the non-shaded channels compared to shaded channels (non-shaded: mean 0.19 ± 0.21 vs. shaded: mean 0.03 ± 0.01 larvae per dip) (Table 1). Differences (F 1, 424 = 39.95, P < 0.0001) were observed in the mean densities of An. gambiae s.l. between non-shaded (1.61 larvae per dip) and shaded (0.24 larvae per dip) sites (Table 1).
Shading was associated with 78.4% and 23.1% (for An. gambiae s.l. and An. funestus, respectively) reductions in larval densities observed in Lunyerere (Table 1). The overall reduction associated with shading in the combined densities of larvae belonging to An. gambiae s.l. and An. funestus species shading was 75.9% (Table 1).
Monthly densities of anopheline larvae showing observed variations in both the non-shaded and shaded water channels is clearly illustrated in Figure 3. The non-shaded channels in Lunyerere had their highest densities (0.39 larvae per dip) in the month of March and the lowest (0.01 larvae per dip) in December, whereas the shaded channels had their highest densities (0.01 larvae per dip) in the month of March, and other months had no larvae collected (Fig. 3a).
Figure 3.
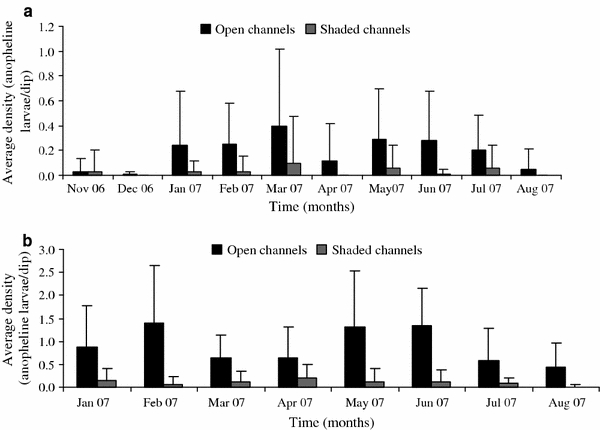
Monthly dynamics of anopheline larval densities in non-shaded and shaded water channels.
Larval Densities (Emutete)
A one-way ANOVA was used to test for the differences in larval densities between non-shaded and shaded channels. The mean densities of anopheline larvae were significantly higher (F 1,262 = 93.87, P < 0.0001) in the non-shaded channels compared to shaded ones (non-shaded: mean 0.93 ± 0.06 vs. shaded: mean 0.11 ± 0.06 larvae per dip) (Table 1). Significant differences (F 1,262 = 91.92, P < 0.0001) were observed in the mean densities of An. gambiae s.l. larvae between non-shaded (0.38 larvae per dip) and shaded channels (0.05 larvae per dip). In the non-shaded channels, the highest densities (1.4 larvae per dip) were observed in the month of February compared to the lowest (0.4 larvae per dip) in August, whereas in the shaded channels, highest densities (0.2 larvae per dip) were observed in April compared to lowest densities (0.01 larvae per dip) in August.
Differences (F = 2.4159, df = 9, P = 0.0134 and F = 1.1081, df = 7, P = 0.0043 for Lunyerere and Emutete sites, respectively) were observed in the monthly densities of anopheline larvae in non-shaded channels unlike in the shaded channels (F = 0.9521, df = 9, P = 0.4806 and F = 1.1081, df = 7, P = 0.3622).
Lunyerere Water Temperatures
A one-way ANOVA was used to test for differences in water temperatures between non-shaded and shaded channels. The mean temperatures were significantly higher, F 1,287 = 204.07, P < 0.0001; F 1,287 = 902.37, P < 0.0001; and F 1,287 = 510.65, P < 0.0001, for maximum, minimum, and average temperatures, respectively, in non-shaded channels compared to shaded ones during the period November–May (Fig. 4). Mean surface water temperatures of 26.2°C, 19.8°C, and 22.2°C for maximum, minimum, and average temperatures, respectively, were observed in non-shaded sites compared to temperatures of 20.5°C, 14.9°C, and 17.6°C observed in shaded channels. Significant differences (P < 0.0001) were observed in monthly mean temperatures between non-shaded and shaded channels. Mean water temperatures of 26.5°C (95% CI: 26.5–26.6°C) and 21.7°C (95% CI: 21.6–21.9°C); 20.0°C (95% CI: 20–20.1°C) and 14.7°C (95% CI: 14.7–14.75°C) for maximum (Fig. 4a) and minimum (Fig. 4b) temperatures, respectively, were observed in non-shaded and shaded channels, respectively.
Figure 4.
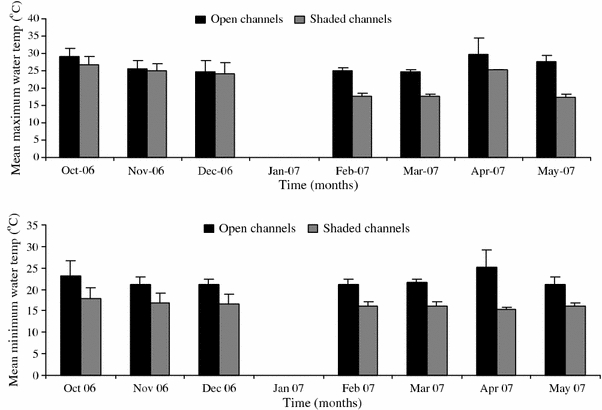
Trends in monthly surface water temperatures in Lunyerere site.
Emutete Water Temperatures
A one-way ANOVA was used to test for differences in water temperature between non-shaded and shaded channels. The mean temperatures were significantly higher, F 1,1887 = 224.3, P < 0.0001; F 1,1887 = 7162.7, P < 0.0001; and F 1,1887 = 2297.6, P < 0.0001, for maximum, minimum, and average temperatures, respectively, in the non-shaded channels compared to shaded channels (Fig. 5). Mean water temperatures of 22.2°C, 21.1°C, and 21.7°C for maximum, minimum, and average temperatures were observed in the non-shaded channels compared to temperatures of 20.9°C, 17.1°C, and 18.9°C observed in shaded channels.
Figure 5.
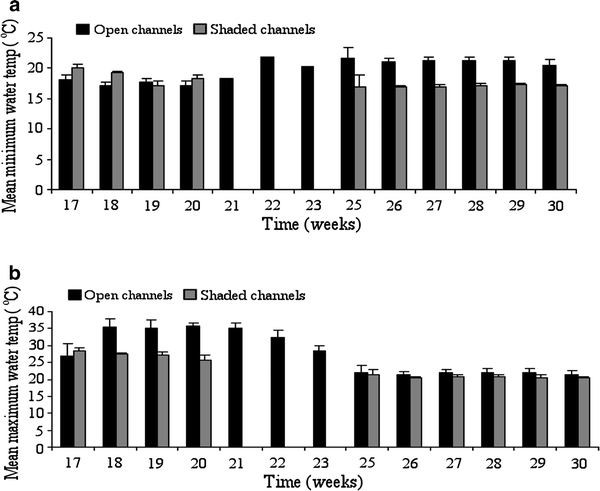
Weekly mean minimum (a) and maximum (b) surface water temperatures in non-shaded and shaded sites in Emutete.
Mean maximum water temperatures of 25.9°C (95% CI: 25.8–26.1°C) were observed in non-shaded sites as opposed to 22.96°C (95% CI: 22.87–23.04°C) in shaded sites (Fig. 5a). Mean minimum water temperature of 19.6°C (95% CI: 19.6–19.7°C) was observed in non-shaded sites compared to17.7°C (95% CI: 17.6–17.7°C) in shaded channels (Fig. 5b).
Temperature and Height of Napier Grass
At the Lunyerere site, a negative association was observed between the water temperature and corresponding height of Napier grass (r = −0.2048, P < 0.5231 and r = −0.7629, P = 0.0039 for mean maximum and minimum temperatures, respectively). There was positive association, though not significant (r = 0.4216, P = 0.1723) between the average water temperature and the height of grass.
In Emutete, a negative association was found between water temperatures and height of grass (r = −0.6346, P = 0.0664; r = −0.6856, P = 0.0415; and r = −0.6764, P = 0.0455 for average maximum, minimum, and average temperatures, respectively).
In both sites, the strongest association was found between the mean minimum water temperature and average height of grass (r = −0.7629 and r = −0.6856 for Lunyerere and Emutete sites, respectively).
An. gambiaes.l. Larval Density and Height of Grass
A negative association was observed between the mean densities of An. gambiae s.l. and the corresponding height of grass in both sites (r = −0.4935, P = 0.1030 and r = −0.7084, P = 0.0046 for Lunyerere and Emutete sites, respectively). The lowest densities of anopheline larvae were observed when Napier grass was 80–99 and 100–119 cm in height for Lunyerere and Emutete sites, respectively.
Discussion
This study demonstrates the impact of the intentional shading of cultivated-swamp habitats in Western Kenya highlands. Shading of these habitats with Napier grass reduces the density of immature mosquitoes, and potentially changes the local population structure of important disease vectors, including An. gambiae s.l. larvae. The only anopheline that could potentially increase due to Napier grass are An. ziemmani, An. coustani, An. christi, An. pharoensis, An. maculipalpis, and An. pretoriensis, all of which are zoophilic and non-vectors in malaria transmission.
Mosquitoes are among the most sensitive insects to environmental change; their survival, density, and distribution are dramatically influenced by small changes in environmental conditions, such as temperature, humidity, and the availability of suitable breeding sites (Martens, 1998; Molyneux, 1998; Grillet, 2000).
When natural swamps are cultivated, pools of water are exposed to direct sunlight, leading to elevated temperature and algae content, and consequent prolific breeding of An. gambiae s.l. (Minakawa et al., 2004; Afrane et al., 2005; Kaufman et al., 2006; Munga et al., 2006).
Shading reduces direct sunlight and lowers the water temperature, an abiotic factor that affects the survival and development rates of Anopheles gambiae s.l. (Paaijmans et al., 2008). An. gambiae s.l. exploits the increased resources in warmer, open habitats that tend to produce more algae, the main food source for An. gambiae s.l., than shaded habitats (Merrit et al., 1992; Gimnig et al., 2002). According to Service (1977), shading reduces the amount of algae. High mortality of An. gambiae s.l. larvae in large and shaded habitats has been suggested as the main reason for the low densities in such habitats, compared to high densities in open and temporary aquatic habitats (Minakawa et al., 2004, 2005b).
Development of 4th instar larvae ceases at water temperature below 16°C (Bayoh and Lindsay, 2004). Low minimum temperatures in the shaded channels habitats are likely to have resulted in delayed larval development and increased mortality (Bayoh and Lindsay, 2003).
Differences in the larval survivorship between the non-shaded and shaded channels may explain the observed differences in their larval densities. The amount of sunlight received by an aquatic habitat and the secondary effects of sunlight on photosynthesis, algal or bacterial growth, may be more important determinants of food chains and larval survivorship in aquatic habitats. Tuno et al. (2005) demonstrated that the survivorship of An. gambiae s.l. larvae was reduced from 55% to 57% in habitats fully exposed to sunlight to 1– 2% in habitats with full or partial forest canopy coverage (forest edge habitats). This was attributed to the high average daily water temperatures experienced in the open habitats which was approximately 3–3.4°C higher than the forests habitats.
In the inverse relationship between heights of the grass and larval densities, water temperatures may be explained by the fact that, as the height of grass increases, less radiative energy reaches the underlying water. According to Muturi et al. (2007), floating and emergent vegetation can obstruct mosquito oviposition and also reduce the amount of sunlight reaching the aquatic habitat, resulting in low water temperatures and interference with microbial growth that forms the main diet for mosquito larvae, leading to extended larval development time and the probability of predation (Ye-Ebiyo et al., 2003).
Abundance of predators or pathogens in the habitats (Service, 1977) constrains the development of adults from larval habitats. In nature, selective pressure from larval predators to complete this early life stage could be tremendous (Service, 1977). According to Carlson et al. (2004), an association of both emergent vegetation and predator diversity with habitat age was suggested as a probable factor to describe the negative association observed between emergent vegetation and presence of malaria vectors in man-made habitats. Carlson et al. (2004) observed a 57% increase in predator taxa found in abandoned, vegetated pits and a >50% reduction of Anopheles larval densities. In ground pools, predators are likely to regulate mosquito larvae density (Washburn et al., 1995; Sunahara et al., 2002).
The oviposition behavior of gravid female mosquitoes may influence the species composition and the productivity of breeding sites. Generally, anopheline larvae prefer open sun-lit waters (Gillies, 1968). Culicine mosquitoes are known to be more opportunistic in selection of oviposition sites and have a broader distribution of immatures in various geographical areas and water body types (Service, 1996). Emergent and floating vegetation in shaded sites may obstruct a gravid female from ovipositing (Rao, 1984). Predators may exert an effect by consuming larvae or through deterring oviposition into an otherwise suitable habitat (Angelon and Petranka, 2002).
Life table analysis studies (Afrane et al., 2005) found that the mean water temperature of aquatic habitats in the deforested area was 4.8–6.1°C degrees higher than in the forested area; larval-to-adult survivorship was increased to 65–82%; larval-to-adult development time was shortened by 8–9 days; and not only was the fraction (4–9%) of first-instar larvae that developed into adults reduced, but the development length exceeded 20 days in larval habitats located in forested areas. The mortality of immatures is affected by several factors, both biotic (food availability, competition, predation) and abiotic (rainfall, temperature) factors.
Our findings suggest that the presence of vegetative cover can play a strong regulatory role on water temperatures and on mosquito populations in larval habitats, and agree with other data (Fischer et al., 2002) which found low mosquito densities in habitats with little shading vegetation. Therefore, canopy/cover affects the temperature of larval habitats directly, and food conditions and other factors indirectly. However, the synergistic effects of these factors may be more significant to larval survivorship and adult mosquito productivity in highlands than the individual factors.
Recent years have seen a reawakening of interest in control of malaria in Africa by targeting the immature stages of mosquito species responsible for malaria transmission and the aquatic habitats in which they live (Utzinger et al., 2001, 2002a, b; Killeen et al., 2002a, b; MacIntyre et al., 2002; Fillinger et al., 2003, 2008; Keating et al., 2003). Fillinger and Lindsay (2006) found that larviciding through application of the larvicidal Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs) reduces Anopheles larval density by 95%, and human exposure to bites from adults by 92%. While Bti gives good control initially (within days of application), it does not appear to persist in most situations. Young mosquito larvae can appear 3–4 days after treatment of habitats (Mulla, 1990), with no residual activity after 2 weeks in a 4-weekly application regime (Mulla, 1985).
The proposed method of using Napier grass as a potential environmental management tool for malaria control in these (and possibly other) areas is based on the widespread practice of Napier grass cultivation and the importance attached to the particular crop due to its multiple uses. The strategy is non-chemical and has potential for sustainability once fully integrated in the community. Community-based participation in vector control programs has proven to be the most sustainable method of vector control (Bryan et al., 1994; Wilson et al., 2005). Napier grass (Pennisetum purpureum) is also known as “elephant grass,” “Sudan grass,” or “king grass,” and is an improved fodder grass that produces a lot of high-protein forage. Napier grass also provides straw used for weaving baskets and provides natural control to stem borers (Busseola fusca), a major pest of maize and sorghum in many countries of tropical Africa. If adopted, the use of Napier grass as a control strategy may also contribute in alleviating poverty in communities living in the malaria epidemic-prone highlands of Western Kenya. Additional studies on the association between anopheline larval occurrence/abundance and Napier grass cover are currently in progress. These studies focus on variables such as water quality and chemistry, molecular analysis of food types and amount present, and predator types and their abundance.
Conclusions
The call for simple, cheap, acceptable, and environmentally friendly mosquito control tools puts this strategy at the forefront in reducing vector breeding in such cultivated areas in the highlands. The habitat management strategy manifests important features which render it distinctively more advantageous than other methods. These include: (1) Its suitability to conditions of mixed agriculture, which is prevalent in Eastern Africa. Grass cultivation can increase both the crop yield and livestock productivity. (2) The proposed technology introduces practices which are already familiar to farmers in Africa. The approach has affinity to the common agricultural practice of multiple cropping and is based on the use of economically valuable plants. For example, the cultivation of Napier grass for livestock fodder and soil conservation is being encouraged in Eastern Africa, and is already widely applied. (3) Its contribution to the conservation of plant biodiversity and in providing a sustainable crop protection system.
This article presents evidence of how simple environmental water management strategies can be used to effectively control the malaria vectors in their larval stages. While there are no known harmful effects associated with the propagation and expansion of rhizomes, it should be noted that the effectiveness of such a strategy would depend strictly upon proper establishment and management of these cover plants (i.e., through proper planting, maintenance, and harvesting regimes) (Orodho, 2006).
Acknowledgments
We thank the individual landowners for allowing us access to the study sites; without their permission, this work would not have been possible. We are grateful to Mr. Gordon Opiyo for his assistance in larval identification. We also appreciate the contributions of Mr. Samuel Akoto and Mr. Nicholas Okonda for their useful assistance in larval sampling work. This study was supported by funds from the Dioraphte Foundation, The Netherlands. This work has been published with permission from the Director of the Kenya Medical Research Institute (KEMRI).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Peter M. Wamae, Email: pmwamai@yahoo.com
Andrew K. Githeko, Email: agitheko@gmail.com, Email: githeko@yahoo.com
Diana M. Menya, Email: dianamenya@gmail.com, Email: dmenya@africaonline.co.ke
Willem Takken, Email: willem.takken@wur.nl.
References
- Afrane YA, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by land use and land cover on duration of gonotrophic cycles of Anopheles gambiae (Diptera: culicidae) in western Kenya highlands. Journal of Medical Entomology. 2005;42:974–980. doi: 10.1603/0022-2585(2005)042[0974:EOMCCB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Angelon KA, Petranka JW. Chemicals of predatory mosquito fish (Gambusia affinis) influence selection of oviposition site by Culex mosquitoes. Journal of Chemical Ecology. 2002;28:797–806. doi: 10.1023/A:1015292827514. [DOI] [PubMed] [Google Scholar]
- Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bulletin of Entomological Research. 2003;93:375–381. doi: 10.1079/BER2003259. [DOI] [PubMed] [Google Scholar]
- Bayoh MN, Lindsay SW. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Medical and Veterinary Entomology. 2004;18:174–179. doi: 10.1111/j.0269-283X.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- Briet OJ, Dossou-Yovo J, Akodo E, van de Giesen N, Teuscher TM. The relationship between Anopheles gambiae density and rice cultivation in the savannah zone and forest zone of Cote d’Ivoire. Tropical Medicine and International Health. 2003;8:439–448. doi: 10.1046/j.1365-3156.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- Bryan R, Balderrama F, Tonn R, Dias J. Community participation in vector control lessons from Chagas’ disease. American Journal of Tropical Medicine and Hygiene. 1994;50:61–71. doi: 10.4269/ajtmh.1994.50.61. [DOI] [PubMed] [Google Scholar]
- Carlson JC, Byrd BD, Omlin FX (2004) Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health 4:33. doi:10.1186/1471-2458-4-33 [DOI] [PMC free article] [PubMed]
- Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in a rural Kenyan town. Tropical Medicine and International Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Knols B, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical Anophelines in western Kenya. Tropical Medicine and International Health. 2003;11:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A toolbox for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malaria Journal. 2008;7:20. doi: 10.1186/1475-2875-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide treated nets in the western Kenyan highlands: a controlled trial. Bulletin of the World Health Organization. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Marinone MC, Schweigmann N. Ochlerotatus albifasciatus in rain pools of Buenos Aires: seasonal dynamics and relation to environmental variables. Memórias do Instituto Oswaldo Cruz. 2002;97:767–773. doi: 10.1590/S0074-02762002000600002. [DOI] [PubMed] [Google Scholar]
- Geissbuhler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayaguya V, et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS ONE. 2009;4:e5107. doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT. The Anophelinae of Africa, South of the Sahara. Publication no. 54, Johannesburg: The South African Institute for Medical Research; 1968. [Google Scholar]
- Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa, South of the Sahara. Publication no. 55, Johannesburg: South African Institute for Medical Research; 1987. [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomolology. 2002;39:11162–11172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, et al. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malaria Journal. 2006;5:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet ME. Factors associated with distribution of Anopheles aquasalis and Anopheles oswaldoi (Diptera: Culicidae) in a malarious area, northeastern Venezuela. Journal of Medical Entomolology. 2000;37:231–238. doi: 10.1603/0022-2585-37.2.231. [DOI] [PubMed] [Google Scholar]
- Gu W, Novak RJ. Habitat based modeling of impacts of mosquito larval interventions on entomological inoculations rates, incidence and prevalence of malaria. American Journal of Medicine and Tropical Hygiene. 2005;73:546–552. [PubMed] [Google Scholar]
- Hahn T, Hill P, Kay B, Quy T. Development of a framework for evaluating the sustainability of community-based dengue control projects. American Journal of Tropical Medicine and Hygiene. 2009;80:312–318. [PubMed] [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM, Walker ED. Importance of algal biomass to growth and development of Anopheles gambiae larvae. Journal of Medical Entomolology. 2006;43:669–676. doi: 10.1603/0022-2585(2006)43[669:IOABTG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Keating J, MacKintyre K, Mbogo CM, Githeko A, Regens JL, Swalm C, et al. A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. American Journal of Topical Medicine and Hygiene. 2003;68:357–365. [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Gouagna LC, Knols BGJ. Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malaria Journal. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BGJ. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? The Lancet Infectious Diseases. 2002;2:618–627. doi: 10.1016/S1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Ross A, Smith T. Infectiousness of malaria-endemic human populations to vectors. American Journal of Medicine and Tropical Hygiene. 2006;75(Suppl):1–10. doi: 10.4269/ajtmh.2006.75.2_suppl.0750038. [DOI] [PubMed] [Google Scholar]
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land-use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Medicine and International Health. 2000;5:263–274. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko A, et al. Examining the determinants of mosquito avoidance practices in two Kenyan cities. Malaria Journal. 2002;1:14. doi: 10.1186/1475-2875-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens P. Modelling global environmental change health impacts. The Globe. 1998;42:13–15. [Google Scholar]
- Merrit RW, Dadd RH, Walker ED. Feeding behaviour, natural food and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of anopheline mosquito larvae in western Kenya. American Journal of Tropical Medicine and Hygiene. 1999;61:1010–1016. doi: 10.4269/ajtmh.1999.61.1010. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Githeko AK, Yan G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. Journal of Medical Entomology. 2002;39:833–841. doi: 10.1603/0022-2585-39.6.833. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Medical and Veterinary Journal. 2004;18:301–305. doi: 10.1111/j.0269-283X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko A, et al. Spatial distribution of anopheline larval habitats in western Kenyan highlands: effects of land cover types and topography. American Journal of Tropical Medicine and Hygiene. 2005;73:157–165. [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Yan G. Relationships between occurrence of Anopheles gambiae s.l. (Diptera: Culicidae) and size and stability of larval habitats. Journal of Medical Entomolology. 2005;42:295–300. doi: 10.1603/0022-2585(2005)042[0295:RBOOAG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Molyneux DH. Vector-borne parasitic diseases—an overview of recent changes. International Journal of Parasitology. 1998;28:927–934. doi: 10.1016/S0020-7519(98)00067-8. [DOI] [PubMed] [Google Scholar]
- Mulla MS. The future of insect growth regulators in vector control. Journal of American Mosquito Control Association. 1985;11:269–273. [PubMed] [Google Scholar]
- Mulla MS. Activity, field efficacy and use of Bacillus thuringiensis H-14 against mosquitoes. In: de Barjac H, Southerland DJ, editors. Bacterial Control of Mosquitoes and Black Flies: Biochemistry, Genetics, and Applications of Bacillus thuringiensis and Bacillus sphaericus. New Brunswick NJ: Rutgers University Press; 1990. pp. 134–160. [Google Scholar]
- Munga S, Minakawa N, Zhou G, Barrack OJ, Githeko AK, Yan G. Oviposition site preference and egg hatchability of Anopheles gambiae: effects of land cover types. Journal of Medical Entomology. 2005;42:993–997. doi: 10.1603/0022-2585(2005)042[0993:OSPAEH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OJ, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. American Journal of Tropical Medicine and Hygiene. 2006;74:69–75. [PubMed] [Google Scholar]
- Mushinzimana E, Munga S, Minakawa N, Li L, Feng CC, Bian L, et al. (2006) Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malaria Journal 5:13 [DOI] [PMC free article] [PubMed]
- Mutuku FMM, Alaii JA, Bayoh N, Gimnig JE, Vulule JM, Walker ED, et al. Distribution, description, and local knowledge of larval habitats of Anopheles gambiae s.l. in a village in western Kenya. American Journal of Tropical Medicine and Hygiene. 2006;74:44–53. [PubMed] [Google Scholar]
- Mutuku FMM, Bayoh N, Gimnig JE, Vulule JM, Kamau L, Walker ED, et al. Pupal habitat productivity of Anopheles gambiae complex mosquitoes in a rural village in western Kenya. American Journal of Tropical Medicine and Hygiene. 2006;74:54–61. [PubMed] [Google Scholar]
- Muturi EJ, Shililu JI, Gu W, Jacob BG, Githure JI, Novak R. Larval habitat dynamics and diversity of Culex mosquitoes in rice agro-ecosystem in Mwea, Kenya. American Journal of Tropical Medicine and Hygiene. 2007;76:95–102. [PubMed] [Google Scholar]
- Orodho AB (2006) The role and importance of Napier grass in the smallholder dairy industry in Kenya. Available: http://www.fao.org/ag/AGP/AGPC/doc/Newpub/napier/napier_kenya.htm [accessed Jan 3, 2010]
- Paaijmans KP, Jacobs AFG, Takken W, Heusinkveld BG, Githeko AK, Dicke M, et al. Observations and model estimates of diurnal water temperature dynamics in mosquito breeding sites in western Kenya. Hydrological Processes. 2008;22:4789–4801. doi: 10.1002/hyp.7099. [DOI] [Google Scholar]
- Rao TR. The Anophelines of India. New Delhi, India: Malaria Research Centre (ICMR); 1984. [Google Scholar]
- Rozendaal JA (1997) Vector Control—Methods for Use by Individuals and Communities, Geneva: World Health Organization, 411 pp
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase reaction. American Journal of Tropical Medicine and Hygiene. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Service MW. Mortalities of the immature stages of species b of the Anopheles gambiae complex: comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. Journal of Medical Entomology. 1977;13:535–545. doi: 10.1093/jmedent/13.4-5.535. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquito Ecology: Field Sampling Method. 2. London: Chapman and Hall; 1993. p. 988. [Google Scholar]
- Service MW. Medical Entomology for Students. London: Chapman and Hall; 1996. p. 278. [Google Scholar]
- Sunahara T, Ishizaka K, Mogi M. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. Journal of Vector Ecology. 2002;27:8–20. [PubMed] [Google Scholar]
- Tuno N, Okeka W, Minakawa N, Takagi M, Yan G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in western Kenya highland forest. Journal of Medical Entomology. 2005;42:270–277. doi: 10.1603/0022-2585(2005)042[0270:SOAGSS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Tozan Y, Singer BH. Efficacy and cost effectiveness of environmental management for malaria control. Tropical Medicine and International Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Tanner M, Kammen DM, Killeen GF, Singer BH. Integrated programme is key to malaria control. Nature. 2002;419:431. doi: 10.1038/419431a. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Tozan Y, Doumani F, Singer BH. The economic payoffs of integrated malaria control in the Zambian copper belt between 1930 and 1950. Tropical Medicine and International Health. 2002;7:657–677. doi: 10.1046/j.1365-3156.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- Washburn JO. Regulatory factors affecting larval mosquito populations in container and pool habitats: implications for biological control. Journal of the American Mosquito Control Association. 1995;11:279–283. [PubMed] [Google Scholar]
- Wilson SD, Varia M, Lior LY. West Nile virus: the buzz on Ottawa resident’s awareness, attitudes and practices. Canadian Journal of Public Health. 2005;96:109–113. doi: 10.1007/BF03403672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Manual on Practical Entomology in Malaria. Part. II: Methods and Techniques. Geneva: WHO Division of Malaria and Other Parasitic Diseases; 1975. [Google Scholar]
- Ye-Ebiyo Y, Pollack RJ, Kiszewki A, Spielman A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. American Journal of Tropical Medicine and Hygiene. 2003;68:748–752. [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko A, Yan G. Spatial distribution patterns of malaria vectors and sample size determination in spatially heterogeneous environments: a case study in the west Kenyan highland. Journal of Medical Entomology. 2004;41:1001–1009. doi: 10.1603/0022-2585-41.6.1001. [DOI] [PubMed] [Google Scholar]


