Abstract
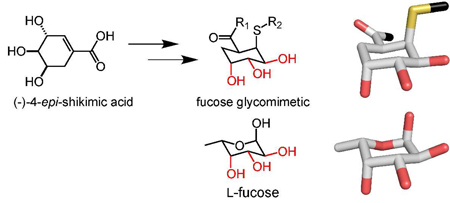
A divergent synthesis of (−)-4-epi-shikimic acid was developed. This route features a one-pot zinc-mediated reductive ring opening of an arabinofuranose followed by a Barbier reaction and culminates in a ring-closing metathesis. Functionalization of (−)-4-epi-shikimic acid via conjugate addition of a thiol occurs in high diastereoselectivity to afford a product with the features of fucosylated glycans.
Carbohydrate–protein interactions are critical for both physiological and pathological processes, including inflammation, immune system function, and host-pathogen interactions.1 These diverse functions are evident in the activities of members of one large class of mammalian carbohydrate-binding proteins–the C-type lectins (CLECs). CLECs have many beneficial roles, as they mediate cell–cell adhesion and facilitate the detection and capture of foreign pathogens. Still, CLEC function can be deleterious. CLECs participate in inflammation,2 and pathogens can co-opt them for dissemination and evasion of immune responses.3 Consequently, compounds that inhibit glycan-CLEC binding events can serve as probes of CLEC function.4
CLECs are targets for glycomimetic design because of their well-defined carbohydrate binding mode. Specifically, CLECs bind calcium ions to which carbohydrate hydroxyl groups chelate.3 This binding mode is not highly specific, as different CLECs can interact with similar oligosaccharides.5 As a result, it is difficult to use naturally occurring glycans to dissect the function of a particular CLEC. Glycomimetics could fill this need if they are selective. Still, the development of CLEC ligands is inherently difficult.4 The carbohydrate binding sites typically are broad, shallow pockets on the lectin surface and binding affinities for monovalent carbohydrate ligands typically are weak (Kd ~ 10−3–4 M). These features offer significant challenges for the development of glycomimetics that are both potent and selective. Despite these challenges, a variety of inhibitors have been identified for CLECs.6 Most approaches involve the use of modified carbohydrates; however, these compounds are challenging to synthesize and they are likely to bind to many CLECs.
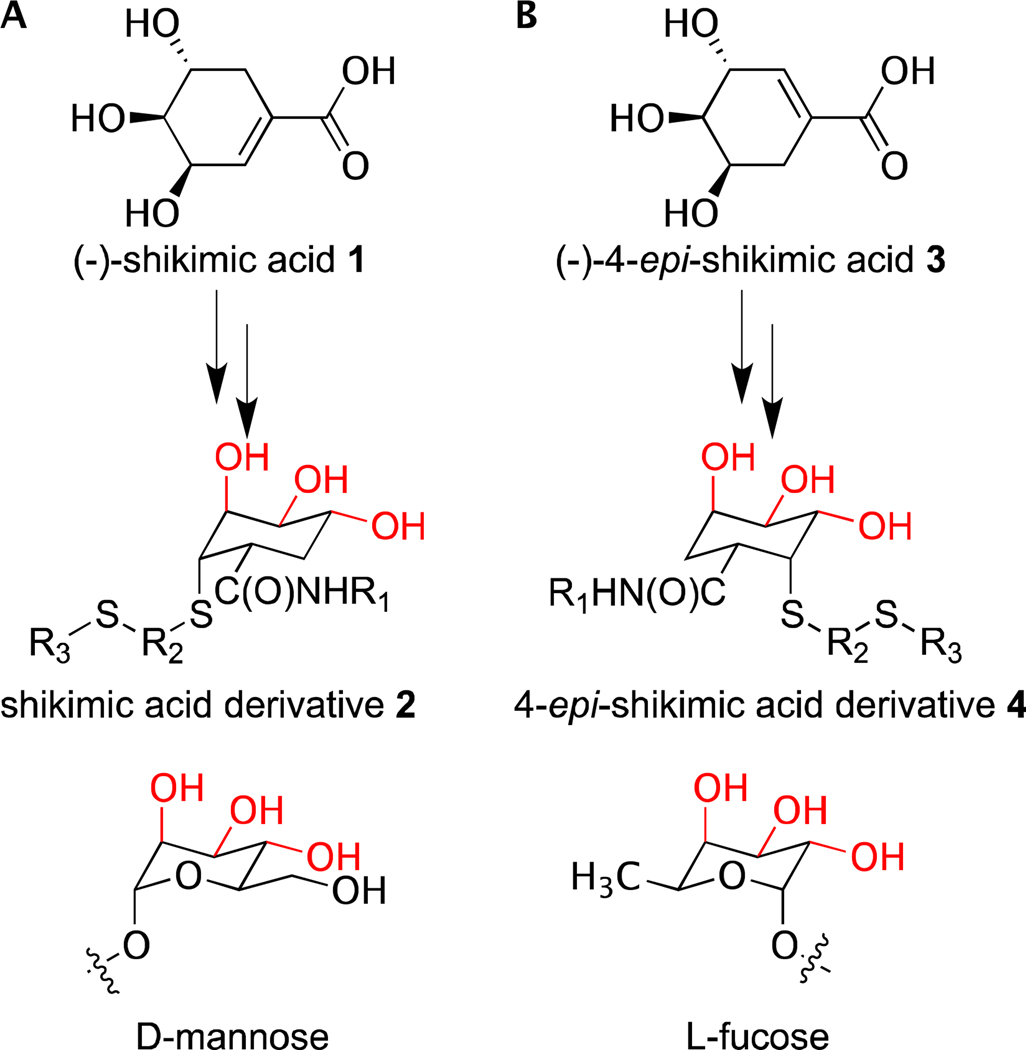
Given the utility of CLEC probes, we sought to develop a general synthetic strategy to assemble non-carbohydrate glycomimetics as CLEC ligands. (−)-Shikimic acid 1 has served as a building block in the synthesis of biologically active compounds.7 Previously, we demonstrated that (−)-shikimic acid 1 can be converted into α-D-mannose mimics.8 We generated active compounds by coupling amino acids to the shikimic acid carboxylate followed by thiol conjugate addition to install a pseudoanomeric group. This approach afforded glycomimetics 2 that have the requisite axial-equatorial-equatorial display of hydroxyl groups present in mannosides (Figure 1a).8a These mimetics also possess additional substituents that can enhance CLEC binding affinity and selectivity. We postulated that epimers of shikimic acid could be used to access scaffolds with the necessary hydroxyl group stereochemistry to mimic other carbohydrates. Like d-mannose, l-fucose posseses an axial-equatorial-equatorial display of hydroxyl groups that it can use for CLEC binding.9 As described, this arrangement mirrors that of shikimic acid, yet shikimic acid falls short as a scaffold for l-fucose mimetics. Shikimic acid lacks a simple means of installing a group that corresponds to the fucose anomeric substituent. Thus, to generate l-fucose surrogates, a different building block is required. We reasoned that (−)-4-epi-shikimic acid 3 could serve in this capacity, if it could be converted to compounds like 4 (Figure 1b). Accordingly, we sought a facile synthesis of (−)-4-epi-shikimic acid.
Figure 1.
Glycomimetic scaffolds designed to mimic (A) mannose and (B) fucose. Hydroxyl groups typically involved in C-type lectin binding are highlighted in red.
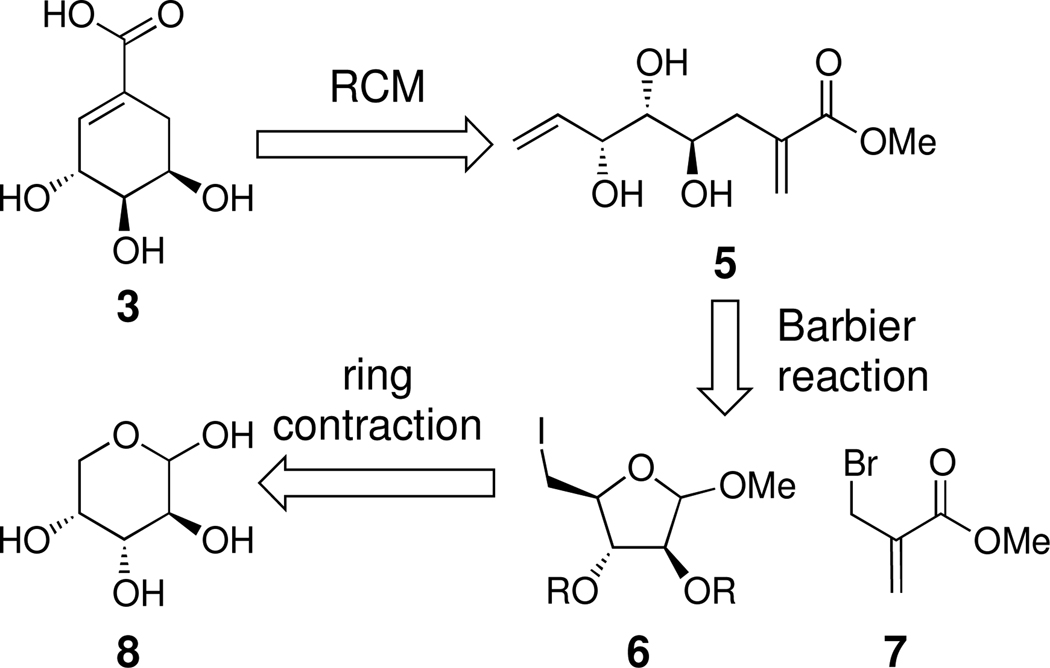
While routes to (−)-4-epi-shikimic acid 3 have been reported,10 our goal was to devise a divergent synthetic strategy that could access any desired epimer. We envisioned using a ring-closing metathesis (RCM) of a trihydroxy precursor such as 5 (Scheme 1). We postulated that 5 could be attained from iodosugar 6 via a tandem reductive ring opening followed by Barbier reaction.11 The iodosugar needed to generate (−)-4-epi-shikimic acid could be obtained from d-arabinose 8. The feasibility of this approach depended upon developing effective conditions for generating the iodosugar derivative and the efficiency of the key steps. Indeed, a concern at the outset was the ring-closing metathesis, as 1,1-disubstituted acrylates are relatively unreactive.
Scheme 1.
Retrosynthetic analysis of (−)-4-epi-shikimic acid.
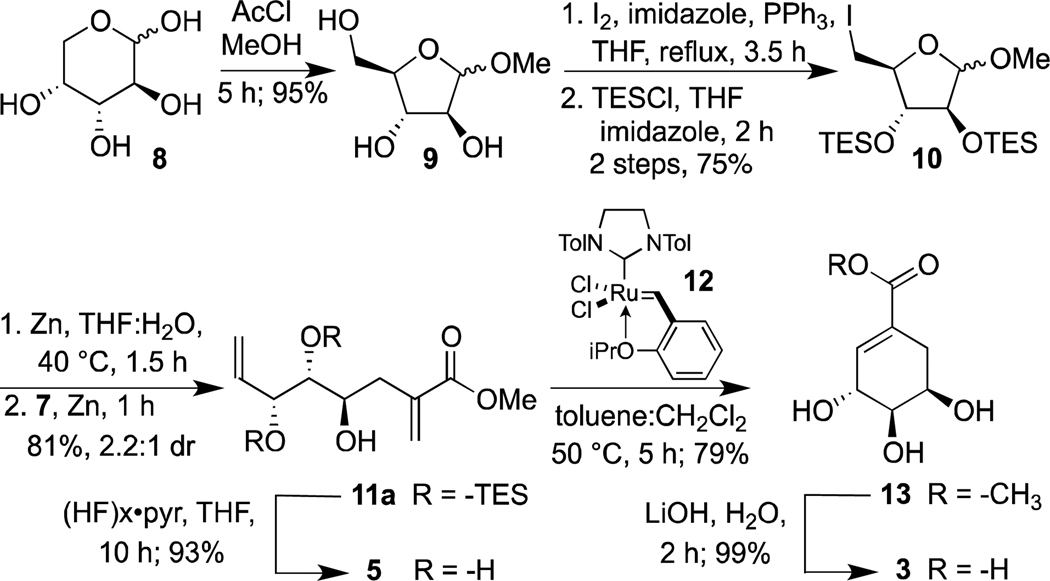
Our first objective was to synthesize the necessary precursor for the Barbier reaction. We initiated our route with a high yielding acid-catalyzed ring-contraction, in which the pyranose form of d-arabinose is converted to furanose 9 (Scheme 2). The primary 5-hydroxyl group was converted into an iodide using Mitsunobu conditions. All remaining hydroxyl groups were masked by triethylsilyl group protection, so that they would not interfere with the subsequent organometallic transformation. In a two-step, one-pot sequence, zinc-mediated reductive ring opening affords an intermediate aldehyde, which is trapped by a Barbier reaction to afford 11a. We examined several conditions to promote this sequence. Reaction sonication improved yields, presumably because it facilitated zinc insertion into the carbon-halogen bond. Derivatives of 10 with alternative hydroxyl protecting groups were tested for increased diastereoselectivity, but triethylsilyl groups afforded the best yields of compound 11a. Upon optimization, the diene was obtained as a mixture of isomers in 81% yield over two steps.
Scheme 2.
Synthesis of (−)-4-epi-shikimic acid. The structure of compound 7 is depicted in Scheme 1.
We next focused on the ring-closing metathesis (RCM) step. Our concerns regarding its potential difficulty were justified. We were unable to find conditions under which 11a would undergo this transformation. We hypothesized that steric demands of the triethylsilyl protecting groups might contribute to the poor reactivity of compound 11a. Ruthenium carbene-catalyzed metathesis reactions can be conducted on substrates with multiple free hydroxyl groups,12 which would remove any steric bias. Moreover, substrates with allylic alcohols undergo more rapid reactions with ruthenium carbene catalysts than do allylic ethers.13 Thus, we removed the protecting groups to afford triol 5 as our metathesis substrate.
Although this substrate did undergo metathesis reactions, it remained reticent toward the desired RCM. When one alkene of the substrate reacts, the high effective molarity of the ruthenium carbene and the remaining alkene should favor a six-membered ring forming intramolecular RCM reaction over an intermolecular cross metathesis (CM).14 The disparity in reactivity of the two alkenes in substrate 5, however, led to CM. Relative alkene reactivities, derived from testing the propensity of a model substrates’ ability to homodimerize, classify the allylic alcohol as a type II alkene (somewhat unreactive) while the 1,1-disubstituted acrylate is considered a much less reactive type III alkene.15 Thus, the type II alkene within substrate 5 can initiate first and then undergo an intermolecular cross metathesis reaction to afford the dimer. This outcome also might be favored by stereoelectronic effects. Specifically, if the substrate adopts a conformation in which the hydroxyl groups are gauche, the intermediate ruthenium carbene and the acrylate would be oriented away from each other. Thus, two features of the substrate might contribute to the sluggishness of the desired ring-closing reaction.
We postulated that if we could drive the reaction of the ruthenium carbene with the type II alkene to completion, we would prevent alkene homodimerization. Indeed, when stoichiometric levels of ruthenium carbene were employed, high yields of the ring-closed product 13 were obtained. Although these conditions were unattractive, they supported our mechanistic hypotheses. Accordingly, we sought conditions that would address this issue as well as a solubility problem. The latter arose because diene 5 is polar and therefore sparingly soluble in toluene, the solvent that gave rise to optimal catalyst reactivity. The conditions that circumvented both of these issues involved adding a solution of diene 5 in dichloromethane (a more effective solvent for the substrate) dropwise to a solution of the to Hoveyda-Grubbs catalyst 12 in toluene.16 These conditions favored the RCM reaction. They allowed for decreased catalyst loading, eliminated the cross-metathesis side reaction, and afforded the product 13 in 79% yield. Saponification proceeded quantitatively to give (−)-4-epi-shikimic acid 3. The overall yield of the synthetic route is 32%.
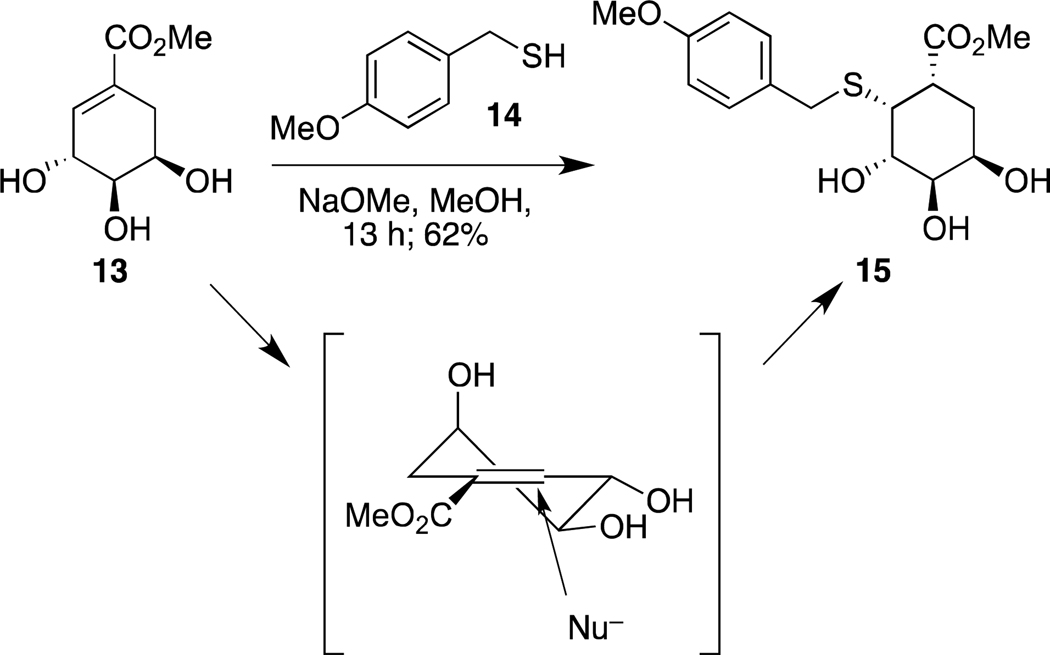
With access to (−)-4-epi-shikimic acid, we investigated its utility for generating compounds that resemble fucose. A key step in our route to shikimic acid-based glycomimetics involves conjugate addition of a thiol.8a The diasteroselectivity of both the addition and the protonation of the resulting enolate determines whether the product will mimic its carbohydrate counterpart. We predicted the thiolate nucleophile would approach the alkene from the re face. The incipient enolate would then be protonated to afford the glycomimetic with the desired stereochemical display to mimic α-l-fucosides. To test this production, we subjected compound 13 to 4-methoxylbenzylthiolate 14. We obtained triol 15 with the correct stereochemical display to mimic α-l-fucosides. The other stereoisomer was not detected. These results provide support for using (−)-4-epi-shikimic acid to access fucose glycomimetics (Scheme 3).
Scheme 3.
Thiolate nucleophiles (Nu) add to compound 13 to give rise to a single diastereomeric product.
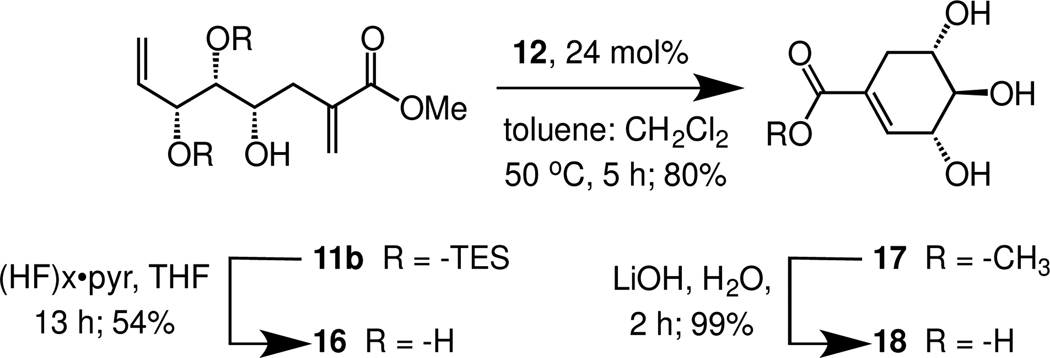
A goal in devising our route to (−)-4-epi-shikimic acid was that it could be used to generate other epimers that might give rise to compounds that mimic other sugars. To verify the adaptability of our approach, we generated (+)-3-epi-shikimic acid 18. We tested the reactivity of 11b in our sequence, as we wanted to ensure that the conditions for RCM that we had developed would be effective for related triols (Scheme 4). Minimal optimization was required, and compound 18 was readily obtained. This observation highlights the robustness of our synthetic route.
Scheme 4.
Synthesis of (+)-3-epi-shikimic acid.
In summary, we synthesized (−)-4-epi-shikimic acid 3 in 32% overall yield. Our 7-step route is comparable to the most efficient synthesis reported,8b but it also has the key advantage of adaptability; it can be used to generate a variety of epimers of shikimic acid. Our interest in shikimic acid dervatives is rooted in their abilities to serve as building blocks for glycomimetics. To lay the groundwork for producing compounds that mimic fucose, we demonstrated that conjugate addition of a thiolate to (−)-4-epi-shikimic acid 3 proceeds with high diastereoselectivity to afford a compound with the critical features of α-l-fucosides.
Supplementary Material
Acknowledgment
This research was supported by the NIGMS (R01 GM049975). In addition, we acknowledge support for the UW-Madison Chemistry NMR facility (National Science Foundation, CHE-9208403) and the Mass Spectrometry Facility (NSF, CHE-9974839). KCAG was supported by a NSF Graduate Research Fellowship and the NIH Chemistry– Biology Interface Training Grant (T32 GM008505). We thank A. Zwicker (UW-Madison) and M. Martinez (UW-Madison) for carrying out preliminary experiments.
Footnotes
Supporting Information Available. Detailed experimental procedures and full characterization of compounds is provided online in the supplemental notes. his material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Varki A. Glycobiology. 1993;3:97. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasky LA. Annu. Rev. Biochem. 1995;64:113–140. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 3.Geijtenbeek TB, van Vliet SJ, Engering A, A'T Hart BA, van Kooyk Y. Annu. Rev. Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 4.Kiessling LL, Splain RA. Annu. Rev. Biochem. 2010;79:619–653. doi: 10.1146/annurev.biochem.77.070606.100917. [DOI] [PubMed] [Google Scholar]
- 5.Lee RT, Hsu T, Huang SK, Hsieh S, Wong C, Lee YC. Glycobiology. 2011;21:512–520. doi: 10.1093/glycob/cwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Ernst B, Magnani JL. Nat. Rev. Drug Discovery. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sattin S, Daghetti A, Thepaut M, Berzi A, Sanchez-Navarro M, Tabarani G, Rojo J, Fieschi F, Clerici M, Bernardi A. ACS Chem. Biol. 2010;5:301–312. doi: 10.1021/cb900216e. [DOI] [PubMed] [Google Scholar]; (c) Pagé D, Roy R. Bioconjugate Chem. 1997;8:714–723. doi: 10.1021/bc970126u. [DOI] [PubMed] [Google Scholar]; (d) Smelt KH, Harrison AJ, Biggadike K, Müller M, Prout K, Watkin DJ, Fleet GWJ. Tetrahedron Lett. 1999;40:3259–3262. [Google Scholar]; (e) Andreini M, Anderluh M, Audfray A, Bernardi A, Imberty A. Carbohydr. Res. 2010;345:1400–1407. doi: 10.1016/j.carres.2010.03.012. [DOI] [PubMed] [Google Scholar]; (f) Imberty A, Chabre YM, Roy R. Chem. Eur. J. 2008;14:7490–7499. doi: 10.1002/chem.200800700. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tan DS, Foley MA, Stockwell BR, Shair MD, Schreiber SL. J. Am. Chem. Soc. 1999;121:9073–9087. [Google Scholar]; (b) Nie L, Shi X, Ko K, Lu W. J. Org. Chem. 2009;74:3970–3973. doi: 10.1021/jo900218k. [DOI] [PubMed] [Google Scholar]; (c) Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY. J. Am. Chem. Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]; (d) Srinivas R, Karmali PP, Pramanik D, Garu A, Venkata Mahidhar Y, Majeti BK, Ramakrishna S, Srinivas G, Chaudhuri A. J. Med. Chem. 2010;53:1387–1391. doi: 10.1021/jm901295s. [DOI] [PubMed] [Google Scholar]
- 8.(a) Schuster MC, Mann DA, Buchholz TJ, Johnson KM, Thomas WD, Kiessling LL. Org. Lett. 2003;5:1407–1410. doi: 10.1021/ol0340383. [DOI] [PubMed] [Google Scholar]; (b) Garber KCA, Wangkanont K, Carlson EE, Kiessling LL. Chem. Commun. 2010;46:6747–6749. doi: 10.1039/c0cc00830c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Nat. Struct. Mol. Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 10.(a) Snyder CD, Rapoport H. J. Am. Chem. Soc. 1973;95:7821–7828. doi: 10.1021/ja00782a067. [DOI] [PubMed] [Google Scholar]; (b) Pornpakakul S, Pritchard RG, Stoodley RJ. Tetrahedron Lett. 2000;41:2691–2694. [Google Scholar]; (c) Sánchez-Abella L, Fernández S, Armesto N, Ferrero M, Gotor V. J. Org. Chem. 2006;14:5397–5399. doi: 10.1021/jo0606249. [DOI] [PubMed] [Google Scholar]
- 11.Hyldtoft L, Madsen R. J. Am. Chem. Soc. 2000;122:8444–8452. [Google Scholar]
- 12.Kanai M, Mortell KH, Kiessling LL. J. Am. Chem. Soc. 1997;119:9931–9932. [Google Scholar]
- 13.Hoye TR, Zhao H. Org. Lett. 1999;1:1123–1125. doi: 10.1021/ol990947+. [DOI] [PubMed] [Google Scholar]
- 14.Samojlowicz C, Bieniek M, Grela K. Chem. Rev. 2009;109:3708–3742. doi: 10.1021/cr800524f. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee AK, Choi T, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 16.(a) Stewart IC, Ung T, Pletnev AA, Berlin JM, Grubbs RH, Schrodi Y. Org. Lett. 2007;9:1589–1592. doi: 10.1021/ol0705144. [DOI] [PubMed] [Google Scholar]; (b) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.