Abstract
In this investigation several HIV protease inhibitors (PIs) altered the virally associated, dsRNA- stimulated, innate immune response. Lopinavir, the most potent inducer of IL-8 expression, also inhibited dsRNA-induced MCP-1 expression. Further analyses demonstrated that NF-κB is required for lopinavir’s induction of IL-8. These findings demonstrate that PIs, such as lopinavir, differentially dysregulate innate immune signaling in a manner that could affect immune (reconstitution) inflammatory responses in oral epithelium.
HIV protease inhibitors (PIs) are components of highly active antiretroviral therapy (HAART) that contribute significantly to the reduction of HIV-associated morbidity and mortality [1, 2]. Despite the benefits, severe and/or atypical symptoms of previously subclinical infections occur in > 20% of patients on HAART [3]. This phenomenon, referred to as immune reconstitution inflammatory syndrome, frequently manifests at epithelial sites [4, 5]. Epithelial eruptions associated with this syndrome often result from the emergence of persistent viruses, such as human papillomavirus (HPV) or human herpesviruses [6]. One possible explanation is that components of HAART may exacerbate inflammatory responses to viruses in epithelial cells. We therefore evaluated the impact of PIs on innate immune signaling in oral epithelial cells following exposure to double-stranded RNA (dsRNA), a virus-associated ligand for several cellular pattern recognition receptors, including Toll-like receptor (TLR)3.
Immortalized oral keratinocyte OKF6/hTERT-2 (OKF6) cells, which have normal growth and differentiation characteristics that represent a model of oral epithelium [7], were cultured as previously described [8]. Cells were treated with varying doses of PIs, 1 μg/ml poly I:C, a synthetic dsRNA, and/or 10 μM Bay11-7082 as indicated. RNA was extracted, reverse transcribed and quantified by quantitative real-time RT-PCR (qRT-PCR) [9]. Primers and probes for IL-8, RANTES, TNF and GAPDH were previously described [9]. TLR3 and MCP-1 TaqMan gene expression assays were purchased from Applied Biosystems (Foster City, CA). Relative mRNA levels were calculated by (2−(CT Test - CT GAPDH)) X 100% using GAPDH as the reference gene. Secreted protein levels were measured using Luminex IS-100 and kits from Beadlyte Technology (Millipore, Billerica, MA) as previously described [10]. NF-kB activity was analyzed as previously described [9], by transfection of an NF-κB reporter plasmid that contains four copies of a consensusNF-κB-binding site upstream of a minimal promoter and the firefly luciferase gene (Agilent Technologies, Inc., Santa Clara, CA). Statistical differences were determined by ANOVA and Fisher’s protected least significant difference test.
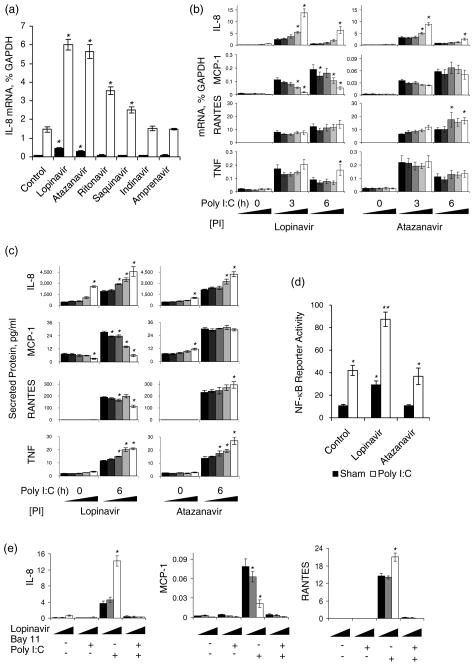
Expression of the chemokine IL-8, an early and sensitive indicator of innate immune responses, was evaluated in response to treatment with HIV PIs and dsRNA. OKF6 cells were treated for 30 h with PIs at concentration ranges found in human plasma [11,12], then treated with dsRNA for the final 3 h (Fig. 1). At the highest dose, lopinavir and atazanavir increased IL-8 mRNA 4.4-fold and 2.8-fold, respectively, and synergized with dsRNA (Fig. 1a). Although ritonavir and saquinavir alone did not alter IL-8 expression, these PIs significantly enhanced the response to dsRNA. In contrast, indinavir and amprenavir did not alter IL-8 expression.
Fig. 1. HIV protease inhibitors alter dsRNA-induced expression of innate immune modulators in oral epithelial cells.
(a) Analysis of IL-8 mRNA following treatment of OKF6 for 30 h with the indicated PI (20 μM), in the presence (open bars) or absence (filled bars) of poly I:C for the final 3 h. (b and c) Analysis of mRNA (b) and secreted protein (c) for innate immune modulators following treatment of OKF6 cells for 30 h with 0, 2.5, 5.0, 10 or 20 μM of lopinavir or atazanavir in the presence and absence of poly I:C for the final 3 and 6 h. (d) Analysis of NF-κB-dependent reporter gene activity following treatment of OKF6 cells for 30 h with 20 μM lopinavir or atazanavir in the presence or absence of poly I:C for the final 6 h . (e) Analysis of chemokine mRNA expression following treatment of OKF6 cells for 30 h with 0, 5 or 20 μM of lopinavir, in the presence and absence of the NF-κB inhibitor Bay 11 for the final 3.5 h and poly I:C for the final 3 h. Data are expressed as mean +/− SEM (n = 4, for panels a, b, c and e, n≥8 for panel d). *The mean for cells cultured in the presence of PIs is significantly different from the mean for cells cultured in the absence of PIs, under the same conditions of dsRNA and/or Bay 11 treatment (P < 0.05). **The mean for cells treated with the combination of dsRNA and lopinavir is significantly greater than the mean for cells treated with either stimulus alone (P < 0.05) (panel d).
We further characterized dose- and time-dependent effects of lopinavir and atazanavir on additional mediators of the innate immune response. Expression of TLR3 was unaffected by these PIs, indicating that up-regulation of this receptor for dsRNA was not responsible for the PI-enhanced expression of IL-8 (data not shown). IL-8 expression increased rapidly in a dose-dependent manner following stimulation with dsRNA and declined by 6 h of treatment (Fig. 1b), as previously demonstrated in intestinal epithelial cells [9]. The dsRNA-stimulated increase in MCP-1 and RANTES mRNA was slower than that of IL-8, consistent with the delayed response of these chemokines in innate immune responses [13]. In contrast to its effect on IL-8, lopinavir but not atazanavir inhibited dsRNA-induced MCP-1 expression in a dose-dependent manner. The highest dose of lopinavir also resulted in sustained expression of IL-8 and TNF expression at 6 h post-stimulation. Levels of secreted IL-8, MCP-1 and RANTES paralleled changes in their respective mRNAs, whereas PI and dsRNA-stimulated secretion of TNF was enhanced to a greater extent than was TNF mRNA(Fig. 1c).
Because activation of the NF-κB signaling pathway is required for induction of IL-8 by TLR3 signaling [14], we hypothesized that lopinavir synergizes with dsRNA signaling to enhance activation of NF-κB. To test this hypothesis, cells were transfected with an NF-κB reporter plasmid and treated with lopinavir and/or dsRNA. Treatment of OKF6 cells with lopinavir or dsRNA alone, but not atazanavir, caused a significant increase in NF-κB dependent gene expression (Fig. 1d). Importantly, the combination of lopinavir and dsRNA caused a greater increase in NF-κB activity than did either stimulus alone, similar to the synergistic effect of these stimuli on IL-8 mRNA (Fig. 1a). Consistent with this finding, induction of IL-8, RANTES and MCP-1 expression by dsRNA and/or lopinavir was completely blocked by Bay 11-7082 (Fig. 1e), a drug that selectively and irreversibly inhibits inducible phosphorylation of IκBα without affecting constitutive IκBα phosphorylation [15]. The finding that lopinavir causes NF-κB activation may be unique to oral epithelial cells, as PIs have been reported to prevent activation of NF-κB in other cell types [16–19].
These data demonstrate that select PIs alter the pattern of chemokine expression and interfere with the negative regulation of pro-inflammatory factors during dsRNA-induced signaling in oral epithelial cells. Surprisingly, we found that lopinavir in conjunction with dsRNA enhanced expression of IL-8 but inhibited expression of MCP-1. Safronova et al. reported that hypoxia, a condition frequently associated with tissue inflammation, had similar opposing effects on induction of IL-8 and MCP-1 expression in human epithelial cells [20]. These investigators found that NF-κB dependent activation of histone deacetylases was responsible for hypoxia-induced repression of MCP-1 expression. Although extrapolation of these in vitro findings to the in vivo setting should be undertaken with caution, exacerbated IL-8 expression caused by PIs could favor the recruitment of neutrophils, whereas down-regulation of MCP-1 could inhibit recruitment of monocytes to sites of epithelial inflammation [21–23]. Significantly, reduced infiltration of monocytes and impaired monocyte function are features of persistent HPV-associated warts [24–29], which have been reported in HIV+ patients on HAART [30–32]. Furthermore, infiltrating monocytes and tissue macrophages in epithelia are critical for antiviral activity [33] and the development of a Th1-dominated cellular immune response, both of which contribute to clearing of HPV infected cells and resolution of warts [25, 34].
In summary, a novel implication of this research is that individual PIs may dysregulate the innate immune response in ways that could exacerbate epithelial viral infections in patients receiving HAART.
Acknowledgments
This study was supported by grants from the National Institute of Health R21DE018332 (CSM) and R21AI069027 (CSK), and a Senior Research Award from the Crohn’s & Colitis Foundation of America (CSK). HIV PIs were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program: Ritonavir, Amprenavir, Lopinavir, Saquinavir, Indinavir Sulfate and Atazanavir Sulfate.
References
- 1.Miller V, Sabin CA, Phillips AN, Rottmann C, Rabenau H, Weidmann E, et al. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS. 2000;14:2129–2136. doi: 10.1097/00002030-200009290-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, Montaner JS. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 3.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57:167–170. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 4.Feller L, Wood NH, Lemmer J. Herpes zoster infection as an immune reconstitution inflammatory syndrome in HIV-seropositive subjects: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:455–460. doi: 10.1016/j.tripleo.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lehloenya R, Meintjes G. Dermatologic manifestations of the immune reconstitution inflammatory syndrome. Dermatol Clin. 2006;24:549–570. vii. doi: 10.1016/j.det.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Meys R, Gotch FM, Bunker CB. Human papillomavirus in the era of highly active antiretroviral therapy for human immunodeficiency virus: an immune reconstitution-associated disease? Br J Dermatol. 2010;162:6–11. doi: 10.1111/j.1365-2133.2009.09365.x. [DOI] [PubMed] [Google Scholar]
- 7.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danaher RJ, Wang C, Roland AT, Kaetzel CS, Greenberg RN, Miller CS. HIV protease inhibitors block oral epithelial cell DNA synthesis. Arch Oral Biol. 2010;55:95–100. doi: 10.1016/j.archoralbio.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175:376–384. doi: 10.4049/jimmunol.175.1.376. [DOI] [PubMed] [Google Scholar]
- 10.Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: A feasibility study. Clin Chem. 2009;55:1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acosta EP, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retroviruses. 2002;18:825–34. doi: 10.1089/08892220260190290. [DOI] [PubMed] [Google Scholar]
- 12.Justesen US. Therapeutic drug monitoring and human immunodeficiency virus (HIV) antiretroviral therapy. Basic Clin Pharmacol Toxicol. 2006;98:20–31. doi: 10.1111/j.1742-7843.2006.pto_246.x. [DOI] [PubMed] [Google Scholar]
- 13.Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469–1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 14.Berube J, Bourdon C, Yao Y, Rousseau S. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal. 2009;21:448–456. doi: 10.1016/j.cellsig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 16.Equils O, Shapiro A, Madak Z, Liu C, Lu D. Human immunodeficiency virus type 1 protease inhibitors block toll-like receptor 2 (TLR2)- and TLR4-Induced NF-kappaB activation. Antimicrob Agents Chemother. 2004;48:3905–3911. doi: 10.1128/AAC.48.10.3905-3911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62:5230–5235. [PubMed] [Google Scholar]
- 18.Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS, Jr, Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002;99:3771–3779. doi: 10.1182/blood.v99.10.3771. [DOI] [PubMed] [Google Scholar]
- 19.Piccinini M, Rinaudo MT, Chiapello N, Ricotti E, Baldovino S, Mostert M, Tovo PA. The human 26S proteasome is a target of antiretroviral agents. AIDS. 2002;16:693–700. doi: 10.1097/00002030-200203290-00004. [DOI] [PubMed] [Google Scholar]
- 20.Safronova O, Pluemsampant S, Nakahama K, Morita I. Regulation of chemokine gene expression by hypoxia via cooperative activation of NF-kappaB and histone deacetylase. Int J Biochem Cell Biol. 2009;41:2270–2280. doi: 10.1016/j.biocel.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, et al. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol. 2001;70:113–120. [PubMed] [Google Scholar]
- 23.Kleine K, Konig G, Kreuzer J, Komitowski D, Zur Hausen H, Rosl F. The effect of the JE (MCP-1) gene, which encodes monocyte chemoattractant protein-1, on the growth of HeLa cells and derived somatic-cell hybrids in nude mice. Mol Carcinog. 1995;14:179–189. doi: 10.1002/mc.2940140307. [DOI] [PubMed] [Google Scholar]
- 24.Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 25.Tagami H, Oku T, Iwatsuki K. Primary tissue culture of spontaneously regressing flat warts. In vitro attack by mononuclear cells against wart-derived epidermal cells. Cancer. 1985;55:2437–2441. doi: 10.1002/1097-0142(19850515)55:10<2437::aid-cncr2820551023>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Iwatsuki K, Tagami H, Takigawa M, Yamada M. Plane warts under spontaneous regression. Immunopathologic study on cellular constituents leading to the inflammatory reaction. Arch Dermatol. 1986;122:655–659. doi: 10.1001/archderm.122.6.655. [DOI] [PubMed] [Google Scholar]
- 27.Arany I, Tyring SK. Status of local cellular immunity in interferon-responsive and - nonresponsive human papillomavirus-associated lesions. Sex Transm Dis. 1996;23:475–480. doi: 10.1097/00007435-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Viac J, Chardonnet Y, Chignol MC, Schmitt D. Papilloma viruses, warts, carcinoma and Langerhans cells. In Vivo. 1993;7:207–212. [PubMed] [Google Scholar]
- 29.Feng JY, Peng ZH, Tang XP, Geng SM, Liu YP. Immunohistochemical and ultrastructural features of Langerhans cells in condyloma acuminatum. J Cutan Pathol. 2008;35:15–20. doi: 10.1111/j.1600-0560.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001;357:1411–1412. doi: 10.1016/S0140-6736(00)04578-5. [DOI] [PubMed] [Google Scholar]
- 31.Leigh J. Oral warts rise dramatically with use of new agents in HIV. HIV Clin. 2000;12:7. [PubMed] [Google Scholar]
- 32.King MD, Reznik DA, O'Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34:641–648. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- 33.Renn CN, Sanchez DJ, Ochoa MT, Legaspi AJ, Oh CK, Liu PT, et al. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- 34.Hengge UR, Cusini M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. Br J Dermatol. 2003;149 (Suppl 66):15–19. doi: 10.1046/j.0366-077x.2003.05623.x. [DOI] [PubMed] [Google Scholar]