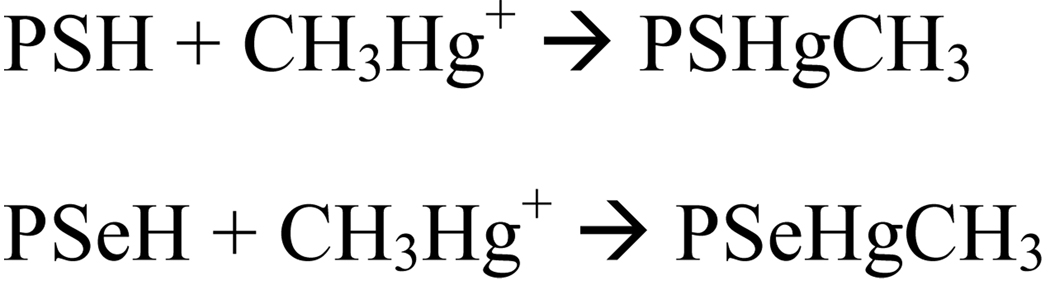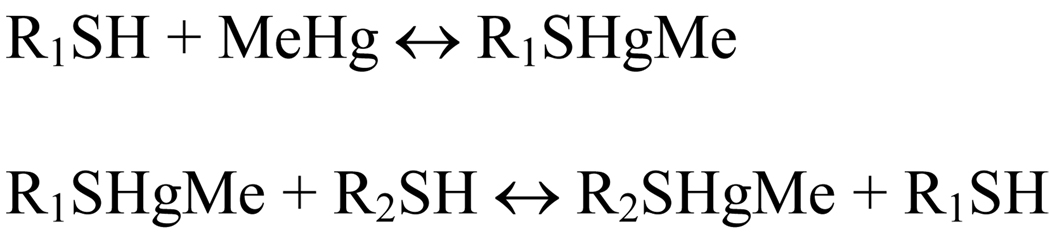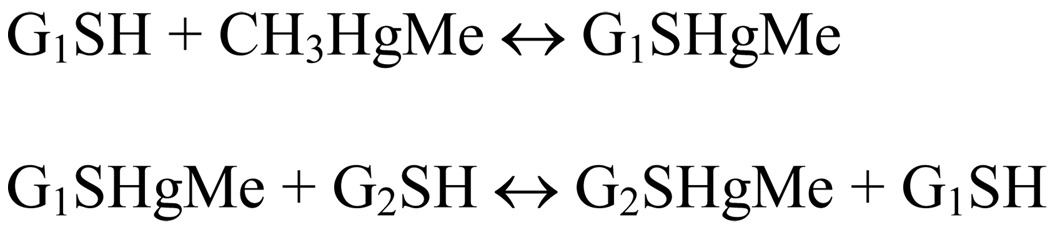Abstract
Methylmercury (MeHg) is an environmental toxicant that leads to long-lasting neurological and developmental deficits in animals and humans. Although the molecular mechanisms mediating MeHg-induced neurotoxicity are not completely understood, several lines of evidence indicate that oxidative stress represents a critical event related to the neurotoxic effects elicited by this toxicant. The objective of this review is to summarize and discuss data from experimental and epidemiological studies that have been important in clarifying the molecular events which mediate MeHg-induced oxidative damage and, consequently, toxicity. Although unanswered questions remain, the electrophilic properties of MeHg and its ability to oxidize thiols have been reported to play decisive roles to the oxidative consequences observed after MeHg exposure. However, a close examination of the relationship between low levels of MeHg necessary to induce oxidative stress and the high amounts of sulfhydryl-containing antioxidants in mammalian cells (e.g., glutathione) have led to the hypothesis that nucleophilic groups with extremely high affinities for MeHg (e.g., selenols) might represent primary targets in MeHg-induced oxidative stress. Indeed, the inhibition of antioxidant selenoproteins during MeHg poisoning in experimental animals has corroborated this hypothesis. The levels of different reactive species (superoxide anion, hydrogen peroxide and nitric oxide) have been reported to be increased in MeHg-exposed systems, and the mechanisms concerning these increments seem to involve a complex sequence of cascading molecular events, such as mitochondrial dysfunction, excitotoxicity, intracellular calcium dyshomeostasis and decreased antioxidant capacity. This review also discusses potential therapeutic strategies to counteract MeHg-induced toxicity and oxidative stress, emphasizing the use of organic selenocompounds, which generally present higher affinity for MeHg when compared to the classically studied agents.
Keywords: methylmercury, oxidative stress, neurotoxicity, selenol, thiol, glutamate, selenoproteins
1 - Introduction
Methylmercury (MeHg; CH3Hg+) is an organomercurial pollutant primarily found in the aquatic environment (Ullrich et al., 2001). Although MeHg has been synthesized in the laboratory (Bancon-Montigny et al., 2004) and used in laboratory-based research (Junghans, 1983) and as a fungicide in seed grains (Bakir et al., 1973), the majority of MeHg present in nature is derived from inorganic mercury biomethylation carried out primarily by aquatic microorganisms (Compeau and Bartha, 1985). Anaerobic sulfate-reducing bacteria exposed to inorganic mercury (released in the aquatic environment mostly from anthropogenic sources) convert it to MeHg, which presents an enormous biomagnification potential: MeHg is accumulated by more than seven orders of magnitude from sub ng/L concentrations in water to over 1 mg/kg in piscivorous fish (Hintelmann, 2010). As a consequence of this phenomenon, human populations whose diets consist largely of fish and shellfish are exposed to high MeHg levels (Clarkson et al., 2003), thus rendering these communities highly vulnerable to MeHg-induced toxicity.
Given that seafood represents a major source of human exposure (Clarkson et al., 2003), the high rate of MeHg absorption in the gastrointestinal tract (around 90–95%) significantly contributes to its high availability and, consequently, toxicity (Nielsen, 1992). Although MeHg is distributed among various tissues after absorption (Zareba et al., 2007), the central nervous system (CNS) is the most sensitive target organ for MeHg, principally when exposures occur during the initial stages of brain development (Marsh et al., 1995; Costa et al., 2004; Johansson et al. 2007; Hassan et al. 2011; Grandjean 2011). Experimental studies with rodents have shown that the developing CNS of fetuses or pups is vastly more susceptible to the neurotoxic effects of MeHg when compared to adult animals (Manfroi et al., 2004; Franco et al., 2006). Corroborating these experimental findings, epidemiological studies have also found severe and permanent neuropsychological outcomes in humans exposed to this pollutant during the prenatal and/or early postnatal period (Grandjean et al., 1997; Murata et al., 2004; Debes et al., 2006).
Several studies performed during the last four decades have contributed to the understanding of pivotal events that mediate MeHg-induced neurotoxicity. Such studies, which were based mainly on in vitro approaches or experimental protocols with animals, have described critical phenomena that mediate MeHg toxicity, such as the depletion of intracellular antioxidants (Fujiyama et al., 1994; Johansson et al. 2007; Franco et al., 2007; Kaur et al., 2006), the inhibition of critical enzymes (Magour et al., 1986; Rocha et al., 1993; Kung et al., 1987; Franco et al., 2009; Valentini et al. 2010; Wagner et al., 2010; Branco et al. 2011) and the modulation of the activity of transporter and neurotransmitter or neuromodulator receptor activity (Aschner et al., 1993; Farina et al., 2003a; Fitsanakis and Aschner, 2005; Yin et al., 2007; 2011; Sakaue et al. 2009). In effect, even a limited targeting of critical proteins by MeHg can initiate a cascade of cellular events that can create long-lasting alterations in normal cell physiology. In addition, recent literature data have clearly indicated that exposure to low levels of MeHg can modify gene expression and can cause long-lasting changes in cell signaling (Toyama et al. 2007 Yin et al. 2007; Onishchenko et al. 2008; Usuki et al. 2008; Rand et al. 2008; Glover et al. 2009; Wang et al. 2009; Jayashankar et al. 2011; Zimmer et al. 2011; Ni et al. 2011; Robinson et al. 2011; Yu et al. 2011; Shimada et al. 2011). However, our understanding of the primary critical targets of MeHg, namely those which trigger MeHg neurotoxicity, remains incipient.
The events mediating MeHg neurotoxicity are largely dependent upon its electrophilic properties, which allows for its interaction with soft nucleophilic groups (mainly thiols and selenols) from either low- or high-molecular-weight biomolecules (Farina et al., 2011). The interaction of MeHg with soft nucleophilic groups from biomolecules is responsible, at least in part, for decreased antioxidant capacity and increased reactive oxygen species (ROS) generation (Aschner et al. 2010; Kaur et al., 2006; Franco et al., 2007, Farina et al., 2009, Farina et al., 2010). These events corroborate observations that oxidative stress, which has been defined as “a disturbance in the pro-oxidant/antioxidant balance in favor of the former” (Sies, 1991), represents a central event in mediating MeHg-induced neurotoxicity (Aschner et al., 2007). In effect, the in vitro and in vivo neurotoxicity of MeHg can be observed in the nM to the low µM range, whereas the concentration of thiol groups in the central nervous system is in the mM range. Consequently, MeHg cannot be expected to stoicheometrically deplete thiols. However, the levels of GSH, which is the most important and abundant low-molecular-weight thiol found in mammals, can be depleted after MeHg intoxication. One plausible explanation for these observations is that MeHg targets some specific thiol- and selenol-containing proteins that trigger secondary molecular mechanism(s) involved in MeHg neurotoxicity. Notably, MeHg can disrupt the activity of thiol- and selenol-containing proteins, such as glutathione peroxidase (Gpx), thioredoxin (Trx) and thioredoxin reductase (TrxR) (Carvalho et al. 2008; 2010; Farina et al. 2009; Franco et al. 2009; Glaser et al. 2010a; Wagner et al. 2010; Branco et al. 2011). These proteins are important components of the cellular antioxidant system, and their inhibition contributes to the disruption of the normal redox balance of brain cells (Farina et al. 2011).
Oxidative stress-mediated damage has been associated with several pathological conditions, such as cancer, atherosclerosis, rheumatoid arthritis, diabetics, post-ischemic perfusion injury, cardiovascular diseases, myocardial infarction, chronic inflammation, stroke, aging and neurodegenerative diseases (Coyle and Puttfarcken, 1993; Fang et al., 2002). Of particular importance, the pro-oxidative properties of several environmental factors have been postulated to play a critical role in the development of neurodegenerative diseases (Potashkin and Meredith, 2006). With emphasis on the neurotoxicity induced by MeHg, experimental evidence indicates that oxidative damage to proteins (Vogel et al., 1985; Rocha et al., 1993), lipids (Wootten et al., 1985; Stringari et al., 2006) and nucleic acids (Belletti et al., 2002) represents an important consequence of exposure to this toxicant. However, although the critical role of oxidative stress in MeHg neurotoxicity has been identified, the precise molecular mechanisms underlying MeHg-mediated oxidative stress are not yet fully understood. As detailed above, the inhibition of antioxidant selenoenzymes, such as GPx and TrxR, can be a primary event in MeHg neurotoxicity, setting in motion a cascade of molecular phenomena culminating in oxidative stress and ultimately leading to cell death (Farina et al. 2011). This review discusses current knowledge on the mechanisms of MeHg-induced oxidative stress and neurotoxicity. The following four major focal points will be addressed: (i) the potential molecular and cellular targets of MeHg; (ii) the main reactive oxygen/nitrogen species that mediate MeHg neurotoxicity; (iii) the molecular oxidative hallmarks; and (iv) the potential use of antioxidant therapy to counteract MeHg-induced neurotoxicity.
2 - MeHg-induced oxidative damage
The initial oxidative damage caused by MeHg in living organisms occurs via its reaction with thiol (-SH) and/or selenol (-SeH) groups from endogenous molecules, resulting in the formation of a very stable complex of the type RSHgCH3 or RSeHgCH3 (Aschner et al. 2011; Farina et al. 2011). In the case of thiol- or selenol-containing proteins and enzymes, the formation of the S-Hg or Se-Hg bond can cause impairment in protein function (Farina et al. 2009; Franco et al. 2009; Glaser et al. 2010a,b; Rocha et al. 1993) or can form inert deposits of cysteyl rich proteins (Barbosa et al. 2001; Dorea 2009). Chemically, MeHg is classified as soft electrophile and, consequently, it reacts preferentially with soft nucleophiles (Pearson and Songstad, 1967). From a physiological point of view, this means that MeHg can react with –SH (sulfhydryl or thiol groups) and with -SeH (selenohydryl or selenol groups), which are the two types of soft nucleophiles found in living organisms. Indeed, MeHg has such high affinity for thiol and selenol groups (Simpson, 1961; Sigiura et al. 1976; Onyido et al. 2004) that finding free MeHg inside a living organism is highly unlikely. It is important to emphasize that selenol groups are softer nucleophiles than thiol groups; consequently, the affinity of MeHg for selenol is expected to be higher than that for an analog thiol (Sigiura et al. 1976; Khan et al. 2009; Khan and Wang, 2009). However, data about the constant affinities of MeHg for thiol- or selenol-containing proteins are rare or lacking in the literature. In effect, there are only limited comparative data regarding the interaction of low-weight molecular thiol and selenol analogs with MeHg (Sigiura et al., 1976). These authors have demonstrated that the affinity of selenocysteamine to MeHg is higher than that of the cysteamine analog (Sigiura et al., 1976). Thus, the identification of the potential in vivo primary high-molecular targets of MeHg, i.e., the proteins that could preferentially be oxidized by MeHg, will require the determination of the affinity constants of MeHg for specific thiolate or selenolate groups in target proteins.
Hg is the softest electrophile from its periodic group or family; consequently, MeHg is a strong soft acid or electrophile that reacts with high affinity with soft bases or nucleophiles. Considering the fact that selenium is a softer element than sulfur, selenol-containing molecules are expected to be softer nucleophiles than thiol- containing molecules. This implies that selenol groups from selenoproteins should be the primary targets of MeHg. Thus, in addition to thiol-containing proteins, which are classically known to be molecular targets of MeHg (Clarkson, 1972; Clarkson and Magos, 2006 ; Farina et al. 2011), selenoenzymes (and possibly other selenoproteins) are also important targets of MeHg (Carvalho et al. 2008; 2010; Farina et al. 2009, 2011; Wagner et al. 2010; Branco et al. 2011). The reaction of MeHg (CH3Hg+) with these targets is schematically represented below (Scheme 1):
Scheme 1.
Reaction of MeHg (CH3Hg+) with its two main cellular high-molecular generic targets, i.e., thiol- and selenol-containing proteins (PSH and PSeH, respectively).
As detailed above, due to its high reactivity with thiols and selenols, MeHg would be expected to be found in living cells bound to these groups. However, thiols are much more abundant than selenols (Nogueira and Rocha, 2010). In fact, thiol groups can be found in low-molecular- (mainly cysteine and reduced glutathione) and high-molecular-weight proteins, whereas selenol groups are found only in a restricted group of selenoproteins (Araie and Shiraiwa, 2009; Lobanov et al. 2009; Lu and Holmgren, 2009). Consequently, in edible fish muscles, which represent the most relevant environmental source of human exposure to MeHg, this neurotoxicant is expected to be found primarily in thiol-containing proteins, and to a much lesser extent in selenol- containing proteins (see Scheme 1, which indicates the stable interaction of either the cysteyl or selenocysteyl residue of proteins with MeHg).
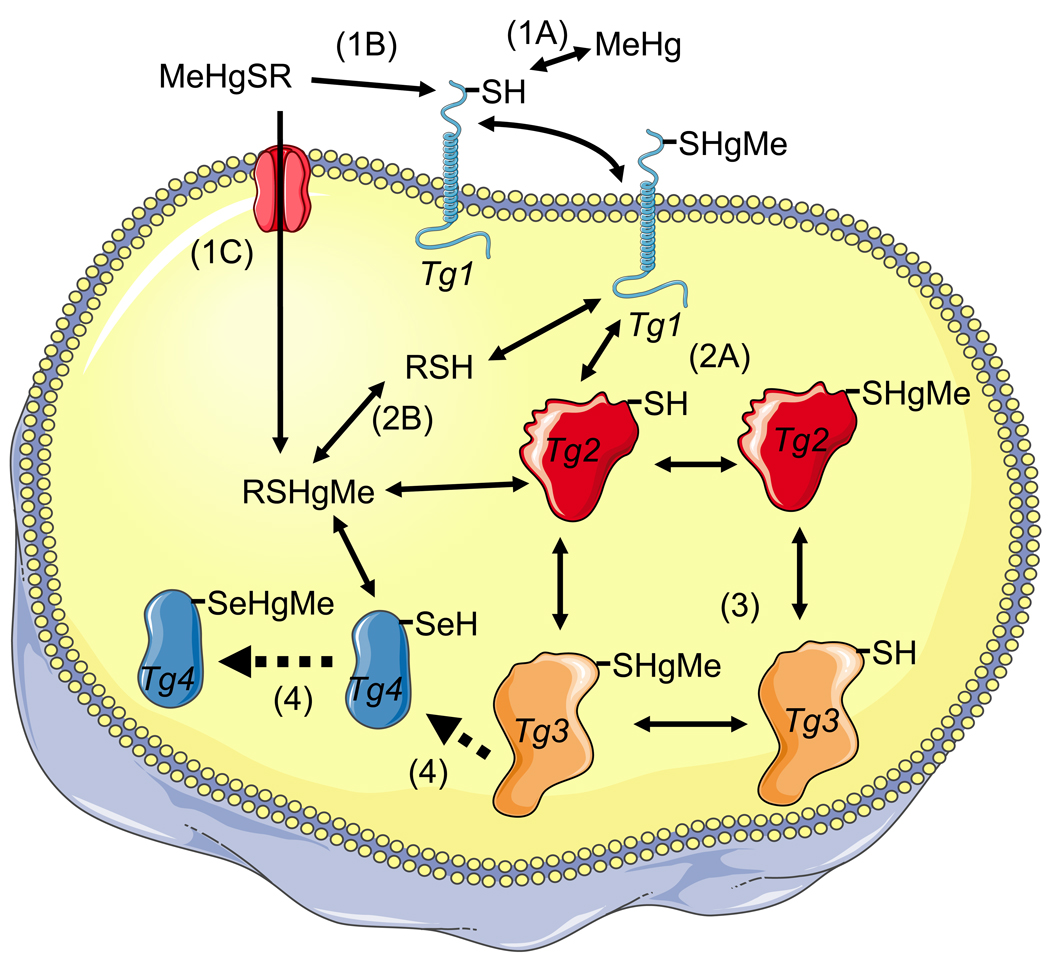
As discussed above, the absorption of MeHg from the gut is high; however, there are no experimental data regarding the speciation of MeHg that is absorbed in mammalian gastrointestinal tract. It is possible that cysteinyl-bound MeHg can be released after the digestion of fish protein, and that it then can be transported as a mimetic of the amino acid methionine (Figure 1, represented by RSHgMe; Yin et al., 2008; Bridges and Zalups, 2011). Alternatively, MeHg could be released by some enzymatic or non-enzymatic process and could subsequently react with thiol groups of proteins found in the plasma membrane of cells from the gastrointestinal tract.
Figure 1. Interaction of MeHg or RSHgMe (a complex of MeHg with a low weight molecular thiol, e.g., cysteine or glutathione) with target cell proteins via exchange reactions.
The first event can be of three types: A) MeHg (free MeHg) can react with a target protein (Tg 1) to oxidize it; B) the low-molecular weight complex of MeHg with a thiol-containing molecule (cysteine or small peptides derived from MeHg contaminated fish muscle proteins) can participate in an exchange reaction with the target protein 1 (Tg 1), oxidizing the Tg 1 and regenerating the free thiol molecule; and C) the complex MeHg-cysteine (MeHgSR) can be transported as a mimetic of methionine. The second event can be of two types: A) the MeHg bound to target protein 1 (Tg 1-HgMe complex) can participate in an exchange reaction with a second target protein (Tg 2) to oxidize it and release the free Tg 1; or B) the exchange can occur with intracellular low-molecular- weight thiols (e.g., cysteine or GSH) to form the MeHgSG or MeHgSCys complex and release the free Tg 1. The third event is similar to that described in the second event (i.e., an exchange reaction between a target protein with a different target protein or with intracellular low-molecular thiols). The fourth event represents the tentative unidirectional (indicated by the broken arrows) reactions of intracellular low-molecular MeHg-thiol complexes (RSHgMe) or of a target protein (Tg 3) with a target selenoprotein (Tg 4). In these cases, the selenocysteyl residue of the Tg 4 selenoprotein is oxidized by MeHg and hypothetically cannot participate in an exchange reaction due to the higher affinity of a selenol group for MeHg than that of a thiol group. The aforementioned events (1–4) are represented as numbers (or numbers plus letters) in parentheses (e.g., 1A).
The fate of MeHg after the ingestion of MeHg-polluted fish meat represents a critical juncture in the cycle of environmental MeHg toxicity which has been largely unexplored. In effect, information about the speciation of MeHg forms that are absorbed after the consumption of contaminated fish remains incipient. Sparse literature data have shown that, in fish muscle, MeHg is bound to cysteine, forming a complex of the type Cys-SHgMe (Harris et al., 2003). Notably, the bioavailability and the neurochemical and neurobehavioral toxicity of the MeHg-cysteine complex from fish meat can be distinct from that of MeHgCl (Harris et al. 2003; Berntssen et al., 2004; Grove et al. 2009).
The question of MeHg speciation is crucial because the first oxidative interaction of MeHg with its molecular targets can occur via the direct interaction of momentarily infinitesimal free MeHg with target proteins in the gastrointestinal tract; or it can be mediated by an exchange reaction of MeHg bound to cysteinyl residues found in reduced glutathione (GSH) secreted by gastrointestinal cells or cysteine derived from fish protein digestion. Of particular importance, Rabeinstein’s group has indicated that MeHg can exchange either from a low-molecular-weight thiol to a low- or high-molecular weight thiol group or from a high-molecular weight to high- or low-molecular weight thiol group (Scheme 2 and Scheme 3; Rabeinstein and Evans, 1978; Rabeinstein et al. 1982, 1983a,b, 1986).
Scheme 2.
Exchange reaction of MeHg from one type of thiol-containing molecule to another class of molecule. R1 and R2 can be either a low- or a high-molecular-weight thiol molecule.
Scheme 3.
Exchange reaction of MeHg between two molecules of the same chemical structure, representing the exchange of MeHg between two thiol-containing glutathione, i.e., a reduced glutathione displaces MeHg from a second GS-HgMe complex. Chemically, this reaction indicates a shift of MeHg from one GSH to another GSH molecule. Biologically, this type of exchange reaction is expected to have little significance.
Specifically, Rabeinstein and colleagues (1983) have demonstrated that the thiol ligands, 2, 3-dimercaptosuccinic acid (DMSA), cysteine, mercaptoacetic acid, D-penicillamine, 2,3-dimercaptopropanesulfonic acid (DMPS), N-acetyl-D,L-penicillamine and D,L-homocysteine can release GSH from its MeHg complex, i.e., these low-molecular-weight thiol ligands can participate in the exchange reaction as depicted in Scheme 2. Of particular biochemical and toxicological significance, Rabeinstein and colleagues have indicated that the potency of the displacement of MeHg from the MeHg-GSH complex occurs in the following order: DMSA > cysteine > mercaptoacetic acid > D-penicillamine > DMPS > N-acetyl-D,L-penicillamine > D,L-homocysteine (Rabeinstein et al., 1983) and in intact erythrocytes. Further, this same group (Rabenstein et al. 1986) has determined the following potency order: 2,3-dimercaptosuccinic acid > 2,3-dimercaptopropane sulfonic acid > dithioerythritol > penicillamine ≈ N-acetylpenicillamine. This implies that the interaction or exchange reactions between the low-weight molecular thiol-MeHg complexes (particularly MeHg-cysteine and MeHg-glutathione complexes, which are the two most abundant low-molecular-weight thiol molecules from mammalians) with specific target proteins can be greatly influenced by steric factors in the intricate structure of the target proteins.
In addition, Rubeistein et al. (1982) have also shown that, in intact erythrocytes, a portion of the MeHg is complexed by intracellular GSH and another portion by hemoglobin. Furthermore, this group has indicated that, in hemolyzed erythrocytes, the sulfhydryl group of GSH binds MeHg more strongly than do the sulfhydryl groups of hemoglobin.
Here it is also important to stress that cysteinyl-bound MeHg could first be absorbed as mimetic of methionine and could then be exchanged after absorption (Figure 1). However, as pointed out by Rabeinstein et al. (1982), the velocity of the removal of MeHg from intact erythrocytes by different exogenous thiol ligands took place far more rapidly than could be accounted for merely by a mechanism in which the ligands cross the membrane, combine with the MeHg (that is bound to intracellular hemoglobin or GSH) and then transport MeHg out of the cell. Thus, the authors proposed that the ligands remove MeHg, which is complexed by sulfhydryl groups of membrane proteins, which, in turn, react with the intracellular MeHg to bring more MeHg into the membrane, where MeHg can react with the added exogenous sulfhydryl ligand. A similar phenomenon could account, at least in part, for the absorption of MeHg from the extracellular medium in mammalian tissues (including cerebral tissues) and from the luminal space of the gastrointestinal tract. This is shown in Figure 1 by the exchange reaction of RSHgCH3 with the target membrane, protein 1 (Tg 1), and then with the intracellular target, protein 2 (Tg 2). The net result of these exchange reactions is the entrance of MeHg into the cell. The fate of exchanged MeHg is complex and, once inside the cell, it can exchange with either low-molecular-weight thiols or with other target proteins (Figure 1). In short, the first oxidative interaction of fish-derived MeHg with mammalian proteins can occur either directly (in the case of the existence of a portion of free MeHg in the gastrointestinal tract) or indirectly via an exchange reaction between MeHg bound to fish muscle-derived cysteine with plasma membrane proteins located in the epithelial cells of the gastrointestinal tract (Figure 1). As pointed out above, similar exchange reactions may occur in all the mammalian tissues and MeHg-thiol exchange reactions may represent an additional and important pathway for MeHg uptake by mammalian cells. Notably, MeHg-thiol group exchanges can distribute MeHg to different subcellular compartments and to target thiol- and selenol-proteins, which can have distinct affinity for MeHg (Figure 1). Hypothetically, the affinity of a given target protein for MeHg can be extremely high so that it can bind MeHg in an irreversible way (this hypothetical reaction is represented by a unidirectional arrow in Figure 1 for a target Selenoprotein, Tg 4. However, as pointed out above, such a reaction could also occur with a specific thiol protein with a high affinity for MeHg).
As previously mentioned, MeHg can potentially target any thiol- or selenol-containing molecule. Indeed, the interaction of MeHg with endogenous thiol groups from proteins located in different subcellular compartments has been extensively suggested in the literature (Clarkson 1972; Atchison and Hare, 1997; Farina et al. 2011). For instance, MeHg can interact with proteins involved in the modulation of intracellular Ca2+ levels (Hare et al. 1995; Deny and Atchison, 1996; Freitas et al. 1996; Gasso et al. 2001; Limke et al. 2003, 2004a,b; Kang et al. 2006; Sirois and Atchison, 1996) and can promote or can block the release of neurotransmitters (Levesque and Atchison, 1988; Gasso et al. 2000; Fitsanakis and Aschner, 2005). Importantly, MeHg can cause sustained increases in the intracellular, cytosolic free Ca2+ concentrations (Deny and Atchison, 1996), which can be secondary to an increase in extracellular glutamate concentrations (Aschner et al. 2007). In addition, MeHg can disrupt mitochondrial functioning (Atchison and Hare, 1997; Shanker et al. 2005; Dreiem and Seegal, 2007; Yin et al. 2007; Usuki et al. 2008; Bourdineaud et al. 2011) by targeting specific thiol-containing proteins, including respiratory chain complexes and mitochondrial Creatine Kinase (mCK) (Glaser et al. 2010a, b). The inhibition of these complexes or enzymes can contribute to mitochondrial depolarization and swelling upon MeHg exposure. Mitochondrial targeting by MeHg has also been associated with an increase in mitochondria oxidative stress production (Franco et al. 2007; Roos et al. 2011). The mitochondrial overproduction of reactive oxygen species (ROS) triggered by MeHg can further exacerbate the neurotoxicity of MeHg by attacking additional nucleophilic centers in mitochondria and in other subcellular compartments. The of this review.
Although data have clearly indicated that the disruptions of Ca2+ homeostasis and mitochondrial functioning play a central role in MeHg neurotoxicity, it has not yet been determined whether these alterations are primary or secondary events triggered by the interaction of MeHg with other cellular components. For example, data have clearly indicated that MeHg can disrupt both intra- and extra-cellular glutamate homeostasis via interaction with glutamate transporters located in the plasma membrane and in synaptic vesicles (Porciuncula et al. 2003; Fitsanakis and Aschner, 2005; Aschner et al. 2007). Of particular significance, glutamate transporters are highly sensitive to oxidant agents, including thiol-blocking agents and ROS (Trotti et al. 1998). Consequently, we can infer that the hierarchical targeting of glutamate transporters in astrocytes can contribute to increased extracellular glutamate levels (Fitsanakis and Aschner, 2005; Aschner et al. 2007). The sustained increase in extracellular levels of glutamate can be the primary event that triggers the elevation in intracellular free Ca2+. However, as explained above, the interaction and exchange of MeHg with different thiol groups seems to occur very rapidly (Rabeinstein et al. 1982, 1983, 1986) and the velocity of exchange between the MeHg-thiol groups could contribute to the prompt distribution of MeHg inside different neural cells, an event which could result in the multifaceted targeting of thiol- and selenol-containing proteins located in different subcellular compartments. In short, the relative ease with which MeHg can move from one thiol to another could allow MeHg to interact in a more stable way with specific and less abundant target proteins for which MeHg has superior affinity as opposed to the most abundant low- (cysteine, GSH) and high-molecular-weight thiol-containing molecules (hemoglobin, albumin). Thus, according to this hypothesis, MeHg would be able to exchange from one thiol target to another with different velocities (Rabeinstein et al. 1978; 1982, 1983; 1986), and the relative time that MeHg would be bound to a given target would depend on the affinity of MeHg for the target considered. As pointed out above, MeHg is expected to have a higher affinity for selenol groups than for thiol groups. Therefore, it is probable that, in addition to bind to specific thiol-containing proteins, MeHg can also bind in a stable way to selenoproteins, such as the antioxidant enzymes, glutathione peroxidase (GPx) and thioredoxin reductase (TrxR). Accordingly, GPx and TrxR can be inhibited after exposure to MeHg (Carvalho et al. 2008; 2010; Farina et al. 2009, Franco et al. 2009; Wagner et al. 2010; Branco et al. 2011). Consequently, the inhibition of these seleno-enzymes (and possibly the disruption of other selenoproteins) could be considered the primary targets of MeHg-induced oxidative stress.
From molecular and developmental points of view, the identification of target proteins that bind MeHg with high affinity is fundamental for better understanding how this electrophile disrupts the tightly regulated developmental molecular processes in the developing brain (Johansson et al. 2007; Farina et al. 2011). In this regard, it is important to note that the developing brain is much more sensitive to MeHg than the mature brain. Further, MeHg concentrations that do not induce overt signs of brain toxicity in mature individuals (Grandjean 2008) or that cause only subtle increases in brain Hg levels (Stringari et al. 2008) can be associated with long-lasting deleterious neurochemical and neurobehavioral changes in mammals exposed to MeHg during critical periods of brain development. This is most likely due to the fact that critical processes that take place in the developing brain do not take place in adult brain, such as cell division, neuronal migration, etc. Furthermore, it is important to note that information regarding the comparative nucleophilic thiol- or selenol-containing targeting by MeHg in the developing vs. the adult brain is extremely limited. Therefore, comparative studies on the differential expression of specific nucleophilic thiol- or selenol-containing targets for MeHg in the mature and developing CNS could provide new insight into the molecular mechanisms implicated in MeHg-induced neurotoxicity. In addition, the identification of “electrophile-sensitive” or “nucleophile-regulated” cellular pathways that could be modified by MeHg exposure during critical periods of development, namely, those involved in brain cell migration and proliferation (Rand et al. 2008; 2009; Vendrell et al. 2010) is particularly important for improving our understanding MeHg neurotoxicity.
3 - Reactive species mediating MeHg-neurotoxicity
Several lines of evidence indicate that MeHg neurotoxicity is associated with increased levels of reactive (oxygen/nitrogen) species (ROS/RNS) (Oyama et al., 1994; Roos et al., 2009; Kaur et al., 2010; Ni et al., 2010). As mentioned previously, MeHg interacts with nucleophilic groups (mainly thiol and selenol) from several low- and high-molecular-weight biomolecules (Farina et al., 2011). Considering that such groups may be critical to the catalytic activity of numerous enzymes involved in intermediate metabolism and antioxidant processes, and that MeHg is able to affect the activity of such enzymes [i.e. glucose-6-phosphate dehydrogenase (Tsuzuki and Yamada, 1979), creatine kinase (Glasser et al., 2010), glutathione reductase (Stringari et al., 2008), glutathione peroxidase (Farina et al., 2009; Franco et al., 2009), thioredoxine reductase (Wagner et al., 2010; Branco et al., 2011), among others], it is reasonable to postulate that this interaction is responsible for imbalances in oxidative metabolism as well as increased levels of reactive species. Although this simplistic idea is indeed correct, the mechanisms mediating MeHg-induced ROS/RNS generation appear to be far more complex, according to the following discussions addressing three specific examples: hydrogen peroxide, superoxide anion and nitric oxide.
3.1 - Hydrogen peroxide
Hydrogen peroxide (H2O2) is quantitatively the most important peroxide generated by cells (Dringen et al., 2005). A microdialysis study revealed extracellular H2O2 concentrations of up to 100 micromolar (Hyslop et al., 1995). The major sources of H2O2 are the disproportionation of mitochondrial superoxide anion (Inoue et al., 2003), primarily catalyzed by superoxide dismutases (SODs) (Fridovich, 1995), and reactions catalyzed by oxidases, such as monoamine oxidases (Nicotra et al., 2004). Although it is known that H2O2 plays important physiological roles in modulating cell function (for a review, see Dröge, 2002), high H2O2 levels are dangerous to the cells, largely due to the generation of hydroxyl radicals via Fenton’s chemistry (McCord and Day, 1978). The main pathways to decompose H2O2 involve catalase (CAT), cytoplasmic glutathione peroxidase (GPx1) and peroxiredoxin (Prx) (Winterbourn and Hampton, 2008). With particular emphasis on the pro-oxidative effects of MeHg, evidence shows that it has the capacity to increase H2O2 levels in several experimental conditions (Manfroi et al., 2004; Shanker et al., 2004; Franco et al., 2007; Mori et al., 2007).
The increased levels of H2O2 observed after MeHg exposure represent consequences of different phenomena, such as MeHg’s inhibitory effects toward glutathione peroxidases (GPxs; Farina et al., 2009, Franco et al., 2009), which are important enzymes involved in peroxide disposal by means of glutathione (GSH; Dringen et al., 2002). GPxs represent a family of selenoproteins whose catalytic activity (peroxide detoxification) depends on the reducing power of a selenol group located at the active site (Brigelius-Flohé, 2006). Due to the extremely high affinity of MeHg for selenol groups (see above, item 2), the decreased GPx activity after MeHg exposures has been attributed to direct inhibitory events (Farina et al., 2009). In addition, a recent study has proposed another molecular mechanism to explain the reduced GPx activity after MeHg exposure: MeHg induces a “selenium-deficient-like” condition, which affects GPx synthesis through a posttranscriptional effect (Usuki et al., 2010), thus leading to decreased GPx levels, reduced peroxidase activity and, consequently, increased H2O2 levels.
Another mechanism related to the increased H2O2 levels after MeHg exposure appears to be the direct hampering effect of this toxicant toward the entire GSH antioxidant system. In addition to the direct depletion of reduced GSH by MeHg, which certainly contributes to the decreased detoxification of H2O2 by GSH-dependent peroxidases, MeHg also impedes the physiological maturation of several enzymes involved with GSH metabolism, thus leading to increased levels of brain H2O2 and lipid peroxidation (Stringari et al., 2008).
In addition to the decreased H2O2 detoxification induced by MeHg (Stringari et al., 2008; Farina et al., 2009; Usuki et al., 2010), increased H2O2 generation also represents an important mechanism by which this toxicant leads to higher ROS levels. In an experimental study with isolated mitochondria from the rat cerebellum, Mori and collaborators (2007) observed that MeHg affects the mitochondrial electron transfer chain (mainly at the level of complex II-III), leading to the increased formation of H2O2. Corroborating these findings, an in vitro experimental study with isolated mitochondria from the mouse brain showed that MeHg toxicity was blunted by catalase, thus indicating that H2O2 is an important factor in the generation of ROS in MeHg-exposed mitochondria (Franco et al., 2007). Although these two studies have demonstrated the increased generation of H2O2 in MeHg-exposed mitochondria, the actual contribution of superoxide anion (a H2O2 precursor) in such an event requires further investigation.
While it is known that increased H2O2 levels represent a consequence of MeHg exposure, the precise role of this molecule in mediating MeHg-induced oxidative damage has not yet been fully determined. However, an interesting experimental study showed that catalase, which detoxifies H2O2, was able to abolish the inhibitory effects of MeHg on glutamate transport in cultured astrocytes (Allen et al., 2001), indicating that H2O2 is responsible, at least in part, for some toxic effects induced by MeHg. This notion was corroborated by a study from Franco and collaborators (2007), who observed that MeHg-induced H2O2 generation was responsible for the mitotoxic effects elicited by this compound.
3.2 - Superoxide anion
Superoxide anion (O2•−), a by-product of normal functioning of the mitochondrial respiratory chain, is a ROS that is produced after the one-electron reduction of molecular oxygen (Chance et al., 1979). It is generated by complexes I and III of the mitochondrial respiratory chain and is readily converted to H2O2 by mitochondrial (manganese-dependent) superoxide dismutase (SOD; Liu et al., 2002). Additionally, superoxide is a product of NADPH-oxidase, which is particularly important in the CNS during microglial activation (Lavigne et al., 2000). Although most of the studies examining the various ROS which are produced as a result of MeHg exposure have focused on H2O2, superoxide anion has also been reported to play an important role in the oxidative damage induced by MeHg. Shanker and collaborators (2004), using a specific probe for superoxide (hydroethidine), observed increased levels of this ROS in MeHg-treated cultured astrocytes. In another study, SOD, which dismutates the superoxide anion radical, was able to attenuate MeHg-induced ROS formation in primary astrocytic cultures (Shanker and Aschner, 2003). Corroborating these findings, Mori and collaborators (2007) reported increased superoxide levels in MeHg-exposed cerebellar mitochondria, and Naganuma and collaborators (1998) observed that the sensitivity of HeLa cells against MeHg was decreased by the overexpression of manganese-SOD (a mitochondrial enzyme), indicating that the formation of superoxide anions in the mitochondria plays a role in the mechanism of the cytotoxicity of MeHg. This notion is reinforced by the fact that MeHg did not decrease SOD activity in different experimental approaches (de Freitas et al., 2009; Grotto et al., 2009).
Another interesting, but currently neglected, research topic seems to be the formation of superoxide by NADPH-oxidase from activated microglia. Although MeHg may induce pro-inflammatory events, as well as toxicity and the activation of microglial cells (Eskes et al., 2002; Chang, 2007; Ni et al., 2010; Ni et al., 2011), a potential relationship between MeHg, microglial activation and increased superoxide production is lacking in the literature.
3.3 - Nitric oxide
Nitric oxide (NO) is a RNS widely used as signaling molecule in cells throughout the body. Although NO plays a number of important roles as the regulator of several biological processes (vascular tone and permeability, platelet adhesion, neurotransmission, and mitochondrial respiration), it can also cause deleterious effects, including the inhibition of enzyme function, the promotion of DNA damage and the activation of inflammatory processes (Hollenberg and Cinel, 2009). NO is synthesized from L-arginine and oxygen in a reaction catalyzed by nitric oxide synthase (NOS), which presents at least three distinct isoforms [neuronal nitric oxide synthase (nNOS, NOS1); inducible nitric oxide synthase (iNOS, NOS2); and endothelial nitric oxide synthase (eNOS, NOS3)]. While iNOS activity depends on transcription, eNOS and nNOS are constitutively expressed and are activated by elevated intracellular calcium (Alderton et al., 2001). Notably, increased NOS activity and increased NO levels have been reported to mediate MeHg neurotoxicity (Himi et al., 1996; Yamashita et al., 1997; Herculano et al., 2006).
Although the mechanisms involved in the interaction between MeHg and NO are not completely understood, intracellular calcium dyshomeostasis appears to play an important role in this scenario. Several lines of evidence point to glutamate dyshomeostasis as a central event in MeHg-induced neurotoxicity (Aschner et al., 2007, Farina et al., 2011). In fact, MeHg has been shown to inhibit glutamate uptake into cultured astrocytes (Brookes and Kristt, 1989; Aschner et al., 2000), inhibit the uptake of glutamate into rat synaptic vesicles (Porciúncula et al., 2003) and cerebral cortical slices (Moretto et al., 2005a), and increase the spontaneous release of glutamate from mouse cerebellar slices (Reynolds and Racz, 1987) and cultured neuronal cells (Vendrell et al., 2007), indicating that increased glutamate levels in the extracellular environment could represent a biochemical consequence of MeHg exposure. These in vitro findings have been confirmed in in vivo studies with microdialysis probes implanted in the frontal cortex of adult rats (Juarez et al., 2002), showing increased levels of extracellular glutamate after MeHg exposure. Moreover, these same researchers observed that MeHg-induced DNA damage in the rat frontal cortex was blocked by the administration of a non competitive N-methyl D-aspartate (NMDA)-type glutamate receptor antagonist (MK-801) (Juarez et al., 2005). In addition, the toxicity elicited by MeHg exposure in cultured neurons (monocultures) was significantly attenuated in the presence of co-culturing with astrocytes (Morken et al., 2005), which are prone to remove excessive glutamate from the synaptic cleft, thus preventing glutamate-induced toxicity. All these evidences reinforce the idea that MeHg toxicity is associated with glutamate dyshomeostasis. With respects to the potential contribution of MeHg to NO formation, it is noteworthy that MeHg-induced increases in extracellular glutamate levels could lead to the overactivation of NMDA-type glutamate receptors, thereby increasing calcium influx into postsynaptic neurons (Lafon-Cazal et al., 1993). In the postsynaptic environment, increased intracellular calcium levels, which have been reported to mediate MeHg toxicity (Atchison, 2005), are prone to activate nNOS, thus increasing NO formation. Increased NOS activity and/or increased NO levels have been reported as consequences of MeHg exposure in different experimental conditions. In vivo studies have demonstrated increased calcium-dependent NOS activity in the CNS of MeHg-exposed rodents (Himi et al., 1996; Yamashita et al., 1997). Corroborating these findings, Herculano and collaborators (2006) observed increased NOS activity in chick retinal cell cultures exposed to MeHg. On the other hand, calcium channel blockers were found to offer protection against MeHg-induced toxicity in rat cerebellar granule neuron cultures (Gasso et al., 2001). Although these observations suggest that increased glutamate levels (a consequence of MeHg exposure) activate NOS by raising intracellular calcium concentrations at postsynaptic neurons, contradictory evidence and divergent experimental results have also been reported. For example, Ikeda and collaborators (1999) showed that the increased activity of cerebellar nNOS observed in MeHg-treated mice was not changed by the co-administration of the NMDA-type glutamate receptor antagonists, MK-801 and 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid. This finding is in agreement with the fact that calcium mobilization from the endoplasmic reticulum appears to be involved in mercury-mediated cytotoxicity (Gasso et al., 2001). Thus, although there are divergences with regard to the exact mechanisms related to MeHg-induced NO generation, it is widely accepted that this reactive species plays an important role in neurotoxicity induced by this toxicant, which can be confirmed by increased nitrotyrosine levels in the CNS of MeHg-treated animals (Miyamoto et al., 2001).
It is important noteworthy that although evidence shows that increased calcium influx can lead to increased generation of reactive species (Himi et al., 1996; Yamashita et al., 1997) and that reactive species (e.g., hydrogen peroxide) are able to inhibit astrocytic glutamate uptake (Allen et al., 2001), the calcium-mediated toxicity is not necessarily directly responsible for glutamate dyshomeostasis in MeHg-exposed systems. In fact, MeHg-induced glutamate release has been reported to be calcium-independent (Reynolds and Racz, 1987) and the inhibition of glutamate uptake by MeHg is unlikely to occur as a consequence of changes in calcium homeostasis (Brookes and Kristt, 1989; Aschner et al., 2000). Thus, the disruption of intracellular calcium homeostasis observed after MeHg exposure seems to represent a consequence (but not a cause) of glutamate dyshomeostasis. This is believed because MeHg-induced increased extracellular glutamate levels can lead to increased calcium influx into postsynaptic neurons via overactivation of NMDA-type glutamate receptors; however, calcium dyshomeostasis seems to play no significant role in the glutamate dyshomeostasis observed after MeHg exposure (Reynolds and Racz, 1987; Brookes and Kristt, 1989; Aschner et al., 2000).
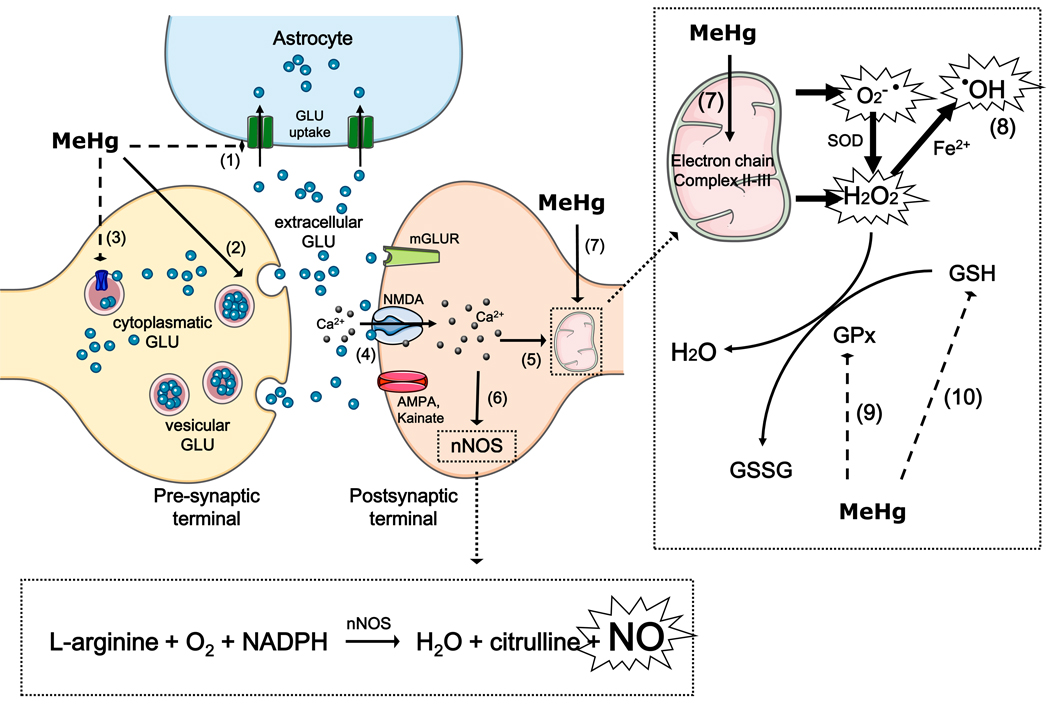
A brief summary on H2O2, superoxide anion and NO as mediators of MeHg-induced neurotoxicity is depicted in Figure 2.
Figure 2. Reactive species as mediators of MeHg induced neurotoxicity.
MeHg leads to increased extracellular glutamate (GLU) levels through the inhibition of astrocytic glutamate uptake (event 1), the stimulation of glutamate release from presynaptic terminals (event 2) and the inhibition of vesicular glutamate uptake (event 3). Increased extracellular glutamate levels overactivate N-methyl D-aspartate (NMDA)-type glutamate receptors, increasing calcium influx into neurons (event 4). Increased levels of intracellular calcium, which can lead to mitochondrial collapse (event 5), activate neuronal nitric oxide synthase (nNOS) (event 6), thus increasing nitric oxide (NO) formation. MeHg affects the mitochondrial electron transfer chain (mainly at the level of complex II-III) (event 7), leading to the increased formation of superoxide anion (O2•−) and hydrogen peroxide (H2O2). H2O2 can produce hydroxyl radical anion (•OH) via Fenton’s Reaction (event 8). MeHg-induced increases in H2O2 levels might be a consequence of decreased glutathione peroxidase (GPx) activity (event 9) and glutathione (GSH) depletion (event 10).
4 - Oxidative hallmarks in MeHg-induced neurotoxicity
Considering the fact that different ROS/RNS mediate MeHg-induced neurotoxicity, it is not surprising to find diverse oxidative hallmarks (i.e. lipid peroxidation, protein oxidation, DNA oxidative damage, protein nitrosylation, among others) in biological systems exposed to this toxicant. Lipid peroxidation has been extensively reported as a consequence of MeHg toxicity (Carvalho et al., 2007; Vendrell et al., 2007; Martins et al., 2009; Franco et al., 2009; Glasser et al., 2010b), and this event is related, at least in part, to the central role of H2O2 (and its precursor superoxide anion) (Mori et al., 2007; Franco et al., 2007). Although the effectiveness of H2O2 or superoxide in inducing lipid peroxidation is relatively low, the formation of the hydroxyl radical by Fenton’s reaction allows for the great tendency to remove hydrogen atoms from the alkylic chain of membrane lipids, which represents a pivotal event in the initiation of lipoperoxidation (Halliwell and Chirico, 1993). In agreement, an in vivo study showed that deferoxamine, an iron chelator, protected against MeHg-induced lipid peroxidation in the rat brain, providing evidence that iron-catalyzed oxygen radical-producing reactions play an important role in MeHg-induced oxidative stress (LeBel et al., 1992). In addition to the important role of H2O2 in mediating MeHg-induced lipid peroxidation, another decisive factor that seems to contribute to the appearance of this hallmark is the large amount of polyunsaturated fatty acids in the CNS, which are highly susceptible to peroxidative damage (Grintal et al., 2009).
4.1 - Lipid peroxidation
MeHg-induced lipid peroxidation seems to be crucial in neurotoxicity. In fact, in addition to the cellular plasma membrane lipids, those from specific cellular organelles also represent important targets of peroxidation. Accordingly, lysosomal rupture has been detected after MeHg-exposure in human astrocytoma D384 cells (Daré et al., 2001). Interestingly, this group observed that D384 cells maintained plasma membrane integrity, and the lysosomal rupture preceded a decrease of the mitochondrial potential, suggesting that lysosomal membranes represent a highly susceptible target of the peroxidative effects of MeHg. The lipoperoxidative effect toward mitochondrial membranes also contributes to the toxicity elicited by MeHg (Franco et al., 2009, 2010), which may be related, at least in part, to the apoptotic characteristics of MeHg-induced cell death (for review, see Ceccatelli et al., 2010).
4.2 - Protein oxidation
Oxidative modifications in proteins represent another important event mediating MeHg-induced toxicity. First, it is imperative to mention that the interaction of MeHg with thiol or selenol groups from a given protein represents the loss its nucleophilic potential, which characterizes an “oxidative event”. As mentioned previously, the interaction of MeHg with the nucleophilic groups of proteins generally leads to loss of protein function, which can be observed by decreased catalytic (Rocha et al., 1993, Farina et al., 2009), transport (Aschner et al., 2000) and binding (Soares et al., 2003) functions. Thus, MeHg-induced changes in the protein thiol or selenol status represent a pro-oxidative effect toward proteins, which significantly contribute to its neurotoxicity. With regard to protein oxidation by MeHg-induced reactive species, NO formed by MeHg-induced nNOS activation is prone to cause the nitrosylation of protein amino acids, such as tyrosine (Miyamoto et al., 2001). This phenomenon has been reported to regulate synaptic activity (LoPachin and Barber, 2006), which mediates MeHg-induced neurotoxicity (Aschner, 1996). Although lipid peroxidation and protein oxidation are major molecular hallmarks of MeHg toxicity, oxidative DNA damage has also been observed in biological systems exposed to this toxicant (Belleti et al., 2002).
4.3 - Changes in the redox thiol state (glutathione and Nrf2)
Alteration in the thiol status, represented mainly by the reduced:oxidized glutathione ratio (GSH/GSSG), is another molecular outcome that occurs as a result of the pro-oxidative properties of MeHg (Kaur et al., 2006). Notably, MeHg is able to interact with the most important thiol antioxidant, GSH, thus leading to the formation of an excretable GS-HgCH3 complex (Ballatori and Clarkson, 1982). This interaction decreases the levels of GSH and, consequently, the GSH:GSSG ratio, which contributes to the occurrence of oxidative stress. Accordingly, decreased GSH levels have been reported after MeHg exposure under several in vitro conditions (Kaur et al., 2006; Franco et al., 2007; Amonpatumrat, 2008; Ni et al., 2011). Moreover, in vivo studies with mice have also reported decreased GSH levels in the CNS after MeHg exposure (Franco et al., 2006; Stringari et al., 2008). In agreement with these observations, GSH precursors, such as N-acetyl cysteine (NAC), have been reported to protect against MeHg-induced neurotoxicity in vitro (Kaur et al., 2006).
Although in vivo studies show that MeHg exposure in mice decreases GSH levels in the CNS (cerebral cortex or cerebellum) (Franco et al., 2006; Stringari et al., 2008), the intracellular levels of GSH in mammalian cells are in the milimolar (mM) range (Cooper and Kristal, 1997). Due to the fact that GSH depletion has been reported in the CNS of MeHg-exposed mice whose mercury levels were found to be in the low micromolar (µM) range, it is difficult to explain the decreased GSH levels after MeHg exposure based solely upon the equimolar interaction between both molecules. A better understanding of this phenomenon is possible when considering that ROS generated mainly in mitochondria after MeHg exposure are detoxified by GSH-dependent systems, thus leading to GSH depletion (Franco et al., 2007).
Another interesting and recently reported event that has been connected to the pro-oxidative effects of MeHg is the activation of NF-E2-related factor 2 (Nrf2) (a major regulator of intracellular antioxidant response). Under physiological conditions, Nrf2 is located in the cellular cytoplasm and is bound to Kelch-like ECH-associating protein 1 (Keap1) (Kensler and Wakabayashi 2009). Experimental evidence has shown that the interaction between Nrf2 and Keap1 is disrupted by oxidative modifications of their cysteine thiol groups (He and Ma, 2009). This event allows for the translocation of Nrf2 to the nucleus (Chen et al. 2009; Li and Kong 2009), where it interacts with an antioxidant response element (ARE) to initiate the transcription of target genes. Subsequently, the encoded proteins of these target genes are used to detoxify xenobiotics and endogenous reactive electrophiles (Itoh et al. 1999; Prestera et al. 1993; Prestera and Talalay 1995). Notably, recent data from studies with cultured neuroblastoma (Toyama et al., 2007) and primary cultures of astrocytes (Wang et al., 2009) have demonstrated that MeHg activates Nrf2 and that this event is essential for reduction of MeHg toxicity. In agreement with these findings, Ni and collaborators showed that MeHg exposure in primary cultures of astrocytes or microglia increases the cytosolic Nrf2 protein level, an event followed by its nuclear translocation (Ni et al., 2010; 2011), which ultimately culminates in increased messenger RNA levels of the antioxidant/protective enzymes, heme oxygenase 1 (Ho-1) and NAD(P)H: quinone oxidoreductase 1 (Nqo1) (Ni et al., 2011). These findings suggest that MeHg-induced Nrf2 activation increases the expression of antioxidant/protective defenses. Of particular importance, MeHg-induced Nrf2 activation was proposed to be linked to changes in the thiol status (Ni et al., 2011), a proposal that is in agreement with the fact that the interaction between Nrf2 and Keap1 is disrupted by oxidative modifications of cysteine thiol groups (He and Ma, 2009). However, the current knowledge regarding this hypothesis remains incomplete; therefore, it is not yet possible to determine whether the oxidative modifications of Nrf2 and/or Keap1 thiols represent a direct effect of MeHg or whether these modifications represent a consequence of the pro-oxidative effects of ROS generated during MeHg exposure. In agreement with this most recent hypothesis, Usuki and collaborators (2011) have demonstrated that Trolox (a soluble analogue of vitamin E with scavenger properties) completely prevents the MeHg-induced overexpression of antioxidant defenses (catalase and thioredoxin reductase), suggesting that MeHg-induced ROS are crucial to the increase in the expression of such enzymes. Nevertheless, additional studies are necessary to better understand the effects of MeHg on Nrf2 signaling.
5 - Antioxidant compounds as potential neuroprotective strategies
Classically, the therapeutic approach to treat mercury intoxication is based on the use of thiol-chelating agents (Hughes and Sparber, 1978; Clarkson et al. 1981; Lund et al. 1984). The basis for the use of thiols in the treatment of mercurial intoxication lies in their potential ability to remove MeHg from biological targets, particularly, from thiol-containing proteins. The general reaction can be summarized as:
2PSHgCH3 + R(SH)2 2PSH + R(S-HgCH3)2
where a dithiol reacts with a target protein-MeHg complex to regenerate the free target protein and an excretable complex of MeHg and the chelating agent (Clarkson et al. 1981; Ruha et al. 2009). This reaction is indeed an exchange reaction (see Scheme 2 in Section 2), where a thiol can displace MeHg from a given protein. From the in vitro experimental data obtained by Rabeinstein’s group, chelation therapy would be expected to be very efficient, particularly in view of the prompt removal of MeHg from erythrocytes by therapeutic chelating agents, such as DMSA, DMPS and penicilamine (Rabeinstein et al. 1982; 1983; 1986). However, under in vivo conditions, the ability of chelating agents to restore normal physiological function in humans with mercurial intoxication is low (Nierenberg et al. 1996), in spite of the fact that thiol-chelating agents can accelerate MeHg excretion (Clarkson et al. 1981; Nierenberg et al. 1996; Drasch et al. 2007). One intriguing aspect that has received little attention and might offer an explanation for this phenomenon is the possibility that the interaction of MeHg with some target proteins could lead to changes in the protein structure, which could prevent MeHg removal via exchange with low-weight-molecular thiols (including therapeutic dithiols). The changes in the structure of particular target proteins could be time-dependent, which would be similar to observations regarding organophosphorous-inhibited acetylcholinestarese (AChE). In the case of AChE, the aging process induced by organophosphorus inhibitors in the enzyme structure prevents oximes from reactivating AChE activity (Jokanovic, 2009).
Nevertheless, despite these inconclusive findings, thiol-chelating therapy can, at least to some extent, ameliorate the symptoms of MeHg intoxication in experimental models (Gomez et al. 1994; Pingree et al. 2001; Carvalho et al. 2007; Bridges et al. 2009). However, it remains unknown whether the protection or recovery afforded by thiol-chelating agents is mediated via the re-establishment of the functionality of MeHg-disrupted protein or via the lowering the MeHg body burden, independent from the restoration of targeted protein function. Furthermore, therapeutic thiol molecules could also exhibit antioxidant activity of their own accord, thus preventing MeHg from reaching the critical target proteins involved in the metabolism of ROS (indirect effect), or by reacting with secondary electrophile species (direct effect) produced indirectly by MeHg via the inhibition of antioxidant selenoenzymes or thiol-containing enzymes involved in the maintenance of the cellular redox state (Farina et al., submitted).
As discussed above, the neurotoxicity of MeHg in developing and mature organisms is associated with changes in a variety of neurochemical processes. In fact, MeHg has a multitude of molecular, subcellular and cellular targets in the central nervous system (Aschner et al. 2007; Johansson et al. 2007) and, to date, our knowledge about the complex hierarchy of molecular events that dictate subcellular and cellular malfunctioning is still incipient. However, oxidative stress is an important hallmark of MeHg neurotoxicity both in the mature and developing brain (Farina et al. 2011), and the protective effect of antioxidants against MeHg toxicity was experimentally demonstrated even before the first observation that oxidative stress plays a central role in MeHg toxicity (Kasuya 1975; Chang et al. 1978; Ganther 1978; LeBel et al. 1990; 1992). Several points of evidence indicate that synthetic and natural antioxidants can protect against both in vitro and in vivo neurotoxicity of MeHg (Farina et al. 2003; 2005; Moretto et al. 2005a; Franco et al. 2007; 2010; Lucena et al. 2007; 2010; Shichiri et al. 2007; Wagner et al. 2010). Therefore, antioxidants could be considered as potential neuroprotective agents against MeHg-induced neurotoxicity. Indeed, recent evidence points to beneficial antioxidant nutrients present in fish as potential confounding factors that have delayed the recognition of MeHg neurotoxicity after low levels of exposure during critical periods of human brain development (Choi et al. 2008; Rice 2008; Grotto et al. 2010; Grandjean and Herz, 2011). Among these nutritional factors, the presence of selenium in fish must be highlighted, as this element was the first to be shown to protect against the toxicity of MeHg in Japanese quail (Ganther et al. 1972). Thereafter, several studies have demonstrated that both inorganic and organic selenium compounds can exhibit in vitro and in vivo protective effects against the neurotoxic effects of MeHg (Ganther 1978; Fredriksson et al. 1993; Choi et al. 2008; Weber et al. 2008; Kaur et al. 2009). However, the neuroprotective mechanism by which selenium compounds decrease MeHg toxicity has not yet been determined. However, as discussed earlier in this review, selenol groups have a stronger affinity for MeHg than do thiol groups (Sugira et al. 1976). Further, during the metabolism of inorganic forms of selenium and selenomethionine, H2Se and CH3SeH are formed and can react with MeHg, creating stable intermediates (Farina et al. 2011). In addition, limited literature data have indicated that Se(IV) and H2Se can accelerate the decomposition of MeHg to inorganic mercury (possibly to the insoluble and inert salt, HgSe). Notably, the Se(IV) was able to stimulate MeHg decomposition only in the presence of reducing thiols (glutathione and cysteine), supporting the notion that in vivo selenium compounds have to be metabolized to selenohydryl-containing intermediates, which react with MeHg to form inert and non-toxic complexes (Iwata et al. 1982). However, the speciation of mercury and selenium in these complexes remains unknown.
In addition to inorganic and naturally occurring organic selenium compounds, synthetic organoselenium compounds can also exhibit neuroprotective effects against MeHg. Accordingly, ebselen and diphenyl diselenide have been shown to exert beneficial effects against in vitro and in vivo MeHg-induced neurotoxicity (Farina et al. 2003a,b; Moretto et al. 2005a,b; Funchal et al. 2006; Roos et al. 2009; Usuki et al. 2010; Yin et al. 2011). In fact, MeHg exposure during lactation caused a disruption in glutamate homeostasis (release and uptake), and co-treatment with ebselen protected developing rats from MeHg neurotoxicity (Farina et al. 2003a). In adult mice, MeHg inhibited glutamate uptake by cortical brain slices and decreased cortical glutathione peroxidase activity; these alterations were blunted in mice co-treated with ebselen (Farina et al. 2003b). Similarly, diphenyl diselenide (which is the simplest of the diaryl diselenides; Nogueira and Rocha, 2010) exhibited neuroprotective activity against MeHg and lowered the Hg burden in the brain, liver and kidneys of adult mice (de Freitas et al. 2009). The molecular mechanism(s) which underlie(s) the neuroprotective effects of ebselen and diphenyl diselenide can be related either to the direct interaction of MeHg with “selenol intermediates” of ebselen and diphenyl diselenide after their reaction with thiols, or indirectly, by the modulation of oxidative stress (via their glutathione peroxidase- and/or thioredoxin reductase-like activities) (Nogueira and Rocha, 2010; Sausen de Freitas et al. 2010). Furthermore, the ability of diphenyl diselenide to decrease cerebral Hg deposition after exposure to MeHg supports the formation of a “selenol intermediate” of diphenyl diselenide that forms a stable and more excretable complex with MeHg. Since ebselen can form both “selenol intermediates” and an ebselen diselenide (Zhao et al. 2002 a, b; Sausen de Freitas et al. 2010), part of the observed neuroprotective effect of ebselen against MeHg could also be a consequence of the reduction in Hg deposition in the brain. In short, the neuroprotective effects of ebselen and diphenyl diselenide against MeHg-induced toxicity can be related to their antioxidant properties and to their abilities to form stable complexes with MeHg, which can increase Hg excretion and decrease the MeHg body burden. Furthermore, diphenyl diselenide can be partially metabolized to inorganic Se (Adams et al. 1989), which may, at least in part, account for its neuroprotective effects against MeHg.
6 - Concluding remarks
Over the past several decades, we have become increasingly aware of the many hazards of industrial chemicals, which have produced detrimental and often devastating effects upon both the natural environment and a multitude of its living inhabitants, including humans. Specifically, the neurotoxicant, MeHg, which was recognized to be the causative factor of “Minamata disease,” a condition that occurred as the direct result of MeHg intoxication via the consumption of fish heavily contaminated with this pollutant, poignantly illustrates the environmental neurotoxicity of mercury. Furthermore, in the Minamata outbreak, the high incidence of cerebral palsy-like disease among infants who had not consumed polluted fish indicated that they were indirectly intoxicated due to the direct intoxication of their mothers. These observations provided an acute warning to the scientific community regarding the neurodevelopmental toxicology of industrial chemicals, particularly in view of the fact that the mothers of the afflicted children were asymptomatic regarding “Minamata disease” (Nelson et al. 1971). Currently, the primary concern about MeHg is related to its ubiquitous presence in edible fish and the absence of a defined “no observable adverse effect level” (NOAEL). There is considerable debate about this subject (Grandjean et al. 2010); however, from the accumulated in vitro and in vivo experimental points of evidence, it is reasonable to suggest that, for a mature organism, a NOAEL can be estimated with relative safety. In sharp contrast, for the developing organism (namely, the central nervous system), the indication of a NOAEL is extremely hazardous and represents the failure to recognize and acknowledge the inherently fragile and uniquely vulnerable state of the developing nervous system. Indeed, definitive scientific evidence has demonstrated that exposure to even undetectable levels of MeHg can disrupt the fine and intricate balance of the developing brain, which can result in long-lasting, deleterious neurophysiological and neurobehavioral consequences. Accordingly, the exposure to low levels of MeHg can affect developing organisms either directly or indirectly, as this toxicant attacks different molecular targets in the brain. Of particular neurotoxicological significance, the literature have indicated that the targeting of thiol- and selenol-containing proteins can be the primary molecular events that trigger secondary and tertiary processes that ultimately culminate in MeHg neurotoxicity. Because several of these primary targets, such as the antioxidant seleno-enzymes, GPx and TrxR, are involved in the maintenance of the redox cell balance, their inhibition by MeHg can predispose a cell to oxidative stress. Furthermore, the concomitant inhibition of astrocytic glutamate transporters by MeHg can increase extracellular glutamate levels to neurotoxic concentrations. The installation of an “excitotoxic state” after exposure to MeHg triggers a complex cascade of events that eventually culminates in oxidative stress. In short, MeHg can simultaneously target a multitude of molecular targets, and the disruption of some of them will converge to common manifestations of neurotoxicity associated with oxidative stress. As discussed above, these deleterious alterations in a mature central nervous system can cause reversible or adaptable neurophysiological changes; however, in the developing brain, the neurophysiological changes are unpredictable. Consequently, the environmental exposure to MeHg during brain development represents a significant human health concern and additional research is necessary to determine the specific, primary targets of MeHg and whether the disruption of these targets triggers the cascade of events that culminates in oxidative stress, neurotoxicity and brain disease.
Acknowledgements
The authors would like to thank the colleagues/co-authors who contributed to several studies referenced in this mini-review. These studies were funded in part by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), FAPERGS/PRONEX and from the National Institute of Environmental Health Sciences (USA). The FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00 and INCT for Excitotoxicity and Neuroprotection-MCT/CNPq are especially appreciated. MA was supported by NIH grant R01 ES07331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams WJ, Jr, Kocsis JJ, Snyder R. Acute toxicity and urinary excretion of diphenyldiselenide. Toxicol Lett. 1989;48:301–310. doi: 10.1016/0378-4274(89)90057-x. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–648. [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H–D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902:92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Amonpatumrat S, Sakurai H, Wiriyasermkul P, Khunweeraphong N, Nagamori S, Tanaka H, Piyachaturawat P, Kanai Y. L-glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2008;108:280–289. doi: 10.1254/jphs.08118fp. [DOI] [PubMed] [Google Scholar]
- Araie H, Shiraiwa Y. Selenium utilization strategy by microalgae. Molecules (Basel, Switzerland) 2009;14:4880–4891. doi: 10.3390/molecules14124880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Astrocytes as modulators of mercury-induced neurotoxicity. Neurotoxicology. 1996;17:663–669. [PubMed] [Google Scholar]
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602:181–186. doi: 10.1016/0006-8993(93)90680-l. [DOI] [PubMed] [Google Scholar]
- Aschner M, Onishchenko N, Ceccatelli S. Toxicology of alkylmercury compounds. Met Ions Life Sci. 7:403–434. doi: 10.1039/BK9781847551771-00403. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol Sci. 2005;26:549–557. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. Faseb J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Developmental changes in the biliary excretion of methylmercury and glutathione. Science. 1982;216:61–63. doi: 10.1126/science.7063871. [DOI] [PubMed] [Google Scholar]
- Bancon-Montigny C, Yang L, Sturgeon RE, Colombini V, Mester Z. High-yield synthesis of milligram amounts of isotopically enriched methylmercury (CH3198HgCl) Appl Organomet Chem. 2004;18:57–64. [Google Scholar]
- Barbosa AC, Jardim W, Dorea JG, Fosberg B, Souza J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River basin, Amazon, Brazil. Arch Environ Contam Tox. 2001;40:439–444. doi: 10.1007/s002440010195. [DOI] [PubMed] [Google Scholar]
- Belletti S, Orlandini G, Vettori MV, Mutti A, Uggeri J, Scandroglio R, Alinovi R, Gatti R. Time course assessment of methylmercury effects on C6 glioma cells: submicromolar concentrations induce oxidative DNA damage and apoptosis. J Neurosci Res. 2002;70:703–711. doi: 10.1002/jnr.10419. [DOI] [PubMed] [Google Scholar]
- Berntssen MH, Hylland K, Lundebye AK, Julshamn K. Higher faecal excretion and lower tissue accumulation of mercury in Wistar rats from contaminated fish than from methylmercury chloride added to fish. Food Chem Toxicol. 2004;42:1359–1366. doi: 10.1016/j.fct.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Branco V, Canário J, Holmgren A, Carvalho C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Tox Appl Pharmacol. 251:95–103. doi: 10.1016/j.taap.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury. Placenta. 2009;30:800–805. doi: 10.1016/j.placenta.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2011;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- Brookes N, Kristt DA. Inhibition of amino acid transport and protein synthesis by HgCl2 and methylmercury in astrocytes: selectivity and reversibility. J Neurochem. 1989;53:1228–1237. doi: 10.1111/j.1471-4159.1989.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Lu J, Zhang X, Arner ES, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. Faseb J. 2010;25:370–381. doi: 10.1096/fj.10-157594. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Franco JL, Ghizoni H, Kobus K, Nazari EM, Rocha JB, Nogueira CW, Dafre AL, Muller YM, Farina M. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239:195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Dare E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact. 2010;188:301–308. doi: 10.1016/j.cbi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chang JY. Methylmercury causes glial IL-6 release. Neurosci Let. 2007;416:217–220. doi: 10.1016/j.neulet.2007.01.076. [DOI] [PubMed] [Google Scholar]
- Chang LW, Gilbert M, Sprecher J. Modification of methylmercury neurotoxicity by vitamin E. Environ Res. 1978;17:356–366. doi: 10.1016/0013-9351(78)90040-3. [DOI] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P. Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res. 2008;107:45–52. doi: 10.1016/j.envres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen TP, Murata K, Nielsen HP, Petersen MS, Askham J, Grandjean P. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW. The pharmacology of mercury compounds. Ann Rev Pharmacol. 1972;12:375–406. doi: 10.1146/annurev.pa.12.040172.002111. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Critl Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Compeau GC, Bartha R. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 1985;50:498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- da Conceição Nascimento M, Nascimento JLMD, de Lima Silveira LC, Da Rocha JBT, Aschner M. Mercury and selenium - a review on aspects related to the health of human populations in the amazon. Environ Bioindic. 2009;4:222–245. doi: 10.1080/15555270903143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare E, Li W, Zhivotovsky B, Yuan X, Ceccatelli S. Methylmercury and H(2)O(2) provoke lysosomal damage in human astrocytoma D384 cells followed by apoptosis. Free Radic Biol Med. 2001;30:1347–1356. doi: 10.1016/s0891-5849(01)00526-3. [DOI] [PubMed] [Google Scholar]
- de Freitas AS, Funck VR, Rotta Mdos S, Bohrer D, Morschbacher V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JB. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res Bull. 2009;79:77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorea JG. Environmental contaminants as biomarkers of fish intake: a case for hair mercury concentrations. Eur J Clin Nut. 2010;65:419–420. doi: 10.1038/ejcn.2010.177. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Seegal RF. Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology. 2007;28:720–726. doi: 10.1016/j.neuro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free Radicals in the Physiological Control of Cell Function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Eskes C, Honegger P, Juillerat-Jeanneret L, Monnet-Tschudi F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia. 2002;37:43–52. doi: 10.1002/glia.10019. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. Oxidative stress and methylmercury-induced neurotoxicity. Indianapolis: John Wiley & Sons; 2011. pp. 357–385. [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Sunol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci. 2003b;73:135–140. doi: 10.1093/toxsci/kfg058. [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol. 2005;57:1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett. 2003a;144:351–357. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005;204:343–354. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47:449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Missau F, Pizzolatti MG, Santos ARS, Souza DO, Aschner M, Rocha JBT, Dafre AL, Farina M. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol. 2010;30:272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, Moro AM, Bohrer D, Bairros AV, Dafre AL, Santos AR, Farina M. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res. 2006;102:22–28. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Freitas AJ, Rocha JB, Wolosker H, Souza DO. Effects of Hg2+ and CH3Hg+ on Ca2+ fluxes in rat brain microsomes. Brain Res. 1996;738:257–264. doi: 10.1016/s0006-8993(96)00781-0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fujiyama J, Hirayama K, Yasutake A. Mechanism of methylmercury efflux from cultured astrocytes. Biochem Pharmacol. 1994;47:1525–1530. doi: 10.1016/0006-2952(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Ganther HE. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: possible mechanisms. Environ Health Perspec. 1978;25:71–76. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. Methylmercury causes oxidative stress and cytotoxicity in microglia: attenuation by 15-deoxy-delta 12, 14-prostaglandin J2. J Neuroimmunol. 2006;171:17–28. doi: 10.1016/j.jneuroim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Gasso S, Cristofol RM, Selema G, Rosa R, Rodriguez-Farre E, Sanfeliu C. Antioxidant compounds and Ca(2+) pathway blockers differentially protect against methylmercury and mercuric chloride neurotoxicity. J Neurosci Res. 2001;66:135–145. doi: 10.1002/jnr.1205. [DOI] [PubMed] [Google Scholar]
- Gasso S, Sunol C, Sanfeliu C, Rodriguez-Farre E, Cristofol RM. Pharmacological characterization of the effects of methylmercury and mercuric chloride on spontaneous noradrenaline release from rat hippocampal slices. Life Sci. 2000;67:1219–1231. doi: 10.1016/s0024-3205(00)00715-3. [DOI] [PubMed] [Google Scholar]
- Glaser V, Leipnitz G, Straliotto MR, Oliveira J, dos Santos VV, Wannmacher CM, de Bem AF, Rocha JB, Farina M, Latini A. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. Neurotoxicology. 2010a;31:454–460. doi: 10.1016/j.neuro.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Glaser V, Nazari EM, Müller YMR, Feksa L, Wannmacher CMD, Rocha JBT, Bem AFd, Farina M, Latini A. Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int J Develop Neurosci. 2010b;28:631–637. doi: 10.1016/j.ijdevneu.2010.07.225. [DOI] [PubMed] [Google Scholar]
- Glover CN, Zheng D, Jayashankar S, Sales GD, Hogstrand C, Lundebye AK. Methylmercury speciation influences brain gene expression and behavior in gestationally-exposed mice pups. Toxicol Sci. 2009;110:389–400. doi: 10.1093/toxsci/kfp105. [DOI] [PubMed] [Google Scholar]
- Gomez M, Sanchez DJ, Colomina MT, Domingo JL, Corbella J. Evaluation of the protective activity of 2,3-dimercaptopropanol and sodium 2,3-dimercaptopropane-1-sulfonate on methylmercury-induced developmental toxicity in mice. Arch Environ Contam Toxicol. 1994;26:64–68. doi: 10.1007/BF00212795. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Herz KT. Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. The Mount Sinai J Med, New York. 78:107–118. doi: 10.1002/msj.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspec. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, SØRensen N, Dahl R, JØRgensen PJ. Cognitive Deficit in 7-Year-Old Children with Prenatal Exposure to Methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: Evidence for a signalling role of docosahexaenoic acid. Neurochem Int. 2009;54:535–543. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Grotto D, de Castro M, Barcelos G, Garcia S, Barbosa F. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: nitric oxide depletion and oxidative damage as possible mechanisms. Arch Toxicol. 2009;83:653–662. doi: 10.1007/s00204-009-0437-8. [DOI] [PubMed] [Google Scholar]
- Grotto D, Vicentini J, Friedmann Angeli JP, Francisco Latorraca E, Pontes Monteiro PA, Mazzaron Barcelos GR, Somacal S, Emanuelli T, Barbosa F., Jr Evaluation of protective effects of fish oil against oxidative damage in rats exposed to methylmercury. Ecotoxicol Environ Saf. doi: 10.1016/j.ecoenv.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. The Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- Hare MF, Atchison WD. Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108-15 cells. J Pharmacol Exp Ther. 1995;272:1016–1023. [PubMed] [Google Scholar]
- Hassan SA, Moussa EA, Abbott LC. The effect of methylmercury exposure on early central nervous system development in the zebrafish (Danio rerio) embryo. J Appl Toxicol. 2011 doi: 10.1002/jat.1675. in press. [DOI] [PubMed] [Google Scholar]
- He X, Ma Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol. 2009;76:1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, Newland MC. Dietary selenium protects against selected signs of aging and methylmercury exposure. Neurotoxicology. 2010;31:169–179. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano AM, Crespo-Lopez ME, Lima SM, Picanco-Diniz DL, Do Nascimento JL. Methylmercury intoxication activates nitric oxide synthase in chick retinal cell culture. Braz J Med Biol Res. 2006;39:415–418. doi: 10.1590/s0100-879x2006000300013. [DOI] [PubMed] [Google Scholar]
- Himi T, Ikeda M, Sato I, Yuasa T, Murota S. Purkinje cells express neuronal nitric oxide synthase after methylmercury administration. Brain Res. 1996;718:189–192. doi: 10.1016/0006-8993(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Hintelmann H. Organomercurials. Their formation and pathways in the environment. Met Ions Life Sci. 7:365–401. doi: 10.1039/BK9781847551771-00365. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Cinel I. Bench-to-bedside review: nitric oxide in critical illness--update 2008. Crit Care. 2009;13:218. doi: 10.1186/cc7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JA, Sparber SB. Reduction of methylmercury concentration in neonatal rat brains after administration of dimercaptosuccinic acid to dams while pregnant. Res Commun Chem Pathol Pharmacol. 1978;22:357–363. [PubMed] [Google Scholar]
- Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Komachi H, Sato I, Himi T, Yuasa T, Murota S-i. Induction of neuronal nitric oxide synthase by methylmercury in the cerebellum. J Neurosci Res. 1999;55:352–356. doi: 10.1002/(SICI)1097-4547(19990201)55:3<352::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Cross talk of nitric oxide, oxygen radicals, and superoxide dismutase regulates the energy metabolism and cell death and determines the fates of aerobic life. Antioxid Redox Signal. 2003;5:475–484. doi: 10.1089/152308603768295221. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Masukawa T, Kito H, Hayashi M. Degradation of methylmercury by selenium. Life Sci. 1982;31:859–866. doi: 10.1016/0024-3205(82)90541-0. [DOI] [PubMed] [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res. 2007;11:241–260. doi: 10.1007/BF03033570. [DOI] [PubMed] [Google Scholar]
- Jokanovic M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107–115. doi: 10.1016/j.toxlet.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Juarez BI, Martinez ML, Montante M, Dufour L, Garcia E, Jimenez-Capdeville ME. Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol. 2002;24:767–771. doi: 10.1016/s0892-0362(02)00270-2. [DOI] [PubMed] [Google Scholar]
- Juárez BI, Portillo-Salazar H, González-Amaro R, Mandeville P, Aguirre JR, Jiménez ME. Participation of N-methyl-D-aspartate receptors on methylmercury-induced DNA damage in rat frontal cortex. Toxicology. 2005;207:223–229. doi: 10.1016/j.tox.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Junghans RP. A review of the toxicity of methylmercury compounds with application to occupational exposures associated with laboratory uses. Environ Res. 1983;31:1–31. doi: 10.1016/0013-9351(83)90059-2. [DOI] [PubMed] [Google Scholar]
- Kasuya M. The effect of vitamin E on the toxicity of alkyl mercurials on nervous tissue in culture. Toxicol Appl Pharmacol. 1975;32:347–354. doi: 10.1016/0041-008x(75)90225-2. [DOI] [PubMed] [Google Scholar]
- Katsuyuki M, PÃ!l W, Esben BJr, Poul JJr, Philippe G. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediat. 2004;144:177–183. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. The in vitro effects of Trolox on methylmercury-induced neurotoxicity. Toxicology. 2010;276:73–78. doi: 10.1016/j.tox.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Asaduzzaman AM, Schreckenbach G, Wang F. Synthesis, characterization and structures of methylmercury complexes with selenoamino acids. Dalton Trans. 2009:5766–5772. doi: 10.1039/b903863a. [DOI] [PubMed] [Google Scholar]
- Kung MP, Kostyniak P, Olson J, Malone M, Roth JA. Studies of the in vitro effect of methylmercury chloride on rat brain neurotransmitter enzymes. J Appl Toxicol. 1987;7:119–121. doi: 10.1002/jat.2550070208. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic requirement of p47phox for superoxide production by murine microglia. FASEB J. 2001;15:285–287. doi: 10.1096/fj.00-0608fje. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ali SF, Bondy SC. Deferoxamine inhibits methyl mercury-induced increases in reactive oxygen species formation in rat brain. Toxicol Appl Pharmacol. 1992;112:161–165. doi: 10.1016/0041-008x(92)90292-z. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ali SF, McKee M, Bondy SC. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol. 1990;104:17–24. doi: 10.1016/0041-008x(90)90278-3. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Effect of alteration of nerve terminal Ca2+ regulation on increased spontaneous quantal release of acetylcholine by methyl mercury. Toxicol Appl Pharmacol. 1988;94:55–65. doi: 10.1016/0041-008x(88)90336-5. [DOI] [PubMed] [Google Scholar]
- Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limke TL, Bearss JJ, Atchison WD. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol Sci. 2004a;80:60–68. doi: 10.1093/toxsci/kfh131. [DOI] [PubMed] [Google Scholar]
- Limke TL, Heidemann SR, Atchison WD. Disruption of intraneuronal divalent cation regulation by methylmercury: are specific targets involved in altered neuronal development and cytotoxicity in methylmercury poisoning? Neurotoxicology. 2004b;25:741–760. doi: 10.1016/j.neuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Limke TL, Otero-Montanez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. The J Pharmacol Exp Ther. 2003;304:949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Lobanov AV, Hatfield DL, Gladyshev VN. Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta. 2009;1790:1424–1428. doi: 10.1016/j.bbagen.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci. 2006;94:240–255. doi: 10.1093/toxsci/kfl066. [DOI] [PubMed] [Google Scholar]
- Lucena GM, Franco JL, Ribas CM, Azevedo MS, Meotti FC, Gadotti VM, Dafre AL, Santos AR, Farina M. Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin Pharmacol Toxicol. 2007;101:127–131. doi: 10.1111/j.1742-7843.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Lucena GM, Porto FA, Campos EG, Azevedo MS, Cechinel-Filho V, Prediger RD, Ferreira VM. Cipura paludosa attenuates long-term behavioral deficits in rats exposed to methylmercury during early development. Ecotoxicol Environ Saf. 73:1150–1158. doi: 10.1016/j.ecoenv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Lund ME, Banner W, Jr, Clarkson TW, Berlin M. Treatment of acute methylmercury ingestion by hemodialysis with N-acetylcysteine (Mucomyst) infusion and 2,3-dimercaptopropane sulfonate. J Toxicol. 1984;22:31–49. doi: 10.3109/00099308409035080. [DOI] [PubMed] [Google Scholar]
- Magour S. Studies on the inhibition of brain synaptosomal Na+/K+-ATPase by mercury chloride and methyl mercury chloride. Arch Toxicol Suppl. 1986;9:393–396. doi: 10.1007/978-3-642-71248-7_77. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Marsh DO, Clarkson TW, Myers GJ, Davidson PW, Cox C, Cernichiari E, Tanner MA, Lednar W, Shamlaye C, Choisy O, et al. The Seychelles study of fetal methylmercury exposure and child development: introduction. Neurotoxicology. 1995;16:583–596. [PubMed] [Google Scholar]
- Martins R, de P, Braga H, de C, da Silva AP, Dalmarco JB, de Bem AF, dos Santos AR, Dafre AL, Pizzolatti MG, Latini A, Aschner M, Farina M. Synergistic neurotoxicity induced by methylmercury and quercetin in mice. Food Chem Toxicol. 2009;47:645–649. doi: 10.1016/j.fct.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Day ED., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron--EDTA complex. FEBS Let. 1978;86:139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto K-i, Nakanishi H, Moriguchi S, Fukuyama N, Eto K, Wakamiya J, Murao K, Arimura K, Osame M. Involvement of enhanced sensitivity of N-methyl--aspartate receptors in vulnerability of developing cortical neurons to methylmercury neurotoxicity. Brain Res. 2001;901:252–258. doi: 10.1016/s0006-8993(01)02281-8. [DOI] [PubMed] [Google Scholar]
- Moretto MB, Funchal C, Santos AQ, Gottfried C, Boff B, Zeni G, Pureur RP, Souza DO, Wofchuk S, Rocha JB. Ebselen protects glutamate uptake inhibition caused by methyl mercury but does not by Hg2+ Toxicology. 2005a;214:57–66. doi: 10.1016/j.tox.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Moretto MB, Funchal C, Zeni G, Pessoa-Pureur R, Rocha JB. Selenium compounds prevent the effects of methylmercury on the in vitro phosphorylation of cytoskeletal proteins in cerebral cortex of young rats. Toxicol Sci. 2005b;85:639–646. doi: 10.1093/toxsci/kfi114. [DOI] [PubMed] [Google Scholar]
- Mori N, Yasutake A, Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol. 2007;81:769–776. doi: 10.1007/s00204-007-0209-2. [DOI] [PubMed] [Google Scholar]
- Morken TS, Sonnewald U, Aschner M, Syversen T. Effects of methylmercury on primary brain cells in mono- and co-culture. Toxicol Sci. 2005;87:169–175. doi: 10.1093/toxsci/kfi227. [DOI] [PubMed] [Google Scholar]
- Murata K, Dakeishi M, Shimada M, Satoh H. Assessment of intrauterine methylmercury exposure affecting child development: messages from the newborn. Tohoku J Exp Med. 2007;213:187–202. doi: 10.1620/tjem.213.187. [DOI] [PubMed] [Google Scholar]
- Naganuma A, Miura K, Tanaka-Kagawa T, Kitahara J, Seko Y, Toyoda H, Imura N. Overexpression of manganese-superoxide dismutase prevents methylmercury tox1city in hela cells. Life Sci. 1998;62:PL157–PL161. doi: 10.1016/s0024-3205(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Nakazawa N, Makino F, Okada S. Acute effects of mercuric compounds on cultured mammalian cells. Biochem Pharmacol. 1975;24:489–493. doi: 10.1016/0006-2952(75)90135-5. [DOI] [PubMed] [Google Scholar]
- Nelson N, Byerly CT, Kolbye AC, Kurland LT, Shapiro RE, Shibko S, Stickel WH, Thompson JE, Van Den Berg LA, Weissler A. Hazards of mercury. Environ Res. 1971;4:1–69. [Google Scholar]
- Ni M, Li X, Yin Z, Jiang H, Sidoryk-WÄ™grzynowicz M, Milatovic D, Cai J, Aschner M. Methylmercury Induces Acute Oxidative Stress, Altering Nrf2 Protein Level in Primary Microglial Cells. Toxicol Sci. 2010;116:590–603. doi: 10.1093/toxsci/kfq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra A, Pierucci F, Parvez H, Senatori O. Monoamine oxidase expression during development and aging. Neurotoxicology. 2004;25:155–165. doi: 10.1016/S0161-813X(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Andersen O. The toxicokinetics of mercury in mice offspring after maternal exposure to methylmercury--effect of selenomethionine. Toxicology. 1992;74:233–241. doi: 10.1016/0300-483x(92)90142-2. [DOI] [PubMed] [Google Scholar]
- Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, Toribara TY, Cernichiari E, Clarkson T. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N Engl J Med. 1998;338:1672–1676. doi: 10.1056/NEJM199806043382305. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Onyido IN, AR; Buncel E. Biomolecule-mercury interactions: Modalities of DNA base-mercury binding mechanisms. Remediation strategies. Cheml Rev. 2004;104:5911–5929. doi: 10.1021/cr030443w. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Tomiyoshi F, Ueno S, Furukawa K, Chikahisa L. Methylmercury-induced augmentation of oxidative metabolism in cerebellar neurons dissociated from the rats: its dependence on intracellular Ca2+ Brain Res. 1994;660:154–157. doi: 10.1016/0006-8993(94)90849-4. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Songstad J. Application of principle of hard and soft acids and bases to organic chemistry. J Am Chem Soc. 1967;89:1827–1838. [Google Scholar]
- Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol Sci. 2001;61:224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- Porciuncula LO, Rocha JB, Tavares RG, Ghisleni G, Reis M, Souza DO. Methylmercury inhibits glutamate uptake by synaptic vesicles from rat brain. Neuroreport. 2003;14:577–580. doi: 10.1097/00001756-200303240-00010. [DOI] [PubMed] [Google Scholar]
- Potashkin JA, Meredith GE. The Role of Oxidative Stress in the Dysregulation of Gene Expression and Protein Metabolism in Neurodegenerative Disease. Antioxidants & Redox Signaling. 2006;8:144–151. doi: 10.1089/ars.2006.8.144. [DOI] [PubMed] [Google Scholar]
- Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1995;92:8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein DL, Arnold AP, Guy RD. 1H–NMR study of the removal of methylmercury from intact erythrocytes by sulfhydryl compounds. J Inorg Biochem. 1986;28:279–287. doi: 10.1016/0162-0134(86)80092-7. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Isab AA, Reid RS. A proton nuclear magnetic resonance study of the binding of methylmercury in human erythrocytes. Biochim Biophys Acta. 1982;720:53–64. doi: 10.1016/0167-4889(82)90038-6. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Reid RS, Isab AA. H nmr study of the effectiveness of various thiols for removal of methylmercury from hemolyzed erythrocytes. J Inorganic Biochem. 1983;18:241–251. doi: 10.1016/0162-0134(83)85006-5. [DOI] [PubMed] [Google Scholar]
- Ralston NV, Raymond LJ. Dietary selenium's protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Rand MD, Bland CE, Bond J. Methylmercury activates enhancer-of-split and bearded complex genes independent of the notch receptor. Toxicol Sci. 2008;104:163–176. doi: 10.1093/toxsci/kfn060. [DOI] [PubMed] [Google Scholar]
- Rand MD, Dao JC, Clason TA. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology. 2009;30:794–802. doi: 10.1016/j.neuro.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Racz WJ. Effects of methylmercury on the spontaneous and potassium-evoked release of endogenous amino acids from mouse cerebellar slices. Can J Physiol Pharmacol. 1987;65:791–798. doi: 10.1139/y87-127. [DOI] [PubMed] [Google Scholar]
- Rice DC. Overview of modifiers of methylmercury neurotoxicity: chemicals, nutrients, and the social environment. Neurotoxicology. 2008;29:761–766. doi: 10.1016/j.neuro.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Robinson JF, Guerrette Z, Yu X, Hong S, Faustman EM. A systems-based approach to investigate dose- and time-dependent methylmercury-induced gene expression response in C57BL/6 mouse embryos undergoing neurulation. Birth Defects Res B Dev Reprod Toxicol. 2010;89:188–200. doi: 10.1002/bdrb.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JB, Freitas AJ, Marques MB, Pereira ME, Emanuelli T, Souza DO. aEffects of methylmercury exposure during the second stage of rapid postnatal brain growth on negative geotaxis and on delta-aminolevulinate dehydratase of suckling rats. Braz J Med Biol Res. 1993;26:1077–1083. [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Farina M, Aschner M, Bohrer D, Rocha JB, de Vargas Barbosa NB. Modulation of methylmercury uptake by methionine: Prevention of mitochondrial dysfunction in rat liver slices by a mimicry mechanism. Toxicol Appl Pharmacol. 2011;252:28–35. doi: 10.1016/j.taap.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Santos MM, Souza DOG, Farina M, Nogueira CW, Aschner M, Burger ME, Barbosa NBV, Rocha JBT. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: Involvement of oxidative stress and glutamatergic system. Toxicol Vitro. 2009;23:302–307. doi: 10.1016/j.tiv.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Ruha AM, Curry SC, Gerkin RD, Caldwell KL, Osterloh JD, Wax PM. Urine mercury excretion following meso-dimercaptosuccinic acid challenge in fish eaters. Arch Pathol Lab Med. 2009;133:87–92. doi: 10.5858/133.1.87. [DOI] [PubMed] [Google Scholar]
- Sausen de Freitas A, de Souza Prestes A, Wagner C, Haigert Sudati J, Alves D, Oliveira Porciuncula L, Kade IJ, Teixeira Rocha JB. Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: a possible novel pathway for their antioxidant activity. Molecules (Basel, Switzerland) 15:7699–7714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Aschner JL, Syversen T, Aschner M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Brain Res Mol Brain Res. 2004;128:48–57. doi: 10.1016/j.molbrainres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Mol Brain Res. 2003;110:85–91. doi: 10.1016/s0169-328x(02)00642-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Atchison WD. Effects of mercurials on ligand- and voltage-gated ion channels: a review. Neurotoxicology. 1996;17:63–84. [PubMed] [Google Scholar]
- Soares FA, Farina M, Santos FW, Souza D, Rocha JB, Nogueira CW. Interaction between metals and chelating agents affects glutamate binding on brain synaptic membranes. Neurochem Res. 2003;28:1859–1865. doi: 10.1023/a:1026175825871. [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res. 2006;31:563–569. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol. 2008;227:147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama T, Sumi D, Shinkai Y, Yasutake A, Taguchi K, Tong KI, Yamamoto M, Kumagai Y. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem Biophys Res Commun. 2007;363:645–650. doi: 10.1016/j.bbrc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Tsuzuki Y, Yamada T. Inhibitory actions of mercury compounds against glucose-6-phosphate dehydrogenase from yeast. J Toxicol Sci. 1979;4:105–113. doi: 10.2131/jts.4.105. [DOI] [PubMed] [Google Scholar]
- Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit Rev Environ Sci Technol. 2001;31:241–293. [Google Scholar]
- Usuki F, Fujita E, Sasagawa N. Methylmercury activates ASK1/JNK signaling pathways, leading to apoptosis due to both mitochondria- and endoplasmic reticulum (ER)-generated processes in myogenic cell lines. Neurotoxicology. 2008;29:22–30. doi: 10.1016/j.neuro.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Usuki F, Yamashita A, Fujimura M. Posttranscriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. J Biol Chem. doi: 10.1074/jbc.M110.168872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini J, Vicentini J, Grotto D, Tonello R, Garcia SC, Barbosa F., Jr Sub-chronic exposure to methylmercury at low levels decreases butyrylcholinesterase activity in rats. Basic Clin Pharmacol Toxicol. 106:95–99. doi: 10.1111/j.1742-7843.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- Vendrell I, Carrascal M, Campos F, Abian J, Sunol C. Methylmercury disrupts the balance between phosphorylated and non-phosphorylated cofilin in primary cultures of mice cerebellar granule cells. A proteomic study. Toxicol Appl Pharmacol. 2011;242:109–118. doi: 10.1016/j.taap.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Vendrell I, Carrascal M, Vilaro MT, Abian J, Rodriguez-Farre E, Sunol C. Cell viability and proteomic analysis in cultured neurons exposed to methylmercury. Hum Exp Toxicol. 2007;26:263–272. doi: 10.1177/0960327106070455. [DOI] [PubMed] [Google Scholar]
- Vogel DG, Margolis RL, Mottet NK. The effects of methyl mercury binding to microtubules. Toxicol Appl Pharmacol. 1985;80:473–486. doi: 10.1016/0041-008x(85)90392-8. [DOI] [PubMed] [Google Scholar]
- Wagner C, Sudati JH, Nogueira CW, Rocha JB. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. Biometals. 2010;23:1171–1177. doi: 10.1007/s10534-010-9367-4. [DOI] [PubMed] [Google Scholar]
- Wagner C, Vargas AP, Roos DH, Morel AF, Farina M, Nogueira CW, Aschner M, Rocha JB. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch Toxicol. 2010;84:89–97. doi: 10.1007/s00204-009-0482-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Jiang H, Yin Z, Aschner M, Cai J. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol Sci. 2009;107:135–143. doi: 10.1093/toxsci/kfn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DN, Connaughton VP, Dellinger JA, Klemer D, Udvadia A, Carvan MJ., 3rd Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Phys Behav. 2008;93:250–260. doi: 10.1016/j.physbeh.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Wootten V, Brown DR, Callahan BG, Vetrano K, Wadman P, Melia J, Mulligan T, Schatz RA. Behavioral and biochemical alterations following in utero exposure to methylmercury. Neurobehav Toxicol Teratol. 1985;7:767–773. [PubMed] [Google Scholar]
- Yamashita T, Ando Y, Sakashita N, Hirayama K, Tanaka Y, Tashima K, Uchino M, Ando M. Role of nitric oxide in the cerebellar degeneration during methylmercury intoxication. Biochim Biophys Acta. 1997;1334:303–311. doi: 10.1016/s0304-4165(96)00108-0. [DOI] [PubMed] [Google Scholar]
- Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, Farina M, Rocha JB, Aschner M. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 32:291–299. doi: 10.1016/j.neuro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JBT, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Robinson JF, Sidhu JS, Hong S, Faustman EM. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and methylmercury in mouse embryonic fibroblast. Toxicol Sci. 114:356–377. doi: 10.1093/toxsci/kfq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun-Zhong F, Sheng Y, Guoyao W. Free radicals, antioxidants, and nutrition. Nutrition (Burbank, Los Angeles County, Calif.) 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Zareba G, Cernichiari E, Hojo R, Nitt SM, Weiss B, Mumtaz MM, Jones DE, Clarkson TW. Thimerosal distribution and metabolism in neonatal mice: comparison with methyl mercury. Jf Appl Toxicol. 2007;27:511–518. doi: 10.1002/jat.1272. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xu Y, Sun J, Li X, Wang L, Jin L. Protection of pyrroloquinoline quinone against methylmercury-induced neurotoxicity via reducing oxidative stress. Free Rad Res. 2009;43:224–233. doi: 10.1080/10715760802677348. [DOI] [PubMed] [Google Scholar]
- Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem. 2002;277:39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Masayasu H, Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc Natl Acad Sci U S A. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Schildknecht S, Kuegler PB, Tanavde V, Kadereit S, Leist M. Sensitivity of dopaminergic neuron differentiation from stem cells to chronic low-dose methylmercury exposure. Toxicol Sci. doi: 10.1093/toxsci/kfr054. [DOI] [PubMed] [Google Scholar]