Abstract
Background/Aims
Obesity tends to be associated with increased mortality and morbidity in acute pancreatitis. However, in Asian populations, higher morbidity and mortality have been reported in patients with low body mass indexes (BMIs). This study was undertaken to evaluate the relation between obesity and outcome, and to investigate the occurrence of complications by overweightedness in acute pancreatitis.
Methods
The medical records of 403 patients with acute pancreatitis were reviewed retrospectively, and Ranson's scores, modified Glasgow scores, Acute Physiology and Chronic Health Evaluation (APACHE) II scores and computed tomography severity indexes were calculated. Patients were categorized by BMI for the analysis.
Results
When compared with normal patients (BMI 18.5 to 22.9), all categories with a BMI ≥23 had an increased risk of developing a severe form of acute pancreatitis (p=0.003) and all categories with a BMI ≥25 significantly predicted severity (p<0.001). Patients with class 1 obesity (BMI 25 to 29.9) developed significantly more systemic and metabolic complications.
Conclusions
Overweightedness and obesity were found to be associated with a higher risk of developing severe pancreatitis. Further studies are needed to establish the precise prognostic value of obesity in members of the population with low BMIs.
Keywords: Acute pancreatitis, Obesity, Overweight
INTRODUCTION
The incidence of acute pancreatitis appears to have increased in South Korea,1 and this increase is attributed to improved diagnostic procedures.2 In the majority of patients, acute pancreatitis is mild. However, in 10% to 20% of patients increased intrapancreatic and extrapancreatic inflammation result in what is generally referred to as systemic inflammatory response syndrome (SIRS). Necrotizing pancreatitis develops in approximately 10% to 20% of patients and mortality is high (range from 14% to 25%). About half of these deaths in patients with acute pancreatitis occur within the first 1-2 weeks and are mainly attributable to multiple organ dysfunction syndrome (MODS).3,4 Hence, early diagnosis and accurate prediction of acute pancreatitis severity are important.
Multifactorial scoring systems like Ranson's prognostic signs, the modified Glasgow, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring systems are widely used to predict the severity of acute pancreatitis.5,6 Morbidity and mortality associated with acute pancreatitis are substantially higher when necrosis is present, especially when the necrotic region is also infected.7 Pancreatic necrosis is diagnosed radiographically by dynamic intravenous contrast-enhanced computed tomography (CT) of the abdomen.8 However, because it is still difficult to establish an immediate prognosis for acute pancreatitis, several studies have been conducted to establish the prognostic usefulnesses of clinical data easily determined on admission, such as, obesity.9 Recently, several studies have identified obesity as a negative prognostic factor in acute pancreatitis.10,11 However, in Asian populations, morbidity and mortality also occur in patients with low body mass indexes (BMIs). Thus Asian studies have failed to demonstrate a meaningful relation between obesity and outcome.12 In the present study, we aimed to evaluate the relation between obesity and the severity of acute pancreatitis and to investigate the occurrence of complications in different BMI classes.
MATERIALS AND METHODS
From January 2000 to February 2007, 574 patients were treated for acute pancreatitis at Kyungpook National University Hospital in Daegu, Korea. This retrospective study was conducted on 403 patients with height and body weight details. Acute pancreatitis was diagnosed based on the presence of typical abdominal pain (the cardinal symptom of acute pancreatitis), a serum amylase or lipase levels threefold or more above upper normal limit, and abdominal CT scan findings compatible acute pancreatitis. We recorded age, gender, height, and body weight at admission, pancreatitis etiology, hospital stay, and hospitalization time in our intensive care unit. For patients with a history of alcohol abuse, pancreatitis was regarded as being alcohol induced. Gallstone pancreatitis was diagnosed based on a radiologic findings of a gallstone or bile duct dilatation and laboratory findings of obstructive jaundice. All patients were assessed using Ranson's, modified Glasgow, and the APACHE II scores. Contrast enhanced CT findings were graded using the Balthazar-Ranson criteria for severity, and CT severity index scores (Balthazar Scoring) were also calculated. Acute pancreatitis was classified using the Atlanta consensus classification system, and severe pancreatitis was defined as pancreatitis associated with organ failure and/or local complications (necrosis, an abscess, or pseudocyst). Organ failure was defined as shock (systolic pressure <90 mm Hg), pulmonary insufficiency (PaO2 ≤60 mm Hg), renal failure (serum creatinine >2.0 mg/dL after hydration), or gastrointestinal bleeding (>500 cc/24 hr). A number of additional systemic complications were identified as being characteristic of severe acute pancreatitis, these were, disseminated intravascular coagulation (platelets ≤100,000/mm3, fibrinogen ≤100 mg/dL, fibrin split products >80 µg/mL), or a severe metabolic disturbance (serum calcium ≤7.5 mg/dL).
Obesity was classified by BMI, which was calculated as weight in kilograms divided by height in meters squared (kg/m2). According to the World Health Organization (WHO) Western Pacific Region, we used BMI cutoff points to define a normal body weight (BMI 18.5 to 22.9 kg/m2), overweightedness (BMI 23 to 24.9 kg/m2), and an obese state (BMI ≥25 kg/m2).
Continuous variables are expressed as means (±SD) and categorical variables as absolutes and relative frequencies. The significances of differences between the distributions of quantitative and qualitative variables were assessed using the Student's t-test or the chi-square test, respectively. SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA) was used throughout, and p-values of <0.05 were considered statistically significant. Non-parametric data was assessed using the Whitney U-test. Relationships between obesity and the incidences of complications were assessed using odds ratios (ORs) with 95% confidence intervals (CIs).
Univariate and multivariate logistic regression analyses were used to investigate relations between age, gender and BMI and a poor prognosis.
RESULTS
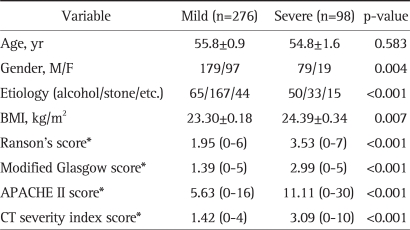
The 29 underweight patients (BMI <18.5 kg/m2), which included 4 cases of severe complications, were excluded. Of the remaining 374 patients (BMI ≥18.5 kg/m2), there were 200 (53.5%) gallstone pancreatitis patients, 115 (30.7%) alcohol-induced pancreatitis patients, and 59 (15.8%) patients with other etiologies, such as, infections and drugs. In addition, there were 276 mild pancreatitis patients and 98 severe pancreatitis patients, 5 of whom died. The characteristics of the patients with mild and severe acute pancreatitis are summarized in Table 1. The incidence of severe pancreatitis was higher in males (OR, 2.253; 95% CI, 1.289 to 3.938). However, 62 of the 65 mild alcohol-induced patients and all 50 patients with severe alcohol-induced pancreatitis were male. No significant gender difference was found for gallstone pancreatitis (p=0.842). Severe pancreatitis developed more frequently in alcohol-induced pancreatitis cases than gallstone-induced pancreatitis cases (OR, 3.893; 95% CI, 2.303 to 6.579). The mean BMI of severe pancreatitis patients was greater than that of mild pancreatitis patients (p=0.007). As was expected, severity index scores were also higher in severe pancreatitis (p<0.001).
Table 1.
Characteristics of Patients with Mild and Severe Acute Pancreatitis
BMI, body mass index; APACHE, acute physiology and chronic health evaluation; CT, computerized tomography.
*Values expressed in mean (range).
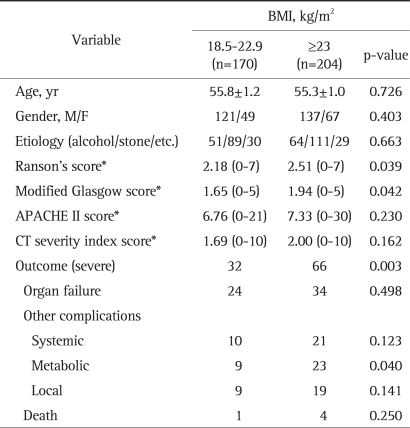
No significant differences in age, gender, and etiology were observed between the normal group (BMI 18.5 to 22.9, n=170) and the overweight and obese group (BMI ≥23, n=204) group (Table 2). In terms of severity index scores, comparing of the normal group and the overweight and obesity groups, Ranson's scores were 2.18 and 2.51 (p=0.039), and modified Glasgow scores were 1.65 and 1.94 (p=0.042), respectively, but APACHE II scores were 6.76 and 7.33 (p=0.230), and CT severity index scores were 1.69 and 2.00 (p=0.162), respectively. The incidence of a severe outcome was higher in the overweight and obese (BMI ≥23) group than normal group (p=0.003; OR, 2.063; 95%CI, 1.272 to 3.345). Metabolic complications developed 5.3% (9/170) in the normal group and 11.3% (23/204) in the overweight and obesity groups (p=0.040), but organ failure, the incidences of systemic and local complications, and death rate were not significantly different in both groups.
Table 2.
Comparison of the Normal Group and the Overweight and Obese Group in Patients with Acute Pancreatitis
BMI, body mass index; APACHE, acute physiology and chronic health evaluation; CT, computerized tomography.
*Values expressed in mean (range).
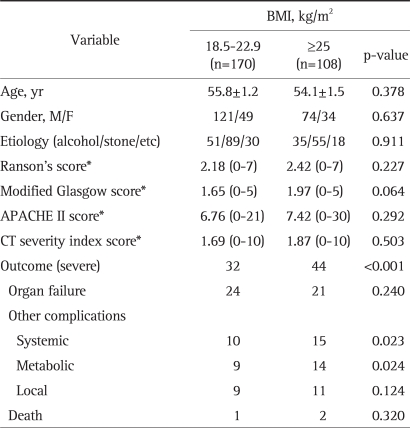
No significant differences in age, gender, etiology, and severity index scores were found between the obese group (BMI ≥25, n=108) and the normal group (BMI 18.5 to 22.9, n=170) (Table 3). The incidence of a severe outcome was higher in the obese (BMI ≥25) group than in the normal group (p<0.001). Systemic complications were 5.9% (10/170) in the normal group and 13.9% (15/108) in the obese group (p=0.023), and metabolic complications were 5.3% (9/170) and 13.0% (14/108), respectively (p=0.024), but organ failure, local complications, and death ratio were not significantly different in both groups. The rate of obesity was 44.9% among those that developed severe pancreatitis, and in the obese group, the odds ratio of severe pancreatitis was 2.965 (95% CI, 1.722 to 5.105), of a systemic complication was 2.581 (95% CI, 1.114 to 5.978), and of a metabolic complication was 2.664 (95% CI, 1.110 to 6.392).
Table 3.
Comparison of the Normal Group and the Obese Group in Patients with Acute Pancreatitis
BMI, body mass index; APACHE, acute physiology and chronic health evaluation; CT, computerized tomography.
*Values expressed in mean (range).
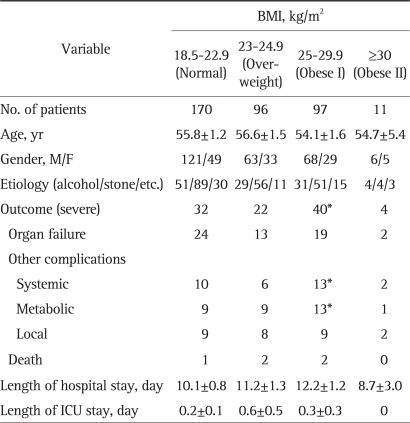
In Table 4, we subclassified obesity into two groups, the obese I (BMI 25 to 29.9) and obese II groups (BMI ≥30). No significant difference in age, gender, or etiology was found between the normal, overweight, obese I, obese II groups. The incidence of severe pancreatitis was higher in the obese I group (p<0.001; OR, 3.026; 95% CI, 1.732 to 5.287), but not in the overweight and obese II group. The obese I group had a higher rate of systemic complications (p=0.035; OR, 2.476; 95% CI, 1.042 to 5.885), and metabolic complications (p=0.020; OR, 2.769; 95% CI, 1.137 to 6.741) than the normal group. We also performed univariate and multivariate logistic regression test to investigate the prognostic values of age, gender, and BMI, and both gender (p=0.004) and BMI (p=0.003) were found to be independent predictive factors of a poor prognosis.
Table 4.
Characteristics of the 374 Patients with Acute Pancreatitis by BMI
BMI, body mass index; ICU, intensive care unit.
*p<0.05.
DISCUSSION
The Atlanta Symposium produced a clinically based classification system for the definition of acute pancreatitis, its severity, and complications.13 According to this system, severe pancreatitis is defined by the presence of organ failure, and or local complications that are associated with an increased risk of mortality. However, results from recent clinical studies indicate that these complications vary in terms of their effects on outcome, and that many are not necessarily life threatening on their own.14 For example, in necrotizing pancreatitis, almost all patients with no sign of organ failure survived, but 47% of patients with multiple organ failure died.
A recent study showed persistent (>48 hours) organ failure, whether present at admission or arising during the first week, was significantly associated with a fatal outcome, and also that a duration of organ failure predicted severe acute pancreatitis and was strongly associated with the risk of death or a local complication.15 In this study, of 254 patients with an APACHE II score that remained stationary or decreased at 48 hours after admission, 55 patients (21.7%) had a severe outcome, however, of 120 patients with an APACHE II score that increased at 48 hours after admission, 43 patients (35.8%) had a severe outcome, and an increase in APACHE II score and the severity of pancreatitis were found to be significantly related (chi-square, p=0.003602; OR, 2.02054; 95% CI, 1.253 to 3.258). Two studies have shown that APACHE II score at 48 hours after admission is a more useful predictor of severe outcome than APACHE II score on admission, and that a deteriorating APACHE II score is significantly associated with mortality in patients with severe acute pancreatitis.16,17
A meta-analysis of all available reports published between 1965 to December 2002, evaluated a total of 81 obese (BMI ≥30) patients and showed that obesity is a prognostic factor of the development of systemic and local complications in acute pancreatitis; however, but mortality among obese patients was only slightly higher.18 In this study, 46 of 170 patients (27.1%) in the normal group showed a deterioration of APACHE II score at 48 hours after admission, and 74 of 204 patients (36.3%) in the overweight and obese group showed deterioration of APACHE II score (p=0.057; OR, 1.534; 95% CI, 0.986 to 2.389).
It has been reported that obesity is associated with a low-grade inflammatory state,19 and it has been suggested that this inflammatory condition predisposes the development of SIRS and organ dysfunction in acute pancreatitis.20 The occurrence of severe pancreatitis in obese patient can also be explained by a lot of fat around the pancreas, fat necrosis and the release of virulence factors, an excessive inflammatory reaction, reduction of respiratory motion, formation of infection foci caused by necrosis. This led to a proposal that the APACHE II scoring system (APACHE-O) be modified to take obesity into account.21 De Waele et al.22 investigated the occurrence of gallstone pancreatitis complications in different BMI classes, and found that numbers and severities of complications increased with BMI. In the present study, Ranson's and modified Glasgow scores at admission in overweight and obese patients were higher than in the normal group, and BMI and the severity of acute pancreatitis were found to be significantly related (p=0.007). Disease severity was greater in the obese group (BMI ≥25) that in normal and overweight group (p<0.001; OR, 2.699; 95% CI, 1.659 to 4.390), but obese II group was similar to obese I group in terms of the incidence of severe outcomes and complications. However, this was probably because the proportion of obese II patients was low in our cohort (2.9%).
In Asians, the incidence of obesity is lower than in Caucasians and this difference causes biases during statistical analyses. Yeung et al.23 showed that obesity is not a prognostic factor when obesity is very uncommon (2.0%). In the present study, the percentage of overweight and obesity patients was 54.5%, and that of obese patients (BMI >25 kg/m2) was 28.9%, which contrast to the high percentage reported in the West, where typically, 55% to 57% of patients have a BMI of >25.9,24
Regional Office for the Western Pacific (WPRO), WHO, International Association for the Study of Obesity, International Obesity Task Force recommend different ranges of cut-offs for overweightedness and obesity in the Asian-Pacific region.12 Asians contain a greater proportion of fat than Caucasians at similar BMI levels, therefore in Asian populations, morbidity and mortality occurring among those with a lower BMI.25,26 Accordingly, before any decisions are made about the relationship between obesity and acute pancreatitis in Asians, further studies are required on other anthropometric indexes, such as waist-hip ratio.
In this study, the incidence of severe pancreatitis was 26.2%, which concurs with a previous report (11.9% to 32.8%).20,21,23 The incidence of alcohol-induced pancreatitis was higher in males than females, but the risk of acute pancreatitis according to the amount of alcohol consumed showed no gender difference. Finally, severe pancreatitis was more frequent in males (OR, 2.253; 95% CI, 1.289 to 3.938).
In conclusion, this study shows that both overweightedness and obesity and severity indexes at admission portend a higher risk of severe pancreatitis. In the overweight and obese group, Ranson's and modified Glasgow scores were significantly more elevated than in the normal group and the obese group developed more complications than the normal group. This study is of clinical significance, because few reports have been previously issued on overweightedness and acute pancreatitis in Asia.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim CD. Current status of acute pancreatitis in Korea. Korean J Gastroenterol. 2003;42:1–11. [PubMed] [Google Scholar]
- 2.McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984-1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Freeman ML Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Sekimoto M, Takada T, Kawarada Y, et al. JPN guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankisch PG, Warnecke B, Bruns D, et al. The APACHE II score is unreliable to diagnose necrotizing pancreatitis on admission to hospital. Pancreas. 2002;24:217–222. doi: 10.1097/00006676-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Perez A, Whang EE, Brooks DC, et al. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229–233. doi: 10.1097/00006676-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130–135. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297–306. doi: 10.1148/radiology.193.2.7972730. [DOI] [PubMed] [Google Scholar]
- 9.Suazo-Baráhona J, Carmona-Sánchez R, Robles-Díaz G, et al. Obesity: a risk factor for severe acute biliary and alcoholic pancreatitis. Am J Gastroenterol. 1998;93:1324–1328. doi: 10.1111/j.1572-0241.1998.442_l.x. [DOI] [PubMed] [Google Scholar]
- 10.Funnell IC, Bornman PC, Weakley SP, Terblanche J, Marks IN. Obesity: an important prognostic factor in acute pancreatitis. Br J Surg. 1993;80:484–486. doi: 10.1002/bjs.1800800426. [DOI] [PubMed] [Google Scholar]
- 11.Martínez J, Johnson CD, Sánchez-Payá J, de Madaria E, Robles-Díaz G, Pérez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology. 2006;6:206–209. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 12.Regional Office for the Western Pacific (WPRO); World Health Organization; International Association for the Study of Obesity; International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 13.Bradley EL., 3rd A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 14.Rau BM. Predicting severity of acute pancreatitis. Curr Gastroenterol Rep. 2007;9:107–115. doi: 10.1007/s11894-007-0004-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan AA, Parekh D, Cho Y, et al. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Acute Physiology and Chronic Health Evaluation. Arch Surg. 2002;137:1136–1140. doi: 10.1001/archsurg.137.10.1136. [DOI] [PubMed] [Google Scholar]
- 17.Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990;77:1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- 18.Martínez J, Sánchez-Payá J, Palazón JM, Suazo-Barahona J, Robles-Díaz G, Pérez-Mateo M. Is obesity a risk factor in acute pancreatitis? A meta-analysis. Pancreatology. 2004;4:42–48. doi: 10.1159/000077025. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 20.Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CD, Toh SK, Campbell MJ. Combination of APACHE-II score and an obesity score (APACHE-O) for the prediction of severe acute pancreatitis. Pancreatology. 2004;4:1–6. doi: 10.1159/000077021. [DOI] [PubMed] [Google Scholar]
- 22.De Waele B, Vanmierlo B, Van Nieuwenhove Y, Delvaux G. Impact of body overweight and class I, II and III obesity on the outcome of acute biliary pancreatitis. Pancreas. 2006;32:343–345. doi: 10.1097/01.mpa.0000220857.55378.7b. [DOI] [PubMed] [Google Scholar]
- 23.Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294–299. [PubMed] [Google Scholar]
- 24.Martínez J, Sánchez-Payá J, Palazón JM, Aparicio JR, Picó A, Pérez-Mateo M. Obesity: a prognostic factor of severity in acute pancreatitis. Pancreas. 1999;19:15–20. [PubMed] [Google Scholar]
- 25.Ko GT, Chan JC, Cockram CS, Woo J. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23:1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- 26.Deurenberg-Yap M, Yian TB, Kai CS, Deurenberg P, van Staveren WA. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pacific J Clin Nutr. 1999;8:177–183. doi: 10.1046/j.1440-6047.1999.00091.x. [DOI] [PubMed] [Google Scholar]