Abstract
Background/Aims
The exclusion of hepatitis B core antibody (HBcAb)-positive donors from liver transplants (LTs) due to the risk of transmitting hepatitis B virus (HBV) does not appear to be practical in Korea, where hepatitis B is endemic. This study assessed the risk of de novo HBV infection in hepatitis B surface antigen (HBsAg)-negative LT recipients receiving a liver from HBcAb-positive donors.
Methods
Of 341 adult living donor LTs conducted at our institution between March 2001 and September 2008, 176 donors (51.6%) were HBcAb-positive, and 26 HBcAb-positive grafts were transplanted to HBsAg-negative recipients. The median follow-up time after LT was 41.9 months.
Results
Without anti-HBV prophylaxis, 2 out of 26 (7.7%) HBsAg-negative recipients who received grafts from HBcAb-positive donors developed de novo HBV infection 20 and 85 months after LT. These patients had been negative for all HBV serologic markers before transplantation. In both cases, there were no abnormalities in liver function tests upon diagnosis of de novo HBV infection.
Conclusions
De novo HBV infection from HBcAb-positive donors after LT does not appear to be of great concern in terms of the number of cases in Korea because high risk patients who are HBV-negative comprise only a small proportion of the recipients. However, HBV-naïve LT recipients still carry the risk of developing de novo HBV infection as in non-HBV endemic areas.
Keywords: De novo hepatitis, Hepatitis B core antibody, Liver transplantation
INTRODUCTION
Owing to the discordance between the increment of potential recipients for liver transplantation (LT) and a lack of available liver donors, there has been increased use of hepatitis B core antibody (HBcAb)-positive liver grafts.1,2 However, liver grafts from HBcAb-positive donors carry the risk of transmitting hepatitis B virus (HBV) to hepatitis B surface antigen (HBsAg)-negative recipients since occult HBV infection in the liver grafts can be reactivated in the recipient through the use of posttransplant immunosuppression.3-6
Because Korea is endemic for HBV and the rate of HBcAb positivity among liver donors reflects the prevalence of HBV infection,3 the prevalence of HBcAb positivity in Korea is greater than that of well-known low prevalence areas.7 Accumulated experiences strongly recommend the administration of preventive therapy for HBV-naïve recipients who receive grafts from HBcAb-positive donors, and anti-HBV prophylaxis is suggested to vaccinated recipients or the ones with isolated HBcAb.8,9 However, it is uncertain if it is suitable to apply these treatments equally in HBV endemic areas. Thus, this study was conducted to evaluate the risk of de novo HBV infection in HBsAg-negative LT recipients who received grafts from HBcAb-positive donors in Korea, where the prevalence of HBcAb positivity is equally high in both donor and recipient groups.
MATERIALS AND METHODS
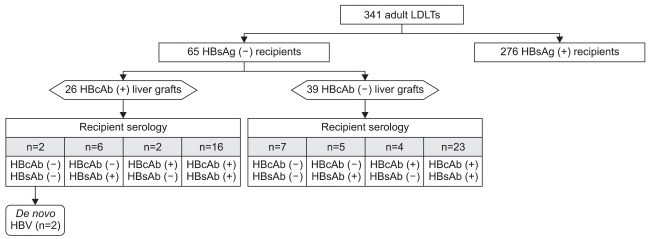
From March 2001 to September 2008, 341 consecutive adult living donor liver transplantations (LDLT) were conducted at our institution. The median age of the 341 donors was 31 years, and 176 donors (51.6%) were HBcAb-positive. Only 65 of the 341 recipients were HBsAg-negative previous to LT. All recipients were followed-up for at least 15 months after LT.
Among 65 HBsAg-negative recipients, nine were naïve for HBV (HBcAb-negative, Hepatitis B surface antibody [HBsAb]-negative), 11 were only HBsAb-positive, indicating that they had been previously vaccinated, 39 were positive for both HBcAb and HBsAb, indicating previous infection, and six were only positive for HBcAb. In the recipient population, the positive rate of HBcAb was 69.2% (45/65). The median age of the 65 HBsAg-negative recipients was 51 years, and 26 of these 65 recipients received HBcAb-positive grafts (Fig. 1). None of the HBsAg-negative recipients receiving liver grafts from HBcAb-positive donors had received preventive therapy against de novo HBV infection at our center.
Fig. 1.
Diagram of the study population. Of 341 adult living donor liver transplantations, 26 HBcAb-positive grafts were transplanted into HBsAg-negative recipients. Without anti-HBV prophylaxis, 2 of the 26 (7.7%) recipients of positive grafts developed de novo HBV infection.
LDLT, living donor liver transplantation; HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; HBV, Hepatitis B virus.
Instead of anti-HBV prophylaxis, recipients were routinely screened for serum HBsAg and HBV DNA at least every 3 months or whenever graft dysfunction was suspected after LT. The median follow-up period after LT was 41.9 months (range, 15 to 103 months).
This study was conducted according to the current declaration of Helsinki, and the protocol was approved by the Institutional Ethics Committee at Seoul St. Mary's Hospital in Korea. Baseline clinical and serologic markers were assessed. Continuous variables were expressed as medians with ranges and were compared using the Mann-Whitney U-test. Categorical variables were expressed as the number of patients with percentage and were compared using the chi-square or the Fisher's exact tests where appropriate. p-value less than 0.05 was considered to be significant. Software package SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
RESULTS
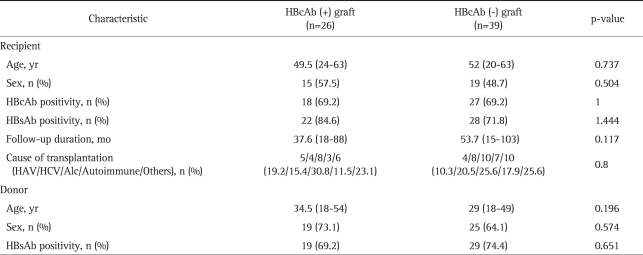
The baseline characteristics of the 65 HBsAg-negative recipients are shown in Table 1. Without any prophylaxis, two out of the 26 (7.7%) HBsAg-negative recipients who received the graft from HBcAb-positive donors developed de novo HBV infection. Both recipients were naïve for all HBV serologic markers (i.e., HBsAg, HBsAb, and HBcAb) preoperatively. In one patient, HbsAg turned out to be positive in serum at 20 months after LT. At the same time, HBsAb and HBeAg were positive, and HBV DNA level was 3.2×105 copies/mL. In the other patient, the positivity for HBsAg and HBeAg came out at 85 months after LT. HBsAb was negative and HBV DNA level was 1.0×109 copies/mL at that time. In both cases, de novo hepatitis B was detected by serological tests incidentally upon routine check-up without abnormality in the liver function test. A liver biopsy was conducted in one of the two patients who developed de novo HBV infection. The results revealed a mild necro-inflammatory reaction, with a minimally positive stain for HBcAg but not for HBsAg in hepatocytes. Both of these two recipients have been followed without administration of any treatment for HBV infection afterwards. About one year has passed since then, there were no significant changes of HBsAg, HBsAb, HBeAg status, and HBV DNA levels. Liver enzyme levels have been kept within normal range.
Table 1.
Baseline Characteristics of 65 HBsAg-Negative Recipients
Continuous variables are expressed as the median (range).
HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; HAV, hepatitis A virus; HCV, hepatitis C virus; Alc, alcohol.
DISCUSSION
It has been reported that de novo HBV infection from HBcAb-positive donors after LT occurs at a high rate especially in HBV-naïve recipients without any prophylaxis. Thus there have been many attempts to minimize the risk for transmission of HBV to HBsAg-negative recipients using immunoprophylaxis regimens such as HBIG, lamivudine and vaccination. To date, accumulated experiences have resulted in immunoprophylaxis being strongly recommended for HBV-naïve recipients receiving grafts from HBcAb-positive donors.8-10 However, most of these studies were conducted in areas with low prevalence (3% to 4%) of HBcAb positivity. The liver donor population reflects the general population to some extent, and it is anticipated that the prevalence of HbcAb positivity in liver donors and the acquisition of HBV infection after LT would be high. As expected, the positive rate of HBcAb was 51.6% (176 out of 341 donors) in the LT donor population and 69.2% (45 out of 65 recipients) in HBsAg-negative recipients in our study, which are very high compared to those reported by Western countries with a low prevalence of HBV.1,6,11,12
Conversely, development of de novo HBV was not as frequent as all that. De novo HBV was detected in two out of 26 (7.7%) HBsAg-negative LDLT recipients receiving HBcAb-positive liver grafts, and both of these were naïve for HBV preoperatively. Our results support those of previous studies that have found HBV-naïve recipients to be more prone to de novo HBV infection, but the total incidence of de novo HBV infection in the present study was lower than formerly reported.8,9 This could be explained by the high prevalence of HBcAb positivity, and therefore small proportion of HBV naïve patients in the adult recipient population in an HBV endemic area like Korea. On the contrary, de novo HBV infection was reported at a relatively high rate in child LDLTs conducted using grafts from adult donors in Korea.13 A possible explanation is that HBV serologic markers are negative in most child LT recipients, while they are positive in half of adult donors.13,14 Therefore, in the clinical setting of child LDLTs in Korea, de novo HBV infection has been issued earlier, and anti-HBV prophylaxis has commonly been used. By contrast, de novo HBV infection in adult LDLTs has not drawn attention, and concensus on treatments for adult patients receiving liver allografts from HBcAb-positive donors has not been established until recently in Korea. Our long-term study clearly suggests that HBsAg-negative LT recipients from HBcAb-positive donors have a high risk of developing de novo HBV infection, particularly in clinical settings in which they are negative for all HBV serologic markers (i.e., HBsAg, HBcAb, HBsAb). Theoretically, among 65 HBsAg-negative recipients, only nine (13.8%) would be considered candidates for anti-HBV prophlylaxis when receiving liver grafts from HBcAb-positive donors.
As described above, there were no abnormalities in liver function tests upon diagnosis for de novo HBV infection, and one biopsy specimen showed mild necroinflammatory reaction with minimal staining for HBcAg. Although a biopsy was only conducted in one case, these findings imply that de novo HBV infection would not result in serious hepatitis if it were diagnosed by regular follow-up tests in HBsAg-negative recipients. Antiviral treatment for HBV was not prescribed to either patient. In the clinical setting of HBsAg-negative recipients receiving HBcAb-positive liver grafts, two strategic options may be taken into consideration: 1) anti-HBV prophylaxis in the recipient group at a high risk for developing de novo HBV infection, 2) routine follow-up for HBV markers and initiation of treatment when clinical hepatitis B is apparent. Only the latter has been applied at our institution since the development of drug resistance in consequence of long-term use of nucleoside analogues for prophylaxis could be of concern, and effects on the graft have not been clearly demonstrated.
While the median follow-up time was 41.9 months with a maximum of 103 months, de novo HBV infection occurred at up to 85 months after LT in our study. This may have great clinical implications since at most 60 months was the longest period in earlier case reports.2,15,16 Our results suggest that surveillance should be conducted for a long period of time, and perhaps throughout the recipient's lifetime.
In Korea, HBsAg-positive patients have mainly comprised adult LT recipient population. Therefore, reports about HBsAg-negative recipients from HBcAb-positive donors has been limited. In our study, 19% (65/341) of adult LDLTs have not been attributed to HBV. Despite the limited cases, this study has the implication that the study population structure was different from that of Western series. About half of LT recipients in other studies conducted in areas with low prevalence of HBV consisted of HBV-naïve recipients. But, only about 15% of LT recipients were HBV-naïve in our series. To draw more conclusive results in different population, multicenter prospective studies will be needed.
In conclusion, de novo HBV infection after LT using HbcAb-positive grafts does not appear to be a significant concern in terms of the number of cases in Korea despite the high prevalence of HbcAb-positivity, since most of the recipients already had positive HBV serologic markers. However, prophylactic strategy should be taken into consideration since HBV-naïve LT recipients poses a particularly high-risk of developing de novo HBV infection after receiving allografts from HbcAb-positive donors as in areas with low prevalence of HBV infection.
ACKNOWLEDGEMENTS
This work was funded by a grant (A050021, Liver Cirrhosis Clinical Research Center) of the Korea Health 21 Research and Development Project, Ministry of Health and Welfare, Republic of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wachs ME, Amend WJ, Ascher NL, et al. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation. 1995;59:230–234. [PubMed] [Google Scholar]
- 2.Donataccio D, Roggen F, De Reyck C, Verbaandert C, Bodeus M, Lerut J. Use of anti-HBc positive allografts in adult liver transplantation: toward a safer way to expand the donor pool. Transpl Int. 2006;19:38–43. doi: 10.1111/j.1432-2277.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 3.Prieto M, Gömez MD, Berenguer M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001;7:51–58. doi: 10.1053/jlts.2001.20786. [DOI] [PubMed] [Google Scholar]
- 4.Rokuhara A, Tanaka E, Yagi S, et al. De novo infection of hepatitis B virus in patients with orthotopic liver transplantation: analysis by determining complete sequence of the genome. J Med Virol. 2000;62:471–478. [PubMed] [Google Scholar]
- 5.Roche B, Samuel D, Gigou M, et al. De novo and apparent de novo hepatitis B virus infection after liver transplantation. J Hepatol. 1997;26:517–526. doi: 10.1016/s0168-8278(97)80416-3. [DOI] [PubMed] [Google Scholar]
- 6.Dickson RC, Everhart JE, Lake JR, et al. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology. 1997;113:1668–1674. doi: 10.1053/gast.1997.v113.pm9352871. [DOI] [PubMed] [Google Scholar]
- 7.Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36(Suppl 1):S33–S44. doi: 10.1016/s1386-6532(06)80007-7. [DOI] [PubMed] [Google Scholar]
- 8.Avelino-Silva VI, D'Albuquerque LA, Bonazzi PR, et al. Liver transplant from Anti-HBc-positive, HBsAg-negative donor into HBsAg-negative recipient: is it safe? A systematic review of the literature. Clin Transplant. 2010;24:735–746. doi: 10.1111/j.1399-0012.2010.01254.x. [DOI] [PubMed] [Google Scholar]
- 9.Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272–279. doi: 10.1016/j.jhep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl. 2010;16:300–307. doi: 10.1002/lt.21998. [DOI] [PubMed] [Google Scholar]
- 11.Dodson SF, Bonham CA, Geller DA, Cacciarelli TV, Rakela J, Fung JJ. Prevention of de novo hepatitis B infection in recipients of hepatic allografts from anti-HBc positive donors. Transplantation. 1999;68:1058–1061. doi: 10.1097/00007890-199910150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Douglas DD, Rakela J, Wright TL, Krom RA, Wiesner RH. The clinical course of transplantation-associated de novo hepatitis B infection in the liver transplant recipient. Liver Transpl Surg. 1997;3:105–111. doi: 10.1002/lt.500030202. [DOI] [PubMed] [Google Scholar]
- 13.Lee KW, Lee DS, Lee HH, et al. Prevention of de novo hepatitis B infection from HbcAb-positive donors in living donor liver transplantation. Transplant Proc. 2004;36:2311–2312. doi: 10.1016/j.transproceed.2004.08.139. [DOI] [PubMed] [Google Scholar]
- 14.Kwon CH, Suh KS, Yi NJ, et al. Long-term protection against hepatitis B in pediatric liver recipients can be achieved effectively with vaccination after transplantation. Pediatr Transplant. 2006;10:479–486. doi: 10.1111/j.1399-3046.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 15.Takemura N, Sugawara Y, Tamura S, Makuuchi M. Liver transplantation using hepatitis B core antibody-positive grafts: review and university of Tokyo experience. Dig Dis Sci. 2007;52:2472–2477. doi: 10.1007/s10620-006-9656-5. [DOI] [PubMed] [Google Scholar]
- 16.Uemoto S, Sugiyama K, Marusawa H, et al. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;65:494–499. doi: 10.1097/00007890-199802270-00007. [DOI] [PubMed] [Google Scholar]