Abstract
Translational molecular imaging with positron emission tomography (PET) and allied technologies offer unrivalled applications in the discovery of biomarkers and aetiological mechanisms relevant to human disease. Foremost among clinical PET findings during the past two decades of addiction research is the seminal discovery of reduced dopamine D2/3 receptor expression in the striatum of drug addicts, which could indicate a predisposing factor and/or compensatory reaction to the chronic abuse of stimulant drugs. In parallel, recent years have witnessed significant improvements in the performance of small animal tomographs (microPET) and a refinement of animal models of addiction based on clinically relevant diagnostic criteria. This review surveys the utility of PET in the elucidation of neuropharmacological mechanisms underlying drug addiction. It considers the consequences of chronic drug exposure on regional brain metabolism and neurotransmitter function and identifies those areas where further research is needed, especially concerning the implementation of PET tracers targeting neurotransmitter systems other than dopamine, which increasingly have been implicated in the pathophysiology of drug addiction. In addition, this review considers the causal effects of behavioural traits such as impulsivity and novelty/sensation-seeking on the emergence of compulsive drug-taking. Previous research indicates that spontaneously high-impulsive rats – as exemplified by ‘Zippy’– are pre-disposed to escalate intravenous cocaine self-administration, and subsequently to develop compulsive drug taking tendencies that endure despite concurrent adverse consequences of such behaviour, just as in human addiction. The discovery using microPET of pre-existing differences in dopamine D2/3 receptor expression in the striatum of high-impulsive rats suggests a neural endophenotype that may likewise pre-dispose to stimulant addiction in humans.
LINKED ARTICLES
This article is part of a themed section on Imaging. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2011.163.issue-8BJP has previously published an Imaging in Pharmacology themed section, edited by A Davenport and C Daly. To view this section visit http://dx.doi.org/10.1111/bph.2010.159.issue-4
Keywords: impulsivity, vulnerability, stimulants, positron emission tomography, cocaine, raclopride, fallypride
Introduction
Misuse of addictive substances including psycho-stimulant drugs and alcohol is a major and growing cause of morbidity and mortality in the United Kingdom (Godfrey and Cave, 2005). Despite extensive research, the aetiology of drug addiction remains remarkably poorly understood in neural terms, even though a detailed knowledge has been obtained of the primary molecular sites in the brain that mediate the reinforcing effects of widely abused drugs such as cocaine and heroin (Nestler et al., 1993; Wise, 1996; Kalivas and Volkow, 2005). The influential discovery by Olds and Milner of intra-cranial self-stimulation (ICSS) marked a major turning point for research on the neuropharmacology of addiction (Olds and Milner, 1954). The subsequent discovery of brain dopamine led to the Nobel Prize in Medicine awarded in the year 2000 to Professors Carlsson, Greengard and Kandel. In the intervening years, it was established that dopaminergic fibres arising in the ventral tegmental area (VTA) of the mesencephalon and projecting to limbic cortico-striatal structures [nucleus accumbens (NAcb), olfactory tubercle, amygdala, orbitofrontal cortex (OFC) and prefrontal cortex (PFC)] were effective substrates for ICSS. This linkage of neurochemical anatomy and behaviour fuelled interest in the brain dpoamine systems as neural substrates for the rewarding properties of both natural (e.g. food) and drug incentives. Indeed, the hypothesis that drugs of abuse mediate their hedonic or pleasurable effects through dpoamine release in mesolimbic regions, including especially the NAcb, has dominated addiction research for more than two decades (Di Chiara and Imperato, 1988; Wise, 1996).
Although the dopamine model continues to influence and inform contemporary theories of drug addiction, supplementary mechanisms have been postulated to explain the emergence of compulsive drug seeking, a defining hallmark of addiction involving continued drug use in the face of mounting negative consequences of such behaviour (Hyman and Malenka, 2001; Everitt et al., 2008). For example, the development of compulsive drug seeking has been posited to represent a failure in top-down, executive control, brought about by the toxic effects of drugs themselves on PFC/OFC functioning (Jentsch and Taylor, 1999; Volkow et al., 2001; Everitt et al., 2008; Garavan et al., 2008; Kalivas, 2008). More specifically, the progression from first drug use to compulsive drug use has been hypothesized to represent a surrender of control over drug seeking by the PFC and ventral striatum to the dorsal striatum, establishing a dysfunctional gradient of neural abnormalities thought likely to encourage the emergence of drug-seeking habits (Everitt and Robbins, 2005).
Increasing evidence also suggests that drug addiction is linked to certain personality traits (e.g. risk-taking, novelty- and sensation-seeking, impulsiveness), and is over-represented in people diagnosed with certain childhood and adolescent psychiatric disorders, including conduct disorder and attention-deficit hyperactivity disorder (ADHD) (Levin and Kleber, 1995; Adams et al., 2003; Chakroun et al., 2004; Dawe and Loxton, 2004; Verdejo-Garcia et al., 2008). Recent research based on prospective studies in adolescents indicates that clinical impulsivity, i.e. rash or risky behaviour and a strong tendency towards sudden poorly judged decisions and actions (Evenden, 1999), may actually pre-date problematic drug use (Nigg et al., 2006; Wong et al., 2006b) and thereby, potentially, contribute causally to drug abuse and addiction (Verdejo-Garcia et al., 2008). Consistent with this view, there is growing evidence for a substantial involvement of genetic variables in complex personality traits related to impulsivity and clinical disorders of impulse control, such as ADHD, which account for a significant proportion of the individual's vulnerability to drug addiction (Uhl, 1999; Kreek et al., 2005). Indeed, twin and family studies yield estimates of inheritability of vulnerability to drug addiction as high as 60% (Kreek et al., 2005). Such influences are hypothesized to have an important bearing on the pathway to drug addiction, including initiation of drug use, severity of drug dependence and risk for relapse (Tsuang et al., 1999; Uhl, 1999).
Impulsivity is a multidimensional behavioural construct encompassing several seemingly distinct classes of behaviour that generally involve a predisposition towards rapid, unplanned or premature actions and which are often risky and result in negative consequences (Evenden, 1999; Moeller et al., 2001). It is often contrasted with compulsivity, which refers to the maladaptive tendency to persist or perseverate responding, despite adverse consequences (Fineberg et al., 2010). In general, brain imaging research involving human drug addicts (with positron emission tomography (PET) and magnetic resonance) is mainly based on impulsive personality traits and cognitive measures of inhibitory response control because, in such settings, compulsive behaviour is difficult to assess in human participants. It is therefore difficult to predict whether volunteers participating in such studies will more likely develop later compulsive drug seeking habits. Research in animals avoids such issues by allowing behavioural and cognitive measures to be precisely assessed prior to drug exposure (Dalley et al., 2005a,b; Briand et al., 2008).
Paradigms used to assess impulsivity can broadly be divided into two categories; those that measure impulsive decision-making and those that measure impulsive action (Winstanley et al., 2006; Dalley et al., 2008; Pattij and Vanderschuren, 2008). Impulsive decision-making is commonly assessed using delay-discounting procedures where subjects must choose between a small, immediate reward and a larger, but delayed reward. Impulsive subjects are intolerant of delayed rewards and consequently opt preferentially for immediate, small magnitude rewards, a deficit also present in rats with selective lesions of the NAcb (Cardinal et al., 2001). Action impulsivity is typically assessed by response inhibition paradigms such as the go/no-go and stop-signal reaction time tasks and, in rodents, by the five choice serial reaction time task (5-CSRTT). Collectively, these tasks require subjects to inhibit a pre-potent motor response or to cancel an already initiated response in the case of the stop-signal reaction time task (Winstanley et al., 2006).
A number of studies in laboratory animals support a causal link between behavioural impulsivity and drug addiction. For example, an increase in delay discounting impulsivity predicts the acquisition or initiation of cocaine self-administration in rats (Perry et al., 2005). In contrast, the maintenance phase of drug use, which is often accompanied by drug bingeing and escalation, is influenced by inter-individual variation in action impulsivity (Dalley et al., 2007; Diergaarde et al., 2008). Specifically, rats selected for spontaneously high levels of impulsivity on the 5-CSRTT, an automated operant procedure to assess sustained visual attention and action impulsivity (Robbins, 2002), subsequently maintain higher rates of intravenous cocaine and nicotine self-administration than low-impulsive rats (Dalley et al., 2007; Diergaarde et al., 2008). Trait-like impulsivity on the 5-CSRTT is exemplified by ‘Zippy’, an intrinsically impulsive rat that shows an additional impairment in delay discounting (Robinson et al., 2009). The relevance of Zippy and his impulsive counterparts to the aetiology of drug addiction will be discussed later in this review.
This review first summarizes the basic principles of molecular imaging as a tool of psychopharmacology. We next present a comprehensive review and synthesis of PET and SPECT studies that have shed light on the neuropharmacology of drug addiction, noting that this approach is most fully developed for the brain dopamine systems. We also discuss how investigating inter-individual differences in dopamine receptor function can help resolve why some individuals are particularly disposed progressively to lose control over their drug intake and ultimately develop drug-seeking habits. Drug and molecular targets cited in this review conform to published guidelines (Alexander et al., 2009).
Principles of molecular imaging
Molecular imaging with PET and also single photon emission computed tomography (SPECT), an allied technology, has been widely used to investigate the different stages of the addiction cycle from the initial site of drug action to the long-term effects of chronic drug exposure on neurotransmitter function and regional brain activity (Volkow et al., 2003; Buchert et al., 2004; Martinez et al., 2004). In common with other imaging modalities, PET allows repeated assessment in the same subject, a feature that can readily be exploited in animal models of addiction where drug exposure is tightly controlled. PET and SPECT are non-invasive autoradiographic methods for molecular and physiological imaging of the living brain. As such, the visualization of a physiological process or molecular target is achieved through the use of a tracer substance, which incorporates in its structure a suitable radionuclide. In the case of nuclear imaging by SPECT, the metastable nuclear isomer technetium-99m can be generated on-site, and incorporated into the structure of a tracer [e.g. [99mTc]hexamethylpropylene amine oxime (HMPAO) for studies of cerebral blood flow (CBF)], whereas iodine-123 must first be produced in a cyclotron prior to radiotracer synthesis (e.g. [123I]iodobenzamide (IBZM), for studies of dopamine D2/3 receptors). The SPECT radionuclides decay in the course of a day or two, with the release of a single energetic photon or γ-ray, which can be mapped to its source in living brain using SPECT.
The brief physical half-lives for decay of common PET radionuclides dictate that they are generated with a cyclotron near the site of use (fluorine-18; t1/2 109 min) or on-site (carbon-11; t1/2 20 min, oxygen-15, t1/2 2 min). Various approaches have been used for the preparation of PET radiopharmaceuticals, where speed is of the essence (Ametamey et al., 2008). Although many tracers commonly used for brain research are conveniently obtained by N-methylation with [11C]methyliodide, more versatile synthetic approaches such as the Huisgen 1,3-dipolar cyclo-addition (‘click’ chemistry) are opening new vistas for radiochemistry (Wangler et al., 2010). However, many potential tracers have failed to find widespread use, due to a variety of factors such as rapid peripheral metabolism, poor passage through the blood–brain barrier, inadequate process specificity, unfavourable binding kinetics, or inadequate specific binding relative to non-specific binding.
Based partly on historical precedents as well as the widespread availability of dopamine D2/3 receptor PET ligands (e.g. [11C]raclopride, [18F]fallypride, see Figure 1), it is perhaps unsurprising that PET studies of drug addiction have tended to focus on the brain dopamine systems. However, the radio-pharmacopeia targeting diverse types of 5-HT receptors is now in a phase of rapid development. The targeting of other biogenic amines and specific opioid peptide receptor subtypes lags lamentably behind, despite their promise for studies of addiction and personality. As in all pharmacological applications, the requirement of strict specificity is seldom met by available radiopharmaceuticals. For example, the study of dopamine receptors is still bedevilled by the lack of agents selecting between D2 and D3 receptor subtypes (Leopoldo et al., 2009), and useful D4 receptor ligands have yet to be developed. The widely used DOPA decarboxylase substrate [18F]fluoro-L-dihydroxyphenylalanine (FDOPA, Figure 1C), while mainly trapped within dopaminergic neurons, also labels those containing 5-HT and noradrenaline (Brown et al., 1999; Kumakura et al., 2010).
Figure 1.
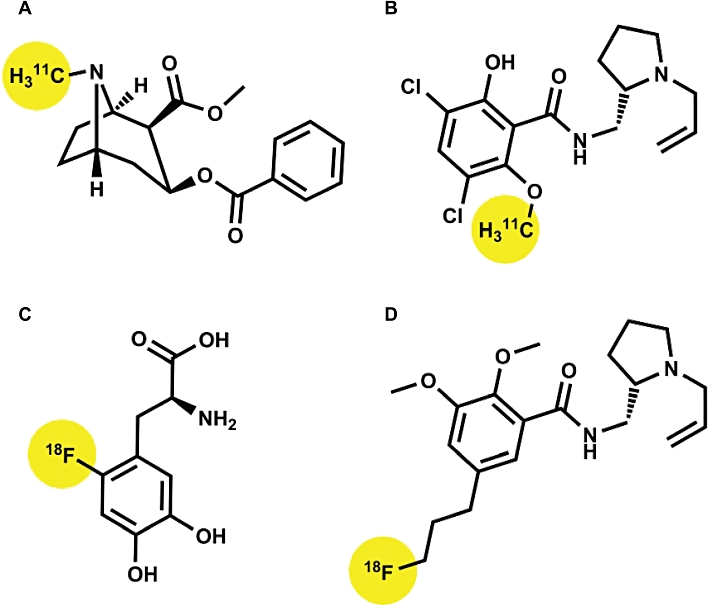
Common PET ligands used to probe dopamine function in drug addiction. Binding sites include dopamine, NA and 5-HT re-uptake transporters (cocaine ‘A’), dopamine D2/3 receptors (raclopride ‘B’; fallypride ‘D’) and dopamine synthesis (FDOPA ‘C’). The molecular positions of carbon-11 and fluorine-18 radioisotopes are highlighted in each case.
General aspects of PET pharmacokinetics
Sequential frames in a dynamic three-hour PET recording with [18F]fallypride show the progressive labelling of dopamine D2/3 receptors in the human striatum (Figure 2); the task of kinetic analysis is to derive from these dynamic measurements an estimate of the specific trapping, either in individual image elements (voxels) or in a defined volume of interest (VOI). This enables the construction of time–activity curves (TACs) containing information about initial clearance across the blood–brain barrier, binding in brain, and washout of the tracer (Figure 2B). Knowing the metabolite-corrected arterial input, as measured in serial blood samples, the brain TACs can be interpreted in terms of a model with binding and non-binding tissue compartments (Figure 2C). However, arterial blood sampling with HPLC correction of labelled metabolites is both invasive and time consuming to carry out. It is far easier to calculate specific binding in the defined VOI relative to the uptake in a reference region devoid of specific binding; this requirement is approximately met by the cerebellum in the case of [18F]fallypride (Figure 2B) and indeed for quite a number of other receptor ligands.
Figure 2.
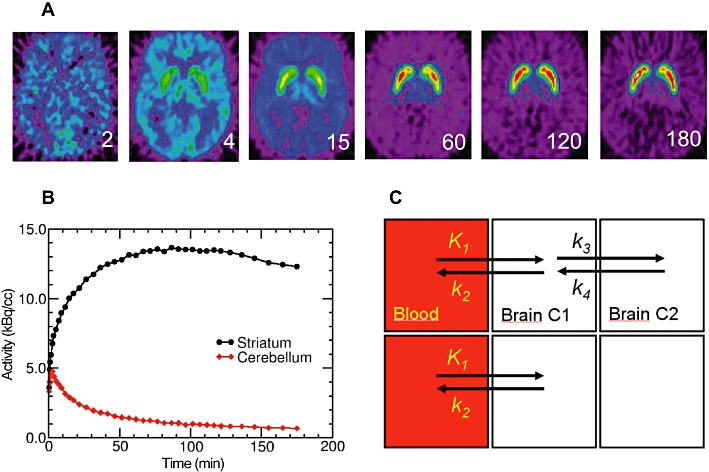
The time course of the uptake, binding and washout of the dopamine D2/3 receptor antagonist [18F]-fallypride in the brain of a healthy human subject. (A) Single frames from the 180 min dynamic recording, (B) the time activity curves extracted from striatum and cerebellum and (C) the compartmental model defining the reversible binding (upper boxes) and non-binding compartments (lower boxes).
The great preponderance of PET tracers is designed to interact with specific enzymes or receptors in brain. The prototype of these is [18F]fluorodeoxyglucose (FDG), which is uniquely suited for measuring the cerebral metabolic rate (CMRglc) in living brain (Kuhl et al., 1980). FDG-PET is an extension of the classical ex vivo autoradiographic method established in rat studies with [14C]deoxyglucose (Sokoloff et al., 1977), whereby the accumulation of radioactivity in brain is determined by the reversible facilitated diffusion of the tracer across the blood–brain barrier (mediated by a glucose transporter) and by the activity in brain of hexokinase, which is the rate-limiting step for glycolysis. As phosphorylated FDG does not proceed further along the glycolytic pathway, its trapping is approximately irreversible during a 45 min dynamic PET recording. This property, and near absence of plasma metabolites, makes FDG (with certain caveats) an ideal PET tracer. FDOPA kinetics is formally very similar to that of FDG, but is mechanistically different; partitioning of FDOPA across the blood–brain barrier is facilitated by an amino acid transporter, and retention within monoaminergic neurons is mediated by the local activity of the enzyme DOPA decarboxylase. Here the formal similarity of FDOPA with FDG ends, as the trapped [18F]fluoropamine is eventually deaminated by monoamine oxidase (MAO); uncorrected diffusion from brain of resultant acidic metabolites can lead to a significant underestimation of the parameter of interest (Kumakura and Cumming, 2009). The penetration of a plasma metabolite into the brain adds further complexity to the interpretation of FDOPA-PET. Thus, FDOPA is a worst case scenario for kinetic modelling.
Whereas the enzymatic trapping of FDG and FDOPA in brain is, to the first approximation, irreversible, some tracers are receptor ligands of such high affinity that their association with the receptor can be considered irreversible for the time course of the PET recording. Thus, the net influx of [11C] 3-N-methylspiperone (NMSP), a butyrophenone antagonist of dopamine D2/3 receptors, can be considered irreversible in human brain during a 60 min dynamic PET recording (Wong et al., 1986), although dissociation of bound [11C]NMSP can be discerned in living pig brain (Rosa-Neto et al., 2004). Most of the widely used PET and SPECT tracers for neuroreceptors share this property of reversibility, enabling the calculation of a steady-state parameter known as binding potential (BP), which is equivalent to the distribution volume ratio (DVR) in a binding region to that in a non-binding reference region, less one. When the mass of tracer is negligible, that is, for tracers of very high specific activity, the BP is proportional to the ratio of the Michaelis–Menten constants, that is, Bmax/KD.
The steady-state estimation of BP is readily obtained with 60 min PET recordings with [11C]raclopride, a benzamide antagonist of dopamine D2/3 receptors. Indeed, benzamides are among the mostly widely used tracers for neuropsychiatric PET studies, and have an especially useful property: vulnerability to competition from endogenous DA, which was first established using [11C]raclopride-PET (Dewey et al., 1993) and [123I]IBZM-SPECT (Innis et al., 1992) studies employing a challenge with the indirect dopamine agonist amphetamine. Other stimulants such as methamphetamine, cocaine, and methylphenidate also reduce the binding of the benzamide [18F]4'-fluoroclebopride in monkey striatum (Mach et al., 1997); even nicotine exerts a certain displacement of [11C]raclopride binding in pig ventral striatum, although to a lesser extent than the psychostimulants (Cumming et al., 2003). Amphetamine-evoked competition at dopamine D2/3 receptors has also been demonstrated in a mouse microPET study with [18F]fallypride and [18F]DMFP (Rominger et al., 2010), and in a rat microPET study with [11C]raclopride (Pedersen et al., 2007), where pre-treatment with a MAO inhibitor failed to produce the expected potentiation of dopamine release.
That the extent of benzamide displacement bears some relationship with the amount of dopamine release evoked by amphetamine is substantiated by animal studies employing microdialysis in conjunction with [123I]IBZM-SPECT (Laruelle et al., 1997) and [11C]raclopride-PET (Endres et al., 1997). However, not all released dopamine is alike with respect to competition. The displacement of [3H]raclopride binding in rat striatum (measured ex vivo) shows a much steeper relationship with the increase in interstitial dopamine evoked by methamphetamine than was the case of nicotine, which releases dopamine by an entirely different mechanism (Kim and Han, 2009). Behavioural sensitization to amphetamine in monkeys has been linked to enhanced displacement of [123I]IBZM by amphetamine challenge (Castner et al., 2000), but behavioural cross-sensitization, as between cocaine and amphetamine (Liu et al., 2007), has not yet been tested in PET or SPECT competition paradigms. In the case of cocaine addiction, the contribution of sensitization to the [11C]raclopride competition paradigm is less clear. Although studies in animals support a pre-synaptic model of sensitization, corresponding studies in cocaine abusers show, on the contrary, an attenuation or blunting of competition in response to a cocaine challenge (Narendran and Martinez, 2008).
Dopaminergic transmission and subjective hedonic experience
It has sometimes been assumed that any pharmacological treatment increasing dopamine release in the ventral striatum should evoke a positive hedonic experience. Consistent with this view, intranasal administration of nicotine to habitual smokers, while not significantly reducing [11C]raclopride binding in the group as a whole, did so in those subjects who reported a positive subjective experience (Montgomery et al., 2007). Likewise, amphetamine-evoked [11C]raclopride displacement in ventral striatum has been found to correlate with the degree of euphoria in normal healthy subjects (Drevets et al., 2001), and subjects who self-report a ‘high’ following systemic administration of methylphenidate also show a significant displacement of [11C]cocaine (Volkow et al., 1999) and [11C]methylphenidate (Volkow et al., 1996) binding in striatum, which is indicative of increased competition from endogenous dopamine. Broadly similar findings have been observed in normal healthy volunteers subjected to psychosocial stress; those subjects with higher blood cortisol responses also reported greater positive subjective drug effects with amphetamine than did subjects with lower stress responses, which correlated positively with [11C]raclopride displacement in the striatum (Wand et al., 2007). In other words, the results of these challenge paradigms are potentially modifiable by behavioural traits and environmental influences. For example, trait impulsivity in human subjects predicts a blunted response to amphetamine in terms of [11C]raclopride displacement in the right ventral striatum but nonetheless is associated with more pleasant subjective effects than experienced by non-impulsive subjects (Oswald et al., 2007). Importantly, however, these effects were modified by history of life stress events, such that low-impulsive subjects showed greater [11C]raclopride displacement (suggestive of greater dopamine release) than did high impulsive subjects under low to moderate stress. Under conditions of high stress, both impulsivity groups showed a blunted displacement of [11C]raclopride compared with subjects with no marked history of life stress. These findings thus suggest that attenuated displacement of [11C]raclopride in the striatum may represent a neural endophenotype associated with risk for drug addiction, in a manner modified by life stress events. This conclusion is broadly compatible with a previous study in non-human primates showing the reinforcing effects of psychostimulant drugs to depend on the sensitivity of individuals to stressful social situations (Morgan et al., 2002).
The imperfect relationship between enhanced dopamine transmission and subjective experience suggests that equating the messenger (dopamine) with the message (reward) is an over-simplification. In particular, cue-evoked craving in cocaine addicts has been linked with reduced [11C]raclopride binding in proportion to the intensity of craving (Wong et al., 2006a). Consistent with this finding, exposure of rats to a cocaine-conditioned environment has recently been found to produce a 20% reduction in striatal [11C]raclopride binding (Schiffer et al., 2009), an effect almost as great as that evoked by the drug itself. Nonetheless, pharmacologically evoked dopamine release is not, per se, a cue for drug seeking in cocaine addicts; although challenge with oral methylphenidate evoked the expected displacement of [11C]raclopride binding in the ventral striatum of cocaine addicts, it did not evoke craving unless paired with cocaine-related cues (Volkow et al., 2008b). Conditioned drug responses are widely believed to powerfully promote drug-seeking behaviour and contribute to drug craving and relapse in drug addicts (Childress et al., 1999; Everitt and Robbins, 2005; Everitt et al., 2008). Indeed, such responses can have a dramatic effect on dopamine release in the striatum. For example, exposing normal healthy male volunteers to a PET scanning suite where previously they had been exposed to amphetamine resulted in a remarkable displacement of [11C]raclopride in the ventral striatum, of a magnitude as great as that produced by the drug itself (Boileau et al., 2007).
PET studies of neural vulnerability mechanisms underlying addiction
Much research indicates that personality traits that encompass impulsivity and novelty/sensation-seeking can predispose to drug use and have a detrimental impact, speeding the development of drug addiction (Chakroun et al., 2004; Dawe and Loxton, 2004; Nigg et al., 2006; Verdejo-Garcia et al., 2008). How in neural terms such traits could influence human drug addiction is poorly understood, but research in animals confirms that high levels of impulsivity is a strong predictor of intravenous cocaine and nicotine self-administration (Perry et al., 2005; Dalley et al., 2007; Diergaarde et al., 2008). Our own research has focussed on a naturally occurring form of impulsivity in an out-bred strain of Lister hooded rats, measured by the increased propensity of some rats to ‘jump the gun’ and respond before the presentation of a visual cue on a 5-CSRTT (see Figure 3). The basic task requires rats to detect the spatial location of a brief light stimulus presented on a discrete trial basis at the rear of five open apertures (Robbins, 2002). Rats are rewarded with a small food pellet for a correct response and punished for responses made either in an adjacent aperture or too early, in the case of an impulsive response. The impulsive phenotype is present in a small but stable proportion of rats tested (∼8–12%) and is exemplified by ‘Zippy’; one of the original high-impulsive rats identified. Zippy was subsequently used as one of the founders in the establishment of a multi-generational inbred colony of high-impulsive rats (Schumann et al., 2010).
Figure 3.
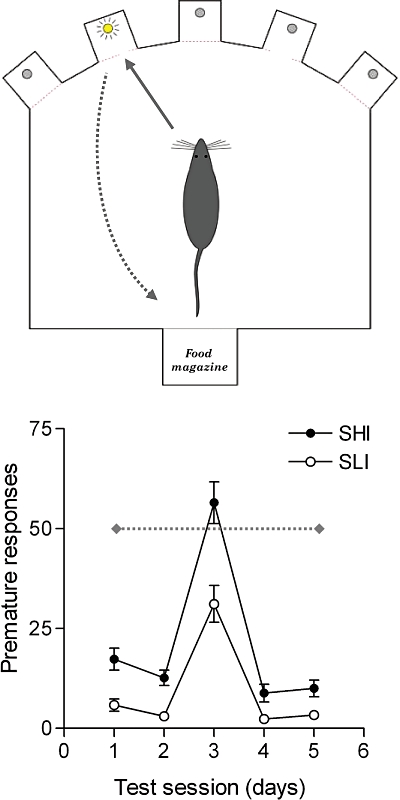
A schematic illustration of the five choice serial reaction time task (5-CSRTT) used to assess sustained visual attention and impulsivity in rats. Subjects are trained to locate the spatial position of a brief light stimulus with a nose-poke response and subsequently collect food reward from a forward facing food magazine. Nose-poke responses in an adjacent, non-illuminated aperture (deemed an ‘incorrect’ response) or responses made before the onset of the visual cue (deemed a ‘premature’ or ‘impulsive’ response) are signalled by the house-light being extinguished and the loss of food reward on that trial. Rats are selected as spontaneously high impulsive (SHI) if they make more than 50 premature responses during sessions where they are required to wait for 7 s (test day 3), rather than the expected 5 s (test days 1,2, 4 and 5), before the onset of the visual cue. Rats making 30 or fewer premature responses on such challenge sessions are categorized as spontaneously low impulsive (SLI).
A remarkable feature of high impulsivity in rats is that it predicts not only the escalation of intravenous cocaine and nicotine self-administration (Dalley et al., 2007; Diergaarde et al., 2008), but also a greatly increased propensity to develop compulsive cocaine taking (Belin et al., 2008), and to relapse after a period of abstinence (Economidou et al., 2009). Of relevance to a key hallmark of addiction specified in DSM-IV, we defined compulsive cocaine taking as drug intake that persists despite negative or adverse consequences, that is, the occasional delivery of positive punishment in the form of a mild electric foot shock (Everitt et al., 2008; Belin et al., 2008). Thus, trait-like impulsivity in rats appears to confer increased risk for drug addiction consistent with the clinical literature (Verdejo-Garcia et al., 2008).
In a previous microPET study, impulsive rats showed significantly reduced [18F]fallypride BP in the ventral striatum (including the NAcb), but not the caudate putamen (Dalley et al., 2007). In addition, the [18F]fallypride BP correlated inversely with impulsivity on the 5-CSRTT. This finding led to the suggestion that earlier reports of low dopamine D2/3 receptor availability among detoxified cocaine users (Volkow et al., 1997; Martinez et al., 2004) may, to some extent at least, indicate a pre-existing trait, imparting a particular vulnerability for cocaine dependence, rather than a state acquired by chronic drug exposure. This conclusion is supported by the observation that low baseline availability of striatal dopamine D2/3 receptors predicts high rates of cocaine self-administration in monkeys, which in turn further reduces the binding (Nader et al., 2006), to an extent similar to what is observed in abstinent cocaine users (Volkow et al., 1997; Martinez et al., 2004) and rats with a previous history of intravenous amphetamine self-administration (see Figure 4). Conversely, high levels of [11C]raclopride binding in abstemious relatives of patients with alcoholism appear to afford protection against the development of alcohol dependence (Volkow et al., 2006). In addition, adenoviral-mediated over-expression of dopamine D2 receptors attenuates the self-administration of alcohol in rats (Thanos et al., 2001).
Figure 4.
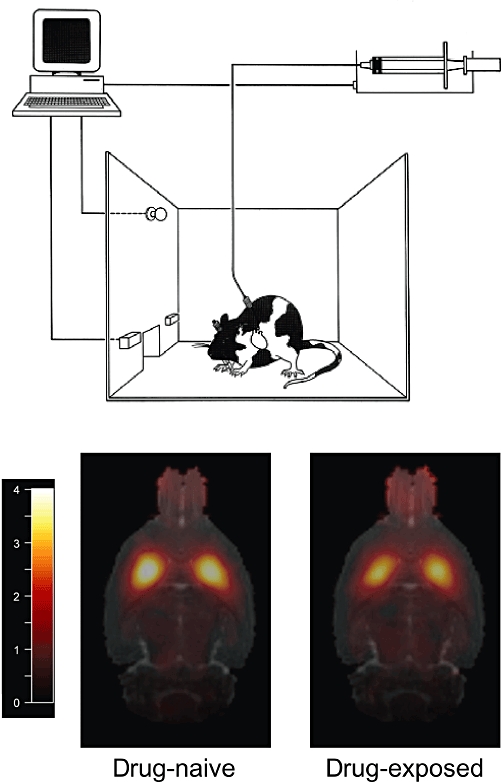
The self-administration paradigm used widely to assess the reinforcing effects of abused drugs like cocaine and amphetamine. In this procedure the intravenous delivery of drug is contingent on the rat pressing a lever while pressing a second lever on the opposite side of the chamber has no consequence. Rats withdrawn from intravenous d-amphetamine self-administration show a reduced uptake of the dopamine D2/3 receptor antagonist [11C]-raclopride in the caudate putamen, compared with rats administered saline. Shown are horizontal magnetic resonance images of the rat brain with co-registered PET. The scale bar denotes binding potential (BP) (reproduced with permission from Dalley et al., 2009).
In general, these observations indicate that a given neurochemical state can be a predisposing factor in the development of drug addiction. Indeed, the converse experiment of the amphetamine challenge, in which endogenous dopamine is transiently depleted by administration of the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine, showed a blunted unmasking of striatal [11C]raclopride binding among chronic cocaine users (Martinez et al., 2009a). This observation of low basal dopamine tonus suggests that cocaine-dependent subjects may seek to self-medicate with cocaine an underlying deficiency state brought about by the drug itself. Indeed, amphetamine-evoked [11C]raclopride binding changes are severely blunted in detoxified cocaine users (Volkow et al., 1997; Martinez et al., 2007). Moreover, higher social status is associated with higher baseline [11C]raclopride binding in healthy volunteers (Martinez et al., 2010), suggesting that social ‘success’ could mitigate against vulnerability towards drug dependence. Conversely, impulsive and antisocial psychopathic traits, which predispose towards drug seeking and addiction (Verdejo-Garcia et al., 2008) are associated with greater reductions in [11C]raclopride binding in the NAcb evoked by monetary or pharmaceutical rewards (Buckholtz et al., 2010). Such findings have parallels with the treatment of Parkinson's disease patients with dopamine receptor agonists, which can sometimes provoke episodes of pathological gambling and other forms of compulsive behaviour (Steeves et al., 2009). In these patients, performance of a gambling task evoked a greater decline in [11C]raclopride binding in the ventral striatum than was seen in control subjects, consistent with greater dopamine release in this region (Steeves et al., 2009).
Insufficiency of dopamine D2/3 receptors in the NAcb, whether pre-existing or acquired, may contribute to the risk for relapse to drug seeking, which is intimately related to the experience of craving in response to drug-related cues (Childress et al., 1999; Everitt et al., 2008; Economidou et al., 2009). Using a multi-modal imaging approach, Heinz and colleagues showed that the severity of cue-evoked craving, and cue-evoked fMRI activation of the anterior cingulate cortex was correlated with low availability of dopamine D2/3 receptors measured with [18F]DMFP-PET in the ventral striatum of detoxified alcoholics (Heinz et al., 2004). In an extension of this work, the uptake of the presynaptic dopamine tracer FDOPA, while not reduced in a group of alcoholics, correlated inversely with the extent of craving and risk for relapse (Heinz et al., 2005).
Dopamine in the frontal cerebral cortex operates quite differently from that in the basal ganglia where it is hypothesized to modulate switching between programmed behaviour and cognitive processes (van Schouwenburg et al., 2010). Recently, it has been shown that a dopamine D2/3 receptor agonist (bromocriptine) can improve flexible updating (switching) in high-impulsive individuals, supporting a top-down regulation of this cognitive process by the PFC (Cools et al., 2007). However, molecular imaging of the contribution of cortical dopamine to fronto-executive cognition is technically challenging. Nevertheless, dopamine D2/3 receptors can be detected in cerebral cortex and other regions of low abundance using the high affinity PET ligands [11C]FLB 457 and [18F]fallypride, or [123I]epidepride-SPECT. In previous research, inventory measures of novelty-seeking were found to correlate negatively with [11C]FLB 457 binding in the right insular cortex of normal subjects (Suhara et al., 2001), and also in patients with Parkinson's disease (Kaasinen et al., 2004). However, such findings are difficult to interpret as relatively little is known about the functional significance of dopamine in the insular cortex. A more tractable method to investigate cortical substrates of relevance to drug addiction and co-morbid brain disorders is with FDG-PET, which assesses cerebral metabolic rate (Kuhl et al., 1980). Here, FDG uptake has been shown to be reduced in the anterior cingulate gyrus and OFC of opiate, methamphetamine and cocaine users (Volkow et al., 1992; Galynker et al., 2000; Kim et al., 2009), as well as in alcoholic patients (Adams et al., 1993). Such findings support the notion of impaired PFC function in human drug addicts leading to the loss of inhibitory control over maladaptive drug-seeking habits (Everitt et al., 2008; Kalivas, 2008; Volkow et al., 2008a).
With respect to novelty-(sensation-) seeking there has been some controversy about the involvement of brain dopamine and 5-HT. For example, in an [123I]β-CIT-SPECT study of biogenic amine transporters in nearly 200 healthy young subjects, neither the early post-injection binding phase, said to reveal preferentially the 5-HT reuptake transporter (SERT), nor the binding at 24 h, which reveals mainly the dopamine uptake transporter (DAT), proved to correlate with sensation-seeking (Burke et al., 2010). We have characterized the behavioural response to novelty of a mixed sex group of Göttingen minipigs, which were also investigated with [11C]raclopride-PET (Lind et al., 2005). The duration of contact with the novel objects proved to correlate with the amphetamine-evoked decline in striatal [11C]raclopride BP; analysis by gender revealed this association to be driven by the male sub-group. In healthy human male subjects, scores in a test of sensation-seeking showed an inverted-U relationship with baseline [11C]raclopride binding in the striatum (Gjedde et al., 2010). This observation may be related to the formal ambiguity of the PET end point, as low BP could arise from low receptor abundance (Bmax) or from high apparent affinity (Kdapp, a function of basal occupancy by dopamine). Whereas striatal dopamine receptors are mostly post-synaptic, much of the binding of dopamine D2 receptor ligands in mesencephalon reveals presynaptic autoreceptors, which regulate the activity of dopaminergic neurons. Given that mesencephalic [18F]fallypride binding has been shown to correlate inversely with novelty-seeking in a large group of healthy volunteers (Zald et al., 2008), tight feedback control of dopaminergic neurons may be unfavourable for the expression of the novelty-seeking trait. However, this conjecture serves as an instance of the need for more selective tracers.
Molecular imaging of ADHD and impulse control disorders
Insofar as ADHD is a predisposing factor for acquiring substance use disorders, ADHD might be considered a natural experiment in the biology of addiction. The contribution of brain dopamine to impulsivity in ADHD has been a matter of some controversy. As in all molecular imaging studies, the possible effects of medication on dopaminergic markers must be considered. Thus, for example, behavioural sensitization to amphetamine in monkeys has been linked to enhanced displacement of [123I]IBZM in the amphetamine challenge paradigm (Castner et al., 2000). Likewise, pre-exposure to amphetamine has been shown to enhance the amphetamine-evoked reduction in [11C]raclopride binding in healthy human volunteers, especially in the ventral striatum (Boileau et al., 2006). With this in mind, scores of inattention and impulsivity in drug-free adolescents with ADHD were found to correlate with the methylphenidate-evoked reduction in striatal [11C]raclopride BP (Rosa-Neto et al., 2005), particularly in low birth weight individuals with ADHD (Rosa Neto et al., 2002). This intriguing finding was interpreted to reveal a functional over-activity, if not an actual superabundance, of DAT. In a study of never-medicated ADHD adults, baseline [11C]raclopride BP in caudate was slightly lower than in the control group, and the methylphenidate-evoked decline was blunted, in proportion to the severity of self-reported symptoms (Volkow et al., 2007b). Several differences might account for the disagreement of these two rather similar studies, such as the association of ADHD with low birth weight and the use of a more objective index of inattention in the former study. Nevertheless, the blunted response to methylphenidate does resonate with similar findings in human cocaine addicts (Narendran and Martinez, 2008), suggesting that partially overlapping neurobiological mechanisms may underlie ADHD and drug addiction.
In a recent [11C]cocaine PET study of ADHD adults with no history of medication, the abundance of DAT was moderately reduced in the left NAcb and caudate nucleus, and, although [11C]cocaine binding in the putamen did not differ between controls and ADHD subjects, it did correlate with inattentiveness in the ADHD group, and to a lesser extent also in the control group (Volkow et al., 2007a). A subsequent study of a larger cohort of never-medicated ADHD patients (n = 53) replicated the finding of reduced [11C]raclopride and [11C]cocaine binding in the left NAcb and caudate, which extended to the midbrain as well in this study (Volkow et al., 2009). This latter finding, which presumably involves somatodendritic sites on dopamine neurons, might suggest desensitization of autoreceptors regulating dopamine synthesis and release. If so, this would concur with a earlier report of increased FDOPA uptake in the mesencephalon of ADHD patients (Ernst et al., 1999), although a subsequent FDOPA study found no such reductions in non-medicated patients, whereas reductions were observed throughout the basal ganglia of the methylphenidate-treated patients (Ludolph et al., 2008), as noted above.
The clinical benefits of methylphenidate are likely mediated via blockade of DAT and/or noradrenaline transporters (NET) (Solanto, 2002; Fone and Nutt, 2005). Indeed, clinically useful doses of methylphenidate occupy approximately 50% of [11C]-cocaine binding sites in the striatum of normal subjects (Volkow et al., 1998), resulting in a 16% decline in the availability of [11C]raclopride binding sites (Volkow et al., 2002). In a SPECT study of 14 never-treated ADHD patients, [123I]FP-CIT binding to DAT was 15% lower in the striatum, but did not differ in the thalamus, where the preponderant binding is to SERT (Hesse et al., 2009). There was also no difference in DAT availability in a [11C]PE2I-PET study of a group of 10, mostly never-medicated ADHD patients (Jucaite et al., 2005). In contrast, DAT availability was 40% elevated in the left striatum in a [123I]IPT-PET study of a relatively small ADHD group (Cheon et al., 2003). Age-corrected DAT availability measured with [11C]altropane-PET was, however, 15% higher in the caudate of a group of 21 never-medicated ADHD patients (Spencer et al., 2007), and in a [99mTc]TRODAT-SPECT study (Dresel et al., 2000). The same group subsequently claimed that responders to methylphenidate therapy had low baseline DAT availability, while responders (12/18) had elevated DAT availability (Krause et al., 2005). It is difficult to imagine a more inconsistent literature, which doubtless reflects the confounding effects of clinical heterogeneity, age and treatment effects in ADHD studies.
The role of dopamine D1 receptors has been little investigated in the impulsivity and addiction literature. Although the binding of the dopamine D1 antagonist [11C]SCH23390 was reduced in the striatum of smokers (Dagher et al., 2001), this observation was not corrected for previous alcohol consumption. More recently, the availability of striatal dopamine D1 receptors in the striatum was shown not to differ between cocaine-dependent subjects and normal healthy volunteers (Martinez et al., 2009b). Nonetheless, there was an inverse relationship between D1 receptor binding in the ventral striatum and choice of cocaine over a monetary reward. Thus, dopamine D1 receptor-mediated processes may confer vulnerability for cocaine addiction but further research would be needed to substantiate this notion.
Non-dopaminergic systems
Dopamine synthesis, receptors and transporters are the best studied neurochemical substrates in impulsivity and drug-seeking, but are not the sole players in this relationship. Efforts to develop PET ligands for NET have recently yielded useful agents of marginal utility, given the low abundance and specific binding (this may be an insuperable problem). In the first clinical application of NET-PET, the age-corrected binding of (S,S)-[11C]O-methylreboxetine was found to be 50% higher in the thalamus and locus coeruleus of cocaine users (Ding et al., 2010). In general, contributions of 5-HT to impulsivity and drug addiction are poorly documented. In a [11C]DASB PET study of SERT, midbrain binding was reduced in patients with obsessive compulsive disorder and correlated inversely with symptom severity (Reimold et al., 2007). In another study, significant reductions in SERT were found in the insular cortex and OFC (Matsumoto et al., 2010). Serotonergic function can also be assessed using [18F]altanserin, which binds to the post-synaptic 5HT2a receptors that are abundantly present in cerebral cortex. However, to our knowledge, there have been no [18F]altanserin-PET studies in the context of drug addiction. Such studies are justified by the fact that brain serotonergic mechanisms are strongly implicated in the form of impulsivity expressed by ‘Zippy’ (Dalley et al., 2002; Puumala and Sirvio, 1998).
Very recently it has become possible to visualize cannabinoid CB1 receptors in the living brain. In a large mixed gender population, a highly significant inverse relationship was found between cerebral binding of the CB1 receptor inverse agonist [18F]MK-9470 and novelty-seeking, as assessed by the Cloninger personality inventory (Van Laere et al., 2009). This phenomenon extended throughout the grey matter, but was most significant in the right amygdala. In rat microPET studies, chronic injection of a high dose of nicotine was without effect on CB1 receptor binding (Gerard et al., 2010), whereas the anticonvulsant drug sodium valproate (but not levetiracetam) increased global [18F]MK-9470 binding by one third (Goffin et al., 2008); these studies serve as a model for longitudinal microPET studies of neurochemical interactions.
Molecular imaging studies of opioid receptors and indeed all G-protein-coupled receptors is complicated by the effects of affinity states. Thus, binding in living brain of the opioid receptor antagonist [11C]diprenorphine is generally resistant to displacement by a number of opioid receptors agonists with a variety of subtype specificities, revealing the existence of a considerable reserve of non-functional opioid binding sites (Hume et al., 2007). This property of agonists may impart the particular sensitivity of a dopamine D2/3 agonist ligand to competition from endogenous dopamine (Cumming et al., 2002; Narendran et al., 2004), although the case for receptor reserve is less clearly established for dopamine receptors.
In normal subjects investigated with the non-selective opioid receptor antagonist ligand [18F]fluoroethyl-diprenorphine, a positive correlation was found between binding in the bilateral ventral striatum and scores on the Cloninger personality dimension of reward dependence, an inventory that predicts drug-seeking propensity (Schreckenberger et al., 2008). In a recent study, the distribution volume of [11C]diprenorphine tended to be globally elevated in acutely abstinent alcoholics, in whom there was a positive correlation with alcohol craving, even after prolonged abstinence (Williams et al., 2009). Others have shown the selective µ-opioid receptor agonist ligand [11C]carfentanil can be almost completely displaced in heroin addicts treated with the partial µ receptor agonist, and κ/δ receptor antagonist buprenorphine (Greenwald et al., 2003), and that high cortical binding of [11C]carfentanil during acute abstinence predicts rapid relapse in cocaine users (Gorelick et al., 2008).
In another of the lamentably few PET or SPECT studies of heroin addiction, striatal binding of the selective DAT ligand [11C]CFT was found to be substantially reduced in patients on methadone maintenance, which showed partial recovery with prolonged abstinence (Shi et al., 2008). However, this need not indicate direct actions of heroin on dopamine transmission, as heroin does not appear to affect striatal [11C]raclopride binding in heroin addicts (Daglish et al., 2008).
While the modulation of drug addiction and impulsivity by the biogenic amines has been extensively examined by molecular imaging, corresponding methods for the excitatory amino acid receptors are poorly developed. The NMDA type ionotropic glutamate receptor, a key element in synaptic plasticity, is a heterotetramer, which presents at least three potential targets for molecular imaging: the cation channel, the modulatory glycine site, and specific NR2B subunits, which have been of particular interest in the study of neuroprotection. As recently reviewed, the search for adequate PET or SPECT tracers for these components of NMDA receptors has yet to yield useful agents (Sobrio et al., 2010); ion channel agents tend only to recognize the open pores, which comprise only a tiny fraction of the total pool of NMDA receptors under normal physiological conditions. Glycine binding sites have been detected in vivo, but available imaging agents have very poor brain penetration (Matsumoto et al., 2007), thus giving low specific signals. NR2B ligands tend to have poor solubility and although one PET ligand yielded specific labeling in vivo, it suffered from very rapid metabolism (Arstad et al., 2006). Efforts to develop ligands for the metabotropic glutamate receptors (mGluR) have recently met with some success, perhaps reflecting the common structural elements of seven transmembrane domain G-protein receptors. Promising agents have been developed for the mGluR5 type receptor, which is highly expressed in striatum and hippocampus (Wyss et al., 2007). There are very recent reports on mGluR1 receptor ligands for PET, one of which has proven adequate for occupancy studies in monkey brain (Hostetler et al., 2010). Given the preliminary state of this research, it can only be anticipated that NMDA-type and MGluR ligands will eventually emerge as key agents for studies of drug-induced adaptation and synaptic plasticity in living brain (Jones and Bonci, 2005; Kauer and Malenka, 2007).
Conclusion
Although the development of molecular imaging has presented formidable technical problems, the effort is offset to a large degree by the provision of a privileged perspective on the neurochemical status of an individual. After 20 years of clinical and basic PET research of addiction, there has emerged a consensus that the transition to compulsive drug taking must entail pre-existing individual neurochemical risk factors, modified and exacerbated by drug exposure and environmental interactions, in the manner of a positive feedback loop with negative consequences for the individual. This model may be best tested in longitudinal molecular imaging studies of experimental animals with well-characterized behavioural traits, and well-documented exposure to psychostimulant drugs; as much as possible, future animal PET studies of addiction should emulate the natural history of human addiction, with consideration of factors thought to mediate the transition to habitual drug use by humans, such as trait-impulsivity, social stress, and environmental cues. Molecular imaging in addiction models has been bedevilled by the low spatial resolution of microPET (and microSPECT) relative to the size of relevant structures in the rodent brain. However, recent improvements in instrumentation now permit the distinction of functional and anatomical divisions of the striatum and PFC/OFC of humans and even rats. This is important because, within the last decade, interest has switched from the primary brain sites mediating the initial reinforcing effects of abused drugs such as the NAcb, to dorsal striatal systems underlying habitual drug use (Belin and Everitt, 2008; Everitt et al., 2008; Belin et al., 2009). However, very little is known of the neural and neurochemical substrates of compulsivity, which represents the final stage of drug addiction, and which may involve dysfunctional prefrontal cortical-striatal circuitry (Vanderschuren and Everitt, 2004; Everitt and Robbins, 2005; Porrino et al., 2007; George and Koob, 2010). The shift from initial drug use to habitual and ultimately compulsive drug seeking appears to be critically influenced by pre-disposing neural and behavioural endophenotypes (e.g. low dopamine D2/3 receptors, impulsivity) and by the progressive effects of repeated drug use on the network of topographically organized glutamatergic inputs to the basal ganglia from the PFC and OFC (Volkow et al., 1992; Dalley et al., 2007; Verdejo-Garcia et al., 2008; Kalivas, 2009). Such changes presumably underlie the progressive decline in behavioural and cognitive control over drug-seeking behaviour that accompanies chronic drug exposure and therefore are major targets for future scientific investigation.
Great emphasis has been placed historically on the indisputable contributions of the brain dopamine systems to behavioural traits such as impulsivity as a predisposing variable in drug addiction. By turning the searchlight of molecular imaging towards biomarkers of other neurotransmitter systems, especially 5-HT, noradrenaline, cannabinoids and opioid peptides, we should eventually obtain a more comprehensive and heuristically useful understanding of the underlying neurobiology of Zippy, which would be of inestimable translational value in deciphering the aetiology of human drug addiction.
Acknowledgments
This research was funded by an MRC Programme Grant to BJ Everitt, TW Robbins, A Dickinson and JW Dalley (G0600196), an MRC Research Grant to JW Dalley, FI Aigbirhio, JC Baron, BJ Everitt, TD Fryer and TW Robbins (G0701500) and a European Communities Sixth Framework Programme Grant (‘Imagen’ LSHM-CT-2007-037286).
Glossary
Abbreviations
- 5-CSRTT
five choice serial reaction time task
- 5-HT
5-hydroxytryptamine
- ADHD
attention deficit hyperactivity disorder
- BP
binding potential
- CBF
cerebral blood flow
- CIT
2β-carboxymethoxy-3β-(4-iodophenyl)tropane
- DASB
N,N-dimethyl-2-(2-amino-4-cyanophenylthio)benzylamine
- DAT
dopamine transporter site
- DMFP
desmethoxyfallypride
- DSM
Diagnostic and Statistical Manual
- FDG
fluorodeoxyglucose
- FDOPA
fluoro-L-dihydroxyphenylalanine
- FLB
457, N-(1-ethyl-2-pyrrolidinyl)methyl)-5-bromo-2,3-dimethoxybenzamide
- HMPAO
hexamethylpropylene amine oxime
- HPLC
high performance liquid chromatography
- IBZM
iodobenzamide
- ICSS
intra-cranial self-stimulation
- IPT
N-(3-iodopropen-2-yl)-2beta-carbo-methoxy-3beta- (4-chlorophenyl)tropane
- MAO
monoamine oxidase
- NAcb
nucleus accumbens
- NET
noradrenaline reuptake inhibitor
- NMSP
3-N-methylspiperone
- OFC
Orbitofrontal cortex
- PET
positron emission tomography
- PFC
prefrontal cortex
- SERT
serotonin transporter site
- SPECT
single photon emission computer tomography
- TAC
time–activity curve
- TRODAT
[2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2,N2',S2,S2']oxo-[1R-(exo-exo)]
- VOI
volume of interest
- VTA
ventral tegmental area
Conflict of interest
The authors have no conflicts of interest to declare.
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse. 2003;29:691–712. doi: 10.1081/ada-120023465. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. 2009;158:S1–S254. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Arstad E, Platzer S, Berthele A, Pilowsky LS, Luthra SK, Wester HJ, et al. Towards NR2B receptor selective imaging agents for PET-synthesis and evaluation of N-[11C]-(2-methoxy)benzyl (E)-styrene-, 2-naphthyl- and 4-trifluoromethoxyphenylamidine. Bioorg Med Chem. 2006;14:6307–6313. doi: 10.1016/j.bmc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, et al. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WD, Taylor MD, Roberts AD, Oakes TR, Schueller MJ, Holden JE, et al. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999;53:1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, et al. A voxel-based PET investigation of the long-term effects of ‘Ecstasy’ consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, van de Giessen E, de Win M, Schilt T, van Herk M, van den Brink W, et al. Serotonin and dopamine transporters in relation to neuropsychological functioning, personality traits and mood in young adult healthy subjects. Psychol Med. 2010;2010:1–11. doi: 10.1017/S0033291710000486. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Castner SA, al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB, Goldman-Rakic PS. Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to subchronic amphetamine. Neuropsychopharmacology. 2000;22:4–13. doi: 10.1016/S0893-133X(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Chakroun N, Doron J, Swendsen J. [Substance use, affective problems and personality traits: test of two association models.] L'Encephale. 2004;30:564–569. doi: 10.1016/s0013-7006(04)95471-1. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Wong DF, Gillings N, Hilton J, Scheffel U, Gjedde A. Specific binding of [(11)C]raclopride and N-[(3)H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J Cereb Blood Flow Metab. 2002;22:596–604. doi: 10.1097/00004647-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Cumming P, Rosa-Neto P, Watanabe H, Smith D, Bender D, Clarke PB, et al. Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage. 2003;19:1127–1136. doi: 10.1016/s1053-8119(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Williams TM, Wilson SJ, Taylor LG, Eap CB, Augsburger M, et al. Brain dopamine response in human opioid addiction. Br J Psychiatry. 2008;193:65–72. doi: 10.1192/bjp.bp.107.041228. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005a;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ, et al. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005b;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Aigbirhio FI, Brichard L, Richards HK, Hong YT, et al. Modelling human drug abuse and addiction with dedicated small animal positron emission tomography. Neuropharmacology. 2009;56(Suppl 1):9–17. doi: 10.1016/j.neuropharm.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse. 2010;64:30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27:1518–1524. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, et al. Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Nutt DJ. Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Curr Opin Pharmacol. 2005;5:87–93. doi: 10.1016/j.coph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Galynker II, Watras-Ganz S, Miner C, Rosenthal RN, Des Jarlais DC, Richman BL, et al. Cerebral metabolism in opiate-dependent subjects: effects of methadone maintenance. Mt Sinai J Med. 2000;67:381–387. [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard N, Ceccarini J, Bormans G, Vanbilloen B, Casteels C, Goffin K, et al. Influence of chronic nicotine administration on cerebral type 1 cannabinoid receptor binding: an in vivo micro-PET study in the rat using [(18)F]MK-9470. J Mol Neurosci. 2010;42:126–127. doi: 10.1007/s12031-010-9340-2. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci USA. 2010;107:3870–3875. doi: 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C, Cave J. Economics of addiction and drugs. 2005. Brain Sciences, Addiction and Drugs, UK Government Foresight Programme.
- Goffin K, Bormans G, Casteels C, Bosier B, Lambert DM, Grachev ID, et al. An in vivo[18F]MK-9470 microPET study of type 1 cannabinoid receptor binding in Wistar rats after chronic administration of valproate and levetiracetam. Neuropharmacology. 2008;54:1103–1106. doi: 10.1016/j.neuropharm.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, et al. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200:475–486. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hesse S, Ballaschke O, Barthel H, Sabri O. Dopamine transporter imaging in adult patients with attention-deficit/hyperactivity disorder. Psychiatry Res. 2009;171:120–128. doi: 10.1016/j.pscychresns.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Eng W, Joshi AD, Sanabria-Bohorquez S, Kawamoto H, Ito S, et al. Synthesis, characterization, and monkey PET studies of [(18)F]MK-1312, a PET tracer for quantification of mGluR1 receptor occupancy by MK-5435. Synapse. 2010 doi: 10.1002/syn.20826. (in press) [DOI] [PubMed] [Google Scholar]
- Hume SP, Lingford-Hughes AR, Nataf V, Hirani E, Ahmad R, Davies AN, et al. Low sensitivity of the positron emission tomography ligand [11C]diprenorphine to agonist opiates. J Pharmacol Exp Ther. 2007;322:661–667. doi: 10.1124/jpet.107.121749. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005;57:229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Rinne JO. Insular dopamine D2 receptors and novelty seeking personality in Parkinson's disease. Mov Disord. 2004;19:1348–1351. doi: 10.1002/mds.20191. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim SE, Han SM. Nicotine- and methamphetamine-induced dopamine release evaluated with in vivo binding of radiolabelled raclopride to dopamine D2 receptors: comparison with in vivo microdialysis data. Int J Neuropsychopharmacol. 2009;12:833–841. doi: 10.1017/S1461145708009826. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SW, Kwon DH, Seo JH, Ahn BC, Lee J. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res. 2009;43:1166–1170. doi: 10.1016/j.jpsychires.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Krause J, la Fougere C, Krause KH, Ackenheil M, Dresel SH. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255:428–431. doi: 10.1007/s00406-005-0602-x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Engel J, Jr, Phelps ME, Selin C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol. 1980;8:348–360. doi: 10.1002/ana.410080403. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Danielsen EH, Gjedde A, Vernaleken I, Buchholz HG, Heinz A, et al. Elevated [(18)F]FDOPA utilization in the periaqueductal gray and medial nucleus accumbens of patients with early Parkinson's disease. NeuroImage. 2010;49:2933–2939. doi: 10.1016/j.neuroimage.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, De Giorgio P, Contino M, Berardi F, Perrone R. Design, synthesis, and binding affinities of potential positron emission tomography (PET) ligands with optimal lipophilicity for brain imaging of the dopamine D3 receptor. Part II. Bioorg Med Chem. 2009;17:758–766. doi: 10.1016/j.bmc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- Lind NM, Gjedde A, Moustgaard A, Olsen AK, Jensen SB, Jakobsen S, et al. Behavioral response to novelty correlates with dopamine receptor availability in striatum of Gottingen minipigs. Behav Brain Res. 2005;164:172–177. doi: 10.1016/j.bbr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Morgan D, Roberts DC. Cross-sensitization of the reinforcing effects of cocaine and amphetamine in rats. Psychopharmacology (Berl) 2007;195:369–375. doi: 10.1007/s00213-007-0909-6. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, et al. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. NeuroImage. 2008;41:718–727. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, et al. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57:477–486. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009a;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, et al. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009b;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Haradahira T, Ito H, Fujimura Y, Seki C, Ikoma Y, et al. Measurement of glycine binding site of N-methyl-D-asparate receptors in living human brain using 4-acetoxy derivative of L-703,717, 4-acetoxy-7-chloro-3-[3-(4-[11c] methoxybenzyl) phenyl]-2(1H)-quinolone (AcL703) with positron emission tomography. Synapse. 2007;61:795–800. doi: 10.1002/syn.20415. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Ichise M, Ito H, Ando T, Takahashi H, Ikoma Y, et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: a [11C]DASB PET study. NeuroImage. 2010;49:121–126. doi: 10.1016/j.neuroimage.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse. 2007;61:637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, et al. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (-)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52:188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, et al. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. NeuroImage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Simonsen M, Ostergaard SD, Munk OL, Rosa-Neto P, Olsen AK, et al. Mapping the amphetamine-evoked changes in [11C]raclopride binding in living rat using small animal PET: modulation by MAO-inhibition. NeuroImage. 2007;35:38–46. doi: 10.1016/j.neuroimage.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puumala T, Sirvio J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Zimmer A, Batra A, Knobel A, Solbach C, et al. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity – a [11C]DASB PET study. J Neural Transm. 2007;114:1603–1609. doi: 10.1007/s00702-007-0785-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, et al. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Rominger A, Wagner E, Mille E, Boning G, Esmaeilzadeh M, Wangler B, et al. Endogenous competition against binding of [(18)F]DMFP and [(18)F]fallypride to dopamine D(2/3) receptors in brain of living mouse. Synapse. 2010;64:313–322. doi: 10.1002/syn.20730. [DOI] [PubMed] [Google Scholar]
- Rosa Neto P, Lou H, Cumming P, Pryds O, Gjedde A. Methylphenidate-evoked potentiation of extracellular dopamine in the brain of adolescents with premature birth: correlation with attentional deficit. Ann NY Acad Sci. 2002;965:434–439. doi: 10.1111/j.1749-6632.2002.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Gjedde A, Olsen AK, Jensen SB, Munk OL, Watanabe H, et al. MDMA-evoked changes in [11C]raclopride and [11C]NMSP binding in living pig brain. Synapse. 2004;53:222–233. doi: 10.1002/syn.20053. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, et al. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. NeuroImage. 2005;25:868–876. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Liebling CN, Reiszel C, Hooker JM, Brodie JD, Dewey SL. Cue-induced dopamine release predicts cocaine preference: positron emission tomography studies in freely moving rodents. J Neurosci. 2009;29:6176–6185. doi: 10.1523/JNEUROSCI.5221-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des. 2010;16:2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Klega A, Grunder G, Buchholz HG, Scheurich A, Schirrmacher R, et al. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J Nucl Med. 2008;49:1257–1261. doi: 10.2967/jnumed.108.050849. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.4. (in press) [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, Tian J, et al. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008;579:160–166. doi: 10.1016/j.ejphar.2007.09.042. [DOI] [PubMed] [Google Scholar]
- Sobrio F, Gilbert G, Perrio C, Barre L, Debruyne D. PET and SPECT imaging of the NMDA receptor system: an overview of radiotracer development. Mini Rev Med Chem. 2010;10:870–886. doi: 10.2174/138955710791608299. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, et al. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. NeuroImage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, et al. Genetic and environmental influences on transitions in drug use. Behav Genet. 1999;29:473–479. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: a current approach. Neuropsychopharmacology. 1999;20:3–9. doi: 10.1016/S0893-133X(98)00061-X. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Bormans G, Casteels C, Mortelmans L, de Hoon J, et al. Relationship of type 1 cannabinoid receptor availability in the human brain to novelty-seeking temperament. Arch Gen Psychiatry. 2009;66:196–204. doi: 10.1001/archgenpsychiatry.2008.530. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, et al. Relationship between psychostimulant-induced ‘high’ and dopamine transporter occupancy. Proc Natl Acad Sci USA. 1996;93:10388–10392. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of ‘high’. J Pharmacol Exp Ther. 1999;288:14–20. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. NeuroImage. 2007a;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007b;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008a;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008b;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Wangler C, Schirrmacher R, Bartenstein P, Wangler B. Click-chemistry reactions in radiopharmaceutical chemistry: fast and easy introduction of radiolabels into biomolecules for in vivo imaging. Curr Med Chem. 2010;17:1092–1116. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, et al. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur Neuropsychopharmacol. 2009;19:740–748. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wong DF, Gjedde A, Wagner HN., Jr Quantification of neuroreceptors in the living human brain. I. Irreversible binding of ligands. J Cereb Blood Flow Metab. 1986;6:137–146. doi: 10.1038/jcbfm.1986.27. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006a;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, et al. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006b;77:1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Ametamey SM, Treyer V, Bettio A, Blagoev M, Kessler LJ, et al. Quantitative evaluation of 11C-ABP688 as PET ligand for the measurement of the metabotropic glutamate receptor subtype 5 using autoradiographic studies and a beta-scintillator. NeuroImage. 2007;35:1086–1092. doi: 10.1016/j.neuroimage.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, et al. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


