Abstract
BACKGROUND AND PURPOSE
Lipopolysaccharide (LPS)-induced expression of cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) has been implicated in several respiratory diseases. HuR is known to enhance the expression of genes by binding to 3′-untranslated region (3′-UTR) of mRNA and stabilizing mRNA. However, the exact mechanisms by which HuR affects the stability of mRNA and modulates LPS-induced COX-2 and cPLA2 expression in human tracheal smooth muscle cells (HTSMCs) are not known.
EXPERIMENTAL APPROACH
The expression of prostaglandin E2 (PGE2) was measured by ELISA, and pro-inflammatory proteins were determined by use of a promoter assay, PCR or Western blot analysis. Overexpression of siRNAs to knock down the target components was used to manipulate the expression of HuR. Release of reactive oxygen species (ROS) was detected by fluorescence dye. The activation of signalling components was assessed by comparing phosphorylation levels, localization of protein kinases or coimmunoprecipitation assay.
KEY RESULTS
LPS induced COX-2 and cPLA2 expression via post-translational regulation of mRNA stabilization, which were attenuated by transfection with HuR siRNA in HTSMCs. In addition, LPS-stimulated NADPH oxidase activation and ROS generation were attenuated by the NADPH oxidase inhibitors diphenyleneiodonium chloride (DPI) and apocynin (APO). Generation of ROS induced phosphorylation of p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK and JNK1/2, which was attenuated by DPI and APO and the ROS scavenger N-acetylcysteine.
CONCLUSIONS AND IMPLICATIONS
These results suggested that in HTSMCs, LPS-induced COX-2 and cPLA2 expression is mediated through NADPH oxidase/ROS-dependent MAPKs associated with HuR accumulation in the cytoplasm. Activated MAPKs may regulate the nucleocytoplasmic shuttling of HuR, and thus induce the cytoplasmic accumulation of HuR.
Keywords: human tracheal smooth muscle cells, lipopolysaccharide, HuR, cPLA2, COX-2
Introduction
Human tracheal smooth muscle cells (HTSMCs) are thought to be end-response effector cells that regulate regional differences in ventilation by contracting in response to various neurotransmitters, pro-inflammatory mediators and exogenous substances released under homeostatic or pathological conditions such as asthma (Hirst et al., 2004). On the other hand, HTSMCs are also important sources of pro-inflammatory cytokines, chemokines and growth factors and extracellular matrix (ECM) components (Lazaar and Panettieri, 2006), which contribute to the development and perpetuation of airway inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD) (Johnson et al., 2001). Cytosolic phospholipase A2 (cPLA2) and cyclooxygenase-2 (COX-2) are pro-inflammatory proteins; cPLA2 catalyses the hydrolysis of the sn-2 position of membrane glycerophospholipids, leading to produce lysophospholipids and free fatty acids including arachidonic acid (AA) (Kudo and Murakami, 2002), which in turn are converted by COX-2 to form various bioactive lipophilic compounds called eicosanoids, such as prostaglandins (PGs) (Samad et al., 2002). The characters of cPLA2 and COX-2 in inflammation have been proved in several studies. In cPLA2-null mice, pulmonary edema, neutrophil sequestration and deterioration of gas exchange induced by lipopolysaccharide (LPS), zymosan administration and acid aspiration are markedly reduced (Nagase et al., 2000). Enhanced levels of COX-2-related prostaglandin E2 (PGE2) have been found to correlate with the severity of airflow limitation in COPD (Chen et al., 2008). Thus, the inhibition of cPLA2-COX-2 related mechanisms may also provide a therapeutic approach for acute lung injury.
Allergen exposure is important in host allergic sensitization and as a common precipitant of asthmatic symptoms in both children and adults (Lemanske and Busse, 2006). Bacterial LPS, present in allergic dust, seems to be one of major candidates for the induction of inflammatory reaction (Thorn, 2001; Schroder and Arditi, 2007). LPS, a key component of outer membranes of gram-negative bacteria, is composed of three structural elements including a core oligosaccharide, an O-specific chain made up of repeating sequences of LPS and a lipid A component, which is responsible for the pro-inflammatory properties of LPS (Miller et al., 2005). In previous studies, we have demonstrated that stimulation with LPS leads to the infiltration of leucocytes into the lung via transcriptional up-regulated expression of adhesion molecules (Lin et al., 2009). However, the exact mechanisms underlying LPS-induced expression of cPLA2 and COX-2 in HTSMCs have not been completely elucidated.
Gene regulation can occur at the level of proteins (selective degradation), DNA (differential transcription) and RNA (mRNA stability) (Brennan and Steitz, 2001). HuR (Hu antigen R; ELAVL1) is a member of the embryonic lethal, abnormal vision, Drosophila-like ELAV family of RNA-binding proteins and is ubiquitously expressed. HuR is involved in a variety of physiological and pathological processes, including cell growth, differentiation and inflammation (Abdelmohsen et al., 2007). HuR contains three RNA-recognition motifs (RRM) through which it binds to adenylate- and uridylate (AU)-rich elements (AREs)-containing mRNAs and induces a marked increase in the half-life of many short-lived mRNAs, possibly by antagonizing the recruitment of competitive mRNA-destabilizing factors. As HuR is mainly located within the nucleus, cytosolic export is an important prerequisite for its protective effects on mRNAs and this is regulated by an HuR nucleocytoplasmic shuttling sequence (HNS) on its structure (Fan and Steitz, 1998). The nuclear export of HuR proteins is also regulated by various stimuli through several intracellular signalling pathways including MAPKs (Subbaramaiah et al., 2003), AMP-activated kinase (AMPK) (Wang et al., 2002), and different members of the PKC family (Doller et al., 2007; 2008a;). Therefore, we investigated whether HuR plays a key role in the regulation of LPS-induced expression of cPLA2 and COX-2 in HTSMCs.
HuR has been found to stabilize COX-2 mRNA in human mesangial cells, ovarian cancer cells and human keratinocytes exposed to various stimuli (Doller et al., 2008a; Oyesanya et al. 2008; Zhang and Bowden, 2008). Anti-rheumatic drugs, such as aurothiomalate, reduce the expression of COX-2 and PGE2 production in chondrocyte cultures and in human cartilage by enhancing the degradation of COX-2 mRNA through mechanisms involving reduced HuR expression (Nieminen et al., 2008). In contrast, little is known about the stabilization of cPLA2 mRNA, although the existence of putative HuR-binding sites on 3′-UTR of human cPLA2 mRNA has been suggested (Tsou et al., 2008). Thus, whether LPS stimulates cPLA2 or COX-2 expression in HTSMCs through mechanisms involving HuR-mediated stabilization of mRNA expression needs to be determined.
Several studies have also revealed that stimulation of LPS may lead to reactive oxygen species (ROS) production in human umbilical vein endothelial cells and macrophage (Al Laham et al., 2010; Kong et al. 2010). Generation of ROS may result from the activation of NADPH oxidase in response to various extracellular stimuli (Frey et al., 2009). The structure of NADPH oxidase including the catalytic centre of membrane-integrated protein gp91phox, which tightly complexed with p22phox, and the activation-related association components of p47phox, p67phox and the small GTPase Rac, which normally reside in the cytoplasm (Sumimoto et al., 2005). However, whether NADPH oxidase-dependent generation of ROS affects HuR-mediated COX-2 and cPLA2 expression in LPS-stimulated HTSMCs remains to be defined. Here we report that LPS-induced COX-2 and cPLA2 expression is mediated through manipulation of the HuR level in HTSMCs. We also provide evidence that LPS regulates the physical interaction between HuR and activated MAPKs, leading to the nucleocytoplasmic shuttling of HuR via a mechanism dependent on NADPH oxidase, ROS and MAPKs.
Methods
Materials
Dulbecco's modified Eagle medium: Nutrient Mixture F-12 (DMEM/F-12 medium) fetal bovine serum (FBS), and TRIZOL were purchased from Invitrogen (Carlsbad, CA, USA). Hybond C membrane, enhanced chemiluminescence (ECL) Western blotting detection system and Hyperfilms were from GE Healthcare Biosciences (Buckinghamshire, UK). Monoclonal antibodies – cPLA2, COX-2, HuR, p47phox, p67phox, β-actin – and polyclonal antibodies – p44 MAPK, p42 MAPK, p38 MAPK, JNK2 – were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PhosphoPlus p42/p44 MAPK, p38 MAPK and SAPK/JNK antibody kits were from Cell Signalings (Danver, MA, USA). Anti-GAPDH antibody was from Biogenesis (Boumemouth, UK). N-acetylcysteine (NAC), diphenyleneiodonium chloride (DPI), apocynin (APO), U0126, SB202190 and SP600125 were from Biomol (Plymouth Meeting, PA, USA). Bicinchoninic acid (BCA) protein assay kit was from Pierce (Rockford, IL, USA). LPS, enzymes and other chemicals were from Sigma (St. Louis, MO, USA). The nomenclatures of all drugs and molecular targets conform to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2009).
Cell culture
HTSMCs were purchased from ScienCell Research Lab (San Diego, CA, USA) and cultured as previously described (Lin et al., 2001). When the cultures reached confluence, cells were treated with 0.05% (w v−1) trypsin/0.53 mM ethylenediaminetetraacetic acid (EDTA) for 5 min at 37°C. The cell suspension was diluted with DMEM/F-12 containing 10% FBS to a concentration of 2 × 105 cells mL−1. The cell suspension was plated onto (1 mL per well) 12 well culture plates (2 mL per well) 6 well culture plates and (10 mL per dish) 10 cm culture dishes for the measurement of protein expression and mRNA accumulation. Experiments were performed with cells from passages 3 to 8.
Total RNA extraction and gene expression analysis
Total RNA was extracted from HTSMCs using Trizol, as previously described (Lin et al., 2001). The cDNA containing 0.5 µg RNA was used as templates to analyse specific gene mRNA level. Oligonucleotide primers for β-actin, cPLA2 and COX-2 were as follows: for β-actin: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ (Sense), 5′-CTAGAAGCATTTGCGGTGGACGATG-3′ (Anti-sense); for cPLA2: 5′-CTC-ACACCACAGAAAGTTAAAAGAT-3′ (Sense), 5′-GCTACCACAGGCACATCA-CG-3′ (Anti-sense); for COX-2: 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ (Sense), 5′-AGATCATCTCTGCCTGAGTATCTT-3′ (Anti-sense). The amplification profile includes one cycle of initial denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 1 min, primer annealing at 62°C for 1 min, extension at 72°C for 1 min, and then one cycle of final extension at 72°C for 5 min. The expression of β-actin was used as an internal control for the assay of a constitutively expressed gene. Real-time PCR was performed with a TaqMan gene expression assay system (Applied Biosystems, Carlsbad, CA, USA), using primer and probe mixtures for COX-2, cPLA2 and GAPDH as endogenous control. The primer and probe sequences for gene amplification were COX-2 (Cat. no. Hs01573471_m1), cPLA2 (Cat. no. Hs00996915_m1) and GAPDH (Cat. no. Hs99999905_m1). The reactions were performed in a StepOnePlus real-time PCR system (Applied Biosystems) under the following thermal cycling conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The threshold cycle value was normalized to that of GAPDH. Relative gene expression was determined by the ΔΔCt method, where Ct meant threshold cycle. All experiments were performed in triplicate.
Preparation of cell extracts and Western blot analysis
After incubation with LPS, the cells were then rapidly washed with ice-cold phosphate-buffered saline (PBS), scraped and collected by centrifugation at 1000× g for 10 min. The collected cells were lysed with an ice-cold lysis buffer. The lysates were centrifuged at 45 000× g for 1 h at 4°C to yield the whole cell extract. Samples from these supernatant fractions (30 µg protein) were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% running gel. Proteins were transferred to nitrocellulose membrane and then incubated successively at room temperature with 5% bovine serum albumin (BSA) in TTBS for 1 h. Membranes were incubated overnight at 4°C with an anti-cPLA2, COX-2, HuR, β-actin, phospho-p42/p44 MAPK, phospho-p38 MAPK or phospho-JNK1/2 antibody. Membranes were incubated with a 1:2000 dilution of anti-rabbit or anti-mouse horseradish peroxidase Ab for 1 h. The immunoreactive bands were detected by ECL reagents (PerkinElmer, Waltham, MA, USA).
Cell fraction isolation
After stimulation with LPS, the membrane, cytosolic and nuclear fractions were prepared by centrifugation. Samples from various fractions (30 µg protein) were denatured and subjected to SDS-PAGE using a 10% running gel. Proteins were transferred to nitrocellulose membrane and then incubated successively at room temperature with 5% BSA in TTBS for 1 h. The translocation of HuR, p47phox or p67phox was analysed by Western blot using an anti-HuR, p47phox, p67phox or anti-β-actin (as an internal control) polyclonal antibody. The immunoreactive bands were detected by ECL reagents (PerkinElmer).
Immunofluorescence staining
HTSMCs were plated on 6 well culture plates with coverslips. Cells were treated with LPS for the indicated time intervals, washed twice with ice-cold PBS, fixed with 4% paraformaldehyde in PBS for 30 min, and then permeabilized with 0.3% Triton X-100 in PBS for 15 min. The staining was performed by incubating with 10% normal goat serum in PBS for 30 min, followed by incubating with an anti-HuR monoclonal antibody for 1 h in PBS with 1% BSA, washing three times with PBS, incubating for 1 h with FITC-conjugated goat anti-rabbit antibody in PBS with 1% BSA, washing three times with PBS, and finally mounting with aqueous mounting medium. The images were observed under a fluorescence microscope (Axiovert 200M, Carl Zeiss light Microscopy, Gottingen, Germany).
Luciferase activity assay
The COX-2-luciferase plasmids were constructed with a region spanning −459 to +9 bp of COX-2 promoter into pGL3-basic vector. The cPLA2-luciferase plasmids were constructed with a region spanning −595 to +75 bp of cPLA2 promoter into a pGL3-basic vector. The plasmids were prepared by using QIAGEN plasmid DNA preparation kits. COX-2-luciferase or cPLA2-luciferase plasmid was transfected into HTSMCs using Genejammer transfection reagent (Stratagene, La Jolla, CA, USA) according to the instructions of the manufacturer. To assess promoter activity, cells were collected and disrupted by sonication in a lysis buffer (25 mM Tris-phosphate, pH 7.8, 2 mM EDTA, 1% Triton X-100 and 10% glycerol). After centrifugation, aliquots of the supernatants were tested for luciferase activity using a luciferase assay system (Promega, Madison, WI, USA) according to the instructions of the manufacturer. Firefly luciferase activities were normalized to β-galactosidase activity.
Overexpression of HuR plasmid or HuR siRNA
To generate the mammalian expressed HuR plasmid, human HuR cDNA was PCR-amplified using the following pair of oligonucleotides: 5′-CGGGAATTCATACAATGTCTAATGGTTATGAAGACC (sense) and 5′-CGGTCTAGAGAGCGTTATTTGTGGGACTTG (antisense); the PCR fragment was then digested with restriction enzymes EcoRI and XbaI, and cloned into the eukaryotic expression vector pCDNA3 (Invitrogen) as described by Fan and Steitz (1998). ON-TARGETplus siRNA of HuR (Accession no. NM_001419) was purchased from Dharmacon Research (Lafayette, CO, USA).
HTSMCs were plated at 3 × 105 cells mL−1 (1 mL per well) in 12 well culture plates for 24 h, reaching about 80% confluence, and 0.4 mL of DMEM/F-12 containing 10% FBS was added to each well. The DNA Metafectene reagent complex was prepared according to the instructions of the manufacturer (Biontex, Martinsried/Planegg, Germany). The amount of transfected DNA was kept constant with 1 µg of HuR plasmid or with 100 nM of siRNA for each well. The DNA Metafectene complex (0.1 mL) was added to each well and then incubated at 37°C for 24 h. The cells were washed twice with PBS and maintained in DMEM/F-12 containing 1% FBS for 24 h (transfection with HuR plasmid or pcDNA3) or 72 h (transfection with siRNA) before treatment with LPS for the indicated time intervals.
ROS measurement by 7′-dichlorofluorescein-diacetate (DCF-DA) fluorescence
Formation of ROS in HTSMCs was determined by the DCF-DA fluorescence method. HTSMCs (∼90% confluence in 12 well plates) were loaded with 1 µM DCF-DA for 30 min in phenol red-free medium at 37°C in a 95% air–5% CO2 environment. The medium containing DCF-DA was aspirated, and the cells were washed twice with PBS, and replenished with 0.5 mL of phenol red- and serum-free RPMI medium. Some cells were pretreated with the inhibitors for 1 h followed by exposure to LPS (100 µg·mL−1) for the indicated time intervals. The cells were washed twice with ice-cold PBS and scraped using lysis buffer (1X PBS containing 20% alcohol and 0.1% Tween 20). The cell lysates were transferred to 1.5-mL Eppendorf vials and centrifuged at 10 000× g for 1 min at 4°C. Fluorescence of oxidized DCF-DA in cell lysates, an index of formation of ROS, was measured by a fluorimeter (AppliskanR, Thermo, Vantaa, Finland) with excitation and emission set at 490 and 530 nm respectively.
Determination of NADPH oxidase activity
Cells were grown on 6 well culture plates and incubated with LPS for the indicated time intervals. Cells were gently scraped and centrifuged at 400× g for 10 min at 4°C. The cell pellet was resuspended in 35 µL of ice-cold RPMI 1640 medium per vial and then was kept on ice. Five microliters of the cell suspension (0.2 × 105 cells) were added to a final 200 µL volume of pre-warmed (37°C) RPMI 1640 medium containing either NADPH (1 µM) or lucigenin (20 µM), to initiate the reaction, followed by immediate measurement of chemiluminescence using a luminometer (Appliskan, Thermo) in out-of-coincidence mode. Appropriate blanks and controls were established, and chemiluminescence was recorded.
Coimmunoprecipitation assay
Cell lysates containing 1 mg of protein were incubated with 2 µg of anti-HuR, anti-p42/p44 MAPK, anti-p38 MAPK or anti-JNK1/2 Ab at 4°C for 1 h, and then 10 µL of 50% protein A-agarose beads was added and mixed for 16 h at 4°C. The immunoprecipitates were collected and washed three times with lysis buffer without Triton X-100, 5X Laemmli buffer was added, and then subjected to Western blot analysis using an anti-HuR, p42/p44 MAPK, p38 MAPK or JNK1/2 Ab.
Measurement of PGE2 generation
HTSMCs were cultured on 6 well culture plates. The cells were incubated with LPS (100 µg mL−1) for the indicated time intervals at 37°C. The medium was collected and stored at −80°C. PGE2 was assayed using a PGE2 enzyme immunoassay kit (Cayman, Ann Arbor, MI, USA) according to the instructions of the manufacturer.
Statistical analysis of data
All data are expressed as the mean ± standard error of the mean using the GraphPad Prism Program (GraphPad, San Diego, CA, USA). Quantitative data were analysed by one-way analysis of variance followed by Tukey's post hoc test. A value of P < 0.05 was considered significant.
Results
LPS induces de novo cPLA2 and COX-2 gene expression and PGE2 synthesis
LPS functioning as a major pro-inflammatory factor has been shown to induce airway inflammation. Here we determined whether COX-2 and cPLA2 expression were up-regulated by LPS. Serum-starved HTSMCs deprived of serum were incubated with 100 µg·mL−1 LPS for various time intervals. As shown in Figure 1A, LPS induced cPLA2 and COX-2 protein expression in a time-dependent manner. LPS-induced cPLA2 expression was observed to be significant at 6 h, reached a peak within 16 h, and lasted up to 24 h. In addition, LPS-induced COX-2 expression was significant within 2 h, reached a maximum within 8 h, and thereafter declined close to the basal level. The blot was stripped and re-probed with an anti-β-actin antibody to demonstrate equivalent amounts of GAPDH expression.
Figure 1.
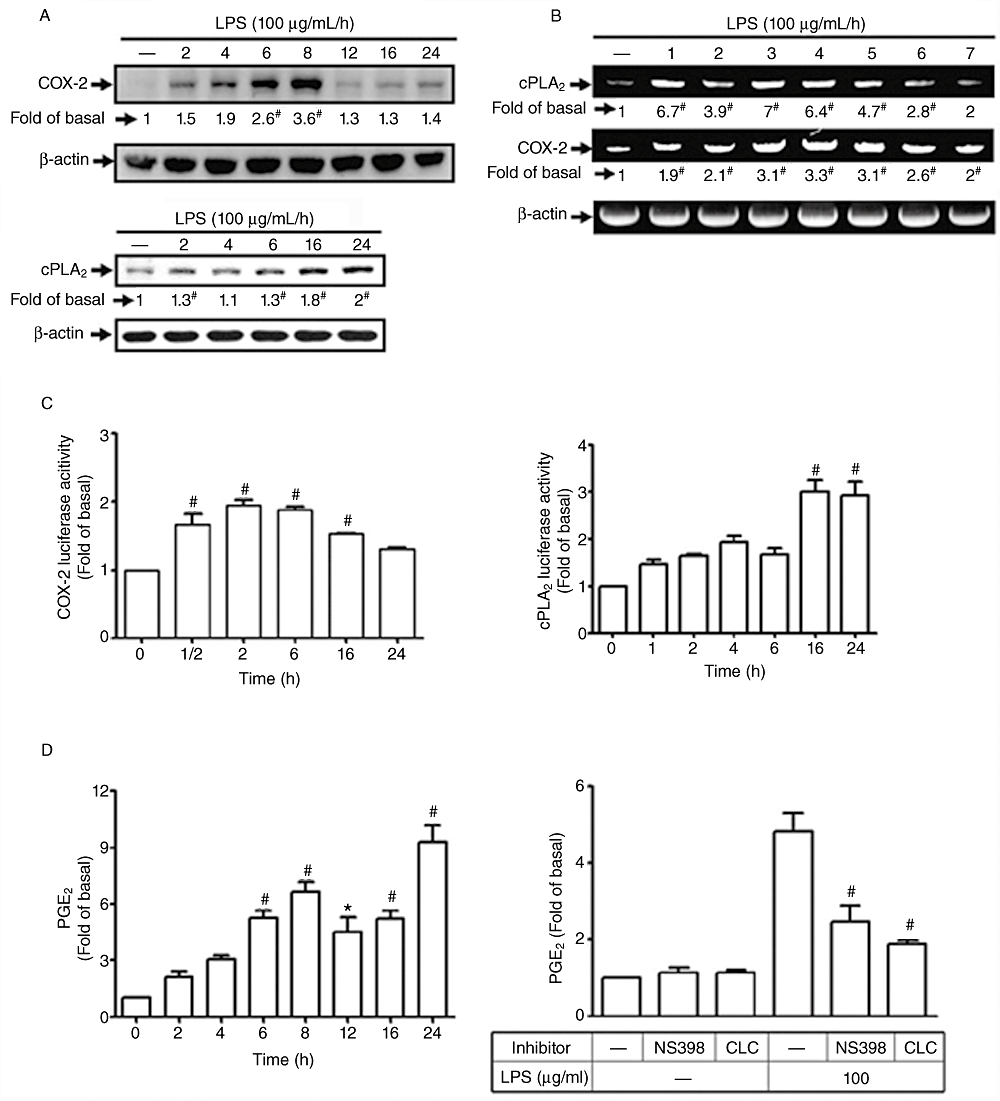
Lipopolysaccharide (LPS) induced cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression and prostaglandin E2 (PGE2) synthesis in human tracheal smooth muscle cells (HTSMCs). (A) HTSMCs were incubated with 100 µg·mL−1 of LPS for the indicated time intervals. COX-2 and cPLA2 protein expression were determined by Western blot analysis. (B) For mRNA expression, HTSMCs were incubated with LPS (100 µg·mL−1) for the indicated time intervals. mRNA was extracted and analysed by RT-PCR. (C) HTSMCs were transiently transfected with either cPLA2-luc or COX-2-luc reporter gene together with β-galactosidase plasmid and then incubated with 100 µg·mL−1 of LPS for the indicated time intervals. Luciferase activity was determined and normalized with β-galactosidase activity. (D) HTSMCs were pretreated without or with NS-398 (10 µM) or celecoxib (CLC; 10 µM) for 1 h and then incubated with LPS for the indicated time intervals or 6 h respectively. The culture supernatants were collected to measure PGE2 concentration. The basal level of PGE2 was approximately 256 pg·mL−1. Data are expressed as mean ± SEM of five independent experiments (n = 5). *P < 0.05, #P < 0.01 as compared with the cells exposed to vehicle alone.
To further examine whether the effects of LPS on cPLA2 and COX-2 expression occurred at the level of transcription, cPLA2 and COX-2 mRNA expression were determined by use of RT-PCR. As shown in Figure 1B, LPS induced cPLA2 or COX-2 mRNA accumulation in a time-dependent manner. The maximal response of cPLA2 mRNA was obtained within 3 h and lasted up to 6 h during the period of observation. The maximal response of COX-2 mRNA occurred within 3 to 6 h after stimulation with LPS. Moreover, the promoter activities of cPLA2 and COX-2 were also enhanced by LPS stimulation, with maximal responses within 16 h and 2 h respectively (Figure 1C). The newly synthesized cPLA2 and COX-2 were biologically functional, indicated by the increased release of PGE2 in response to LPS (Figure 1D). The synthesis of PGE2 induced by LPS was found to be biphasic with the first phase maximum occurring within 8 h and the second maximal response within 24 h. LPS-induced PGE2 synthesis was attenuated by the COX-2 inhibitors, NS-398 and celecoxib (Figure 1D), suggesting that the release of PGE2 was mediated through COX-2 expression. These data suggest that LPS-stimulated PGE2 synthesis is mediated through up-regulation of cPLA2 and COX-2 expression in HTSMCs.
LPS induces cPLA2 and COX-2 expression via the generation of ROS
To investigate whether ROS participates in LPS-induced cPLA2 and COX-2 expression, HTSMCs were pretreated with various concentrations of an ROS scavenger (NAC) for 1 h and then incubated with 100 µg·mL−1 of LPS for 24 h. Cell lysates were analysed by Western blot using an anti-cPLA2 or anti-COX-2 Ab. We found that LPS-induced expression of cPLA2 and COX-2 proteins was attenuated by pretreatment with NAC in a concentration-dependent manner (Figure 2A). LPS-induced expression of cPLA2 and COX-2 mRNA was also reduced by pretreatment with NAC (Figure 2B), revealing the involvement of ROS generation in LPS-induced cPLA2 and COX-2 expression. Moreover, the levels of ROS production were determined by using DCF-DA. LPS-stimulated accumulation of intracellular ROS was significant within 15 min and lasted up to 3 h (Figure 2C). Pretreatment with NAC significantly attenuated LPS-induced ROS generation (Figure 2D). Taken together, these data suggest that ROS generation contributes to the expression of cPLA2 and COX-2 in response to LPS in HTSMCs.
Figure 2.
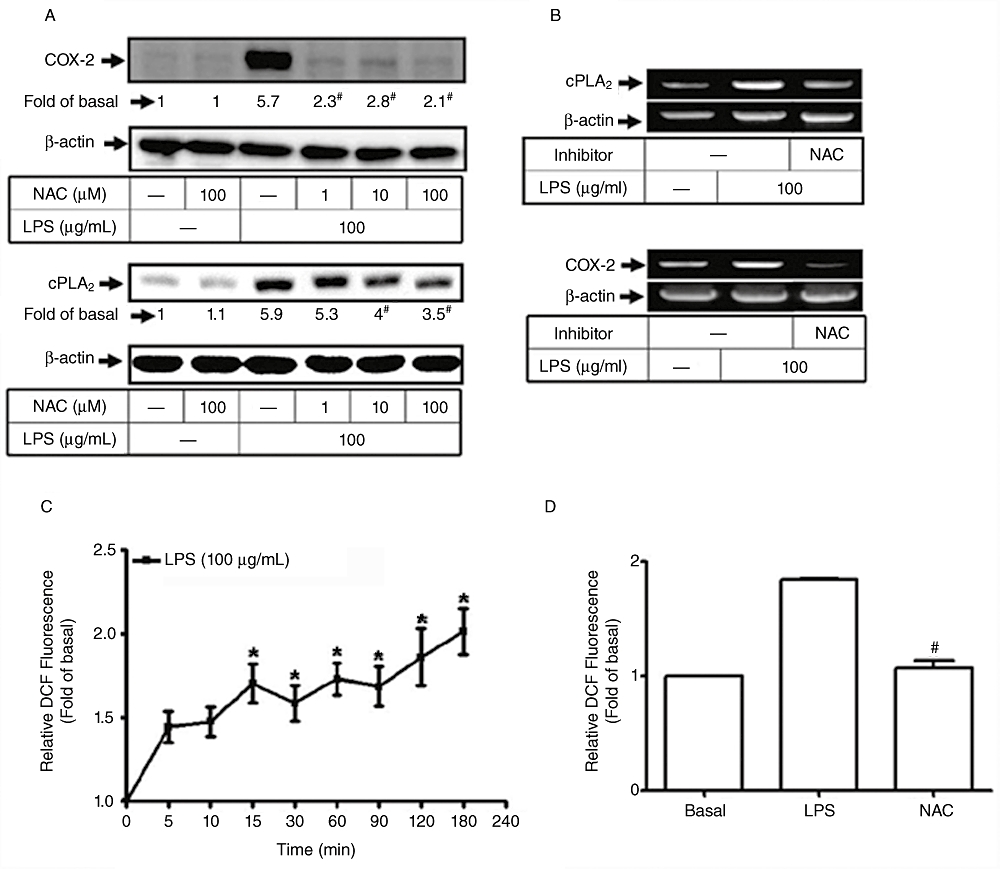
Involvement of reactive oxygen species (ROS) in lipopolysaccharide (LPS)-mediated cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression. (A) Human tracheal smooth muscle cells (HTSMCs) were pretreated with various concentrations of N-acetylcysteine for 2 h and then incubated with 100 µg·mL−1 of LPS for 6 h or 24 h. Cell lysates were analysed by Western blot using an anti-cPLA2, anti-COX-2 or anti-β-actin Ab. (B) Cells were pretreated with 100 µM of N-acetylcysteine and then incubated with 100 µg·mL−1 LPS for 4 h. Expression of cPAL2 or COX-2 mRNA was analysed by RT-PCR. (C, D) HTSMCs deprived of serum were pretreated with or without N-acetylcysteine (NAC; 100 µM) for 2 h and then incubated with 100 µg·mL−1 LPS for the indicated time intervals or 15 min. The amount of intracellular ROS was determined by using H2DCFDA fluorescence dye. Data are expressed as mean ± SEM of five independent experiments (n = 5). *P < 0.05, #P < 0.01 as compared with the cells exposed to vehicle alone.
Participation of NADPH oxidase in LPS-induced cPLA2 and COX-2 expression
Several lines of evidence have demonstrated that ROS contribute to the expression of inflammatory proteins in various cell types (Bedard and Krause, 2007). NADPH oxidase is an important enzymatic source for the production of ROS under various pathological conditions. Thus, the role of NADPH oxidase in ROS generation associated with cPLA2 and COX-2 expression was investigated in HTSMCs challenged with LPS. As shown in Figure 3A, pretreatment of HTSMCs with inhibitors of NADPH oxidase (DPI and APO) significantly attenuated LPS-induced cPLA2 and COX-2 protein expression. Moreover, pretreatment with either DPI or APO also inhibited cPLA2 and COX-2 mRNA expression induced by LPS (Figure 3B). These results suggest that NADPH oxidase plays a key role in LPS-induced cPLA2 and COX-2 expression in HTSMCs.
Figure 3.
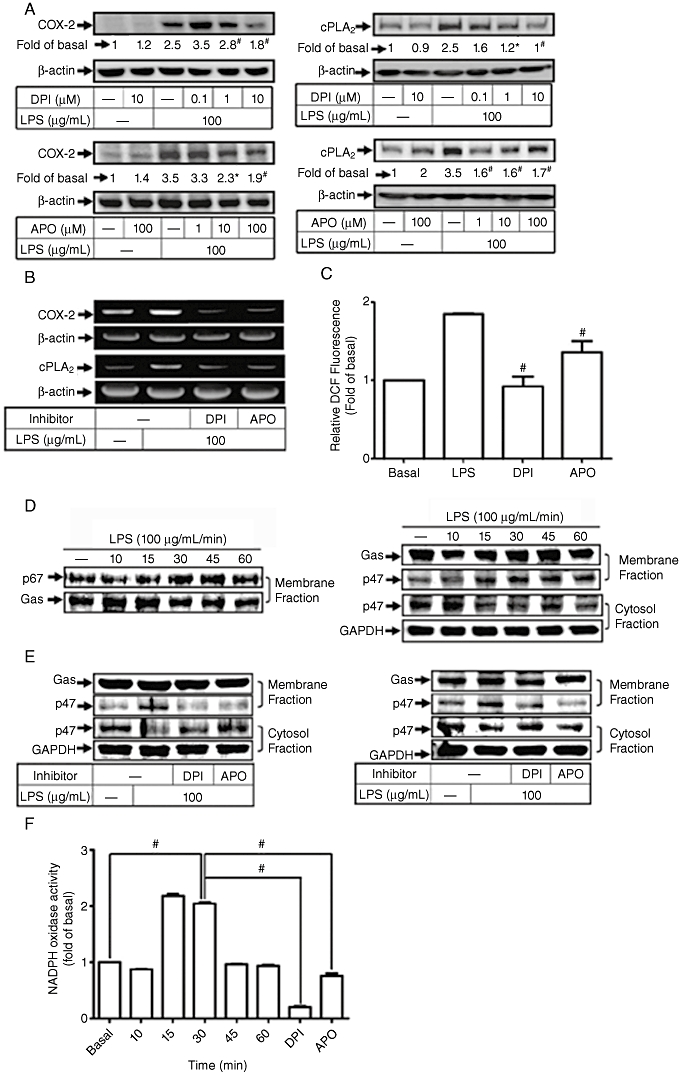
Involvement of NADPH oxidase in lipopolysaccharide (LPS)-stimulated cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression. (A) Human tracheal smooth muscle cells (HTSMCs) were pretreated with various concentrations of diphenyleneiodonium chloride (DPI) or apocynin (APO) for 1 h and then incubated with 100 µg·mL−1 LPS for 6 h or 24 h. Cell lysates were analysed by Western blot using an anti-cPLA2, anti-COX-2 or anti-β-actin Ab. (B) Cells were pretreated with DPI (10 µM) or APO (100 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for another 4 h. mRNA expression of cPLA2 and COX-2 was detected by RT-PCR. (C) HTSMCs were pretreated with DPI (10 µM) or APO (100 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for 15 min. The amount of intracellular reactive oxygen species (ROS) was determined by using H2DCFDA fluorescent dye. (D, E) HTSMCs were pretreated without or with DPI (10 µM) or APO (100 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for the indicated time intervals or 15 min. Cytosolic and membrane fractions of cell lysates were prepared and analysed by Western blot using an anti-p47, anti-p67, anti-Gαs or anti-GAPDH Ab. Gαs and GAPDH were used as marker proteins for membrane and cytosolic fractions respectively. (F) HTSMCs were stimulated with 100 µg·mL−1 LPS for the indicated time intervals or pretreated with DPI (10 µM) or apocycin (100 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for 30 min. The activity of NADPH oxidase was determined. Data are expressed as mean ± SEM of five independent experiments (n = 5). #P < 0.01 as compared with the cells exposed to LPS or vehicle alone, as indicated.
The involvement of NADPH oxidase in these responses may be due to the generation of ROS. As shown in Figure 3C, LPS-induced ROS generation was reduced by pretreatment with inhibitors of NADPH oxidase (DPI and APO). Generation of intracellular ROS may result from the activation of NADPH oxidase, which is composed of p47phox, p67phox, p40phox, p22 and gp91. It has been demonstrated that p47phox organizes the translocation of other cytosolic factors, hence its designation as ‘organizer subunit’ (Barbieri et al., 2004). We found that LPS induced a significant increase in translocation of p67phox and p47phox from the cytosol to the membrane (Figure 3D) and these effects were attenuated by pretreatment with DPI or APO (Figure 3E). Moreover, the enhanced activity of NADPH oxidase induced by LPS was significantly attenuated by pretreatment with DPI or APO (Figure 3F). These results indicate that the activation of NADPH oxidase and generation of ROS have critical roles in LPS-induced cPLA2 and COX-2 expression in HTSMCs.
LPS-induced cPLA2 and COX-2 expression via NADPH oxidase/ROS-dependent MAPKs cascade
To investigate whether p42/p44 MAPK, p38 MAPK or JNK1/2 participates in LPS-induced cPLA2 and COX-2 expression, cells were pretreated with inhibitors of MEK1/2 (PD98059 and U0126), p38 MAPK (SB20190) or JNK (SP600125) for 1 h and then incubated with 100 µg·mL−1 of LPS for 24 h or 6 h. As shown in Figure 4A and B, pretreatment with PD98059, U0126, SB202190 or SP600125 inhibited LPS-induced cPLA2 or COX-2 protein and mRNA expression in a concentration-dependent manner. Further LPS-stimulated phosphorylation of MAPKs was shown to be mediated by NADPH oxidase-dependent generation of ROS, as pretreatment with NAC, DPI or APO attenuated LPS-induced phosphorylation of p42/p44 MAPK, p38 MAPK and JNK1/2 (Figure 4C). Collectively, these results reveal that in HTSMCs, LPS-induced cPLA2 and COX-2 expression is mediated through an NADPH oxidase/ROS-dependent activation of MAPKs cascade.
Figure 4.
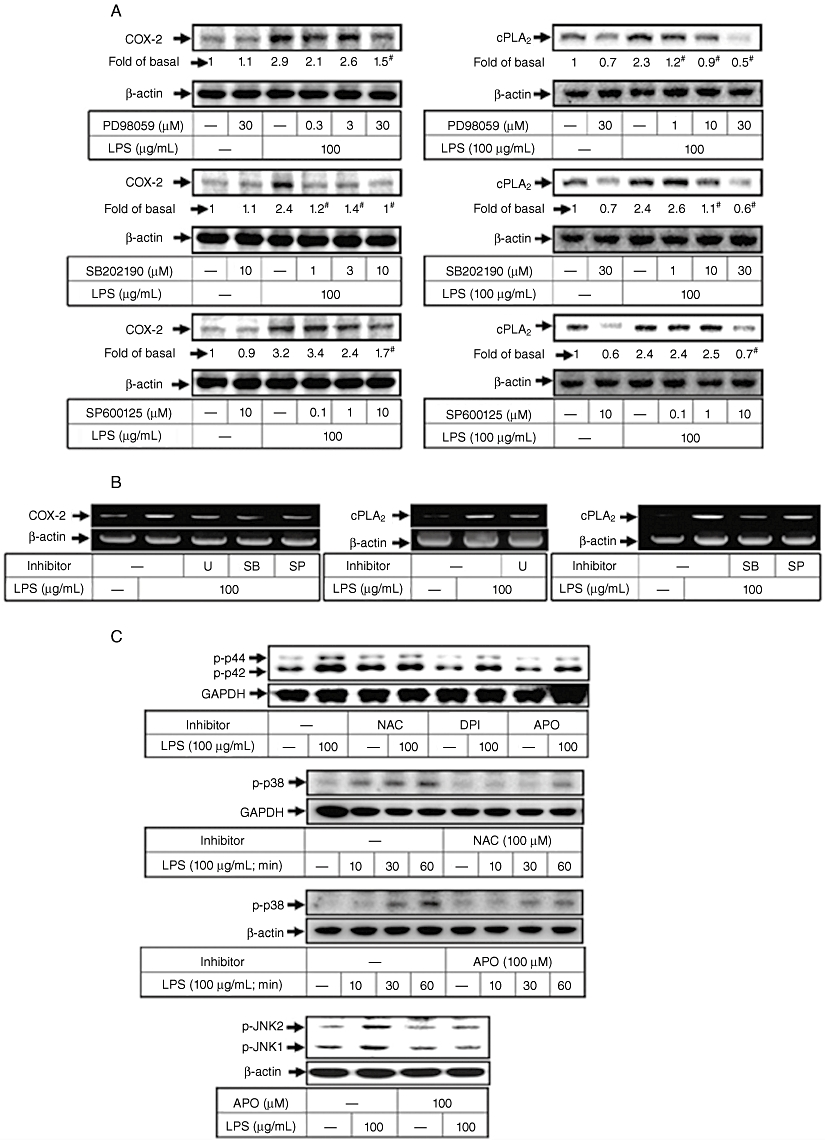
Lipopolysaccharide (LPS) induces cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression through reactive oxygen species (ROS)-activated MAPKs in human tracheal smooth muscle cells (HTSMCs). (A) HTSMCs were pretreated with various concentrations of PD98059, SB203580 or SP600125 for 1 h and then incubated with 100 µg·mL−1 LPS for 6 h or 24 h. Cell lysates were analysed by Western blot using an anti-COX-2, anti-cPLA2 or anti-β-actin Ab. (B) Cells were pretreated with 30 µM PD98059, 30 µM SB202190 or 10 µM SP600125 for 1 h and then incubated with 100 µg·mL−1 LPS for 4 h. mRNA expression of COX-2 and cPLA2 was determined by RT-PCR. (C) HTSMCs were pretreated with N-acetylcysteine (100 µM), diphenyleneiodonium chloride (DPI) (10 µM) or apocynin (APO) (100 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for 10 min or the indicated time intervals. MAPKs in cell lysates were determined by Western blot analysis using an anti-phospho-p42/p44 MAPK, anti-phospho-p38 MAPK, anti-phospho-JNK or anti-β-actin Ab. Similar results were obtained with five independent experiments (n = 5).
LPS stimulates nucleocytoplasmic shuttling of HuR in HTSMCs
Several studies have suggested the involvement of HuR in mRNA stabilization of inflammatory genes. We investigated whether HuR participates in the process of cPLA2 or COX-2 expression in LPS-stimulated HTSMCs. HTSMCs were transiently transfected with HuR siRNA and then incubated with 100 µg·mL−1 LPS for 24 or 6 h. Cell lysates were subjected to Western blot analysis using an anti-cPLA2 or anti-COX-2 antibody. Transfection with HuR siRNA significantly reduced HuR expression and also attenuated LPS-induced expression of cPLA2 and COX-2 (Figure 5A), suggesting that HuR is involved in cPLA2 and COX-2 expression in HTSMCs. The cytosolic export of HuR needs to occur for it to become biologically active and involved in gene transcriptional regulation, hence, cytosolic and nuclear fractions were prepared from LPS-challenged HTSMCs. We found that LPS decreased the amount of HuR in the nuclear fraction and increased that in the cytosolic fraction (Figure 5B). These data indicate that LPS stimulates the cytosolic accumulation of HuR in HTSMCs. To determine whether NADPH oxidase/ROS generation and MAPKs activation participate in this LPS-induced activation of HuR, HTSMCs were pretreated with an ROS scavenger (NAC), inhibitors of NADPH oxidase (DPI and APO), MEK1/2 (PD98059 and U0126), p38 MAPK (SB202190) or JNK1/2 (SP600125) for 1 h and then incubated with LPS (100 µg·mL−1) for 90 min. The cytosolic fraction was prepared and analysed by Western blot using an anti-HuR antibody. We found that LPS-induced cytosolic accumulation of HuR was significantly inhibited by pretreatment with NAC, DPI, APO, PD98059, U0126, SB202190 or SP600125 (Figure 5C). To confirm these results, immunofluorescent staining also revealed that LPS-stimulated accumulation of HuR was significantly attenuated by pretreatment of HTSMCs with NAC, DPI, APO, PD98059, U0126, SB202190 or SP600125 (Figure 5D). Taken together, these data suggest that LPS-induced cPLA2 and COX-2 expression is mediated through an NADPH oxidase/ROS/MAPKs-dependent activation of HuR.
Figure 5.
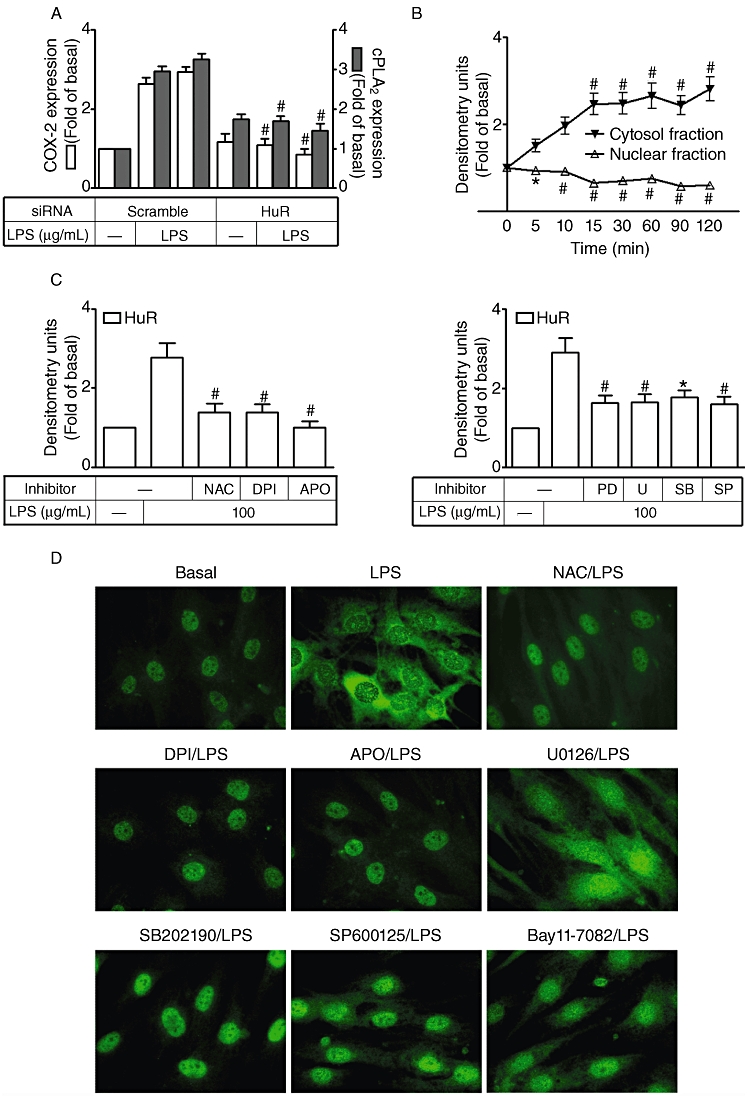
Involvement of HuR in lipopolysaccharide (LPS)-induced cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression in human tracheal smooth muscle cells (HTSMCs). (A) HTSMCs were transfected with HuR siRNA for 24 h, deprived of serum for 48 h, and then incubated with 100 µg·mL−1 LPS for 6 h or 24 h. Expression of cPLA2, COX-2 and HuR was determined by Western blot analysis using an anti-COX-2, anti-cPLA2, anti-HuR or anti-β-actin Ab. (B) HTSMCs deprived of serum were stimulated with 100 µg·mL−1 LPS for the indicated time intervals. Nuclear and cytosolic fractions were prepared and analysed by Western blot using an anti-HuR, anti-lamin A or anti-β-actin Ab. (C) Cells were pretreated with N-acetylcysteine (100 µM), diphenyleneiodonium chloride (DPI) (10 µM), apocynin (APO) (100 µM), PD98059 (30 µM), U0126 (10 µM), SB202190 (30 µM) or SP600125 (10 µM) for 1 h and then incubated with 100 µg·mL−1 LPS for another 90 min. Cell fraction assays were performed and cytosolic fractions of cells were analyzed by Western blot with anti-HuR and anti-β-actin actin. Data are expressed as mean ± SEM of five independent experiments (n = 5). *P < 0.05, #P < 0.01 as compared with the cells exposed to LPS. (D) HTSMCs were pretreated with N-acetylcysteine (NAC; 100 µM), DPI (10 µM), APO (100 µM), U0126 (10 µM), SB202190 (30 µM) or SP600125 (10 µM) for 1 h and then stimulated with 100 mg·mL−1 LPS for 60 min. Immunofluorescence staining was performed using an anti-HuR Ab. Similar results were obtained with five independent experiments (n = 5).
LPS stimulates the physical association between HuR and MAPKs
To further demonstrate that activation of p42/p44 MAPK, p38 MAPK or JNK1/2 is associated with the activation of HuR, an immunoprecipitation assay was performed using an anti-HuR, anti-p44, anti-p38 or anti-JNK2 Ab. Firstly, HTSMCs were incubated with 100 µg·mL−1 LPS for the indicated time intervals and the immunoprecipitation assay was performed using an anti-HuR Ab. We found that LPS stimulated a physical interaction between HuR and p42, p38 or JNK2 in a time-dependent manner with the maximal response occurring within 60 min (Figure 6A). To determine whether the interaction between HuR and MAPKs was attenuated by their respective inhibitors, cells were pretreated with inhibitors of MEK1/2 (U0126), p38 MAPK (SB202190) or JNK1/2 (SP600125) for 1 h and then incubated with 100 µg·mL−1 LPS for 60 min. As shown in Figure 6B–D, LPS-stimulated physical association between HuR and p42/p44 MAPK, p38 MAPK or JNK2 was reduced by U0126, SB202190 or SP600126. Moreover, pretreatment with an ROS scavenger (NAC), or NADPH oxidase inhibitors (DPI or APO), also significantly reduced the physical interaction between HuR and p42/p44 MAPK, p38 MAPK or JNK (Figure 6E). These data suggest that the LPS-induced physical association between HuR and MAPKs is mediated through an NADPH oxidase/ROS generation-dependent cascade.
Figure 6.
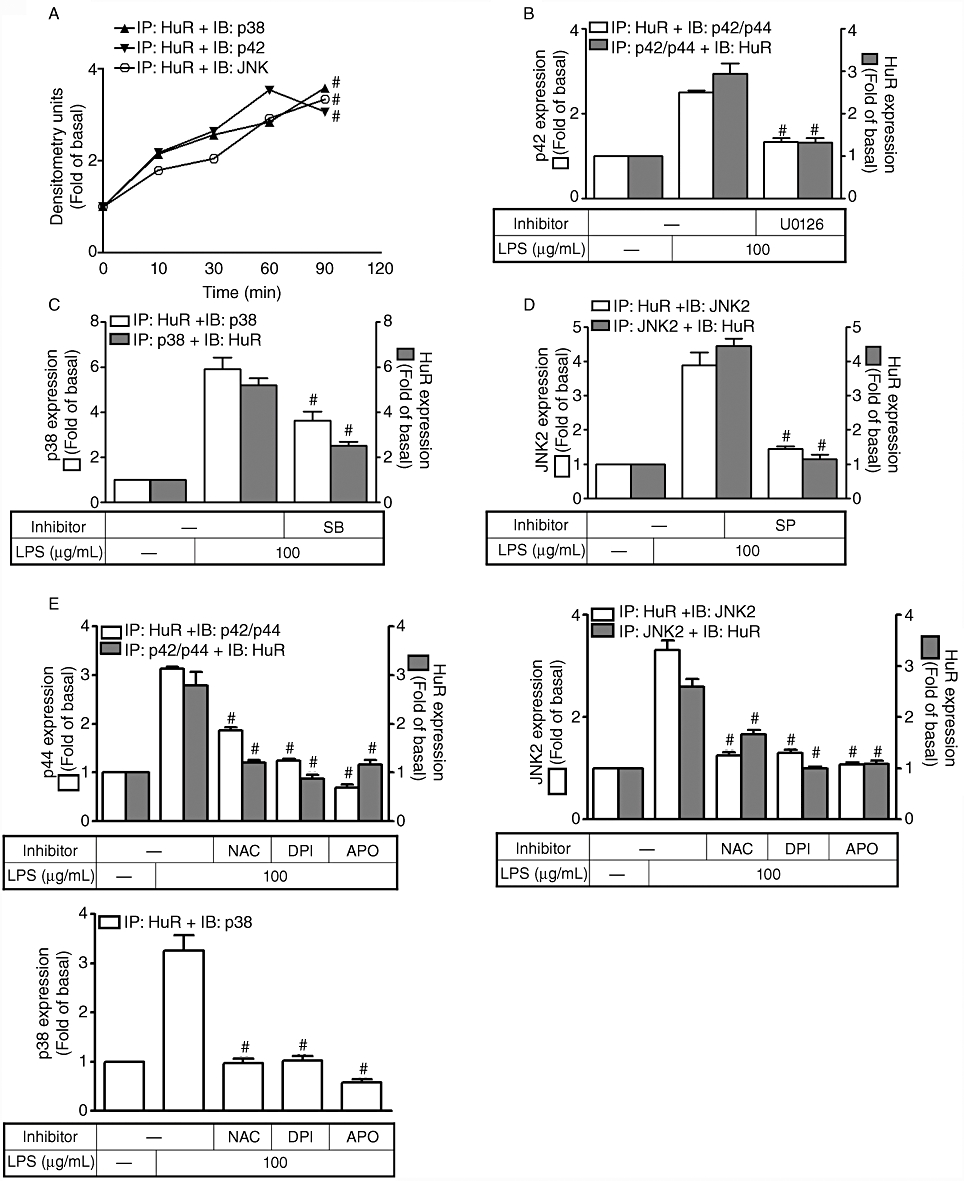
Lipopolysaccharide (LPS) stimulated the physical interaction between HuR and MAPKs in human tracheal smooth muscle cells (HTSMCs). (A–D) HTSMCs deprived of serum were incubated with 100 µg·mL−1 LPS for the indicated time intervals or pretreated with 10 µM U0126, 30 µM SB202190 or 10 µM of SP600125 for 1 h and then incubated with LPS for another 1 h. The cell lysates were subjected to immunoprecipitation with an anti-HuR, anti-p42/p44 MAPK, anti-p38 MAPK or anti-JNK2 Ab, and then the immunoprecipitates were analysed by Western blot using an anti-HuR, anti-p42 MAPK, anti-p38 MAPK or anti-JNK2. (E) HTSMCs were pretreated with N-acetylcysteine (NAC; 100 µM), diphenyleneiodonium chloride (DPI; 10 µM) or apocynin (APO; 100 µM) for 1 h and then stimulated by LPS (100 µg·mL−1) for 60 min. The cell lysates were analysed as described in (A–D). Data are expressed as mean ± SEM of five independent experiments (n = 5). #P < 0.01 as compared with the cells exposed to LPS or vehicle alone.
The involvement of HuR in LPS-induced cPLA2 and COX-2 expression
To further confirm the involvement of HuR in the expression of cPLA2 and COX-2 induced by LPS, an HuR plasmid was constructed and transiently transfected into HTSMCs. Transfection with HuR plasmid led to an increased expression of HuR protein and subsequently enhanced LPS-induced expression of COX-2 and cPLA2 protein, as revealed by Western blot analysis (Figure 7A). Similarly, the expression of COX-2 and cPLA2 mRNA was also up-regulated in the HTSMCs transfected with HuR plasmid (Figure 7B). The involvement of HuR in the LPS-induced responses was further confirmed by transfection with HuR siRNA. As shown in Figure 7C and D, transfection with HuR siRNA significantly reduced LPS-induced COX-2 and cPLA2 mRNA expression, as determined by RT-PCR and real-time PCR respectively. The stabilities of cPLA2 and COX-2 mRNA were not significantly affected by addition of actinomycin D 4 h after treatment with LPS (Figure 7D). These data further suggest that HuR plays an important role in modulating LPS-stimulated COX-2 and cPLA2 expression in HTSMCs.
Figure 7.
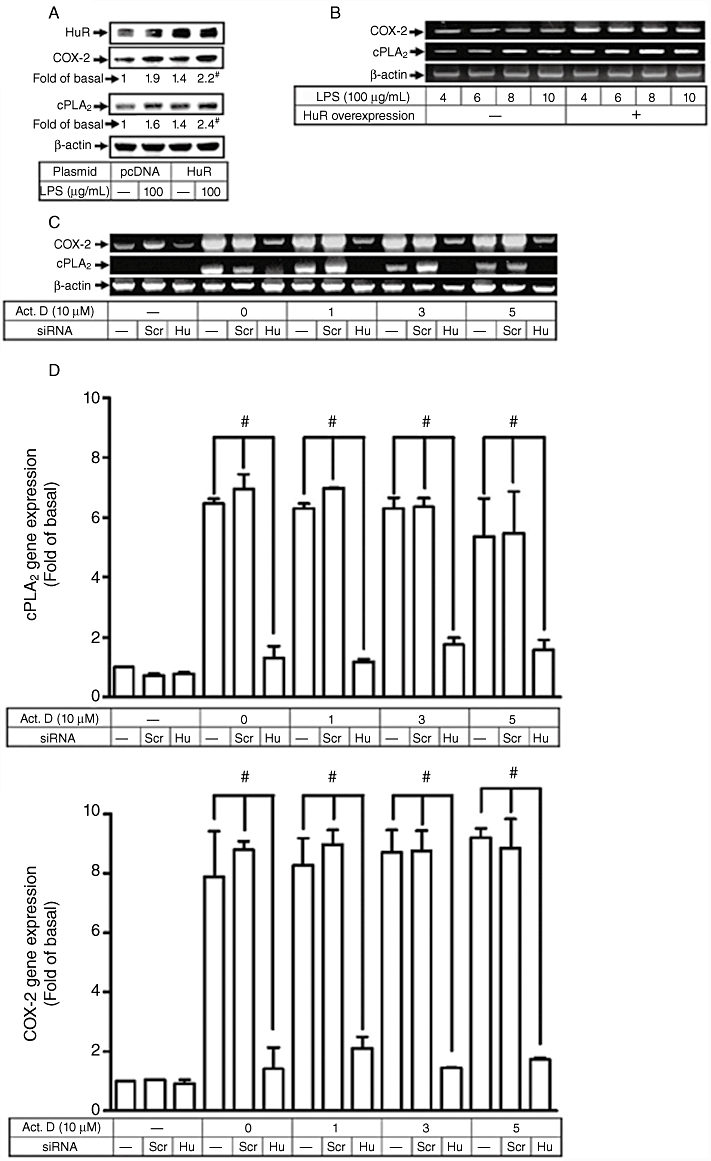
Involvement of HuR in lipopolysaccharide (LPS)-induced cyclooxygenase-2 (COX-2) and cytosolic phospholipase A2 (cPLA2) expression. (A) Human tracheal smooth muscle cells (HTSMCs) were transfected with HuR plamid or pcDNA for 24 h, serum-starved for 48 h, and then incubated by 100 µg·mL−1 of LPS for 6 h or 24 h. Expression of cPLA2, COX-2 and HuR was determined by Western blot using an anti-COX-2, anticPLA2 or anti-HuR Ab. (B) HTSMCs were transfected with HuR plasmid and then stimulated with 100 µg·mL−1 of LPS for the indicated time periods. mRNA expression of COX-2 and cPLA2 was determined by RT-PCR. (C, D) HTSMCs were transfected with HuR siRNA for 24 h, serum-starved for 48 h, incubated with LPS (100 µg·mL−1) for 4 h, and then added with 1 µM of transcription inhibitor, actinomycin D (Act.D) for the indicated time intervals. mRNA was extracted, and the expression of COX-2 and cPLA2 were analysed by RT-PCR and real-time PCR respectively. Data are expressed as mean ± SEM of five independent experiments (n = 5). #P < 0.01, as compared with the respective values of cells incubated with vehicle alone.
Discussion and conclusions
Inflammation is an innate immunity of numerous airway diseases including asthma and COPD. COX-2 and cPLA2 are key enzymes in mediating the conversion of lipids to PGs and LTs which further regulate the process of inflammation (Samad et al., 2002). However, the action of LPS, a candidate of these bacteria-induced allergies, in regulating the expression of COX-2 and cPLA2 in HTSMCs has not been completely elucidated. Here we showed that LPS stimulates the expression of COX-2 and cPLA2 genes via the activation of NADPH oxidase, generation of ROS and phosphorylation of MAPKs. In addition, we showed that overexpression of HuR positively up-regulated COX-2 and cPLA2 expression in LPS-challenged HTSMCs knocked-down HuR expression, induced by transfection with HuR siRNA significantly reduced COX-2 and cPLA2 expression in these HTSMCs. Our results obtained by cell fraction assay, immunofluorescent staining and immunoprecipitation assays also revealed that p42/p44 MAPK, p38 MAPK and JNK participate in LPS-mediated nucleocytoplasmic shuttling of HuR. Western blot analysis revealed that activation of these MAPKs was regulated by activated NADPH oxidase-dependent generation of ROS. Thus, we conclude that in HTSMCs, LPS induces COX-2 and cPLA2 expression by promoting the cytosolic export of HuR via NADPH oxidase/ROS-dependent phosphorylation of MAPKs.
HTSMCs have been demonstrated to play important roles in orchestrating and perpetuating airway inflammation, remodelling and fibrosis in chronic airway diseases (Tliba and Panettieri, 2008). Phenotype transition of HTSMCs from contractile units to synthetic function is a major signature of the pathology of airways during disease. Here we showed that LPS induces HTSMCs to express COX-2 and cPLA2, which accompanied an increased secretion of PGE2 (Figure 1). This accords with the higher PGE2 levels found in the sputum of patients with eosinophilic bronchitis (Sastre et al., 2008), and also with results obtained with RAW264.7 macrophages showing that LPS induces the production of inflammatory mediators such as PGE2 and COX-2 (Ci et al., 2009). However, the functions of PGs in regulating the outcome of inflammation are still controversial. Thus, understanding the mechanisms associated with the generation of PGs will help to improve the development of specific drug targets.
HuR, a ubiquitously expressed membrane of ELAV family, has been shown to participate in mediating the stabilization of a broad spectrum of mRNAs coding for cell-cycle regulators, cytokines, growth factors, tumour suppressors, protooncogenes, apoptosis regulatory proteins and various inflammatory enzymes (Doller et al., 2008b). In this study, induction of COX-2 and cPLA2 expression by LPS were found to be dependent on the mRNA-binding protein HuR, which accumulated in the cytoplasm upon exposure of HTSMCs to LPS (Figure 5). Both mRNA binding activity and nucleocytoplasmic shuttling of HuR are known to be regulated by several protein kinases such as MAPKs. In the present study, these MAPKs were demonstrated to enhance HuR-dependent mRNA stabilization in LPS-challenged HTSMCs (Figure 6). In fact, it was recently demonstrated that HuR is a direct substrate of p38 MAPK which stimulates phosphorylation of HuR and thus enhances its mRNA stabilizing activity (Lafarga et al., 2009). Here we found that overexpression of HuR siRNA significantly attenuated LPS-induced COX-2 and cPLA2 expression, while overexpression of HuR protein significantly promoted LPS-mediated COX-2 and cPAL2 expression (Figures 5A and 7), suggesting that HuR contributes to the inflammatory effect of LPS in HTSMCs. Moreover, treatment with actinomycin D had no effect on LPS-mediated COX-2 and cPLA2 mRNA expression, which was reduced in HTSMCs transfected with HuR siRNA (Figure 7C and D), indicating the involvement of HuR in these LPS-mediated responses. Similarly, HuR have been shown to be involved in the expression of plasminogen activator inhibitor-1 and COX-2 in response to angiotensin-II in kidneys (Doller et al., 2009). In addition, ultraviolet B stimulates COX-2 expression by modifying the activity of HuR in a human keratinocyte cell line HaCaT (Fernau et al., 2009).
Moreover, the localization of HuR in cytoplasm is important for the stabilization of target gene mRNA. However, the exact mechanisms that mediate the nucleocytoplasmic shuttling of HuR are still not known. Here we found that in HTSMCs, LPS induces the nuclear export of HuR via MAPKs (Figure 5B and D). In addition, we also showed that HuR may complex with p42/p44 MAPK, p38 MAPK or JNK1/2, which was attenuated by pretreatment with the inhibitors of MAPKs, as revealed by an immunoprecipitation assay (Figure 6). These results suggest that activated MAPKs interact with HuR and regulate the cytosolic localization of HuR, and are consistent with the findings of Lafarga et al. (2009) who by investigating the effects of γ-radiation on p21-related cell cycle arrest showed that activated p38 MAPK regulates the cytoplasmic accumulation of HuR. In addition, the increase in the accumulation of HuR in cytoplasma has also been found to be regulated by activated JNK and related to tamoxifen resistance in breast cancer cells (Hostetter et al., 2008).
Recently, oxidative stress has been found to have a role in various inflammation-related pathologies including asthma. Here we found that pretreatment of HTSMCs with NADPH oxidase inhibitors or an ROS scavenger also attenuated the phosphorylation of MAPKs and inhibited the interaction between HuR and MAPKs (Figures 4C and 6E), indicating that NADPH oxidase and ROS contribute to the association between MAPKs and HuR and induce the nuclear export of HuR. In addition, LPS stimulation directly induced membrane localization of NADPH oxidase subunits, p47 and p67, and increased NADPH oxidase activity, which was associated with an increased intracellular ROS level (Figures 3D, F and 2C). LPS-mediated membrane localization of p67phox and p47phox was attenuated by pretreatment with either DPI or APO (Figure 3E), consistent with the results obtained by Barbieri et al. (2004) and Nishikawa et al. (2007). In addition, pretreatment with NADPH oxidase inhibitors or an ROS scavenger significantly attenuated the LPS-induced COX-2 and cPLA2 expression (Figures 2A, B and 3A, B). These results indicate that NADPH oxidase-dependent generation of ROS may mediate COX-2 and cPLA2 expression by activating MAPKs and promoting the cytoplasmic accumulation of HuR, consistent with results obtained in human keratinocyte HaCaT cells and hepatocellular carcinoma cells (Lim et al., 2009; Liu et al. 2009). Results obtained with macrophage RAW264.7 cells have also indicated that LPS mediates the activation of the NADPH oxidase/superoxide production that results in innate immune responses (Check et al., 2009).
In conclusion, our results indicate that LPS promotes COX-2 and cPLA2 gene expression via an increase in the cytoplasmic accumulation of HuR, which is involved in mediating the stability of mRNA. The mechanisms underlying LPS-stimulated nucleocytoplasmic shuttling of HuR were mediated through the activation of NADPH oxidase, generation of ROS and phosphorylation of MAPKs. We showed that in HTSMCs LPS induces the membrane localization of p47 and p67 as well as the activation of NADPH oxidase. Moreover, the activation of NADPH oxidase increased intracellular ROS levels in LPS-challenged HTSMCs and the increased ROS led to p42/p44 MAPK, p38 MAPK and JNK phosphorylation and their physical association with HuR, which resulted in cytoplasmic accumulation of HuR. Thus, in HTSMCs, the expression of COX-2 and cPLA2 may be regulated by NADPH oxidase/ROS/MAPKs-dependent cytoplasmic accumulation of HuR in response to LPS. Manipulation of HuR expression may provide a novel strategy to deal with inflammation-related pathologies.
Acknowledgments
This work was supported by grants NSC98-2314-B-182-021-MY3 (CCL), NSC98-2321-B-182-004 and NSC98-2320-B-182-004-MY3 (CMY) from National Science Council, Taiwan; and CMRPD32043, CMRPD170332, CMRPG381521, CMRPG360153 and CMRPG350653 from Chang Gung Medical Research Foundation. The authors appreciate Mr Li-Der Hsiao for his technical assistance during the preparation of paper.
Glossary
Abbreviations
- AA
arachidonic acid
- AMPK
AMP-activated kinase
- APO
apocynin
- BCA
bicinchoninic acid
- COPD
obstructive pulmonary disease
- COX-2
cyclooxygenase-2
- cPLA2
cytosolic phospholipase A2
- DPI
diphenyleneiodonium chloride
- ECL
enhanced chemiluminescence
- ECM
extracellular matrix
- HNS
HuR nucleocytoplasmic shuttling sequence
- HTSMCs
human tracheal smooth muscle cells
- HuR
Hu antigen R
- LPS
lipopolysaccharide
- LTs
leukotrienes
- NAC
N-acetylcysteine
- PGs
prostaglandins
- RRM
RNA-recognition motifs
Conflicts of interest
The authors have no financial conflict of interest.
Supporting Information
Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6:1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- Al Laham F, Kalsch AI, Heinrich L, Birck R, Kallenberg CG, Heeringa P, et al. Inhibition of neutrophil-mediated production of reactive oxygen species (ROS) by endothelial cells is not impaired in anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitis patients. Clin Exp Immunol. 2010;161:268–275. doi: 10.1111/j.1365-2249.2010.04171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn (2009) Br J Pharmacol. 2009;158:S1, S254. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, et al. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check J, Byrd CL, Menio J, Rippe RA, Hines IN, Wheeler MD. Src kinase participates in LPS-induced activation of NADPH oxidase. Mol Immunol. 2009;47:756–762. doi: 10.1016/j.molimm.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13:1014–1021. doi: 10.1111/j.1440-1843.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- Ci X, Ren R, Xu K, Li H, Yu Q, Song Y, et al. Schisantherin a exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2009;33:126–136. doi: 10.1007/s10753-009-9166-7. [DOI] [PubMed] [Google Scholar]
- Doller A, Huwiler A, Muller R, Radeke HH, Pfeilschifter J, Eberhardt W. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2. Mol Biol Cell. 2007;18:2137–2148. doi: 10.1091/mbc.E06-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller A, Akool EL, Huwiler A, Muller R, Radeke HH, Pfeilschifter J, et al. Posttranslational modification of the AU-rich element binding protein HuR by protein kinase Cdelta elicits angiotensin II-induced stabilization and nuclear export of cyclooxygenase 2 mRNA. Mol Cell Biol. 2008a;28:2608–2625. doi: 10.1128/MCB.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008b;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Doller A, Gauer S, Sobkowiak E, Geiger H, Pfeilschifter J, Eberhardt W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am J Pathol. 2009;174:1252–1263. doi: 10.2353/ajpath.2009.080652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernau NS, Fugmann D, Leyendecker M, Reimann K, Grether-Beck S, Galban S, et al. Role of HuR and p38-MAP kinase in ultraviolet B-induced posttranscriptional regulation of cyclooxygenase-2 expression in the human keratinocyte cell line HaCaT. J Biol Chem. 2009;235:3896–3904. doi: 10.1074/jbc.M109.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Hostetter C, Licata LA, Witkiewicz A, Costantino CL, Yeo CJ, Brody JR, et al. Cytoplasmic accumulation of the RNA binding protein HuR is central to tamoxifen resistance in estrogen receptor positive breast cancer cells. Cancer Biol Ther. 2008;7:1496–1506. doi: 10.4161/cbt.7.9.6490. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol. 2009;29:4341–4351. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaar AL, Panettieri RA., Jr Airway smooth muscle as a regulator of immune responses and bronchomotor tone. Clin Chest Med. 2006;27:53–69. doi: 10.1016/j.ccm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lemanske RF, Jr, Busse WW. 6. Asthma: Factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006;117:S456–S461. doi: 10.1016/j.jaci.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Kwon SH, Cho H, Kim S, Lee S, Ryu WS, et al. HBx targeting to mitochondria and ROS generation are necessary but insufficient for HBV-induced cyclooxygenase-2 expression. J Mol Med. 2009;88:359–369. doi: 10.1007/s00109-009-0563-z. [DOI] [PubMed] [Google Scholar]
- Lin CC, Shyr MH, Chien CS, Wang CC, Chiu CT, Hsiao LD, et al. Mechanisms of thrombin-induced MAPK activation associated with cell proliferation in human cultured tracheal smooth muscle cells. Cell Signal. 2001;13:257–267. doi: 10.1016/s0898-6568(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Lin WN, Luo SF, Lin CC, Hsiao LD, Yang CM. Differential involvement of PKC-dependent MAPKs activation in lipopolysaccharide-induced AP-1 expression in human tracheal smooth muscle cells. Cell Signal. 2009;21:1385–1395. doi: 10.1016/j.cellsig.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Liu S, Mizu H, Yamauchi H. Photoinflammatory responses to UV-irradiated ketoprofen mediated by the induction of ROS generation, gnhancement of cyclooxygenase-2 expression and regulation of multiple signaling pathways. Free Radic Biol Med. 2009;48:772–780. doi: 10.1016/j.freeradbiomed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, et al. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat Immunol. 2000;1:42–46. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- Nieminen R, Vuolteenaho K, Riutta A, Kankaanranta H, van der Kraan PM, Moilanen T, et al. Aurothiomalate inhibits COX-2 expression in chondrocytes and in human cartilage possibly through its effects on COX-2 mRNA stability. Eur J Pharmacol. 2008;587:309–316. doi: 10.1016/j.ejphar.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Wakano K, Kitani S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochem Biophys Res Commun. 2007;362:504–509. doi: 10.1016/j.bbrc.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Oyesanya RA, Lee ZP, Wu J, Chen J, Song Y, Mukherjee A, et al. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. FASEB J. 2008;22:2639–2651. doi: 10.1096/fj.07-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–396. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- Sastre B, Fernandez-Nieto M, Molla R, Lopez E, Lahoz C, Sastre J, et al. Increased prostaglandin E2 levels in the airway of patients with eosinophilic bronchitis. Allergy. 2008;63:58–66. doi: 10.1111/j.1398-9995.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- Schroder NW, Arditi M. The role of innate immunity in the pathogenesis of asthma: evidence for the involvement of Toll-like receptor signaling. J Endotoxin Res. 2007;13:305–312. doi: 10.1177/0968051907084652. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxgenase-2 mRNA stability by taxanes: evidence for involvement of p38, MAPKAPK-2, and HuR. J Biol Chem. 2003;278:37637–37647. doi: 10.1074/jbc.M301481200. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Thorn J. The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflamm Res. 2001;50:254–261. doi: 10.1007/s000110050751. [DOI] [PubMed] [Google Scholar]
- Tliba O, Panettieri RA., Jr Regulation of inflammation by airway smooth muscle. Curr Allergy Asthma Rep. 2008;8:262–268. doi: 10.1007/s11882-008-0043-5. [DOI] [PubMed] [Google Scholar]
- Tsou JH, Chang KY, Wang WC, Tseng JT, Su WC, Hung LY, et al. Nucleolin regulates c-Jun/Sp1-dependent transcriptional activation of cPLA2alpha in phorbol ester-treated non-small cell lung cancer A549 cells. Nucleic Acids Res. 2008;36:217–227. doi: 10.1093/nar/gkm1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fan J, Yang X, Fürer-Galban S, Lopez de Silanes I, von Kobbe C, et al. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bowden GT. UVB irradiation regulates Cox-2 mRNA stability through AMPK and HuR in human keratinocytes. Mol Carcinog. 2008;47:974–983. doi: 10.1002/mc.20450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


