Abstract
The Regulator of G Protein Signaling 1 [RGS1] gene is associated with celiac disease, multiple sclerosis (MS) and Type I diabetes (T1D), which are all T cell-mediated pathologies. And yet there is no reported analysis of RGS1 biology in human T cells. This study shows that RGS1 expression is substantially higher in T cells from human gut versus peripheral blood, and that this can be exaggerated in intestinal inflammation. Elevated RGS1 levels profoundly reduce T cell migration to lymphoid-homing chemokines, whereas RGS-1 depletion selectively enhances such chemotaxis in gut T cells, and impairs their colitogenic potential. These findings provide a revised framework in which to view the linkage of RGS1 to inflammatory disease.
Introduction
For T cells to make regulated responses to infections within tissues, they must enter, reside within, and then egress from relevant sites. Chemokines play pivotal roles in such processes. In chronic inflammation, it may be argued that T cell egress in response to lymphoid-homing chemokines is impaired. Hence, the value of elucidating the factors regulating the selective chemotaxis of T cells. Being G-protein coupled receptors (GPCRs), chemokine receptors may be regulated by RGS molecules which bind Gα proteins, increasing their intrinsic GTPase activity up to 100 fold, and thereby attenuating signaling (1). Rgs1 mRNA is highly enriched in murine gut versus lymphoid T cells (2-4), suggesting that it might likewise be enriched in human gut T cells where it might selectively regulate chemokine responses. However, while RGS1 was shown to regulate B cell chemotaxis, there has been no substantive study of it in human T cells.
Perhaps discouraging such analysis, splenic and lymph node T cells from Rgs1-/- mice show seemingly normal chemotaxis (5). Nonetheless, the strong associations of RGS1 SNPs with T cell-mediated diseases including T1D, celiac disease, and MS (4,6-8) argue for it being an important T cell regulator. Therefore, this study investigates RGS1 biology in human intestinal T cells, with supporting studies of Rgs1-/- mice. The data argue for a pivotal role of RGS1 in T cell trafficking and tissue immunopathologies.
Materials and Methods
Human Samples
Mucosal biopsies and paired blood samples were from consented patients with ulcerative colitis (UC) or Crohn’s disease (CD) undergoing ileocolonoscopy, or from control patients undergoing colonoscopy for polyp follow-up or diagnosis. PBMC were isolated using Ficoll (GE Healthcare) and gut intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) isolated as described (9). Cells were stained and flow-sorted into discrete populations. RNA was isolated, cDNA synthesis performed, and RGS1, RGS2, and RGS10 mRNA assayed by Q-RT-PCR relative to cyclophilin A, using the following primers:
RGS1: TGTGCATTCAGATGCTGCTA and CTCGAGTGCGGAAGTCAATA;
RGS2: AAGATTGGAAGACCCGTTTG and GATGAAAGCTTGCTGTTTGC;
RGS10: CCCTGGAGAATCTGCTGGAAC and TTTCCTGCATCTGAGTCTTA;
cyclophilin A: TGGTGGACCCAACACAAATC and TGCCATCCAACCACTCAGTCT.
Mice
Wild type (WT) and Rgs1-/- C57BL/6 mice have been described (5); C57BL/6 Rag2-/- mice were from Taconic Farms. Mice were age-matched in all experiments.
T cell transfer model of colitis
0.5×106 naïve, CD4+CD45RBhi cells from either WT or Rgs1-/- C57BL/6 mice were injected intraperitoneally into Rag2-/- mice. Mice were weighed weekly, and culled 7-8 weeks post injection. Colons were removed, measured and subject to histology.
Transient transfections
Jurkat cells were transiently transfected with IRES-EGFP (Clontech) or RGS1-EGFP by electroporation, alone or with CCR7-tomato (pMIT-tomato, kind gift of H. Jumaa, Freiburg). Cells were left for 24-48hrs and washed before use. Primary human T cell blasts were transfected with IRES-EGFP, RGS1-EGFP or RGS10-EGFP (B isoform) using Amaxa II nucleofection.
Migration assays
Transfected Jurkat cells were placed in Transwell migration assays (5μM pore, Corning) for 2-3hrs at 37°C, and migrated cells enumerated by FACS Canto II (BD) analysis of 150μl harvested from the bottom chamber. Cells migrated to 200ng/ml human CXCL12 (ImmunoTools) or 500ng/ml human CCL19 (R&D Systems). Percentages of migrated cells were calculated by dividing the number of transmigrated EGFPhi, EGFPlow, or EGFP(-) cells by the number of input cells with comparable EGFP levels. Murine intestinal T cells were isolated and placed in Transwell migration assays, and migrated cells (CD45+, TCR+) enumerated as above in response to recombinant murine CCL19 (50ng/ml), CCL20 (50ng/ml), CXCL12 (100ng/ml) all from ImmunoTools or CCL25 (50ng/ml, R&D Systems).
Confocal imaging
Transfected primary human T cell blasts were incubated overnight, washed and equilibrated on an ICAM-1 pre-coated μVI slide (Ibidi, Germany). 2×105 cells were added to each slide chamber and imaged every 10 seconds for 20 minutes. Prior to image acquisition, 10μl of medium or recombinant human CXCL12 (10μg/ml) (R&D Systems) was applied to filter paper at one end of the chamber. Time-lapse films were analysed by Volocity (Improvision, UK), and EGFP+ cells tracked automatically.
Results and Discussion
RGS1 over-expression in normal and inflamed gut
To determine whether human intestinal T cells, like their mouse counterparts, are enriched in RGS1 expression, RGS1 RNA was quantified in purified TCRαβ+CD4+ and TCRαβ+CD8αβ+ cells from peripheral blood (light diamonds) or gut biopsies (dark diamonds) (Fig 1A). Notwithstanding inter-individual variation common in human studies, RGS1 was over-expressed in human gut T cells by an average of ~100 fold, as occurs in mice (2-4). RGS2 was less overtly over-expressed (~15-fold), and RGS10 was generally under-represented in gut T cells (Supplemental Figure S1A,B), again as is true in mice. RGS1 can regulate B cell migration to chemokines by targeting Gαi (10) whereas RGS2 preferentially targets Gαq (11), not commonly utilised by chemokine receptors. Hence, RGS1 became the primary focus for further studies.
Fig 1.
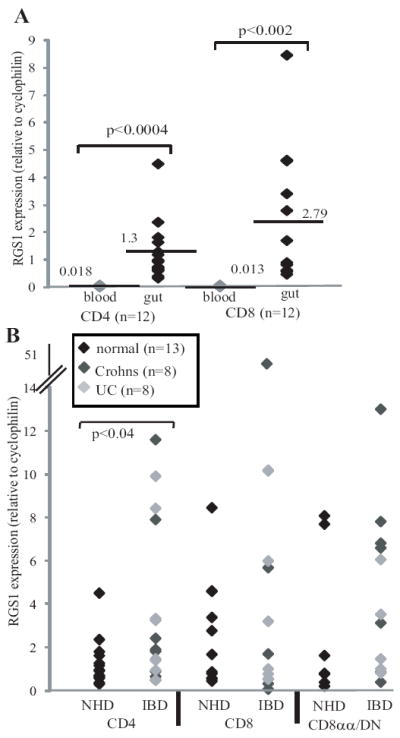
(A) Paired samples of TCRαβ+ CD4+ or CD8αβ+ cells from blood (light diamonds) or gut (black diamonds) of normal patients, were assessed for RGS1 mRNA relative to cyclophilin A. Significance (by Student’s T test) are shown for comparisons of blood and intestinal T cells. Note that RGS1 mRNA levels were not statistically different between intestinal IEL and LPL (data not shown). (B) RGS1 mRNA levels in gut CD4+, or CD8αβ+ or unconventional CD8αα+/DN cells from normal donors (NHD, black diamonds) or patients with UC (light grey diamonds) or CD (dark grey diamonds).
Conventional (CD4+; CD8αβ+) and pooled unconventional (CD4(-)CD8(-) “double negative” [DN], and CD4(-) CD8αα(+)) αβ T cells from normal, CD, and UC gut mucosa (Fig 1B) revealed consistently high RGS1 levels, and while many CD/UC samples fell within the normal range, some showed highly elevated expression, particularly for intestinal CD4+ T cells (p<0.04). This is noteworthy, given that many such cells will have infiltrated the inflamed gut from the blood and lymphoid organs where RGS1 levels are low (Fig 1A). Although such cells will be activated in the gut, activation alone of peripheral blood T cells evoked only a transient increase in RGS1 RNA, with peak levels ~10-fold less than in gut T cells (Supplemental Figure S1C). Moreover, RGS1 expression was not significantly different in naive versus memory CD4 T cells (Supplemental Figure S1D).
RGS1 decreases T cell migration to lymphoid-homing chemokines
To assess the functional significance of RGS1 over-expression, transwells were employed to assay T cell migration to chemokines. Because two human RGS1 transcripts, differing in coding potential by 13 amino acids, have been reported (5), Jurkat cells (which do not express RGS1) were independently transfected with each species linked via an internal ribosome entry site (IRES) to EGFP. Relative to control, EGFP+, empty-vector transfectants, EGFP+ RGS1 transfectants showed dose-dependent inhibition of migration to CXCL12, a lymphoid chemokine for the receptor CXCR4. Moreover, transfectants expressing lower levels of RGS1 (EGFPlow) were less compromised than EGFPhi cells expressing higher RGS1 (Fig 2A). From data collated from ten independent experiments, RGS1 significantly decreased migration to CXCL12 by >35% (p<0.01). Primary human peripheral blood T cells which express very little RGS1 were then engineered to over-express either RGS1 or RGS10, and their migration assessed by time-lapse microscopy (Fig 2B). Addition of CXCL12 to the bottom of the ICAM-1-coated slide redirected the random migration of empty-vector control or RGS10-transfected cells along the chemokine gradient (Fig 2B), with coincident decreases in migration velocity (p~0.001, Fig 2C). Conversely, RGS1-transfected cells did not align with the chemokine gradient (Fig 2B,C). Thus, elevating RGS1 limited CXCL12 responsiveness without grossly affecting normal kinesis.
Fig 2.
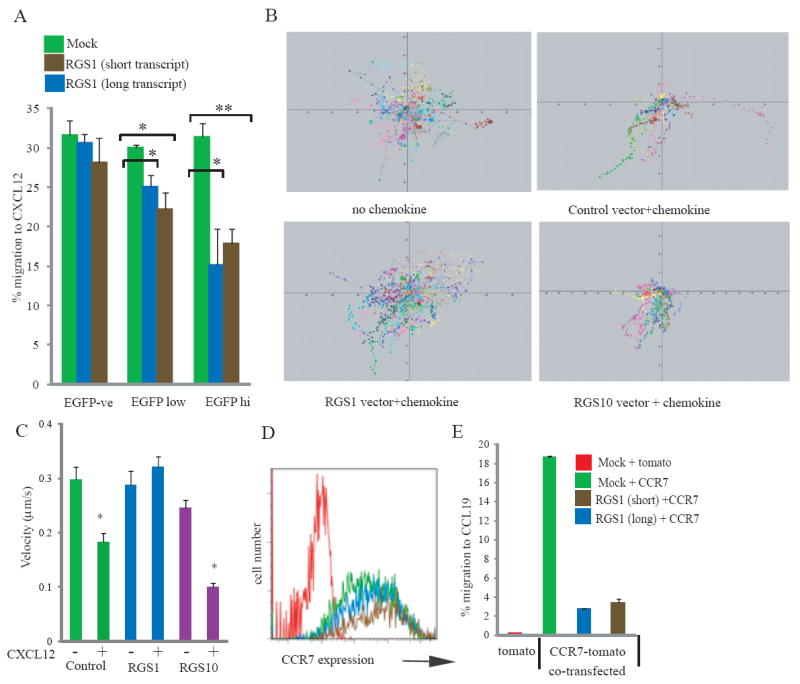
(A). Jurkat cells transfected with EGFP control vector (green bars), short RGS1-EGFP (brown bars), or long RGS1-EGFP (blue bars) assessed in duplicate CXCL12-responsive migration assays. Specific migration for EGFP-ve, EGFPlow or EGFPhigh cells is shown from a representative experiment. Statistically significant differences (Student’s T test) are highlighted: *p<0.05, **p<0.02.
(B, C) Peripheral blood T cells nucleofected with RGS1-EGFP, RGS10-EGFP, or control vector and assessed for CXCL12 responses: (B) migration tracks measured by time-lapse confocal imaging- cells migrated to chemokine added to the base of the slide; (C) velocities of cells transfected with the different vectors in the presence or absence of CXCL12. Statistics were performed using Kolmogorov-Smirnov comparisons, *p<0.001. (D) CCR7 expression levels on Jurkat cells transfected with control tomato vector and control EGFP vector (red line); CCR7-tomato and control EGFP vector (green); or CCR7-tomato co-transfected with either “short-RGS1”-EGFP (brown) or “long-RGS1”-EGFP (blue). (E) Specific migration to CCL19 of Jurkat cells transfected with CCR7-tomato in the presence or absence of RGS1 isoforms.
To assess the capacity of RGS1 to regulate CCR7-mediated chemotaxis to CCL19, that promotes T cell egress from tissues to lymph nodes, Jurkat cells (which express very low levels of CCR7) were transfected with CCR7 (upstream of a tomato-tracker gene), resulting in CCR7 surface expression (Fig 2D) and migration to CCL19 (Fig 2E). Co-transfection with RGS1 did not effect CCR7 levels (Fig 2D), but across ten independent experiments reduced migration to CCL19 by ~54% (p<0.003) (Fig 2E).
Having established that RGS1 is elevated in human gut T cells and can limit directional chemotaxis, the requirement for RGS1 in T cell migration was re-examined. Of note, the comparable migration to CCL19 reported for WT and Rgs1-/- splenic T cells (5) might simply reflect the negligible expression of RGS1 by splenic T cells. Consistent with this, intestinal T cells from Rgs1-/- mice showed significantly enhanced migration to CCL19 and CXCL12 relative to WT intestinal T cells. By contrast, migration to the gut-homing chemokine CCL25 (CCR9 ligand) or to CCL20 (CCR6 ligand) was unaffected (Figure 3). Hence, RGS1 specifically limited gut T cell responsiveness to the lymphoid-homing chemokines tested. Also, in two housing facilities, the gut-associated integrin, CD103, was found on significantly more CD4+ T cells (p<0.008) (Supplemental Figure S2A) at significantly higher levels (p<0.037), in the spleens of Rgs1-/- versus WT mice, perhaps reflecting enhanced intestinal egress.
Fig 3.
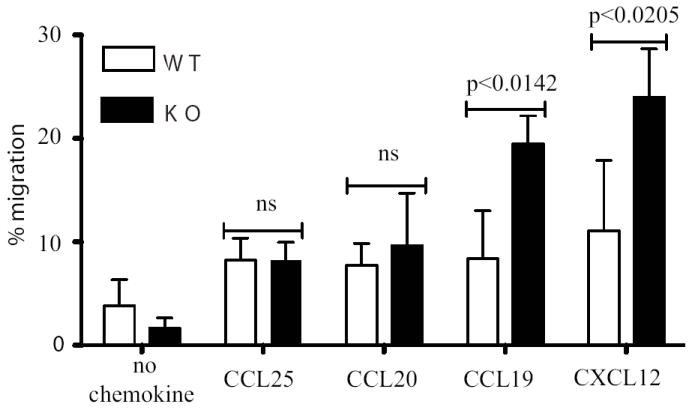
Migration of intestinal T cells (mean of three independent experiments) isolated from WT (open bars) or Rgs1-/- mice (dark bars) in response to different chemokines.
To determine if these effects extrapolated to a role for Rgs1 in intestinal immunopathology, three experiments in two housing facilities on two continents compared the colitogenic potentials of Rgs1-/- versus WT T cells after transfer to Rag2-/- mice (12). Invariably, colitis was ameliorated by Rgs1 deficiency, as judged by significantly less increase in colon weight/thickness and by histopathology (Fig 4A, B). Recipients of Rgs1-/- cells usually lost less weight (Supplemental Fig 2B) although as has been noted previously (13) overall weight loss does not correlate well with colitis in this system. Reduced disease did not reflect generally impaired engraftment, since by contrast to the gut, spleens of recipient mice showed comparable accumulation of Rgs1-/- and WT T cells (Supplemental Figure S2C).
Fig 4.
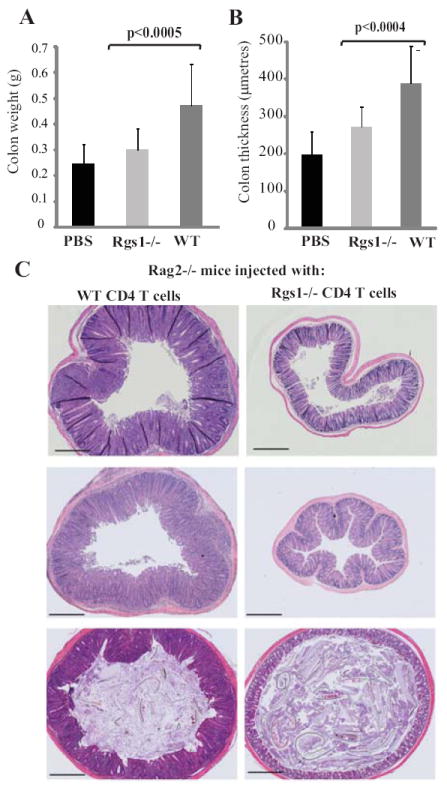
Rag2-/- mice were injected in three separate experiments with either PBS (n=6) or CD4+ CD45RBhi T cells extracted from WT (n=13) or Rgs1-/- mice (n=11). Colons were removed 7-8 weeks later, weighed and sectioned. Mean colon weight (A) and medial colon thickness (B) in recipients of WT (dark grey bars) or Rgs1-/- T cells (light grey bars) or PBS (black bars): statistics by Student’s T test. (C) Representative mid colon histologies at 7 weeks for recipients of WT (left) or Rgs1-/- cells (right). One colon for each group is shown from each of three independent experiments. Scale bar =500μm.
In sum, this first study of RGS1 biology in human T cells has identified overtly high expression in the gut; exaggerated expression in some IBD cases; a significant, non-redundant capacity to limit gut T cell responses lymphoid-homing chemokines, and a profound contribution to the colitogenic potential of T cells. These findings could be consistent with RGS1 ordinarily repressing T cell egress from the gut, possibly to sustain local immunoprotection and/or immunoregulation vis-à-vis commensals. Nonetheless, an analogous capacity to limit egress of inflammatory and/or autoimmune cells could clearly promote immunopathology. Such a key contribution of regulated T cell migration to pathology seems consistent with spontaneous colitis occurring in mice lacking Gαi2, that reportedly opposes the effects of RGS1 in B cells (14,15). Additionally, the selective importance of RGS1 is strongly suggested by evidence that mice transgenic for Rgs16, which does not affect responses to CCL19 (16,17) and which is not substantively expressed in the gut (data not shown), display T cell hyper-responsiveness (as do Gαi2 mutant mice) but no colon pathology.
Notwithstanding practical difficulties, it would seem appropriate to assess whether RGS1 is elevated in relevant tissue-associated T cells in T1D, MS, and celiac disease, all of which are associated with RGS1 SNPs (4, 6-8). That such SNPs have not yet been associated with IBD may merely reflect the complex, multi-factorial nature of IBD, and should not diminish the prospect of RGS1 regulating disease initiation and/or progression. Indeed, RGS1 has been highlighted as a biomarker for undifferentiated spondyloarthritis (18), often associated with gastrointestinal lesions (19). Clearly, RGS1 appears a valid, tissue-associated target for further biological and clinical investigation.
Supplementary Material
Acknowledgments
We thank T Chan for sample collection; R Ellis and T Hayday for flow cytometry; N Powell for assistance with colitis experiments; and Experimental Histopathology Unit at Cancer Research UK
We acknowledge financial support from: the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust; the Wellcome Trust; the Marie Curie fellowship programme; and the NIH.
References
- 1.Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006;17:363–376. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Shires J, Theodoridis E, Hayday AC. Biological insights into TCR gamma delta+ and TCR alpha beta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 3.Pennington DJ, Vermijlen D, Wise EL, Clarke SL, Tigelaar RE, Hayday AC. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv Immunol. 2005;87:27–59. doi: 10.1016/S0065-2776(05)87002-6. [DOI] [PubMed] [Google Scholar]
- 4.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, Romanos J, Dinesen LC, Ryan AW, Panesar D, Gwilliam R, Takeuchi F, McLaren WM, Holmes GK, Howdle PD, Walters JR, Sanders DS, Playford RJ, Trynka G, Mulder CJ, Mearin ML, Verbeek WH, Trimble V, Stevens FM, O’Morain C, Kennedy NP, Kelleher D, Pennington DJ, Strachan DP, McArdle WL, Mein CA, Wapenaar MC, Deloukas P, McGinnis R, McManus R, Wijmenga C, van Heel DA. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40:395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moratz C, Hayman JR, Gu H, Kehrl JH. Abnormal B-cell responses to chemokines, disturbed plasma cell localization, and distorted immune tissue architecture in Rgs1-/- mice. Mol Cell Biol. 2004;24:5767–5775. doi: 10.1128/MCB.24.13.5767-5775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–2777. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson BA, Wang J, Taylor EM, Caillier SJ, Herbert J, Khan OA, Cross AH, De Jager PL, Gourraud PA, Cree BC, Hauser SL, Oksenberg JR. Multiple sclerosis susceptibility alleles in African Americans. Genes Immun. 2010;11(4):343–350. doi: 10.1038/gene.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito F. IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis loci. Genes Immun. 2010;11:397–405. doi: 10.1038/gene.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundqvist C, Hammarstrom ML, Athlin L, Hammarstrom S. Isolation of functionally active intraepithelial lymphocytes and enterocytes from human small and large intestine. J Immunol Methods. 1992;152:253–263. doi: 10.1016/0022-1759(92)90147-l. [DOI] [PubMed] [Google Scholar]
- 10.Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, Kozasa T, Kehrl JH. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol. 2000;164:1829–1838. doi: 10.4049/jimmunol.164.4.1829. [DOI] [PubMed] [Google Scholar]
- 11.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci U S A. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1(7):553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin 23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33(2):279–88. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 15.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Estes JD, Thacker TC, Hampton DL, Kell SA, Keele BF, Palenske EA, Druey KM, Burton GF. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 17.Lippert E, Yowe DL, Gonzalo JA, Justice JP, Webster JM, Fedyk ER, Hodge M, Miller C, Gutierrez-Ramos JC, Borrego F, Keane-Myers A, Druey KM. Role of regulator of G protein signaling 16 in inflammation-induced T lymphocyte migration and activation. J Immunol. 2003;171:1542–1555. doi: 10.4049/jimmunol.171.3.1542. [DOI] [PubMed] [Google Scholar]
- 18.Gu J, Wei YL, Wei JC, Huang F, Jan MS, Centola M, Frank MB, Yu D. Identification of RGS1 as a candidate biomarker for undifferentiated spondylarthritis by genome-wide expression profiling and real-time polymerase chain reaction. Arthritis Rheum. 2009;60:3269–3279. doi: 10.1002/art.24968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlando A, Renna S, Perricone G, Cottone M. Gastrointestinal lesions associated with spondyloarthropathies. World J Gastroenterol. 2009;15:2443–2448. doi: 10.3748/wjg.15.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


