Abstract
Chagas’ disease is caused by Trypanosoma cruzi infection and is characterized by chronic fibrogenic inflammation and heart dysfunction. Chemokines are produced during infection and drive tissue inflammation. In rats, acute infection is characterized by intense myocarditis and regression of inflammation after control of parasitism. We investigated the role of CCL3 and CCL5 during infection by using DNA vaccination encoding for each chemokine separately or simultaneously. MetRANTES treatment was used to evaluate the role of CCR1 and CCR5, the receptors for CCL3 and CCL5. Vaccination with CCL3 or CCL5 increased heart parasitism and decreased local IFN-γ production, but did not influence intensity of inflammation. Simultaneous treatment with both plasmids or treatment with MetRANTES enhanced cardiac inflammation, fibrosis and parasitism. In conclusion, chemokines CCL3 and CCL5 are relevant, but not essential, for control of T. cruzi infection in rats. On the other hand, combined blockade of these chemokines or their receptors enhanced tissue inflammation and fibrosis, clearly contrasting with available data in murine models of T. cruzi infection. These data reinforce the important role of chemokines during T. cruzi infection but suggest that caution must be taken when expanding the therapeutic modulation of the chemokine system in mice to the human infection.
Keywords: Chemokines, CCR5, Trypanosoma cruzi, Myocarditis
1. Introduction
Chagas’ heart disease is caused by infection with Trypanosoma cruzi and is characterized by chronic fibrogenic inflammation and loss of function of the heart [1]. It is estimated that 200,000 new cases and 21,000 disease-associated deaths occur annually in Latin America [2]. Specific treatment of the infection with benznidazole is effective in the acute phase but not shown to halt progression of the chronic forms of Chagas’ disease [2].
Chemokines are a group of mediators of the inflammatory process thought to play an essential role in the recruitment and activation of leukocytes in various models of inflammatory diseases [3]. Specific chemokines are produced in tissue in response to T. cruzi infection and are crucial to define the leukocyte subtypes that compose the inflammatory infiltrate in the heart of infected animals [4–6]. CC-chemokines preferentially attract mononuclear cells to sites of chronic inflammation and mononuclear cells predominate in lesions of patients with Chagas’ disease and in experimental T. cruzi infection [7–9]. Although some studies have evaluated the role of chemokines in experimental T. cruzi infection [4,5,10], few studies have addressed the role of specific CC-chemokines, especially in the rat model. In rats, acute infection is characterized by intense myocarditis, with many mononuclear cells surrounding amastigote nests. Interestingly, high levels of IL-10 are found in the myocardium and myocarditis regresses after control of parasitism [6].
It has been shown that chemokine-encoding DNA vaccines are able to induce high titers of neutralizing antibodies against the targeted chemokine in rats [11,12,6]. For example, we have previously shown that Holtzman rats immunized with a CCL4/MIP-1β-encoding DNA vaccine had enhanced heart inflammation but unchanged heart parasite load when infected with T. cruzi. The latter study suggested that CCL4 could be recruiting regulatory cells to the infected tissue and, hence, regulating the intensity of the inflammatory response to the infection [6]. The aim of the present work was to study the role of the chemokines CCL3 and CCL5 in rats infected with T. cruzi. We also investigated the effect of MetRANTES treatment as this drug is a functional antagonist of the receptors at which CCL3 and CCL5 act; i.e. CCR1 and CCR5.
2. Materials and methods
2.1. Animals and infection
Holtzman rats, 90 days old and 300–400 g of weight, were obtained from CEBIO/UFMG (Minas Gerais, Brasil) and maintained in the animal facilities of the Laboratório de Imunofarmacologia, with filtered water and food ad libitum. Animals were infected intraperitoneally with 104 blood forms of the CL-Brener clone of T. cruzi per 50 g of body weight. All procedures had prior approval from the local animal ethics committee (CETEA/UFMG) and are in accordance with international guidelines for animal procedures. The myocardium was obtained in days corresponding to the acute (15, 20 and 30) and chronic (65) phases of infection, transversally sectioned and divided in 3 defined parts to detection of cytokines by ELISA, to histopathology and to collagen quantification of hydroxyproline, an indirect measurement of tissue fibrosis.
2.2. Rat CCL3/MIP-1α and CCL5/RANTES cloning and construction of the vaccination plasmid
The genes coding for CCL3 and CCL5 were amplified using primers to CCL3 and CCL5 by RT-PCR reactions in myocardial samples from acutely T. cruzi-infected rats using high-fidelity platinum Taq polymerase (GIBCO BRL). The signal peptide-encoding sequence to secretion present in the chemokine genes was identified by using the program “Signal Peptide” and specific primers to amplify the chemokine genes lacking signal-sequence to secretion were constructed, as the VR2001-TOPO DNA plasmid vector (Vical) has this sequence [13]. The PCR products were immediately cloned into TOPO TA cloning vector PCRII (Invitrogen) following the manufacturer’s specifications. The construction of vaccination plasmids was done as previously described [13]. After visualization of the PCR products on a 2% agarosis gel, we sequenced the positive PCR products using CEQ2000 DNA (Beckman Coulter). For the vaccine construct, we chose a sample that contained the entire sequence of CCL3 or CCL5 in the right orientation and in the correct open-reading frame after the tissue plasminogen activator signal peptide. Cells containing the CCL3 gene on VR2001 and CCL5 gene on VR2001 were grown overnight at 37 °C on 1L of Luria broth with kanamycin (100 µg/ml) and plasmid isolation was performed using Wizard Maxiprep kit. After plasmid isolation, the constructs containing VR2001-CCL3, VR2001-CCL5 and VR2001 alone (control) were washed three times with ultrapure water using an Amicon-100 (Millipore). The concentration of the samples was measured by UV absorbance.
2.3. Immunization
Immunizations with CCL3 or CCL5 constructs or control plasmid were performed by intradermal injection of 30 µg of DNA, suspended in 30 µl of sterile and apyrogenic ultrapure water, per immunization. In addition, some groups of rats were immunized with both CCL3 and CCL5 constructs (total of 60 µg of DNA). Intradermal immunization was made into the foot of rats after anesthesia with ketamine–xylazine. Boosters were given twice a week for 2 weeks – a total of 4 boosters. Two weeks after the last immunization, rats were infected as described above and euthanized at 20 (acute phase) or 65 days (chronic phase) after infection.
2.4. In vivo readout assay for screening the neutralizing activity of rat anti-CCL5 sera
Sera obtained from CCL5- or control plasmid-vaccinated animals (20 days after infection, n = 5) were pooled, diluted at 1:20 in PBS and given s.c. to rats. After 1 h, 300 ng of recombinant rat CCL5 (Peprotech) or sterile PBS was injected i. p. and animals killed 18 h later. Cells were harvested with 15 ml PBS, total cell counts performed in a Neubauer chamber using Turk’s stain and differential cell counts on cytospin preparations (Shandon III) stained with May–Grumwald–Giemsa using standard morphologic criteria to identify cell types. There was no effect of the sera on baseline (PBS-induced) recruitment of cells (data not shown).
2.5. Treatment with MetRANTES
MetRANTES (donated by Dr. Amanda Proudfoot, Merck-Serono Pharmaceuticals, Switzerland) is a modified form of human CCL5/RANTES that works as a functional antagonist of CCR1 and CCR5 receptors, the receptors at which CCL3 and CCL5 bind. Rats were treated subcutaneously with 150 µg of MetRANTES or saline daily, from days 10–20 after infection. Rats were euthanized at day 20.
2.6. Cytokine and chemokine detection by ELISA
A portion of the heart was processed in a solution containing protease inhibitors and centrifuged, as previously described [14]. The supernantant was used to investigate the concentration of the cytokines IFN-γ and IL-4 and the chemokines CCL3 and CCL5 by ELISA (Peprotech, USA), according to the instructions of the manufacturer.
2.7. Histopathological analysis
A portion of the heart was fixed in 4% buffered paraformaldehyde and 7 µm sections stained with hematoxylin and eosin to quantify inflammation and infection. Cardiac parasitism and inflammation were analyzed with a Zeiss integrating eyepiece with 100 hits (Öberkohen, Germany) at a final magnification of 320×. A total of 4000 hits were evaluated in each section of cardiac tissue. The infection and inflammation indices represent the number of hits covered by amastigote nests and inflammatory cells, respectively.
2.8. Hydroxyproline quantification
Fragments of 100 mg of myocardium were removed for hydroxyproline determination, as an indirect measure of collagen content [15]. Briefly, tissues were homogenized in saline 0.9%, frozen at −70 °C and lyophilized. The assay was performed with 20 mg of dry tissue, which was subjected to alkaline hydrolysis in 300 µl H2O plus 75 µl NaOH 10 M at 120 °C for 20 min. An aliquot of 50 µl of the hydrolyzed tissue was added to 450 µl of Chloramine T oxidizing reagent (Chloramine T 0.056 M, n-propanol 10% in acetate/citrate buffer pH 6.5) and allowed to react for 20 min. A hydroxyproline standard curve was prepared likewise. Color was developed by addition of 500 µl of 1 M p-dimethyl-aminebenzaldehyde diluted in n-propanol/perchloric acid 2:1 v/v. Absorbance was read at 550 nm.
2.9. Statistical analyses
Results are shown as means ± S.E.M.. Differences between groups were compared using Student’s t test (two sets of data) or one-way ANOVA (three or more sets of data), followed by Student–Newman–Keuls post hoc test. Differences were considered significant at p < 0.05.
3. Results
3.1. CCL3 and CCL5 are produced in the myocardium of T. cruzi-infected rats
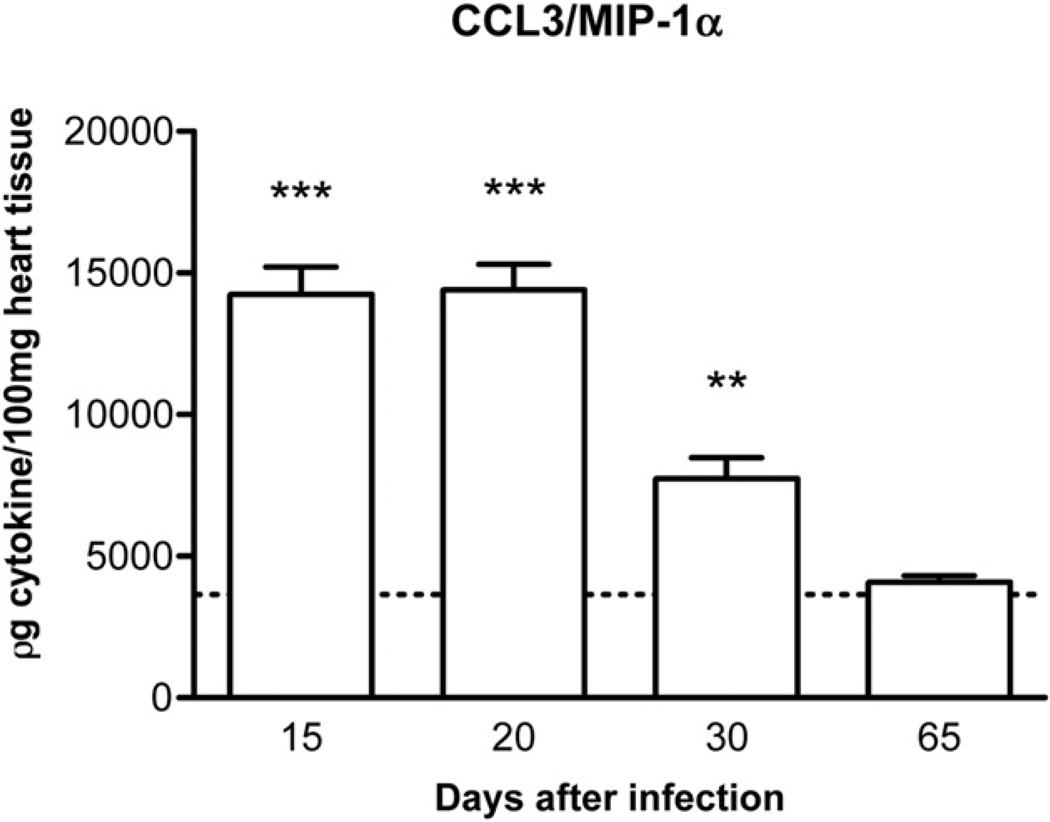
Initial experiments were conducted to characterize the kinetics of chemokine expression in infected rats. A previous study from our group has shown that the chemokine CCL5 was detectable in the myocardium of infected rats from day 15 till day 30 after infection. Levels dropped to background at 65 days after infection [6]. Likewise, CCL3 was already detectable in high levels in the myocardium of infected rats at day 15. Levels of CCL3 dropped by day 30 after infection and reached basal levels at day 65 (Fig. 1), a time at which infection and inflammation had subsided [6]. Based on the data above and on evaluation of tissue sections of infected animals, days 20 and 65 were used to study the acute and chronic phases of infection, respectively.
Figure 1.
Kinetics of CCL3 production in the cardiac tissue of T. cruzi-infected animals. The organs were excised and processed for the measurement of CCL3 by ELISA. The dotted line across the bars represents the background value in non-infected animals. Results are the means ± S.E.M. of 5–7 animals in each group. ** and *** for P < 0.01 and 0.001, respectively, when comparing infected versus non-infected animals.
3.2. Effects of CCL3- and CCL5-encoding DNA vaccines in T. cruzi-infected rats
Initial experiments evaluated the efficacy of the CCL3- and the CCL5-encoding vaccines. The administration of commercially available rat CCL3 failed to induce the recruitment of leukocytes in the peritoneal cavity of rats (data not shown). In order to define the efficacy of the vaccine at inhibiting CCL3 production in vivo, we measured CCL3 levels in the hearts of infected animals given CCL3 or control plasmids. Levels of CCL3 were significantly reduced in rats given CCL3 plasmid as compared to animals given control plasmid (Levels of CCL3 in the heart of infected rats: control plasmid-treated, 4517 ± 472 pg/100 mg heart tissue; CCL3 plasmid-treated, 2696 ± 228 pg/100 mg heart tissue, n = 6, P < 0.001).
We also analyzed the effect of a DNA vaccine encoding CCL5 in T. cruzi-infected rats. The efficacy of the CCL5-encoding DNA vaccination was evaluated by assessing the ability of sera from vaccinated rats to neutralize the chemo-attractant activity of CCL5. Sera from animals immunized with CCL5 DNA decreased the migration of leukocytes to the peritoneal cavity after injection of 300 ng of rat CCL5 when compared to treatment with sera from animals immunized with control plasmid (rat CCL5 + control plasmid serum, 3780 ± 877 × 104 cells/cavity; rat CCL5 + CCL5 plasmid serum, 1140 ± 311 × 104 cells/cavity; n = 5, P < 0.01).
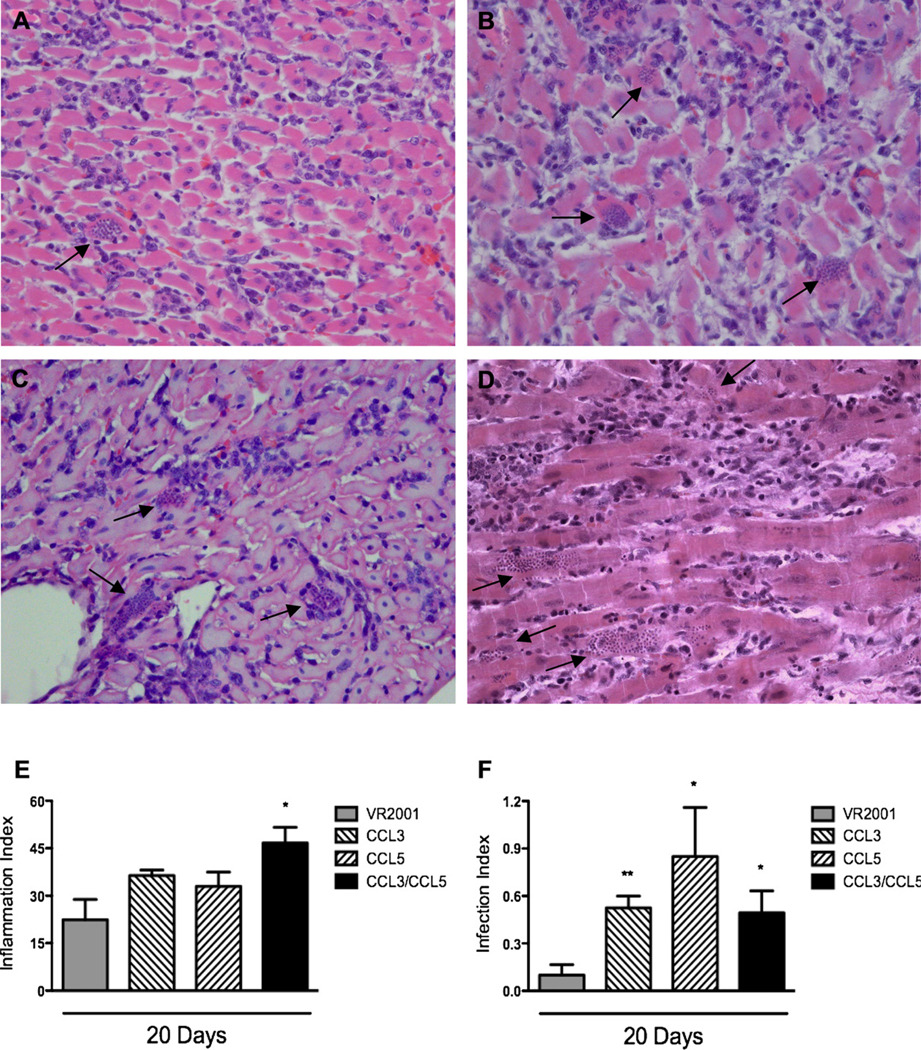
In T. cruzi-infected animals, myocarditis was clearly observed at day 20 after infection. Inflammatory lesions were predominantly characterized by mononuclear cells that infiltrated cardiac fibers (Fig. 2A). The inflammatory infiltrates were diffuse (Fig. 2A) and amastigote pseudocysts were present, accompanied or not by inflammatory cells (Fig. 2A). Inflammatory and infection indices were not different in T. cruzi-infected animals that received or not the control plasmid, indicating that vaccination with empty plasmid had no effect in the course of infection (data not shown).
Figure 2.
Histopathological alterations in myocardium of T. cruzi-infected animals previously immunized with control plasmid (A) or plasmids encoding for CCL3 (B), CCL5 (C) or both CCL3 and CCL5 (D). Myocardia were excised at days 20 (A–F) and 65 (E–F) after infection, included in paraffin, and stained with hematoxylin and eosin. The arrows show amastigote nests. Final magnification of 200×. Semi-quantitative analysis of Inflammation (E) and Infection (F) is also shown. A total number of 40 microscopic fields were analyzed per section, in a total of 4000 points. Inflammation and infection indices were quantified using an ocular containing 100 points/microscopic field in a final magnification of 320×. Results are the means ± S.E.M. of 5–7 animals in each group. * and ** for P < 0.05 and 0.01 respectively, when comparing chemokine- versus control plasmid-immunized animals.
In both CCL3- and CCL5-vaccinated infected animals, there was no significant increase of inflammation in the heart at day 20 after infection (Fig. 2B and C, respectively). The inflammatory lesions were also quantitatively similar to the ones found in control-plasmid vaccinated group (Fig. 2E). In contrast, number of amastigote nests was greater in both CCL3- (Fig. 2B and F) and CCL5-vaccinated animals (Fig. 2C and F) in comparison to control plasmid-vaccinated animals (Fig. 2A and F).
Next, we evaluated levels of the cytokines IFN-γ and IL-4 in the heart of infected rats. The administration of CCL3 and CCL5 plasmids was accompanied by a significant inhibition in the local production of IFN-γ (810 ± 200 pg/100 mg of tissue, plasmid-vaccinated group; 427 ± 191 pg/100 mg of tissue, CCL3-vaccinated group; 401 ± 54 pg/100 mg of tissue, CCL5-vaccinated group), while levels of IL-4 were similar to those of control plasmid-treated rats (4039 ± 1468 pg/100 mg of tissue, plasmid-vaccinated group; 3182 ± 912 pg/100 mg of tissue, CCL3-vaccinated group; 4448 ± 2327 pg/100 mg of tissue, CCL5-vaccinated group).
3.3. Effects of a cocktail of CCL3/CCL5-encoding DNA vaccines in T. cruzi-infected rats
To evaluate the effect of simultaneous blockade of CCL3 and CCL5 in T. cruzi-infected animals, we used a cocktail of plasmids containing CCL3 and CCL5 DNA in a separate group of rats. CCL3/CCL5-vaccinated rats had increased cardiac inflammation compared to control plasmid-vaccinated animals or animals vaccinated with either CCL3 or CCL5 plasmid separately (Fig. 2D and E). Multifocal inflammation, with high predominance of mononuclear cells was found in animals vaccinate with the combined vaccines (Fig. 2D). Moreover, enhanced heart parasitism was observed (Fig. 2F). However, administration of the combined CCL3/CCL5 vaccine did not enhance cardiac parasitism above that observed when either CCL3 or CCL5 vaccines were given alone (Fig. 2F). So, there was no synergistic or additive effect of CCL3 and CCL5 in terms of parasitism.
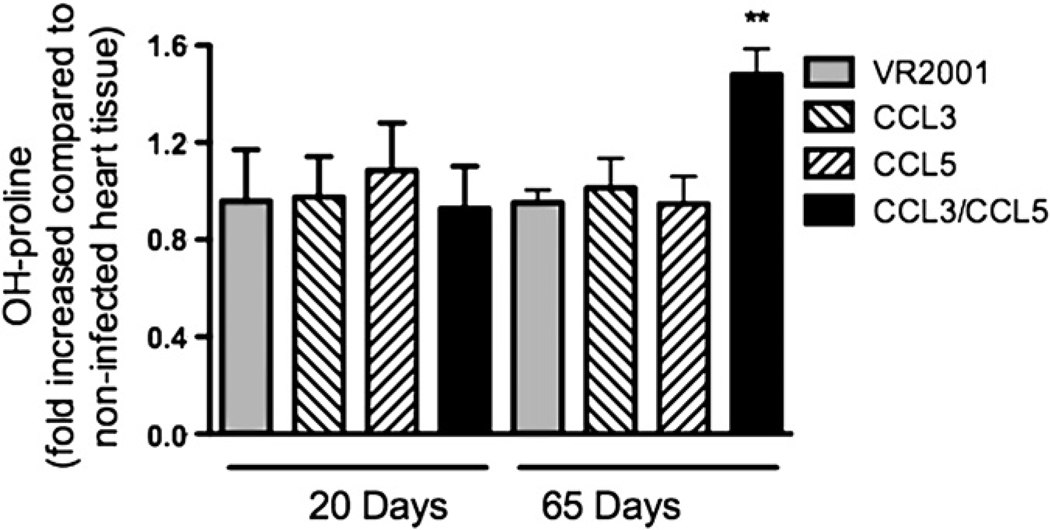
Collagen deposition in tissues and fibrosis are important characteristics of chronic T. cruzi infection [16]. Infection of rats with T. cruzi resulted in no increase of cardiac content of hydroxyproline, a marker of tissue collagen deposition, at days 20 or 65 after infection (Fig. 3). This is consistent with the idea that infection in rats usually develops without much chronic damage [17]. Vaccination with plasmids encoding for CCL3 or CCL5 resulted in no increase of hydroxyproline content at days 20 or 65 after infection. However, the combined vaccination with both CCL3 and CCL5 plasmids resulted in a significant increase of hydroxyproline levels at day 65 after infection (Fig. 3).
Figure 3.
Hydroxyproline content in myocardium of T. cruzi-infected animals previously immunized with control plasmid, or plasmids encoding for CCL3, CCL5 or both CCL3 and CCL5. Myocardia were excised at days 20 and 65 after infection. Hydroxyproline content was evaluated by colorimetric assay. Results are the means ± S.E.M. of 5–7 animals in each group. ** for P < 0.01 when comparing chemokine- versus control plasmid-immunized animals.
3.4. Effects of the treatment with MetRANTES in T. cruzi-infected rats
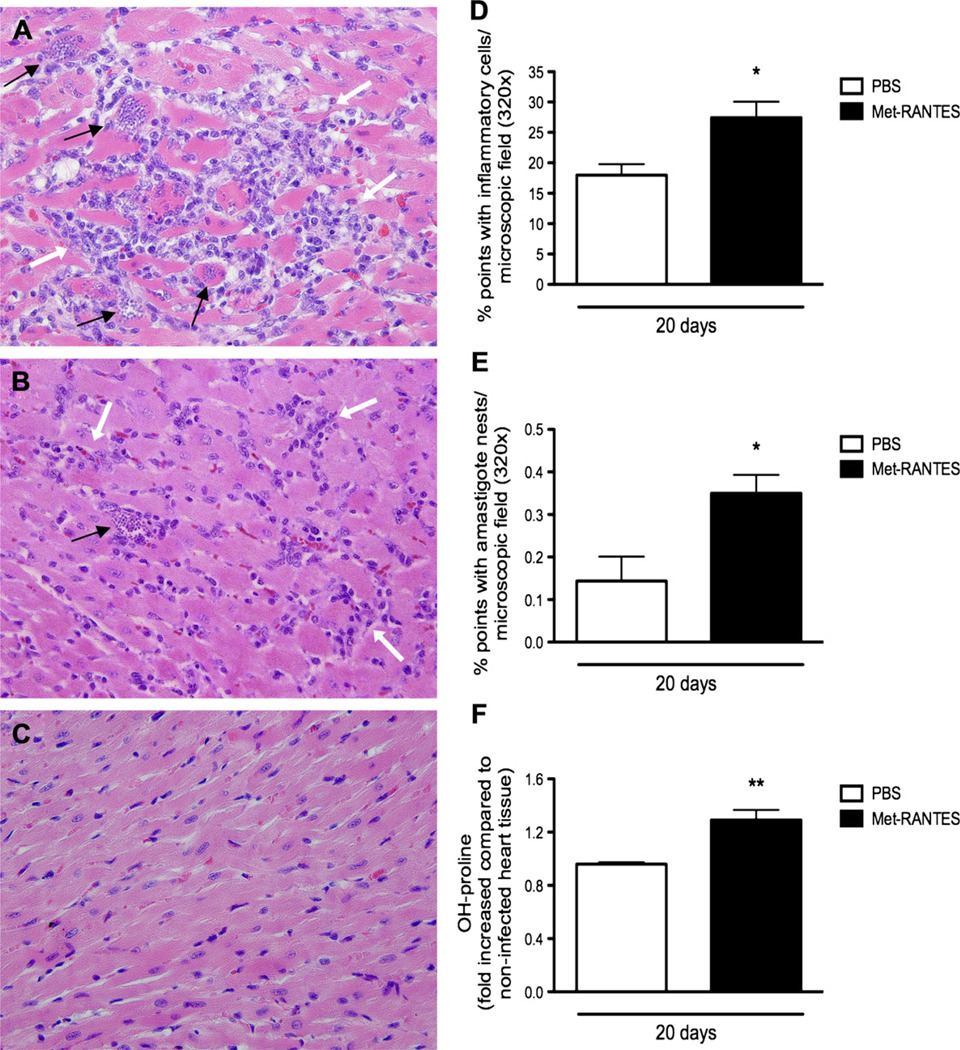
MetRANTES is a modified human CCL5/RANTES that acts in vivo as an inhibitor of CCR1 and CCR5, the receptors for CCL3 and CCL5. Overall, the inflammatory infiltrates in MetRANTES-treated rats were predominantly mononuclear and qualitatively similar to the inflammation found in vehicle-given animals (Fig. 4A and B). However, daily treatment with MetRANTES between days 10 and 20 after infection significantly increased inflammatory (Fig. 4A and D) and infection indices (Fig. 4A, E). The enhanced inflammatory response observed in treated animals was accompanied by significant higher collagen deposition in the heart of MetRANTES- than vehicle-treated rats, as assessed by hydroxyproline assay (Fig. 3F).
Figure 4.
Histopathology of myocardium from T. cruzi-infected animals treated with MetRANTES. The myocardium was excised and evaluated 20 days after infection. Animals were infected and treated with MetRANTES (150 µg/rat per day, subcutaneous) (black bar) or vehicle (open bar), once a day, between days 10 and 20 after infection. Hearts were included in paraffin and stained with hematoxylin and eosin in infected rats treated with vehicle (B) or MetRANTES (A) or in non-infected rats (C). The black arrows show amastigote nests, while the white arrows show inflammatory infiltrates at a final magnification of 200×. Inflammation (D) and infection (E) indices were quantified using an ocular containing 100 points/microscopic field in a final magnification of 320×. A total number of 40 microscopic fields were analyzed per section, in a total of 4000 points. Hydroxyproline content (F) was measured using a colorimetric assay. Results are the means ± S.E.M. of 5–7 animals in each group. * and ** for P < 0.05 and 0.01 respectively, when comparing MetRANTES versus vehicle-treated animals.
MetRANTES treatment reduced levels of IFN-γ in the heart of infected animals (3418 ± 1035 pg/100 mg of tissue, saline-treated group; 1911 ± 678 pg/100 mg of tissue, MetRANTES-treated group). IL-4 levels in the heart were also significantly reduced in MetRANTES-treated rats (1035 ± 238 pg/100 mg of tissue, saline-treated group; 697 ± 283 pg/100 mg of tissue, MetRANTES-treated group).
4. Discussion
The data reported in the present study show that CCL3 and CCL5 play a non redundant role in controlling the initial phases of T. cruzi replication in rats. This was evidenced by an increase in number of amastigote nests and reduction in levels of IFN-γ in the heart of animals vaccinated with DNA vaccines encoding for CCL3 or CCL5. Vaccination with a cocktail containing both CCL3 and CCL5 or treatment with MetRANTES resulted in greater parasitism that was of similar intensity to that observed in animals vaccinated with CCL3 or CCL5 alone. However, combined vaccination or treatment with MetRANTES resulted in an increase in the inflammatory response and collagen deposition in the heart of infected animals.
Previous data have shown the relevance of chemokines and chemokine receptors in controlling T. cruzi burden in vivo, including studies evaluating the role of CXCL9/CXCL10 [10], CCR2 [18] and CCR5 [5,19]. Treatment of T. cruzi-infected mice with anti-CCL5 and/or anti-CXCL10 antibodies did not alter T. cruzi burden in mice [10]. In contrast, CCL5 (or CCL3)-encoding DNA vaccination increased parasite burden in the heart of rats. The differences between these studies could reflect the use of different animal models/species (mice vs rats). Alternatively, the antibody schedule may have not been able to deplete completely the relevant ligand. It is clear, however, that single blockade of CCL3 or CCL5 is sufficient to impair temporarily the control of parasite replication in rats. The ability of chemokines to activate macrophages [20] and cardiomyocytes [21] to produce NO and kill T. cruzi may be an explanation for our in vivo observations.
An alternative explanation derives from the finding that there was lower production of IFN-γ in myocardium of CCL3- and CCL5-vaccinated rats. The role of CCL5 in inducing IFN-γ production and recruiting polarized type 1, activated T cells, has been previously described [22–24]. Administration of CCL3 may exacerbate inflammatory bowel disease in mice and this was associated with increased IFN-γ production by lymphocytes in lamina propria [25]. CCL3 and CCL5 may attract and activate type 1 polarized T cells, increasing IFN-γ production, which is important to control T. cruzi replication. CCR5-binding chemokines are postulated to have a role in recruiting polarized type 1 T cells [26]. Whatever the mechanism responsible for the decreased IFN-γ production, the diminished levels of this cytokine can provide an alternative explanation for the enhanced tissue parasitism observed after chemokine DNA vaccination.
It is of note that treatment with CCL3 or CCL5-encoding DNA did not modify total leukocyte influx in the heart of infected animals. However, when both CCL3 and CCL5 were blocked by using CCL3/CCL5-encoding DNA vaccines, inflammation was significantly enhanced. In rats vaccinated with a plasmid encoding CCL4, which binds solely to CCR5, exacerbated myocarditis was also found [27]. Similarly, increased parasitism, exacerbated myocarditis and increased collagen content were observed when rats were treated with MetRANTES. In contrast, MetRANTES treatment reduced T lymphocyte infiltration in the heart of T. cruzi-infected mice without affecting parasite load [4] and CCR5 deletion was also shown to reduce dramatically cardiac inflammation in mice [5]. The latter studies highlight the importance of CCR5-mediated events for leukocyte recruitment in murine, but not rat, models of T. cruzi infection. Therefore, whereas in the mouse, CCR5 appears to be essential for the recruitment of leukocytes to the heart, in rats, CCL3 and CCL5 and their receptors CCR1 and CCR5 appear not to be relevant for total leukocyte infiltration.
An important finding was that despite the initial increase of parasitism, control of infection was eventually attained in all rats. These results suggest that CCL3 and CCL5 are important but not essential in the system to control parasite replication. Alternatively, the degree of blockade attained with the DNA vaccination or MetRANTES treatment was not sufficient to blunt an anti-parasite response. For example, in mice, treatment with MetRANTES [4] or anti-CCL5 [10] had little effect on parasite burden. In contrast, experiments in CCR5-deleted mice showed that parasite levels were greatly enhanced [5]. Therefore, it is possible that partial CCR5 blockade is sufficient to control parasitism both in mice [4,10] and in rats (here). Despite the eventual control of parasitemia and tissue parasitism, there was an increase in cardiac fibrosis in rats that received the CCL3/CCL5 vaccine or were treated with MetRANTES. The results with MetRANTES once again contrast with the situation in mice where administration of MetRANTES significantly decreased fibrosis in chronically-infected animals [27]. In humans, CCR5 expression on peripheral blood mononuclear cells was decreased in patients with more severe disease and correlated with the degree of heart dysfunction [28]. The latter study suggests that loss of CCR5 correlates and may be important for heart dysfunction in patients. This is similar to our findings in rats in which administration of MetRANTES or treatment with CCL3/CCL5 vaccine led to worsening of disease. In that regard, results from mice suggesting that blockade/antagonism of chemokine receptors may be useful in chronically-infected patients should be taken with great care, as the disease in these animals may not mimic the human situation. This is not to say that rats are more useful than mice to study T. cruzi infection, but results do highlight the need to expand the immunopathology of experimental T. cruzi infection to species other than mice.
In summary, our data show that the chemokines CCL3 and CCL5 are relevant, but not essential, for the control of T. cruzi infection in rats. On the other hand, combined blockade of these chemokines or their receptors enhanced tissue inflammation and fibrosis, clearly contrasting with available data in murine models of T. cruzi infection [4,10]. Altogether these data reinforce the important role of chemokines in the context of T. cruzi infection but suggest that caution must be taken when expanding the therapeutic modulation of the chemokine system in mice to the human infection.
Acknowledgements
We are grateful to Rosana Oliveira Alves (CPqRR/Fiocruz, Minas Gerais, Brasil) for infecting the animals, to Dr. Geovanni D. Cassali (UFMG, Minas Gerais, Brasil) for helping with the histopathological description), and to Valdinéria Borges, Luíza Silva and Carlos Henrique Silva, by the technical support.
This work was supported by Conselho Nacional de Pesquisa (CNPq, Brazil), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Fogarty International Research Collaboration Award (NIH/FIRCA, 1 R03-TW006857-01A1, USA), and NIH (AI-076248, USA).
References
- 1.Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:44–54. doi: 10.2741/926. [DOI] [PubMed] [Google Scholar]
- 2.Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Marino APMP, Silva AA, Santos PVA, Pinto LMO, Gazzinelli RT, Teixeira MM, Lannes-Vieira J. Regulated on activation, normal T cell expressed and secreted (RANTES) antagonist (Met-RANTES) controls the early phase of Trypanosoma cruzi-elicited myocarditis. Circulation. 2004;110:1443–1449. doi: 10.1161/01.CIR.0000141561.15939.EC. [DOI] [PubMed] [Google Scholar]
- 5.Machado FS, Koyama NS, Carregaro V, Ferreira BR, Milanezi CM, Teixeira MM, Rossi MA, Silva JS. CCR5 plays a critical role in the development of myocarditis and host protection in mice infected with Trypanosoma cruzi. J. Infect. Dis. 2005;191:627–636. doi: 10.1086/427515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roffê E, Souza ALS, Caetano BC, Machado PP, Barcelos LS, Russo RC, Santiago HC, Souza DG, Pinho V, Tanowitz HB, Camargos ER, Bruña-Romero O, Teixeira MM. A DNA vaccine encoding CCL4/MIP-1beta enhances myocarditis in experimental Trypanosoma cruzi infection in rats. Microbes Infect. 2006;8:2745–2755. doi: 10.1016/j.micinf.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Reis DD, Jones EM, Tostes S, Jr, Lopes ER, Gazzinelli G, Colley DG, McCurley TL. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am. J. Trop. Med. Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi MD, Reis MM, Aiello VD, Benvenuti LA, Gutierrez PS, Bellotti G, Pileggi F. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am. J. Trop. Med. Hyg. 1997;56:485–489. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 9.Molina HA, Kierszenbaum F. Kinetics of development of inflammatory lesions in myocardial and skeletal muscle in experimental Trypanosoma cruzi infection. J. Parasitol. 1988;74:370–374. [PubMed] [Google Scholar]
- 10.Hardison JL, Wrightsman RA, Carpenter PM, Lane TE, Manning JE. The chemokines CXCL9 and CXCL10 promote a protective immune response but do not contribute to cardiac inflammation following infection with Trypanosoma cruzi. Infect. Immun. 2006;74:125–134. doi: 10.1128/IAI.74.1.125-134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youssef S, Wildbaum G, Maor G, Lanir N, Gour-Lavie A, Grabie N, Karin N. Long-lasting protective immunity to experimental autoimmune encephalomyelitis following vaccination with naked DNA encoding C–C chemokines. J. Immunol. 1998;161:3870–3879. [PubMed] [Google Scholar]
- 12.Youssef S, Maor G, Wildbaum G, Grabie N, Gour-Lavie A, Karin N. C–C chemokine-encoding DNA vaccines enhance breakdown of tolerance to their gene products and treat ongoing adjuvant arthritis. J. Clin. Invest. 2000;106:361–371. doi: 10.1172/JCI9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira F, Kamhawi S, Seitz AE, Pham VM, Guigal PM, Fischer L, Ward J, Valenzuela JG. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 14.Souza DG, Cara DC, Cassali GD, Coutinho SF, Silveira MR, Andrade SP, Poole SP, Teixeira MM. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy GK, Enwemeka CS. A simplified method for the analysis of hidroxyproline in biological tissues. Clin. Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 16.Pereira Barretto AC, Mady C, Arteaga-Fernandez E, Stolf N, Lopes EA, Higuchi ML, Bellotti G, Pileggi F. Right ventricular endomyocardial biopsy in chronic Chagas’ disease. Am. Heart J. 1986;111:307–312. doi: 10.1016/0002-8703(86)90144-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez LE, Silva VD, Lages-Silva E, Chapadeiro E. In: Doença de Chagas – Manual Para Experimentação Animal, Fiocruz/Instituto Oswaldo Cruz, Rio de Janeiro. Araújo-Jorge TC, Castro SL, editors. 2000. pp. 140–142. [Google Scholar]
- 18.Hardison JL, Kuziel WA, Manning JE, Lane TE. Chemokine CC receptor 2 is important for acute control of cardiac parasitism but does not contribute to cardiac inflammation after infection with Trypanosoma cruzi. J. Infect. Dis. 2006;193:1584–1588. doi: 10.1086/503812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardison JL, Wrightsman RA, Carpenter PM, Kuziel WA, Lane TE, Manning JE. The CC chemokine receptor 5 is important in control of parasite replication and acute cardiac inflammation following infection with Trypanosoma cruzi. Infect. Immun. 2006;74:135–143. doi: 10.1128/IAI.74.1.135-143.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliberti JC, Machado FS, Souto JT, Campanelli AP, Teixeira MM, Gazzinelli RT, Silva JS. Beta-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect. Immun. 1999;67:4819–4826. doi: 10.1128/iai.67.9.4819-4826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- 22.Makino Y, Cook DN, Smithies O, Hwang OY, Neilson EG, Turka LA, Sato H, Wells AD, Danoff TM. Impaired T cell function in RANTES-deficient mice. Clin. Immunol. 2002;102:302–309. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]
- 23.Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells. Blood. 2001;97:1144–1146. doi: 10.1182/blood.v97.4.1144. [DOI] [PubMed] [Google Scholar]
- 24.Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect. Immun. 2004;72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pender SL, Chance V, Whiting CV, Buckley M, Edwards M, Pettipher R, MacDonald TT. Systemic administration of the chemokine macrophage inflammatory protein 1alpha exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54:1114–1120. doi: 10.1136/gut.2004.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros GA, Silvério JC, Marino AP, Roffê E, Vieira V, Kroll-Palhares K, Carvalho CE, Silva AA, Teixeira MM, Lannes-Vieira J. Treatment of chronically Trypanosoma cruzi-infected mice with a CCR1/CCR5 antagonist (Met-RANTES) results in amelioration of cardiac tissue damage. Microbes Infect. 2009;11:264–273. doi: 10.1016/j.micinf.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Talvani A, Rocha MO, Ribeiro AL, Correa-Oliveira R, Teixeira MM. Chemokine receptor expression on the surface of peripheral blood mononuclear cells in Chagas disease. J. Infect. Dis. 2004;189:214–220. doi: 10.1086/380803. [DOI] [PubMed] [Google Scholar]