Abstract
The authors examined the association between menstrual characteristics and time to pregnancy among 2,653 Danish women enrolled in a prospective cohort study (2007–2009). Menstrual characteristics were reported at baseline. Outcome data were updated bimonthly until pregnancy, fertility treatment, loss to follow-up, or end of observation (12 cycles). Adjusted fecundability ratios and 95% confidence intervals were estimated by using discrete-time Cox regression models. Relative to average cycle lengths (27–29 days), fecundability ratios for cycle lengths <25, 25–26, 30–31, 32–33, and ≥34 days were 0.64 (95% confidence interval (CI): 0.49, 0.84), 0.94 (95% CI: 0.77, 1.13), 1.10 (95% CI: 0.97, 1.25), 1.35 (95% CI: 1.06, 1.73), and 1.17 (95% CI: 0.91, 1.49), respectively. Compared with cycles that regularized within 2 years after menarche, fecundability ratios for cycles that regularized 2–3 and ≥4 years after menarche were 0.90 (95% CI: 0.80, 1.02) and 0.89 (95% CI: 0.77, 1.03), respectively. Fecundability ratios were 0.87 (95% CI: 0.72, 1.05) comparing <3 with 3–4 days of menstrual bleeding and 0.70 (95% CI: 0.43, 1.13) comparing very heavy with moderate flow. In the present study, shorter cycle length was associated with delayed time to pregnancy. Age at menarche, time to menstrual regularization, and duration or intensity of menstrual flow were not appreciably associated with fecundability.
Keywords: cohort studies, fertility, menstrual cycle, menstruation, prospective studies
Menstrual cycle patterns are an indicator of endocrine function in reproductive-aged women (1). Variability in menstrual cycle length is determined largely by variation in follicular phase length (2, 3), with shorter cycles reflecting shorter follicular phase length. The most consistent predictor of cycle length is age: Cycles shorten with chronologic age (4) and then increase in variability at perimenopause (5, 6). Independent of age, however, short cycle length may be a marker of poor oocyte quality or abnormal hormonal patterns (7–11). Shorter cycles have been associated with reduced fecundability in 2 prospective studies of women with regular menstrual cycles (7, 12).
Duration and amount of menstrual bleeding may also serve as fertility indicators, reflecting growth of the endometrial lining and the occurrence of ovulation. Shorter bleeds may indicate unsuitable endometrium for implantation, and longer bleeds may indicate anovulatory cycles (1). In the 2 studies that examined duration of menstrual flow in association with fecundability, one reported that bleeds of average length (5 days relative to <5 or >5 days) preceded the most fecund cycles (7), and the other reported that women who conceived had longer-than-average bleeds (13). Neither study reported data on intensity of menstrual flow.
Anovulation and cycle irregularity are common in early menstrual life (14–17), and frequency of ovulation is related to both time since menarche and age at menarche (17–19). The overall trend is toward shorter and more regular cycles with increasing age (15, 20, 21), with a woman’s usual cycle length being established within 6 years of menarche (15, 21). Although cycle irregularity in adulthood is associated with anovulation and infertility (20, 22), it is unclear whether a history of cycle irregularity in early menstrual life—among women with regular cycles in adulthood—also predicts fertility potential across the reproductive years.
We examined the influence of selected menstrual characteristics—including menstrual cycle length, age at menarche, time from menarche to cycle regularity, duration, and intensity of menses—on time to pregnancy among Danish women enrolled in a prospective cohort study.
MATERIALS AND METHODS
Study population
The “Snart Gravid” Study is an Internet-based prospective cohort study of pregnancy planners in Denmark. The study methodology has been described in detail elsewhere (23–25). Briefly, recruitment commenced in June 2007 with placement of an advertisement on a health-related Web site (www.netdoktor.dk) and a coordinated media strategy involving radio, print media, online news sites, and television. Enrollment and primary data collection were conducted via a self-administered questionnaire on the study Web site (www.snart-gravid.dk).
Before enrollment, participants read a consent form and completed an online screening questionnaire to confirm eligibility. Eligible women were aged 18–40 years, residents of Denmark, in a stable relationship with a male partner, and not using any type of fertility treatment. Participants provided a valid e-mail address and their Civil Personal Registration number—a unique 10-digit personal identification number assigned to each resident by the Central Office of Civil Registration (26).
The baseline questionnaire collected information on demographics, reproductive and medical history, and lifestyle and behavioral factors. Participants were randomized to receive either a short- or a long-form baseline questionnaire, with similar completion rates for both versions (24). Follow-up questionnaires evaluated changes in various exposures, frequency of intercourse, and clinically recognized conception. Participants were contacted every 2 months by e-mail for 12 months or until recognized conception. Those who conceived were asked to complete 1 questionnaire during early pregnancy to assess changes in exposures, after which active follow-up was completed. Cohort retention after 12 months of follow-up was approximately 82% (25).
Assessment of menstrual cycle characteristics
On the baseline questionnaire, women reported their age at menarche (in whole years) and the age at which their menstrual periods became regular, defined as “usually being able to predict from one period to the next about when the next period would start.” Women also reported whether their cycles were currently regular and, if so, what their usual cycle length was when not using hormonal contraception (“number of days from the first day of one menstrual period to the first day of the next menstrual period”). A variable on “time to cycle regularity” was created by taking the difference between age at menarche and age at which menstrual cycles became regular. Women also reported their duration of menstrual flow (“How many days does your period usually flow (bleeding, not spotting)?”) in categories of “<3,” “3–4,” “5–6,” and “ >6” days and intensity of menstrual flow (“How would you classify the total amount of your menstrual flow?”) in categories of “light: ≤10 pads or tampons,” “moderate: 11–20 pads or tampons,” “heavy: 21–30 pads or tampons,” and “very heavy: >30 pads or tampons.”
Questions about current menstrual cycle length were added to bimonthly follow-up questionnaires on January 1, 2009, after which 501 women were enrolled (19% of the analytical sample). We compared self-reported cycle length at baseline with reports over follow-up among the women who provided follow-up data on cycle length (13% of the analytical sample). There was moderate agreement between the cycle length reported at baseline versus that at first follow-up (weighted kappa for 6 categories (k) = 0.61, Pearson’s correlation (r) = 0.61) or average of all follow-ups (k = 0.60, r = 0.60). Agreement was similar among those using nonhormonal and hormonal contraception as their last method of contraception (baseline vs. first follow-up: k = 0.62, r = 0.63; vs. k = 0.59, r = 0.59, respectively). Agreement was higher among those reporting 3–6 cycles versus ≤2 cycles of attempt time before study entry (baseline vs. first follow-up: k = 0.74, r = 0.69; vs. k = 0.54, r = 0.56, respectively), consistent with research showing higher accuracy of reporting of cycle length among less fertile women (27).
Assessment of pregnancy and cycles at risk
On each follow-up questionnaire, women reported the date of their last menstrual period, whether they were currently pregnant, and whether they had experienced any other pregnancies since the date of their last questionnaire, including miscarriage, induced abortion, or ectopic pregnancy. The total number of cycles at risk was calculated as follows: (months of attempt time at study entry/usual cycle length) + (((last menstrual period date from most recent follow-up questionnaire − date of baseline questionnaire completion)/usual cycle length) + 1), with observed cycles at risk (rounded to the nearest whole number) defined as those that were contributed after study entry.
Assessment of covariates
Data on age, weight, height, parity, smoking history, current alcohol consumption, last method of contraception, physical activity, and frequency of intercourse were self-reported on the baseline questionnaire and were updated every 2 months by follow-up questionnaire. We estimated the total metabolic equivalents of physical activity per week by summing the metabolic equivalents from moderate exercise (hours/week multiplied by 3.5) and vigorous exercise (hours/week multiplied by 7.0) (28). We calculated body mass index as weight (kg)/height (m)2. Self-reported height and weight among women who delivered babies conceived during our study showed excellent agreement with measures provided by the Danish Medical Birth Registry (29).
After 30 months of recruitment, 5,460 women were enrolled in the study. Of these, we excluded 1,063 women who had been trying to conceive for >6 cycles at study entry, 263 women with insufficient or implausible information about their last menstrual period date or date of first pregnancy attempt, 495 women who did not complete a follow-up survey, 863 reporting current irregular cycles, and 112 women with missing data on menstrual cycle length. After these exclusions, 2,653 regularly cycling women remained and were included in the present analyses. The 453 women who were subsequently lost to follow-up (mean follow-up of 5.4 months) had shorter menstrual cycles (mean: 28.3 vs. 28.7 days), lower parity (30.7% vs. 36.2%), higher body mass index (mean: 24.7 vs. 24.0 kg/m2), and heavier smoking histories (mean: 2.8 vs. 2.1 pack-years) than the 2,200 women who were followed to a study endpoint but were similar with respect to other characteristics (e.g., mean age: 28.4 vs. 28.7 years; mean age at menarche: both 12.9 years; and use of oral contraceptives as their last method of contraception: 59.4% vs. 59.3%).
Data analysis
We divided menstrual cycle length into the following categories: <25, 25–26, 27–29, 30–31, 32–33, and ≥34 days, using 27–29 days as the reference group because the modal value was 28 days. We categorized age at menarche and time to cycle regularity on the basis of their frequency distributions within the cohort. Duration and intensity of menstrual flow were categorized as asked on the baseline questionnaire. We examined the possibility of a nonlinear relation or threshold effect of each menstrual characteristic on fecundability by using restricted cubic splines (30, 31).
The fecundability ratio represents the cycle-specific probability of conception among the exposed divided by that among the unexposed. We used a discrete-time analog of the Cox proportional hazards model to estimate fecundability ratios and 95% confidence intervals for menstrual characteristics in association with time to pregnancy, in cycles (32). We evaluated time to any pregnancy, regardless of pregnancy outcome. Women were censored if they did not conceive after 12 cycles, the typical amount of time after which couples seek medical assistance for infertility (32, 33). Women contributed cycles at risk until they reached a study endpoint—pregnancy, use of fertility treatments, loss to follow-up, or the end of observation (12 cycles), whichever occurred first. The Cox model allowed for “delayed entry” into the risk set—which would have occurred if women entered the study after having tried to conceive for 1 or more cycles. Therefore, risk sets were based only on cycles at risk observed after study entry (29).
We selected potential confounders from a list of variables associated with menstrual characteristics at baseline that met criteria for confounding on the basis of a review of the literature and assessment of a causal graph (34). We then controlled for potential confounders that changed the adjusted fecundability ratio by more than 5% relative to the unadjusted fecundability ratio (34). According to these criteria, we controlled for female age (<25, 25–29, 30–34, ≥35 years), frequency of intercourse (<1, 1, 2–3, ≥4 times/week), pack-years of smoking (never smoked, <5, 5–9, ≥10 pack-years), body mass index (<20, 20–24, 25–29, ≥30 kg/m2), and last method of contraception (barrier methods, oral contraceptives, other hormonal contraceptives, natural family planning). Frequency of intercourse was modeled as a time-varying variable. To assess the effect of each menstrual characteristic independent of cycle length, we further controlled for cycle length in all models. We used multiple imputation methods to impute missing covariate values (35). In SAS software (SAS Institute, Inc., Cary, North Carolina), we used PROC MI to create 5 imputed data sets—including 68 covariates in the imputation (refer to the Web Appendix, which is posted on the Journal’s Web site (http://aje.oxfordjournals.org/))—and then combined results across the imputed data sets using PROC MIANALYZE (36).
In secondary analyses, we evaluated whether the associations were similar when pregnancy losses were excluded from the outcome definition. In these analyses, women who reported an abortion or ectopic pregnancy were censored at their estimated time to pregnancy (37). Analyses were also repeated among the 501 (19%) women enrolled in the study after January 1, 2009, the date we began asking women to report menstrual cycle length prospectively every 2 months over follow-up. We stratified by age, parity status, smoking, body mass index, last method of contraception, and number of cycle attempts before study entry. We assessed departure from the proportional hazards assumption by plotting the log-log survivor functions for each exposure variable in categorical form, where parallel log-log survivor curves indicated proportional hazards. We used SAS, version 9.1, statistical software for all analyses (36, 37).
RESULTS
The mean and median cycle lengths in our sample were 28.6 and 28.0 days, respectively (range: 18–55 days). Baseline characteristics of the study sample according to menstrual cycle length are presented in Table 1. Shorter cycle length was positively associated with physical activity, current smoking, pack-years of smoking, and having a mother who smoked during pregnancy. Longer cycle length was positively associated with time to cycle regularity and inversely associated with smoking, parity, and caffeine intake. Long duration of menstrual flow (≥7 days) was more prevalent among women with short and long cycles relative to those with average cycle lengths (27–29 days).
Table 1.
Baseline Characteristics of 2,653 Women According to Usual Menstrual Cycle Length, Denmark, 2007–2009
| Characteristica | Menstrual Cycle Length, days |
|||||||||||||||||
| <25 |
25–26 |
27–29 |
30–31 |
32–33 |
≥34 |
|||||||||||||
| No. | % | Mean | No. | % | Mean | No. | % | Mean | No. | % | Mean | No. | % | Mean | No. | % | Mean | |
| No. of women | 116 | 229 | 1,482 | 589 | 121 | 116 | ||||||||||||
| Age, years | 28.5 | 29.4 | 28.8 | 28.4 | 27.5 | 27.8 | ||||||||||||
| Partner’s age, years | 31.2 | 31.8 | 30.9 | 30.9 | 30.6 | 30.5 | ||||||||||||
| Age at menarche, years | 12.9 | 13.0 | 12.9 | 13.0 | 12.8 | 12.9 | ||||||||||||
| Body mass index, kg/m2 | 24.6 | 24.1 | 24.0 | 24.2 | 23.7 | 25.0 | ||||||||||||
| Physical activity, MET-hours/week | 27.9 | 26.2 | 24.3 | 25.4 | 24.4 | 23.7 | ||||||||||||
| No vocational training | 12.3 | 9.7 | 11.3 | 12.6 | 10.9 | 13.2 | ||||||||||||
| Parous | 32.4 | 30.8 | 35.8 | 36.1 | 41.0 | 27.7 | ||||||||||||
| Current regular smoker, yes | 18.1 | 16.2 | 13.2 | 11.5 | 11.4 | 9.4 | ||||||||||||
| Pack-years of ever smoking | 2.9 | 2.8 | 2.2 | 2.0 | 2.1 | 2.3 | ||||||||||||
| Mother smoked during pregnancy, yes | 39.8 | 37.1 | 32.8 | 36.8 | 30.3 | 29.7 | ||||||||||||
| Alcohol intake (current), drinks/week | 3.2 | 2.5 | 2.9 | 3.0 | 2.4 | 2.6 | ||||||||||||
| Caffeine intake (current), mg/day | 109.0 | 110.3 | 109.1 | 101.6 | 105.1 | 108.8 | ||||||||||||
| Frequency of intercourse, ≥4 times/week | 21.1 | 25.5 | 18.6 | 20.4 | 15.3 | 17.8 | ||||||||||||
| Last method of contraception | ||||||||||||||||||
| Barrier methods | 27.3 | 28.7 | 28.8 | 29.4 | 43.9 | 31.1 | ||||||||||||
| Hormonal contraceptives | 66.0 | 60.7 | 61.2 | 58.9 | 46.3 | 60.6 | ||||||||||||
| Withdrawal, charting, or other | 6.7 | 10.6 | 10.0 | 11.7 | 9.8 | 8.3 | ||||||||||||
| Time to cycle regularity, years | 2.2 | 2.2 | 2.2 | 2.6 | 3.1 | 3.9 | ||||||||||||
| Duration of flow ≥7 days | 4.5 | 3.5 | 2.9 | 4.1 | 5.1 | 4.2 | ||||||||||||
| Amount of flow heavy or very heavy | 17.3 | 15.9 | 17.9 | 19.7 | 16.4 | 17.3 | ||||||||||||
Abbreviation: MET, metabolic equivalent.
All characteristics, with the exception of age, are age standardized to the cohort at baseline.
Relative to average cycle lengths (27–29 days), multivariable fecundability ratios for cycle lengths of <25, 25–26, 30–31, 32–33, and ≥34 days were 0.64 (95% confidence interval (CI): 0.49, 0.84), 0.94 (95% CI: 0.77, 1.13), 1.10 (95% CI: 0.97, 1.25), 1.35 (95% CI: 1.06, 1.73), and 1.17 (95% CI: 0.91, 1.49), respectively (Table 2). The fecundability ratio comparing ages at menarche of ≥15 with 12–13 years was 0.88 (95% CI: 0.74, 1.05). Compared with cycles that regularized within 2 years after the onset of menses, fecundability ratios for cycles that regularized 2–3 years and ≥4 years after menarche were 0.90 (95% CI: 0.80, 1.02) and 0.89 (95% CI: 0.77, 1.03), respectively. Fecundability ratios were 0.87 (95% CI: 0.72, 1.05) comparing <3 with 3–4 days of menstrual bleeding and 0.70 (95% CI: 0.43, 1.13) comparing very heavy with moderate flow. There was no clear pattern of effect for amount and duration of menstrual flow, when considered jointly, in association with time to pregnancy.
Table 2.
Menstrual Cycle Characteristics and Time to Pregnancy, Denmark, 2007–2009
| No. of Pregnancies | No. of Cycles | Unadjusted Model |
Adjusted Modela |
|||
| FR | 95% CI | FR | 95% CI | |||
| Menstrual cycle length, days | ||||||
| <25 | 65 | 646 | 0.60 | 0.46, 0.78 | 0.64 | 0.49, 0.84 |
| 25–26 | 152 | 996 | 0.92 | 0.77, 1.11 | 0.94 | 0.77, 1.13 |
| 27–29 | 1,051 | 6,411 | 1.00 | Referent | 1.00 | Referent |
| 30–31 | 416 | 2,321 | 1.10 | 0.97, 1.25 | 1.10 | 0.97, 1.25 |
| 32–33 | 92 | 419 | 1.40 | 1.10, 1.78 | 1.35 | 1.06, 1.73 |
| ≥34 | 87 | 467 | 1.17 | 0.92, 1.50 | 1.17 | 0.91, 1.49 |
| Age at menarche, years | ||||||
| <12 | 245 | 1,424 | 1.04 | 0.89, 1.22 | 1.06 | 0.91, 1.24 |
| 12–13 | 1,056 | 6,420 | 1.00 | Referent | 1.00 | Referent |
| 14 | 376 | 2,158 | 1.07 | 0.94, 1.22 | 1.05 | 0.92, 1.20 |
| ≥15 | 186 | 1,258 | 0.89 | 0.75, 1.05 | 0.88 | 0.74, 1.05 |
| Time until cycle regularity, years | ||||||
| <2 | 933 | 5,365 | 1.00 | Referent | 1.00 | Referent |
| 2–3 | 592 | 3,719 | 0.90 | 0.80, 1.02 | 0.90 | 0.80, 1.02 |
| ≥4 | 338 | 2,176 | 0.89 | 0.78, 1.03 | 0.89 | 0.77, 1.03 |
| Duration of menses, days | ||||||
| <3 | 150 | 1,048 | 0.86 | 0.71, 1.04 | 0.87 | 0.72, 1.05 |
| 3–4 | 1,006 | 5,921 | 1.00 | Referent | 1.00 | Referent |
| 5–6 | 636 | 3,938 | 0.94 | 0.84, 1.05 | 0.92 | 0.82, 1.02 |
| ≥7 | 71 | 353 | 1.19 | 0.91, 1.56 | 1.14 | 0.87, 1.51 |
| Intensity of menstrual flow | ||||||
| Light (≤10 pads/tampons) | 449 | 2,814 | 0.94 | 0.83, 1.07 | 0.95 | 0.84, 1.09 |
| Moderate (11–20 pads/tampons) | 1,079 | 6,320 | 1.00 | Referent | 1.00 | Referent |
| Heavy (21–30 pads/tampons) | 313 | 1,936 | 0.91 | 0.76, 1.09 | 0.90 | 0.74, 1.08 |
| Very heavy (>30 pads/tampons) | 22 | 190 | 0.76 | 0.47, 1.22 | 0.70 | 0.43, 1.13 |
| Menstrual flow and duration | ||||||
| Light/moderate, <5 days | 1,052 | 6,263 | 1.00 | Referent | 1.00 | Referent |
| Light/moderate, ≥5 days | 104 | 706 | 0.84 | 0.66, 1.07 | 0.84 | 0.65, 1.09 |
| Heavy/very heavy, <5 days | 476 | 2,871 | 0.97 | 0.86, 1.10 | 0.95 | 0.84, 1.09 |
| Heavy/very heavy, ≥5 days | 231 | 1,420 | 0.94 | 0.77, 1.13 | 0.90 | 0.74, 1.09 |
Abbreviations: CI, confidence interval; FR, fecundability ratio.
Adjusts for age, body mass index, smoking, intercourse frequency, last method of contraception, and menstrual cycle length (when applicable).
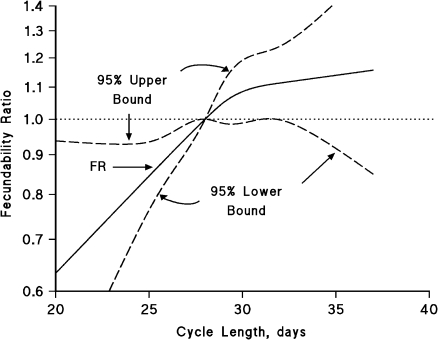
Figure 1 displays the association of menstrual cycle length with fecundability by using restricted cubic splines (38). The fecundability ratio increased monotonically with increasing cycle length (P = 0.31 comparing a linear model with a model containing both linear and spline terms), but the spline model indicated a possible larger influence on fecundability for a 1-unit change in cycle length among shorter cycles compared with longer cycles.
Figure 1.
Association between usual menstrual cycle length and fecundability, fitted by restricted cubic splines, Denmark, 2007–2009. The reference level for the fecundability ratio (FR) is a cycle length of 28 days. The curves are adjusted for age, pack-years of smoking, body mass index, intercourse frequency, and last method of contraception. The spline graph has 4 knot points located at 24, 28, 29, and 32 days.
The effect of short cycle length (<25 days) was relatively uniform across levels of age, smoking history, parity, body mass index, and attempt time at study entry (Table 3). Because menstrual cycles may take several months to “normalize” after cessation of hormonal contraceptives and because women who recently discontinued hormonal contraceptives may have difficulties reporting their menstrual characteristics, we also stratified by use of hormonal methods as their last method of contraception (Table 3). Results for short cycle length were similar for both groups. When we further stratified these results according to whether the hormonal contraceptive users had waited “a few months” after discontinuation before attempting to conceive, fecundability ratios (<25 vs. 27–29 days) were similar among the 254 women who waited (fecundability ratio = 0.58, 95% CI: 0.21, 1.28) and the 1,335 women who did not wait (fecundability ratio = 0.68, 95% CI: 0.48, 0.98) to conceive after discontinuation of hormonal methods.
Table 3.
Usual Menstrual Cycle Length and Time to Pregnancy, by Selected Factors, Denmark, 2007–2009
| Characteristic | Menstrual Cycle Length, days |
|||||
| <25 | 25–26 | 27–29 | 30–31 | 32–33 | ≥34 | |
| Age at baseline, years | ||||||
| <25 | ||||||
| Pregnancies, no. | 10 | 16 | 141 | 61 | 21 | 13 |
| Cycles, no. | 140 | 115 | 1,085 | 406 | 88 | 98 |
| FR (95% CI)a | 0.69 (0.35, 1.37) | 1.09 (0.62, 1.94) | 1.00 (Referent) | 1.20 (0.85, 1.69) | 1.83 (1.07, 3.15) | 0.99 (0.53, 1.85) |
| 25–29 | ||||||
| Pregnancies, no. | 34 | 70 | 487 | 196 | 45 | 46 |
| Cycles, no. | 218 | 395 | 2,831 | 962 | 195 | 236 |
| FR (95% CI)a | 0.88 (0.60, 1.30) | 1.06 (0.80, 1.40) | 1.00 (Referent) | 1.20 (1.00, 1.45) | 1.39 (0.97, 1.98) | 1.21 (0.86, 1.71) |
| ≥30 | ||||||
| Pregnancies, no. | 21 | 66 | 423 | 159 | 26 | 28 |
| Cycles, no. | 288 | 486 | 2,495 | 953 | 136 | 133 |
| FR (95% CI)a | 0.46 (0.29, 0.73) | 0.83 (0.62, 1.11) | 1.00 (Referent) | 0.98 (0.80, 1.21) | 1.13 (0.72, 1.77) | 1.26 (0.81, 1.95) |
| Smoking status | ||||||
| Current regular smoker | ||||||
| Pregnancies, no. | 14 | 27 | 183 | 68 | 19 | 10 |
| Cycles, no. | 162 | 239 | 1,175 | 440 | 85 | 61 |
| FR (95% CI)a | 0.58 (0.32, 1.04) | 0.74 (0.47, 1.15) | 1.00 (Referent) | 1.04 (0.76, 1.41) | 1.56 (0.89, 2.71) | 1.18 (0.58, 2.42) |
| Nonsmoker | ||||||
| Pregnancies, no. | 51 | 125 | 868 | 348 | 73 | 77 |
| Cycles, no. | 484 | 757 | 5,236 | 1,881 | 334 | 406 |
| FR (95% CI)a | 0.65 (0.48, 0.88) | 0.99 (0.80, 1.22) | 1.00 (Referent) | 1.11 (0.97, 1.28) | 1.32 (1.00, 1.74) | 1.17 (0.90, 1.52) |
| Parity status | ||||||
| Parous | ||||||
| Pregnancies, no. | 21 | 56 | 413 | 164 | 38 | 24 |
| Cycles, no. | 232 | 292 | 1,956 | 768 | 115 | 101 |
| FR (95% CI)a | 0.44 (0.27, 0.70) | 0.92 (0.67, 1.27) | 1.00 (Referent) | 0.99 (0.80, 1.22) | 1.64 (1.08, 2.48) | 1.14 (0.70, 1.84) |
| Nulliparous | ||||||
| Pregnancies, no. | 44 | 96 | 638 | 252 | 54 | 63 |
| Cycles, no. | 414 | 704 | 4,455 | 1,553 | 304 | 366 |
| FR (95% CI)a | 0.79 (0.57, 1.10) | 1.01 (0.80, 1.28) | 1.00 (Referent) | 1.16 (0.99, 1.36) | 1.27 (0.93, 1.74) | 1.24 (0.93, 1.65) |
| Body mass index (kg/m2) | ||||||
| <25 | ||||||
| Pregnancies, no. | 48 | 105 | 742 | 289 | 67 | 53 |
| Cycles, no. | 398 | 682 | 4,406 | 1,531 | 271 | 289 |
| FR (95% CI)a | 0.75 (0.55, 1.03) | 0.93 (0.74, 1.16) | 1.00 (Referent) | 1.13 (0.97, 1.32) | 1.41 (1.05, 1.89) | 1.10 (0.81, 1.51) |
| ≥25 | ||||||
| Pregnancies, no. | 17 | 47 | 309 | 127 | 25 | 34 |
| Cycles, no. | 248 | 314 | 2,005 | 790 | 148 | 178 |
| FR (95% CI)a | 0.47 (0.28, 0.78) | 0.92 (0.65, 1.29) | 1.00 (Referent) | 1.05 (0.83, 1.32) | 1.22 (0.77, 1.93) | 1.26 (0.84, 1.88) |
| Last method of contraception | ||||||
| Hormonalb | ||||||
| Pregnancies, no. | 42 | 88 | 635 | 241 | 44 | 54 |
| Cycles, no. | 432 | 614 | 4,092 | 1,394 | 196 | 285 |
| FR (95% CI)a | 0.66 (0.47, 0.92) | 0.96 (0.75, 1.22) | 1.00 (Referent) | 1.14 (0.97, 1.35) | 1.49 (1.05, 2.12) | 1.29 (0.94, 1.77) |
| Nonhormonal | ||||||
| Pregnancies, no. | 23 | 64 | 416 | 175 | 48 | 33 |
| Cycles, no. | 214 | 382 | 2,319 | 927 | 223 | 182 |
| FR (95% CI)a | 0.62 (0.40, 0.98) | 0.90 (0.67, 1.21) | 1.00 (Referent) | 1.04 (0.85, 1.28) | 1.22 (0.86, 1.73) | 1.00 (0.67, 1.49) |
| Cycle attempts before study entry | ||||||
| ≤2 | ||||||
| Pregnancies, no. | 51 | 124 | 806 | 292 | 65 | 55 |
| Cycles, no. | 463 | 713 | 4,566 | 1,529 | 268 | 294 |
| FR (95% CI)a | 0.66 (0.48, 0.89) | 1.00 (0.81, 1.23) | 1.00 (Referent) | 1.05 (0.90, 1.22) | 1.31 (0.97, 1.76) | 1.04 (0.77, 1.42) |
| 3–6 | ||||||
| Pregnancies, no. | 14 | 28 | 245 | 124 | 27 | 32 |
| Cycles, no. | 183 | 283 | 1,845 | 792 | 151 | 173 |
| FR (95% CI)a | 0.60 (0.34, 1.07) | 0.75 (0.49, 1.14) | 1.00 (Referent) | 1.20 (0.95, 1.53) | 1.40 (0.89, 2.18) | 1.37 (0.90, 2.09) |
Abbreviations: CI, confidence interval; FR, fecundability ratio.
Adjusted for age, body mass index, pack-years of smoking, intercourse frequency, and last method of contraception.
Includes oral contraceptives, hormonal implants, and hormonal injections.
The time-varying analysis of cycle length and time to pregnancy (TTP) among the 501 women (18.9%) who provided prospective data on cycle length produced results that were generally consistent with those found in the primary analysis, albeit the effect estimates were less precise: Fecundability ratios for cycle lengths of <25, 25–26, 30–31, 32–33, and ≥34 days, relative to 27–29 days, were 0.39 (95% CI: 0.18, 0.86), 0.90 (95% CI: 0.57, 1.41), 1.41 (95% CI: 1.05, 1.91), 1.33 (95% CI: 0.76, 2.33), and 1.49 (95% CI: 0.89, 2.51), respectively. Finally, results were similar when pregnancy losses were excluded from the outcome definition (data not shown).
It is possible that the results could have differed according to the use of home pregnancy tests, as women using such tests have a higher chance of detecting subclinical losses and reporting shorter TTPs than women having their pregnancies first confirmed by a physician. Data on home pregnancy tests were collected only from women reporting viable pregnancies. When we confined events to the 1,717 women using home pregnancy tests to confirm their pregnancies (96% of viable pregnancies), effect estimates were similar to those found in the primary analyses: Fecundability ratios for cycle lengths of <25, 25–26, 30–31, 32–33, and ≥34 days, relative to 27–29 days, were 0.62 (95% CI: 0.47, 0.83), 0.93 (95% CI: 0.77, 1.14), 1.08 (95% CI: 0.94, 1.23), 1.37 (95% CI: 1.06, 1.77), and 1.16 (95% CI: 0.90, 1.49), respectively.
DISCUSSION
In this prospective cohort study of Danish women aged 18–40 years, short cycle length (<25 days) was associated with delayed time to pregnancy. The effect of short cycle length persisted across categories of age, body mass index, parity, smoking history, and last method of contraception. Age at menarche, time to menstrual regularization, duration of menses, and intensity of menstrual flow were not appreciably associated with fecundability.
Our finding of delayed TTP among women with short cycle lengths agrees with previous retrospective (22) and prospective (7, 12) studies, with the exception of 1 retrospective study (20). Peak fecundability in our study (32–33 days) differed from that found in the 2 other prospective studies (30–31 days) (7, 12) and 1 retrospective cohort study (28 days) (22). In the latter study, both short (<28 days) and long (≥31 days) cycles were associated with reduced fecundability (22). Our data are also consistent with those of 2 studies of women undergoing fertility treatments in which a shorter follicular phase length was predictive of reduced fecundability (10, 11). To rule out the possibility that our results may be explained by chronologic aging as opposed to ovarian aging (or other hormonal factors), we stratified by age at baseline. Short cycle length was inversely associated with fecundability even among the youngest women in our cohort.
We found no clear pattern between duration of menses and fecundability, but results are difficult to compare across studies because of the variety of ways in which duration of menses was categorized. In a study by Small et al. (7), menses that were shorter or greater than 5 days preceded less fecund cycles relative to 5-day bleeds. This finding was partly consistent with a study conducted in rural Bolivia, in which those who conceived had longer menstrual bleeds in the preceding menstrual cycle than those who did not conceive (13). To our knowledge, there are no other studies investigating the joint effects of amount and duration of menstrual flow, or the effect of time to cycle regularity, on fecundability.
Studies that have examined the reliability of retrospectively assessed, self-reported menstrual cycle length against prospective menstrual diaries showed moderate levels of agreement, which were greater among sexually active women (27, 39, 40). In a subset of participants from our cohort (19%), we found moderate agreement between self-reported menstrual cycle lengths at baseline versus follow-up (k = 0.60). Results generated from a time-varying analysis of the 501 women who provided follow-up data on cycle length were consistent with primary results based on usual cycle length reported at baseline. Because not all women entered the study when they were first attempting to conceive, both differential and nondifferential misclassifications of menstrual characteristics was possible. However, the persistence of an association among women with ≤2 cycle attempts before study entry suggests that bias due to left truncation did not have a large influence on our results. Women who recently discontinued hormonal contraception may have experienced a change in menstrual cycle patterns, but our results were similar after accounting for the last method of contraception. Given that women with irregular cycles were excluded and that such women are more likely to have long cycles and experience delayed TTP (20, 22), the effect estimates for longer cycle length may be upwardly biased.
Nearly one quarter of early pregnancies are lost before clinical detection of conception (41). In our cohort, more than 96% of women with a viable pregnancy reported using home pregnancy tests to confirm their pregnancy, suggesting that bias due to differential recognition of early pregnancy loss was unlikely. Although rates of unintended pregnancy are considerably lower in Denmark compared with other developed countries (42), restriction of the cohort to pregnancy planners means that a nonnegligible fraction of total pregnancies was ignored. If pregnancy intention was related to both menstrual patterns and fertility potential, our results would not apply to women with unplanned pregnancies.
Cohort retention was similar to what has been reported for other large volunteer cohort studies (43, 44). The mean cycle length in our sample was consistent with that reported in other population studies (45–48), and similar menstrual patterns were found between the small proportion of women lost to follow-up and the women followed to a study endpoint, which implies minimal bias due to selective losses. Finally, although this study enrolled a self-selected sample of pregnancy planners recruited via the Internet, there is little reason to believe that such women would differ from the general population of women at risk of pregnancy in ways that would lead to biased effect estimates. Furthermore, 2 recent reports from Scandinavian birth cohort studies, in which population registry data were used to compare differences between study participants and all women giving birth in the general population, found that nonparticipation at the study outset had little impact on effect estimates (49, 50).
In summary, we found that women with shorter menstrual cycle lengths had reduced fecundability. The adverse effect of short cycle length on fecundability persisted across levels of age, which indicates that the effect is not solely attributable to aging. No clear pattern was found for age at menarche, time to menstrual regularization, and duration or intensity of menstrual flow in association with TTP. The exact biologic mechanism underlying the link between short menstrual cycles and fertility remains to be elucidated. Our findings suggest that menstrual cycle length may serve as a useful clinical marker of fertility potential, over and above that of chronologic age.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts (Lauren A. Wise, Kenneth J. Rothman, Henrik Toft Sørensen, Krista F. Huybrechts, Elizabeth E. Hatch); Slone Epidemiology Center at Boston University, Boston, Massachusetts (Lauren A. Wise); Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Ellen M. Mikkelsen, Anders H. Riis, Henrik Toft Sørensen); and RTI Health Solutions, Research Triangle Park, North Carolina (Kenneth J. Rothman).
This work was supported by the National Institute of Child Health and Human Development (R21-050264) and the Danish Medical Research Council (271-07-0338).
The authors would like to thank Tina Christensen for her support with data collection; media contact, Dr. Donna Baird, for her feedback on questionnaire development; and Thomas Jensen for his assistance with Web site design.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- TTP
time to pregnancy
References
- 1.Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev. 1995;17(2):265–286. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- 2.Waller K, Swan SH, Windham GC, et al. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147(11):1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- 3.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35(3):376–384. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 4.Lenton EA, Landgren BM, Sexton L, et al. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984;91(7):681–684. doi: 10.1111/j.1471-0528.1984.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, Barbieri RL, Xu H, et al. Determinants of basal follicle-stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79(4):1105–1109. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- 6.Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35(1):3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- 7.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17(1):52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- 8.Windham GC, Elkin E, Fenster L, et al. Ovarian hormones in premenopausal women: variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology. 2002;13(6):675–684. doi: 10.1097/00001648-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Klein NA, Battaglia DE, Fujimoto VY, et al. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81(3):1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 10.Check JH, Liss JR, Shucoski K, et al. Effect of short follicular phase with follicular maturity on conception outcome. Clin Exp Obstet Gynecol. 2003;30(4):195–196. [PubMed] [Google Scholar]
- 11.Fukuda M, Fukuda K, Andersen CY, et al. Characteristics of human ovulation in natural cycles correlated with age and achievement of pregnancy. Hum Reprod. 2001;16(12):2501–2507. doi: 10.1093/humrep/16.12.2501. [DOI] [PubMed] [Google Scholar]
- 12.Kolstad HA, Bonde JP, Hjøllund NH, et al. Menstrual cycle pattern and fertility: a prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertil Steril. 1999;71(3):490–496. doi: 10.1016/s0015-0282(98)00474-9. [DOI] [PubMed] [Google Scholar]
- 13.Vitzthum VJ, Spielvogel H, Caceres E, et al. Vaginal bleeding patterns among rural highland Bolivian women: relationship to fecundity and fetal loss. Contraception. 2001;64(5):319–325. doi: 10.1016/s0010-7824(01)00260-8. [DOI] [PubMed] [Google Scholar]
- 14.Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. American Academy of Pediatrics Committee on Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Pediatrics. 2006;118(5):2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care. 1986;7(4):236–244. [PubMed] [Google Scholar]
- 16.Venturoli S, Porcu E, Fabbri R, et al. Postmenarchal evolution of endocrine pattern and ovarian aspects in adolescents with menstrual irregularities. Fertil Steril. 1987;48(1):78–85. doi: 10.1016/s0015-0282(16)59294-2. [DOI] [PubMed] [Google Scholar]
- 17.Venturoli S, Porcu E, Fabbri R, et al. Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab. 1992;74(4):836–841. doi: 10.1210/jcem.74.4.1548348. [DOI] [PubMed] [Google Scholar]
- 18.Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab. 1983;57(1):82–86. doi: 10.1210/jcem-57-1-82. [DOI] [PubMed] [Google Scholar]
- 19.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20(1):231–236. doi: 10.1016/0022-4731(84)90209-7. [DOI] [PubMed] [Google Scholar]
- 20.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Widholm O, Kantero RL. A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand Suppl. 1971;14(suppl 14):1–36. [PubMed] [Google Scholar]
- 22.Jensen TK, Scheike T, Keiding N, et al. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen EM, Hatch EE, Wise LA, et al. Cohort profile: the Danish Web-based Pregnancy Planning Study—‘Snart-Gravid.’. Int J Epidemiol. 2009;38(4):938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ, Mikkelsen EM, Riis A, et al. Randomized trial of questionnaire length [letter] Epidemiology. 2009;20(1):154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- 25.Huybrechts KF, Mikkelsen EM, Christensen T, et al. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid.’. Eur J Epidemiol. 2010;25(5):297–304. doi: 10.1007/s10654-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank L Epidemiology. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 27.Jukic AM, Weinberg CR, Wilcox AJ, et al. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167(1):25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, et al. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Wise LA, Rothman KJ, Mikkelsen EM, et al. An Internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25(1):253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Hertzmark E, Louie M, et al. The SAS LGTPHCURV8 Macro. Boston, MA: Channing Laboratory; 2003. [Google Scholar]
- 32.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124(3):470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- 33.Bonde JP, Joffe M, Sallmén M, et al. Validity issues relating to time-to-pregnancy studies of fertility. Epidemiology. 2006;17(4):347–349. doi: 10.1097/01.ede.0000210239.80406.46. [DOI] [PubMed] [Google Scholar]
- 34.Greenland S, Rothman KJ. Introduction to stratified analysis: selecting confounders for control. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. New York, NY: Lippincott Williams & Wilkins; 2008. pp. 261–263. [Google Scholar]
- 35.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9-10):1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute, Inc. SAS/STAT User’s Guide. Version 9.1. Cary, NC: SAS Institute, Inc; 2004. [Google Scholar]
- 37.Joffe M, Key J, Best N, et al. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162(2):115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Hertzmark E, Louie M, et al. The SAS LGTPHCURV8 Macro. Boston, MA: Channing Laboratory; 2004. [Google Scholar]
- 39.Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007;17(3):163–170. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Bachand AM, Cragin LA, Reif JS. Reliability of retrospectively assessed categorical menstrual cycle length data. Ann Epidemiol. 2009;19(7):501–503. doi: 10.1016/j.annepidem.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 42.Jones EF, Forrest JD, Henshaw SK, et al. Unintended pregnancy, contraceptive practice and family planning services in developed countries. Fam Plann Perspect. 1988;20(2):53–67. [Google Scholar]
- 43.Russell C, Palmer JR, Adams-Campbell LL, et al. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154(9):845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- 44.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 45.Treloar AE, Boynton RE, Behn BG, et al. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 pt 2):77–126. [PubMed] [Google Scholar]
- 46.Chiazze L, Jr, Brayer FT, Macisco JJ, Jr, et al. The length and variability of the human menstrual cycle. JAMA. 1968;203(6):377–380. [PubMed] [Google Scholar]
- 47.Münster K, Schmidt L, Helm P. Length and variation in the menstrual cycle—a cross-sectional study from a Danish county. Br J Obstet Gynaecol. 1992;99(5):422–429. doi: 10.1111/j.1471-0528.1992.tb13762.x. [DOI] [PubMed] [Google Scholar]
- 48.Florack EI, Zielhuis GA, Rolland R. The influence of occupational physical activity on the menstrual cycle and fecundability. Epidemiology. 1994;5(1):14–18. doi: 10.1097/00001648-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 50.Nohr EA, Frydenberg M, Henriksen TB, et al. Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.