Abstract
During fission yeast mitosis, the duplicated spindle pole bodies (SPBs) nucleate microtubule arrays that interdigitate to form the mitotic spindle. cut12.1 mutants form a monopolar mitotic spindle, chromosome segregation fails, and the mutant undergoes a lethal cytokinesis. The cut12+ gene encodes a novel 62-kD protein with two predicted coiled coil regions, and one consensus phosphorylation site for p34cdc2 and two for MAP kinase. Cut12 is localized to the SPB throughout the cell cycle, predominantly around the inner face of the interphase SPB, adjacent to the nucleus. cut12+ is allelic to stf1+; stf1.1 is a gain-of-function mutation bypassing the requirement for the Cdc25 tyrosine phosphatase, which normally dephosphorylates and activates the p34cdc2/cyclin B kinase to promote the onset of mitosis. Expressing a cut12+ cDNA carrying the stf1.1 mutation also suppressed cdc25.22. The spindle defect in cut12.1 is exacerbated by the cdc25.22 mutation, and stf1.1 cells formed defective spindles in a cdc25.22 background at high temperatures. We propose that Cut12 may be a regulator or substrate of the p34cdc2 mitotic kinase.
Keywords: Mitosis, SPB, cut12, cdc25, MPF, S. pombe
Microtubule organizing centers (MTOCs) play a key role in nucleating microtubule assembly and organizing existing microtubules during the formation of a bipolar mitotic spindle (Kalt and Schliwa 1993). The onset of mitosis is preceded by MTOC duplication and maturation (Balczon 1996). Mitotic onset is accompanied by the nucleation of an increased number of highly dynamic microtubules from the MTOCs (Kirschner and Mitchison 1986; Hyman and Karsenti 1996). The increase in both the number and dynamics of microtubules nucleated by the MTOC is ultimately controlled by mitosis promoting factor (MPF), a dimer consisting of the p34cdc2 kinase subunit and a mitotic B-type cyclin (Nurse 1990). A fraction of cellular MPF has been localized to the MTOCs of organisms as diverse as yeast and man (Alfa et al. 1990; Bailly et al. 1989, 1992; Pines and Hunter 1991; Buendia et al. 1992; Maldonado-Codina and Glover 1992; Ohta et al. 1993; Pockwinse et al. 1997). The MPF component of Xenopus egg extracts is capable of indirectly activating the spindle microtubule nucleation capacity of the interphase spindle pole body (SPB) of permeabilized fission yeast cells (Masuda et al. 1992), and induces the increase in microtubule dynamics in vitro which is characteristic of the mitotic state (Belmont et al. 1990; Verde et al. 1990, 1992). The mitotic p34cdc2 kinase may promote bipolar spindle formation by directly phosphorylating and activating components of the spindle apparatus such as mitotic microtubule based motor proteins (Blangy et al. 1995). Alternately, p34cdc2 may phosphorylate and activate other kinases required for spindle formation, such as the NIMA (never in mitosis) kinase in Aspergillus nidulans (Ye et al. 1995). NIMA activity is essential for progression into mitosis even in the presence of active p34cdc2/cyclin B, and NIMA is phosphorylated and stimulated by p34cdc2 dependent phosphorylation in vitro (Ye et al. 1995, 1996). Thus, NIMA may act downstream of p34cdc2 to promote spindle formation at the onset of mitosis. In addition to p34cdc2 and the NIMA kinase, a family of conserved kinases termed the Polo-like kinases (Plks) play an essential role in bipolar spindle formation in a range of different eukaryotes (Glover et al. 1996). A human Plk is required for MTOC maturation (Lane and Nigg 1996) and a Xenopus Plk can phosphorylate and activate the MPF activator Cdc25 (Kumagai and Dunphy 1996). Thus, the Plks may act to promote the activation of p34cdc2 kinase and spindle formation upon maturation of the duplicated MTOCs (Lane and Nigg 1996).
The rapid changes in the organization of the microtubule cytoskeleton in Schizosaccharomyces pombe that accompany entry into mitosis are similar to those seen in higher eukaryotes and make the fission yeast an excellent model system for the study of mitotic spindle formation (Tanaka and Kanbe 1986; Hagan and Hyams 1988; Ding et al. 1993; Hagan and Yanagida 1997; Saito et al. 1997). Like its higher eukaryotic equivalent, the centrosome, the fission yeast SPB is excluded from the nucleus during interphase (Ding et al. 1997). In prophase, the duplicated SPBs gain access to the chromosomes through a restricted opening of the nuclear envelope and begin to nucleate spindle microtubules (Ding et al. 1997). At the onset of anaphase, the spindle microtubules decrease in number as they increase in length, and the opening in the nuclear envelope adjacent to the SPB begins to close (Ding et al. 1993, 1997). After anaphase, spindle microtubules disassemble, the SPB returns to the cytoplasm, and cytoplasmic microtubule arrays are re-established from MTOCs at the cell equator (Hagan and Hyams 1988; Horio et al. 1991; Pichova et al. 1995) and from the outer face of the SPB (Hagan and Yanagida 1997).
Here we report the characterization of the cut12+ spindle formation gene and its product. In the temperature sensitive cut12.1 mutant only one of the two structures recognized by SPB specific antibodies nucleates microtubules, leading to monopolar spindle formation and asymmetric chromosome segregation. The cut12+ gene encodes a novel 62-kD protein that is essential for bipolar spindle formation and localizes to the SPB throughout the cell cycle. The cut12+ locus is allelic to a previously identified mutation termed stf1.1 for suppressor of cdc twenty-five (Hudson et al. 1990). stf1.1 is a semidominant mutation that bypasses the requirement for Cdc25, a normally essential tyrosine phosphatase that removes an inhibitory phosphate from p34cdc2 (Hudson et al. 1990). We discuss the implications of these findings for spindle formation and cell cycle control.
Results
Isolation and characterization of the cut12.1 mutant
A screen for temperature-sensitive mutants that increase in ploidy at the restrictive temperature has identified several mitotic mutants that undergo asymmetric chromosome segregation such as the temperature-sensitive mutant cut12.1 (Broek et al. 1991; Hayles et al. 1994; A.M. Poziemba, A. Woollard, R.A. Craven, P. Nurse, and I. Hagan, unpubl.). To determine the precise nature of the defect in cut12.1 an asynchronous culture of cut12.1 was shifted from 25°C to the restrictive temperature of 36°C and stained with antibodies against the SPB component Sad1 (Hagan and Yanagida 1995) and tubulin (Woods et al. 1989). At 36°C, the cut12.1 mutant entered mitosis but formed defective mitotic spindles. Although two Sad1 foci were often visible, only one of the two presumptive SPBs in each cell retained all the functions necessary to nucleate microtubules (Fig. 1, cells 1 and 2). The inactive Sad1 focus stained less intensely, and in extreme cases, microtubules arose from a point devoid of Sad1 (Fig. 1, cell 2). The differential staining of the two Sad1 foci is illustrated most clearly in a merged false color immunofluorescence image (Fig. 1g). Hence, the spindles formed in the cut12.1 mutant were monopolar, and the condensed chromatin remained in a single unsegregated mass. In spite of this defect, cells proceeded through cytokinesis, a so-called cut division, generating aneuploid (Fig. 1, cell 3) and diploid cells (Fig. 1, cell 4). As the Sad1 foci were inactive, they were randomly segregated by the central cut division.
Figure 1.
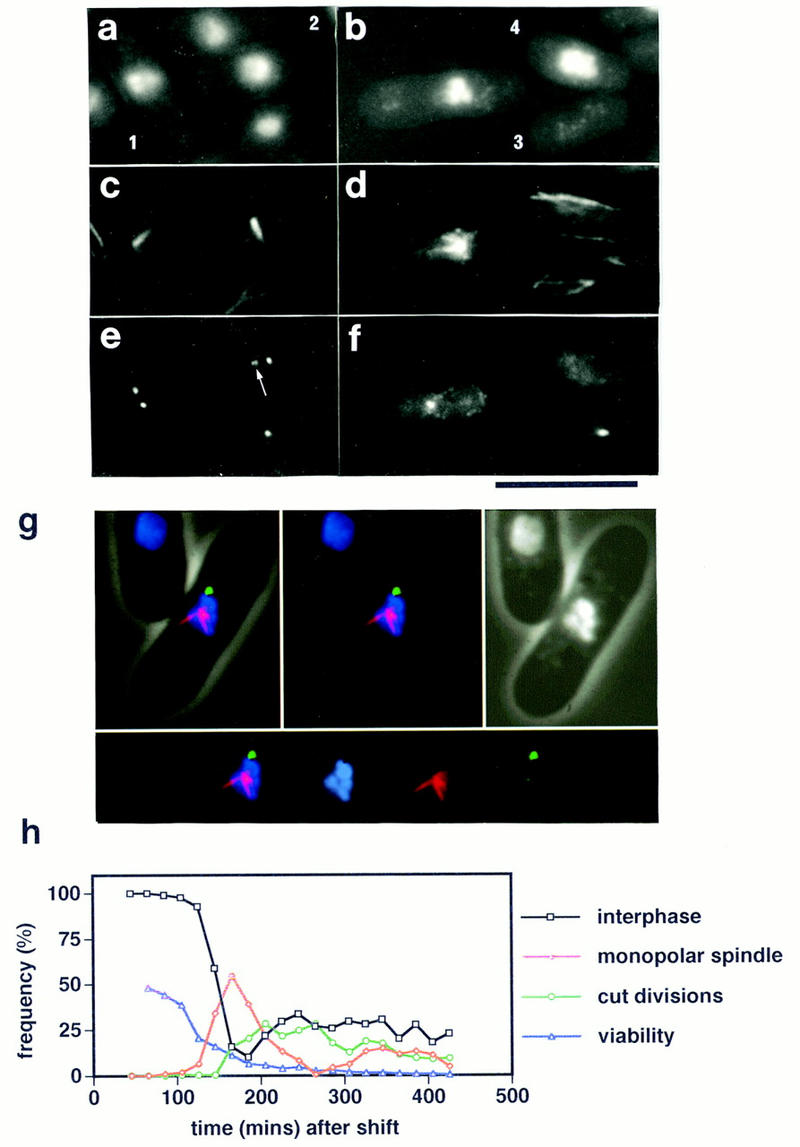
Monopolar mitotic spindle formation in the cut12.1 mutant. (a–f) Immunofluorescence analysis of an asynchronous culture of the cut12.1 mutant at restrictive temperature of 36°C. (a,b) phase/DAPI reveals cell outline and chromatin; (c,d) anti-tubulin staining; (e,f) anti-Sad1 staining. Monopolar mitotic spindles arose from one of the two foci of Sad1 staining (cells 1 and 2). The microtubules normally arose from the less bright staining of the two Sad1 foci (indicated by an arrow in cell 2). Asymmetric segregation of the chromatin and Sad1 staining material led to the formation of aneuploid cells containing Sad1 (cell 3) and diploid cells that lack any detectable Sad1 staining (cell 4). Scale bar, 10 μm. (g) False color immunofluorescence image of a defective monopolar spindle in the cut12.1 mutant. Microtubules are shown in red, Sad1 staining in green, and chromatin in blue. (Top left) Merged image reveals cell outline and spindle structures; (top middle) spindle structure alone; (top right) phase/DAPI staining; (bottom) individual spindle components. The microtubules arose from the less bright staining focus of Sad1 staining. (h) Time course of the loss of viability and the appearance of monopolar spindles and cut divisions in a population of the cut12.1 mutant grown at 25°C, synchronized in early G2 by lactose gradient centrifugation, and then shifted to 36°C. Note that the loss of viability precedes the appearance of defective spindles and cut divisions.
To examine the phenotype of the cut12.1 mutant in more detail, an early G2 population of cut12.1 cells was isolated at 25°C by lactose gradient centrifugation, and an aliquot was shifted to 36°C before samples were taken every 20 min for 6 hr for immunofluorescence. The population of cells entered a synchronous mitosis at both permissive and restrictive temperature, but formed monopolar mitotic spindles at the restrictive temperature and underwent cut divisions. The relative timing of appearance of these phenotypes is shown in Figure 1h. The loss of viability of the cut12.1 mutant on shift to the restrictive temperature preceded the appearance of defective spindles and cut divisions, indicating that the defect in cut12.1 is not reversible or that the Cut12 protein is required in some process prior to spindle formation.
Cloning and sequence analysis of the cut12+ gene
The construction of a heterozygous cut12+/cut12.1 diploid showed that cut12.1 is a recessive mutation (data not shown). The cut12+ gene was therefore cloned by complementation of the temperature-sensitive defect (Fig. 2a). The cut12+ gene encoded a 548 amino acid protein of 62 kD, with no overall homology to known proteins (Fig. 2b). The protein was found to be relatively hydrophilic and basic (pI of 9.4). Two regions (from amino acids 259 to 311 and 521 to 548) were predicted to form coiled coils according to the algorithm of Lupas et al. (1991). Amino acids 61–64 represented a potential phosphorylation target site for p34cdc2 kinase (TPLK; Nigg 1993), which also matched one of the two proposed MPM2 kinase consensus sites (LTPLK; Westendorf et al. 1994). Two potential MAP kinase phosphorylation sites from amino acids 73–76 (LKTP) and 516–519 (LKSP) were also identified (Nigg 1993). The cut12.1 mutation is a single base change in the first nucleotide of codon 536 (CGA to TGA), which would be predicted to produce a truncated form of Cut12 lacking the carboxy-terminal 13 amino acids of the protein. As the minimum length required for the formation of a stable coiled coil is thought to be 28 amino acids (Lupas et al. 1991), the cut12.1 allele would be predicted to encode a protein lacking a coiled coil domain at its carboxyl terminus (Fig. 2c).
Figure 2.
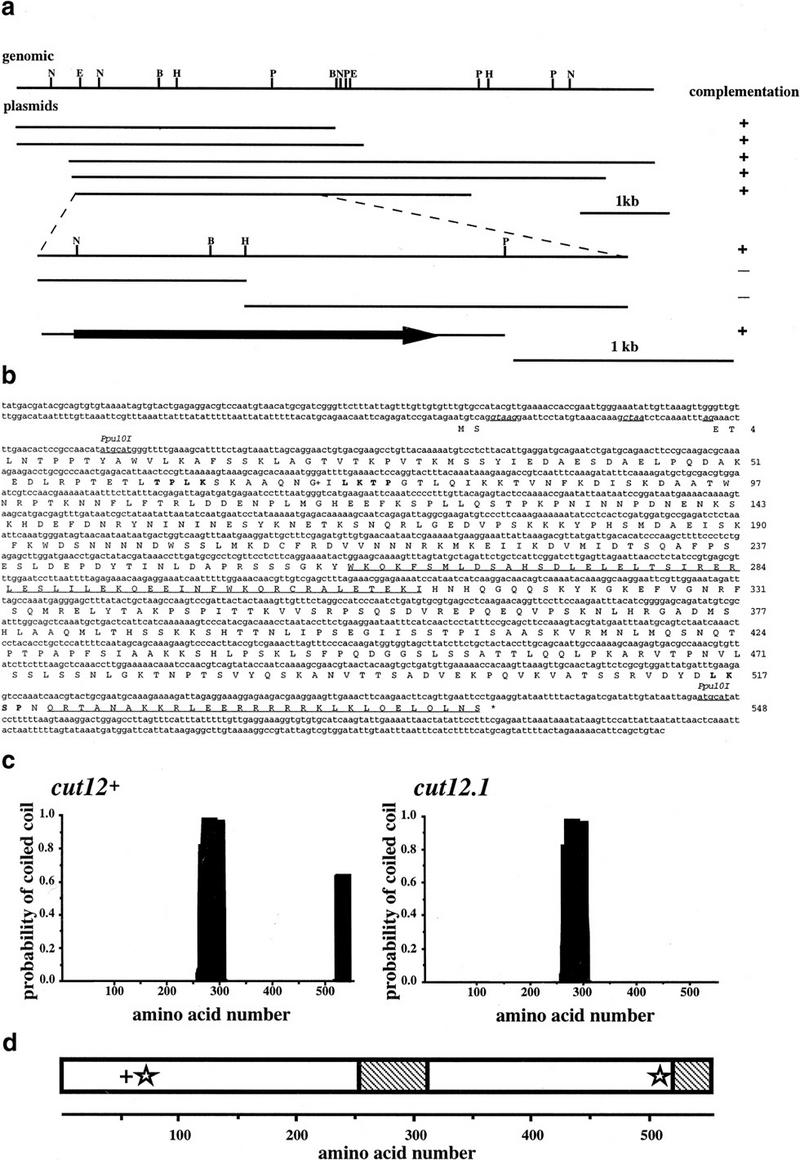
Cloning and molecular analysis of the cut12+ gene. (a) Restriction mapping and complementation analysis of the cut12+ containing clones isolated by complementation of the cut12.1 mutant, and of subclones generated from the overlapping 2.8-kb region found in all cut12.1 complementing plasmids. (b) Nucleotide and predicted amino acid sequence of the cut12+ gene. The cut12+ gene contained 2 exons of 7 and 1637 nucleotides, which together encode a predicted 548 amino acid protein of 62 kD. The two exons are separated by an intron of 42 bp; the consensus intron splice and branch sequences are shown underlined and in italics. The two regions of the Cut12 protein predicted to form coiled coils are underlined. The potential p34cdc2 and MAP kinase consensus sites are indicated in boldface type. (G+) The site of the stf1.1 mutation at glycine 71. The GenBank accession no. for the cut12+ sequence is Y16837. (c) Predicted coiled–coil regions in the Cut12 protein were determined by applying the algorithm of Lupas et al. (1991) with a 28 amino acid window. (d) Schematic representation of the main features of the Cut12 protein. (+) p34cdc2 kinase consensus site; (star) MAP kinase consensus site; (hatched region) predicted region of coiled coil.
cut12+ gene deletion results in defective spindles and cut divisions
The formation of monopolar spindles in the cut12.1 mutant may be caused by the presence of sufficient Cut12 activity to allow activation of one of the two SPBs. Therefore, we examined the effect of a complete loss of Cut12 protein. One copy of the cut12+ gene was deleted in a diploid by replacing all of the coding region between the Ppu10I sites (Fig. 2b) with the ura4+ sequence of S. pombe, thereby removing all save the first 32 nucleotides of the gene and an additional 41 nucleotides downstream of the gene. Tetrad analysis demonstrated that the cut12+ gene is essential for viability at all temperatures tested from 20°C to 36°C (data not shown).
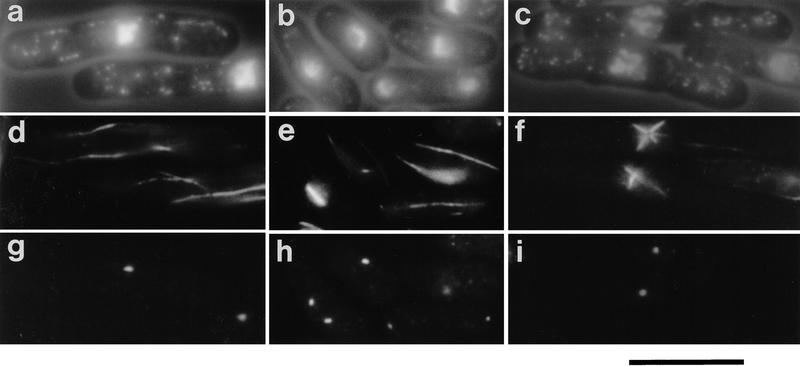
The cellular phenotypes resulting from deletion of cut12+ were examined by spore germination (Fig. 3a–c). Germinating Ura+ spores entered mitosis and disassembled cytoplasmic microtubules and condensed their chromosomes. However, the cut12.d1 spores formed defective mitotic spindles in which microtubules were not associated with either of the two equally intense Sad1 foci. The position of the origin of spindle microtubules is indicated by an arrow in each of the three cells in Figure 3c, and clearly does not correlate with the position of the Sad1 staining foci.
Figure 3.
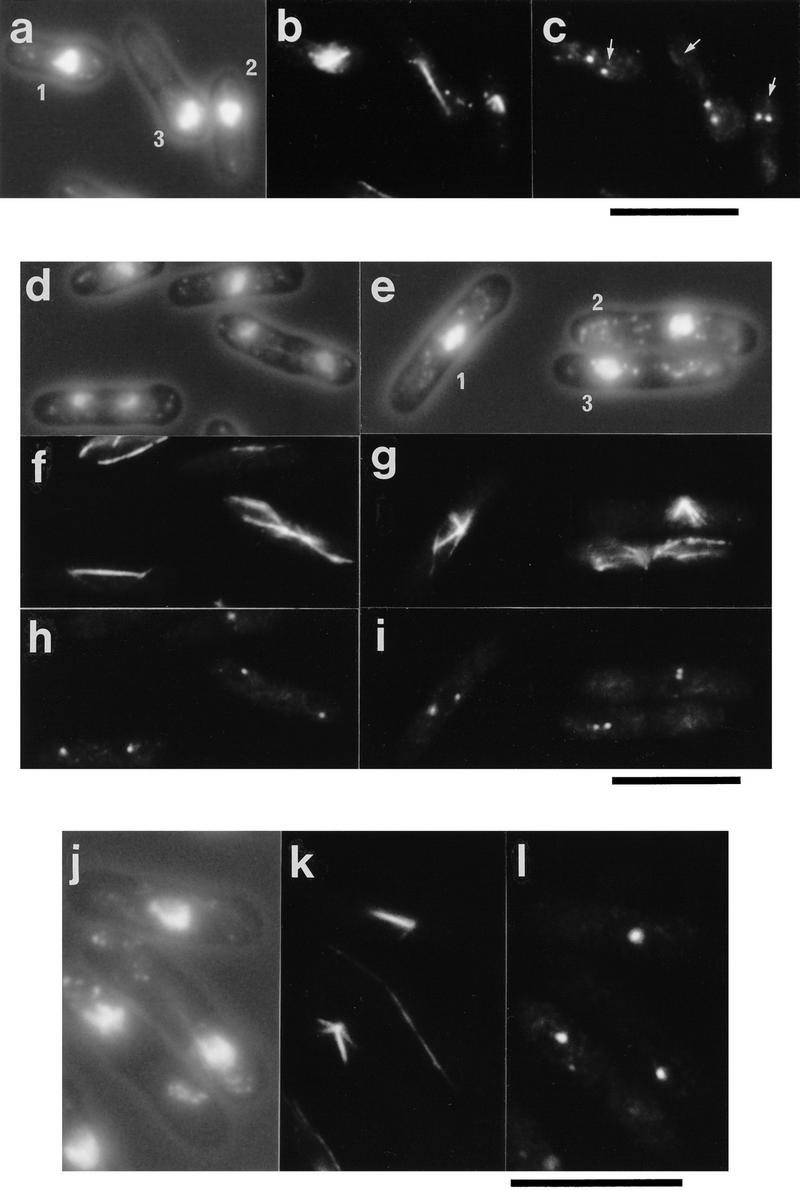
Defective spindle formation in cells deleted for the cut12+ gene and cells overexpressing cut12+. (a–c) Defective spindle formation and cut divisions in germinating cut12.d1 spores. Haploid spores prepared from a cut12+/cut12.d1 diploid were germinated in minimal medium lacking uracil at 30°C and processed for immunofluorescence from 12 to 22 hr after inoculation. Shown are three cells fixed 16 hr after inoculation. (A) Phase/DAPI reveals cell outline and chromatin; (B) anti-tubulin staining; (C) anti-Sad1 staining. Germinating cut12.d1 spores appeared to contain two foci of Sad1 staining, although the microtubules arose from a position distal to both foci, indicated by an arrow for each of the three cells. Cells also underwent cut divisions (cell 3). Scale bar, 10 μm. (d–i) Monopolar spindle formation and cut divisions following the switch off of plasmid borne cut12+ expression in cut12.d1 haploid cells. A culture of the cut12.d1 haploid maintained by the expression of a plasmid borne copy of the cut12+ cDNA from the attenuated thiamine repressible nmt1+ promoter was grown to log phase in minimal medium in the absence of thiamine at 30°C. Aliquots were then inoculated into thiamine-free media to allow continued expression of cut12+, and a second aliquot inoculated into medium containing thiamine to repress cut12+ expression. Samples were processed for immunofluorescence from 14 to 28 hr later. (d,f,h) cut12+ expressed; (e,g,i) cut12+ repressed. (d,e) Phase/DAPI reveals cell outline and chromatin staining; (f,g) anti-tubulin staining; (h,i) anti-Sad1 staining. Defective spindles formed in cells ∼14 hr after repression of cut12+. Monopolar spindles arose from only one of the two Sad1 foci (cell 1 and 2), similar to the ts cut12.1 mutant, and Cut divisions occurred (cell 3). Scale bar, 10 μm. (j–l) Immunofluorescence analysis of cells overexpressing cut12+. Cells carrying the cut12+ cDNA linked to the wild-type nmt1+ promoter of the pREP1 plasmid were grown to mid log phase, the culture was divided in two, and cut12+ expression was derepressed in one half. Samples were taken every hour from 12 to 20 hr following induction. Shown are cells in which cut12+ was derepressed for 16 hr. (j) Phase contrast/DAPI stain; (k) anti-tubulin staining; (l) anti-Sad1 staining. Cells overexpressing the cut12+ gene formed monopolar spindles that seemed to arise from the single focus of Sad1 staining visible within the cell. Cells in which cut12+ expression was repressed formed normal bipolar spindles (not shown). Scale bar, 10 μm.
We also examined the effect of a loss of Cut12 protein from a culture of the cut12.d1 haploid in which the cells were maintained in a viable state by the expression of the cut12+ cDNA from the pREP81 plasmid. The pREP81 plasmid carries an attenuated form of the thiamine repressible nmt1+ promoter (Basi et al. 1993; Maundrell 1993). The repression of cut12+ transcription by the addition of 2 μm thiamine resulted in the formation of defective monopolar spindles identical with those seen in the ts cut12.1 mutant, where the microtubules arose from the less intense of the two foci of Sad1 staining (Fig. 3e,g,i). In control cultures expressing cut12+, bipolar spindle formation was normal (Fig. 3d,f,h). Cells that formed defective spindles and underwent defective cut divisions were elongated relative to cells in which cut12+ expression was not repressed (mean cell length 13.5 μm s.e.m. 0.2 μm; and 12.4 μm, s.e.m. 0.1 μm, respectively; n=50). Thus, the spindles formed in the absence of Cut12 protein are monopolar and unable to support chromosome segregation, because of a defect in SPB duplication and/or activation or maturation.
cut12+ overexpression perturbs bipolar spindle formation
Bipolar spindle formation and cytokinesis were normal when cut12+ expression from the wild-type nmt1+ promoter (Maundrell 1993) was repressed (data not shown), whereas on derepression, V-shaped spindles arising from the single focus of Sad1 staining accumulated (Fig. 3j–l). A similar phenotype has been described for mutations in the kinesin-related gene cut7+ (Hagan and Yanagida 1990) and the S. pombe Plk encoded by the plo1+ gene (Ohkura et al. 1995), and may result either from a defect in SPB duplication or from the inability of cells to resolve a parallel array of microtubules to a bipolar spindle. The cells that formed monopolar spindles were elongated relative to cells in which cut12+ expression was repressed (14.1 μm, s.e.m. 0.2 μm, vs. 13.5 μm, s.e.m. 0.1 μm, respectively; n=50).
Cut12 is an SPB component
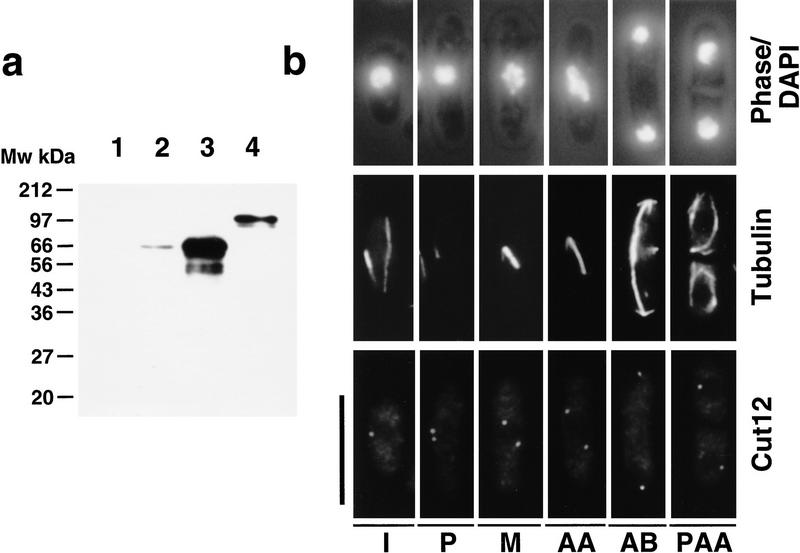
Several approaches were taken to localize the Cut12 protein. Two polyclonal rabbit antisera were raised against amino acids 33 to 548 of the Cut12 protein fused to GST. Affinity-purified antibodies from these sera failed to recognize any bands in a protein extract prepared from germinating cut12+ deletion spores (Fig. 4a, lane 1) but recognized a single band of ∼62-kD by immunoblot of a wild-type S. pombe extract (Fig. 4a, lane 2). Overexpression of a cut12+ cDNA from the wild-type nmt1+ promoter resulted in an increase in the intensity of this band (Fig. 4a, lane 3), whereas replacement of the wild-type cut12+ gene with a GFP-tagged version resulted in an increase in molecular mass to ∼90 kD (Fig. 4a, lane 4). We conclude that p62 is the product of the cut12+ gene.
Figure 4.
Characterization of anti-Cut12 antibodies and immunofluorescence localization of Cut12. (a) Affinity-purified rabbit antibodies against amino acids 33–548 of the Cut12 protein fused to GST were used to probe Western-blotted extracts from cut12.d1 cells (1), wild-type cells (2), cells overexpressing a full length cut12+ cDNA from the nmt1+ promoter (3), or cells in which the wild-type cut12+ gene had been replaced with a version bearing the GFP tag inserted at methionine 33 of the cut12+ sequence (4), thereby causing a shift in predicted molecular weight of the Cut12 protein from 62 to 89 kD. (b) Immunolocalization of the Cut12 protein through the cell cycle. Wild-type fission yeast cells were grown to mid log phase, processed for immunofluorescence, and stained with anti-Cut12 and anti-tubulin antibodies. (Top) Cell outline and position of chromatin as revealed by DAPI; (middle) anti-tubulin staining; (bottom) anti-Cut12 staining. (I) interphase; (P) prophase; (M) metaphase; (AA) anaphase A; (AB) anaphase B; (PAA) postanaphase array. Interphase cells were found to contain a single dot of anti-Cut12 staining associated with the interphase chromatin, whereas in mitotic cells anti-Cut12 staining was localized to the spindle poles. Scale bar, 10 μm.
Exponentially growing wild-type cells were stained with anti-Cut12 and anti-tubulin antibodies. A single spot of anti-Cut12 staining was associated with the interphase chromatin, whereas in mitotic cells two discrete spots were seen at the mitotic spindle poles, consistent with Cut12 localization to the SPB (Fig. 4b). The intensity of these spots did not vary throughout the cell cycle.
A concern with immunolocalization data is that the specificity of antibodies in fixed samples may differ from that observed by Western blot analysis, so we used two additional methods to localize the Cut12 protein. In the first approach, the wild-type cut12+ gene was replaced with a version bearing the green fluorescent protein (GFP) cDNA of Aequorea victoria inserted at methionine 33 (Fig. 5a). The cells were viable, and formed normal spindles, although the frequency of cells with postanaphase arrays (PAAs) was approximately double that in wild-type controls type cells (not shown). One or two distinct dots of GFP fluorescence were seen per cell (Fig. 5b–e). Staining with antibodies to the Sad1 protein confirmed that GFP and Sad1 signals were coincident (Fig. 5f–h). No variation in intensity of GFP signal was detected throughout the cell cycle.
Figure 5.
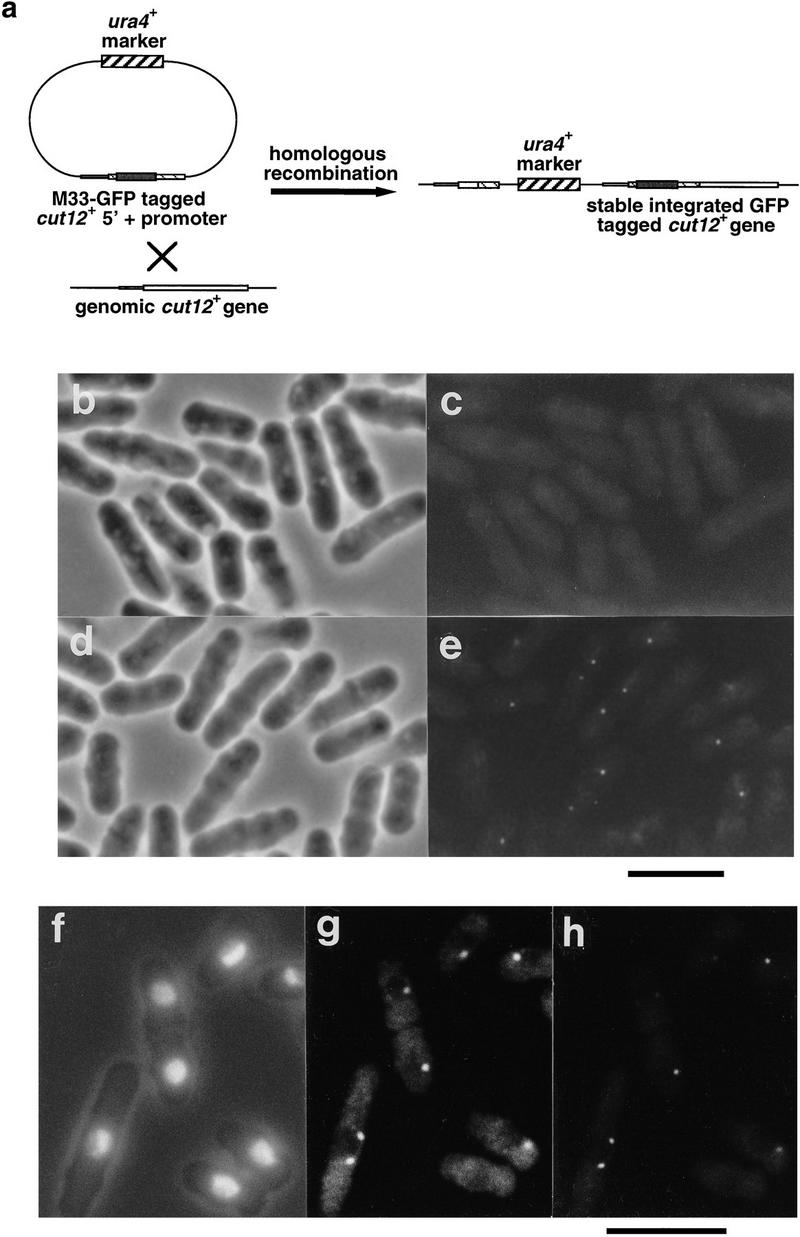
Localization of the Cut12 protein by use of GFP. (a). The wild-type cut12+ gene was replaced with a version bearing the GFP protein by homologous recombination with a plasmid containing the 5′ region of the cut12+ gene tagged at methionine 33 with the GFP cDNA. (b–e). Wild-type cells and cells bearing the GFP-tagged cut12+ gene were removed from culture and examined by phase microscopy (b,d) and fluorescence microscopy with standard FITC filters to reveal GFP fluorescence (c,e). (b,c) Wild-type cells; (d,e) Cut12-GFP tag cells. Cells carrying the integrated GFP-tagged cut12+ gene contained one or two dots of GFP fluorescence that were absent from wild-type cells. Scale bar, 10 μm. (f–h). Cells carrying integrated GFP-tagged cut12+ were processed for immunofluorescence and stained with anti-Sad1 antibodies. (f) Cell outline and chromatin revealed by DAPI; (g) Sad1 localization; (h) GFP-Cut12 localization. The GFP–Cut12 and Sad1 signals were found to colocalize in both interphase and mitotic cells. Scale bar, 10 μm.
Finally, the wild-type cut12+ gene was replaced with a version of the gene bearing two copies of the Pk epitope tag (Hanke et al. 1992) at methionine 33 (S. pombe strain IH744). Staining of cells with anti-Pk and anti-Sad1 antibodies confirmed that the Pk and Sad1 signals were coincident throughout the cell cycle (data not shown). Depolymerization of microtubules by cold shock showed that localization of Cut12 to the SPB as judged by all three procedures was independent of microtubule integrity, indicating that Cut12 is a bona fide SPB component (data not shown).
Cut12 protein localizes to the edge of the inner face of the SPB
Cut12 protein was localized by simultaneous immunogold staining of S. pombe strain IH744 with both monoclonal antibodies against the Pk epitope tag (5 nm gold) and polyclonal anti-Cut12 antibodies (10 nm gold). Both probes localized specifically to the periphery of the face of the cytoplasmic body of the interphase SPB (Fig. 6a). In Figure 6b–f, the relative positions of gold particles in five serial sections through a single interphase SPB are shown; the features of note in these five sections are superimposed in Figure 6g. Whereas the Cut12 epitopes were evenly distributed along the face of the SPB adjacent to the membrane, the Pk epitope was localized to only one side.
Figure 6.
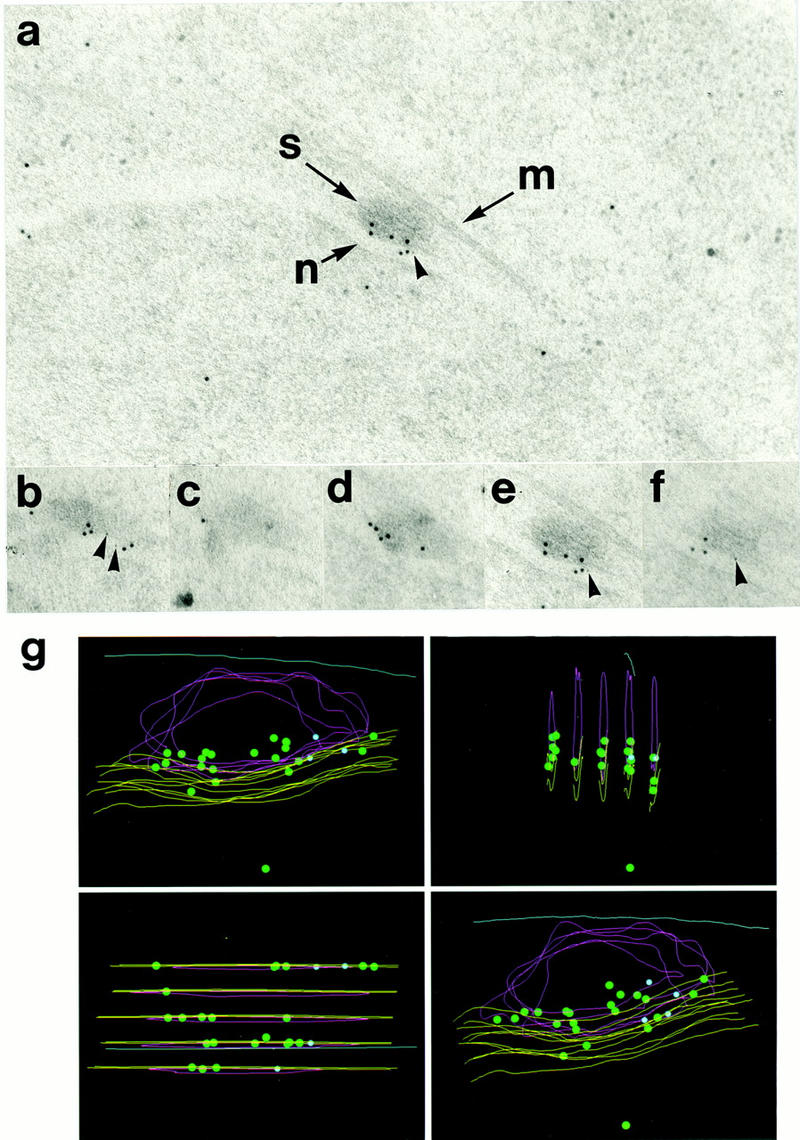
Electron microscopic localization of Cut12 protein within the SPB. (a) Immunogold staining of a section through an SPB by use of antibodies to the Pk epitope tag and a polyclonal anti-Cut12 antibody. Secondary antibodies were conjugated to gold particles of 5 nm (Pk) and 10 nm (Cut12). (S) Spindle pole body; (M) cytoplasmic microtubule laterally associated with the SPB; (N) nuclear envelope. The Cut12 epitopes were localized specifically to the inner face of the main body of the cytoplasmic SPB, adjacent to the nuclear envelope. The Pk epitope tag appeared localized more to one side of the inner face of the SPB. (b–f) Five serial sections through the single SPB shown in a were stained with antibodies to the Pk epitope tag and polyclonal antibodies to the Cut12 protein. Secondary antibodies were conjugated to gold particles of 5 nm (Pk) and 10 nm (Cut12). (g) Immunogold localization of Cut12 epitopes in serial sections through a single SPB in the Pk epitope tagged cut12+ strain. The five serial sections shown in b–f were superimposed. (Green dots) 10 nm-gold particles (anti-Cut12); (blue dots) 5 nm gold particles (anti-Pk); (yellow lines) nuclear envelope; (red lines) SPB; (blue line) cytoplasmic microtubule.
The cut12+ locus is allelic to stf1.1
Database comparisons of the cut12+ nucleotide sequence revealed 100% identity of nucleotides 450–588 downstream of the cut12+ stop codon with nucleotides 1–139 of the gtp1+ gene, which encodes a putative GTP binding protein (Hudson and Young 1993). The gtp1+ gene was cloned by virtue of its lying immediately (449 nucleotides) downstream of the mitotic control gene stf1+ (Hudson et al. 1990; Hudson and Young 1993).
The cdc25+ gene of S. pombe encodes a tyrosine phosphatase that activates the p34cdc2/cyclin B kinase at the G2 to M transition by catalyzing the removal of inhibitory phosphorylation on tyrosine 15 of p34cdc2 (Russell and Nurse 1986; Dunphy and Kumagai 1991; Gautier et al. 1991; Kumagai and Dunphy 1991; Strausfeld et al. 1991). Mutants lacking Cdc25 function replicate their DNA but fail to activate the mitotic p34cdc2/cyclin B kinase, and become arrested in the G2 phase of the cell cycle with an interphase array of microtubules (Russell and Nurse 1986; Hagan and Hyams 1988). stf1.1 is a semidominant mutation that allows loss-of-function mutants in cdc25+, including a cdc25+ gene disruption, to successfully divide (Hudson et al. 1990). Our finding that both cut12+ and stf1+ appear to lie immediately upstream of the same gene indicated that the cut12+ and stf1+ genes may be identical.
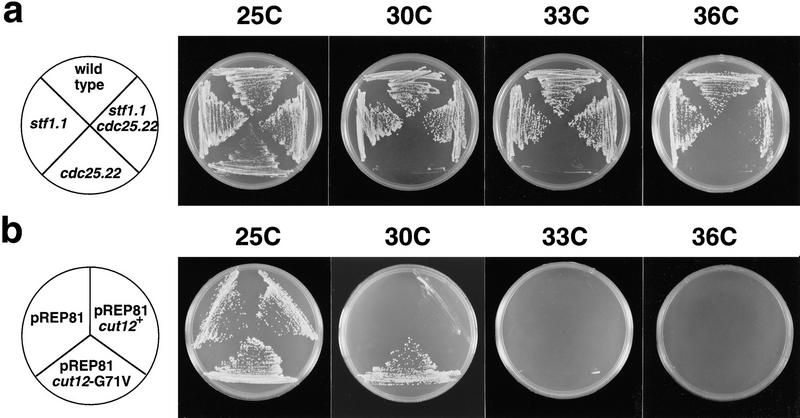
We crossed a cdc25.22 mutant in which the cut12+ locus was marked with the ura4+ gene with a cdc25.22 stf1.1 double mutant. In 30 tetrads we found no Ura+ Cdc+ spores, corresponding to a linkage of within 1.7 cm between the Ura4+-tagged cut12+ locus and the stf1.1 locus. The cut12+ gene was amplified from the stf1.1 strain and the entire ORF of the PCR product sequenced directly. We identified a single base change (G–T) in codon 71 of the cut12+ sequence in four independent PCR reactions, which would result in the replacement of glycine 71 with a valine residue. Although expression of a plasmid borne cut12+ cDNA from the pREP81 plasmid was unable to rescue the ts cdc25.22 defect, expression of the cut12.G71V mutant cDNA suppressed the cdc25.22 defect at 30°C, but not at 33°C or 36°C (Fig. 7b). This level of suppression is equivalent to that seen in a cdc25.22/cdc25.22 stf1+/stf1.1 diploid, which is viable up to 32°C (Hudson et al. 1990). Therefore, cut12+ and stf1+ are allelic, and a single mutation of glycine 71 to valine in cut12+ can suppress the requirement for Cdc25 in mitotic initiation. The cut12.G71V mutation did not alter the localization pattern of Cut12 as determined by immunofluorescence in either a wild-type or cdc25.22 background at 25°C or 36°C (data not shown).
Figure 7.
The cut12+ and stf1+ genes are identical. (a) Temperature sensitivity of the cdc25.22 and stf1.1 single mutants and a cdc25.22 stf1.1 double mutant. Cells were streaked onto complete medium at 25°C, 30°C, 33°C, and 36°C. The stf1.1 mutation allowed growth of a cdc25.22 mutant on complete medium at all four temperatures tested, although at 36°C growth was slow relative to wild-type cells, and colony size is irregular. (b) Suppression of cdc25.22 by a point mutation in the cut12+ gene. cdc25.22 mutant cells carrying a pREP81 plasmid containing no insert, a full-length wild-type cut12+ cDNA, or the mutant cut12.G71V cDNA, were streaked onto minimal medium lacking thiamine at 25°C, 30°C , 33°C, and 36°C. Although cdc25.22 cells carrying a blank plasmid or the wild-type cut12+ gene were unable to form colonies at 30°C, cdc25.22 cells carrying the cut12.G71V mutant cDNA were able to grow at 30°C but not 33°C. This is consistent with the ability of a cdc25.22/cdc25.22 stf1.1/stf1+ diploid to grow at temperatures below 33°C (Hudson et al. 1990).
The stf1.1 cdc25.22 mutant forms defective mitotic spindles at high temperatures
The ability of stf1.1 to bypass the requirement for Cdc25 is incomplete. The genomic mutation stf1.1 could suppress cdc25.22 and a deletion of the cdc25+ gene only up to 35°C, whereas at 37°C, both double mutants formed small colonies that contained aberrantly dividing cells (Hudson et al. 1990). To determine the basis of the lethality at the higher temperature, the single mutants stf1.1 and cdc25.22, and the double mutant stf1.1 cdc25.22, were grown at 25°C and shifted to 37°C. On shift to 37°C, cdc25.22 cells arrested cell cycle progression with interphase microtubules and a single nucleus characteristic of G2 (Fig. 8a,d,g) whereas stf1.1 cells continued to form normal spindles and divide (Fig. 8b,e,h). stf1.1 cdc25.22 cells disassembled the interphase cytoskeleton, condensed their chromosomes, and formed aberrant star shaped spindles that originated from a single large focus of Sad1 staining (Fig. 8c,f,i). The presence of an enlarged Sad1 focus caused a characteristic hole in the center of the microtubule array. Thus, whereas the stf1.1 mutation can bypass the requirement for Cdc25 at all temperatures and allow commitment to mitosis, at 37°C the spindles that are formed in the stf1.1 mutant in the absence of Cdc25 function are defective.
Figure 8.
The stf1.1 mutant has a temperature-sensitive spindle defect in a cdc25.22 background. (a–i) Immunofluorescence analysis of the cdc25.22 and stf1.1 single mutants and the double mutant cdc25.22 stf1.1. Cells were cultured in complete medium to mid-log phase at 25°C and then shifted to 37°C, and processed for immunofluorescence analysis. (a–c) Phase/DAPI; (d–f) anti-tubulin staining; (g–i) anti-Sad1 staining. (a,d,g) cdc25.22; (e,f,h) stf1.1; (c,f,i) cdc25.22 stf1.1. The single cdc25.22 mutant arrested with an interphase cytoskeleton characteristic of a G2 cell, whereas the stf1.1 mutant continued to form normal spindles and divide. The double-mutant cdc25.22 stf1.1 formed aberrant star-shaped spindles arising from a single large Sad1 focus within the cell. Scale bar, 10 μm.
cdc25.22 exacerbates the spindle formation defect in cut12.1
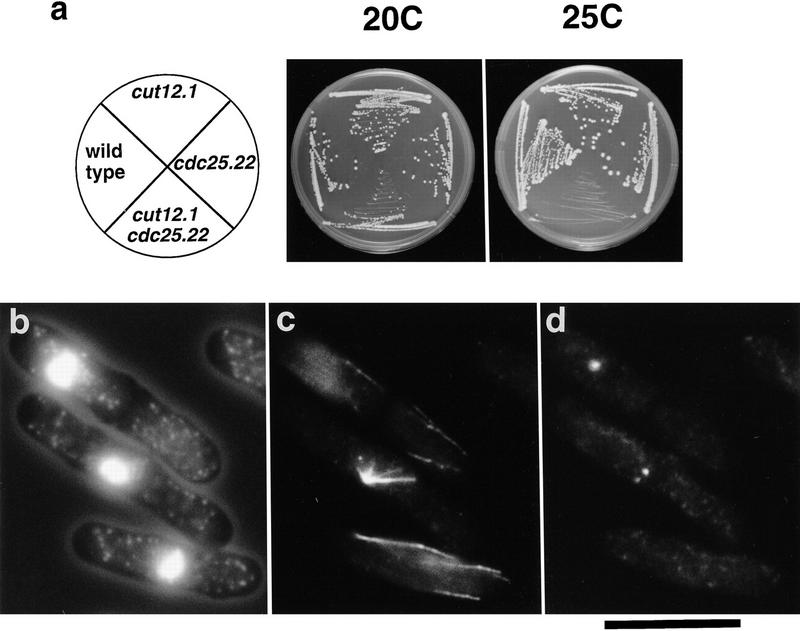
As a semidominant mutation in cut12 can bypass the requirement for Cdc25, we determined the effect of combining the loss-of-function cut12.1 mutation with cdc25.22. Although the single cdc25.22 and cut12.1 mutants were viable up to 30°C, the double mutant cut12.1 cdc25.22 was viable only at 20°C and unable to grow at temperatures of 25°C and above (Fig. 9a). On shift to 25°C, the cut12.1 cdc25.22 cells entered mitosis and formed monopolar spindles that arose from a weakly staining focus of Sad1 staining (Fig. 9b–d). This phenotype is identical to that of the single cut12.1 mutant on shift from 25°C to 36°C (Fig. 1). At 36°C, the double mutant cut12.1 cdc25.22 arrested with interphase microtubules characteristic of the G2 arrest seen in the single mutant cdc25.22 (data not shown). Thus, the presence of the cdc25.22 mutation exacerbates the spindle defect in the cut12.1 mutant.
Figure 9.
Synthetic lethality between cut12.1 and cdc25.22. (a) Temperature sensitivity of cdc25.22, cut12.1, and cdc25.22 cut12.1 mutants. Cells were streaked onto complete medium at 20°C and 25°C. Although the single mutants cdc25.22 and cut12.1 both grew at 25°C, the double mutant cdc25.22 cut12.1 was inviable at this temperature. (b–d) Immunofluorescence analysis of phenotype of the cdc25.22 cut12.1 mutants. Cells were cultured in complete medium at 20°C and then shifted to 25°C and processed for immunofluorescence. (b) Cell outline and chromatin; (c) anti-tubulin staining; (d) anti-Sad1 staining. On shift to 25°C, the cdc25.22 cut12.1 mutant formed defective spindles. Although the cdc25.22 cut12.1 cells appeared to contain two foci of Sad1 staining, cells formed defective monopolar spindles that arose from the less bright staining Sad1 focus, as in the cut12.1 mutant at 36°C. Scale bar, 10 μm.
Discussion
We have shown that loss-of-function of the SPB component Cut12 leads to the formation of defective monopolar spindles. Unexpectedly, a semidominant mutation in cut12+ can bypass the requirement for the essential MPF activating tyrosine phosphatase Cdc25 (Hudson et al. 1990, 1991). This was suprising as Cut12 is therefore an SPB component that is both essential for bipolar spindle formation and capable of influencing the requirement for a normally essential inducer of mitosis. Because the loss-of-function phenotype defines cut12+ as a cut gene, whereas the stf1.1 mutation is a gain-of-function mutation, we will refer to the cut12+/stf1+ gene as cut12+.
Loss of Cut12 function leads to formation of monopolar spindles
The interphase fission yeast SPB consists of two components. The main body of the SPB resides in the cytoplasm and is separated from the mitotic spindle microtubule nucleator γ-tubulin by the nuclear envelope (Ding et al. 1997). Mitotic activation of the SPB is accompanied by localized nuclear envelope breakdown and association of these two elements. In actively dividing cells that lack Cut12 function, Sad1 localization did not correlate with microtubule nucleating activity. This may indicate that these two elements of the mitotic SPB remain independent in the absence of Cut12.
In cut12.1, monopolar spindles arose from the weaker of the two Sad1 staining foci, whereas in germinating cut12.d1 spores monopolar spindles were not associated with either of the two equally staining Sad1 foci. The differential effect of the cut12.1 mutation on the two SPBs suggests that Cut12 is incorporated into the duplicated SPBs in a conservative fashion, and that the Cut12 protein incorporated into the SPB in the cell cycle prior to the temperature shift is stabilized. Differential behavior of the duplicated SPBs is also illustrated by the activation of the cytokinesis regulating GTPase Spg1 on only one of the two anaphase SPBs (Sohrmann et al. 1998). As the two SPBs appear to be generated by fission of a larger precursor to two smaller structures (Ding et al. 1997), the nonequivalence between the two daughter SPBs may be generated by localized incorporation of new material within a particular region of the SPB during growth; fission would then lead to the production of a mature and a new SPB. The apparent asymmetry of exposure of amino-terminal epitopes of Cut12 within the SPB supports this possibility.
cut12.G71V bypasses the requirement for Cdc25
The p34cdc2 kinase is subject to inhibitory phosphorylation on T14 and Y15 residues, and the removal of these inhibitory phosphates determines the timing of entry into mitosis (Gould and Nurse 1989). In fission yeast this inhibitory phosphorylation is catalyzed in by the Wee1 kinase and a redundant Wee1 homolog Mik1 (Russell and Nurse 1987a; Featherstone and Russell 1991; Lundgren et al. 1991; Parker et al. 1993; Lee et al. 1994; Den Haese et al. 1995). The action of the Wee1/Mik1 kinases is opposed by Cdc25 phosphatase (Fantes 1979; Russell and Nurse 1986), which dephosphorylates both T14 and Y15 in vitro (Dunphy and Kumagai 1991; Gautier et al. 1991; Kumagai and Dunphy 1991; Strausfeld et al. 1991).
The activity of Cdc25 is stimulated by cell-cycle specific phosphorylation of certain sites (Ducommun et al. 1990; Moreno et al. 1990; Izumi et al. 1992; Kumagai and Dunphy 1992; Hoffmann et al. 1993; Izumi and Maller 1993). The Wee1 and Mik1 kinases are also subject to cell cycle-dependent phosphorylation that has been reported to inhibit the activity of the kinase (Russell and Nurse 1987b; Coleman et al. 1993; Parker et al. 1993; Tang et al. 1993; Wu and Russell 1993; McGowan and Russell 1995; Mueller et al. 1995; Watanabe et al. 1995). Thus, phosphorylation of both Cdc25 and the opposing Wee1/Mik1 kinases promotes the activation of p34cdc2/cyclin B and onset of mitosis. Phosphorylation and inhibition of Wee1 is catalyzed by the action of the Nim1/Cdr1 kinase (Coleman et al. 1993; Parker et al. 1993; Wu and Russell 1993), and other unrelated kinases including MPM2 epitope kinases (Tang et al. 1993; Mueller et al. 1995). The stimulatory phosphorylation of Cdc25 can be accomplished in vitro by either the p34cdc2/cyclin B kinase (Hoffmann et al. 1993; Izumi and Maller 1993) or by non-p34cdc2 kinases including MPM2 epitope kinases such as the Xenopus Plx kinase (Kuang et al. 1994; Izumi and Maller 1995; Kumagai and Dunphy 1996). Biochemical studies in Xenopus have demonstrated the existence of a positive feedback loop involving p34cdc2 and Cdc25, in which Cdc25 may dephosphorylate and activate p34cdc2/cyclin B, which then phosphorylates and stimulates Cdc25 to, in turn, dephosphorylate further p34cdc2/cyclin B, leading to the rapid amplification of a small quantity of MPF and entry into mitosis (Izumi and Maller 1993; Hoffmann et al. 1993). This amplification loop could be triggered by the p34cdc2 independent phosphorylation and activation of Cdc25, or by the phosphorylation and inhibition of Wee1/Mik1. The loop may also be triggered by the generation of a fraction of p34cdc2/cyclin B that does not require Cdc25 for activation or that is not sensitive to inhibition by Wee1/Mik1.
Within this context, a number of models can be considered that may account for the ability of a semidominant mutation in an SPB component to bypass the requirement for Cdc25. Cut12 is unlikely to solely regulate the activity of Cdc25, as stf1.1 can suppress a complete loss of Cdc25 activity arising from the disruption of the cdc25+ gene (Hudson et al. 1990, 1991). Cut12 is also unlikely to solely regulate Wee1, as stf1.1 is additive with wee1.50 in suppression of cdc25.22 and is sensitive to the overexpression of wee1+ (Hudson et al. 1990). Cut12 might, however, directly or indirectly regulate the activities of both Wee1 and Mik1, or possibly interact with a dual regulator of Cdc25 and Wee1/Mik1 function, a role proposed for the Xenopus Plx MPM2 kinase (Kumagai and Dunphy 1996; Lane and Nigg 1996). In both these models, Cut12 may promote mitosis in the absence of Cdc25 function by down-regulating both Wee1 and Mik1, thereby relieving the negative restraint that holds a cdc25.22 mutant in G2. Cut12 could also relieve this restraint by promoting Y15 dephosphorylation of p34cdc2 by another tyrosine phosphatase, such as the tyrosine phosphatase Pyp3 (Millar et al. 1992). Pyp3 is unrelated to Cdc25 but may act cooperatively with Cdc25 to dephosphorylate Y15 of p34cdc2 (Millar et al. 1992). However, the stf1.1 mutation is unlikely to bypass loss of Cdc25 function by increasing pyp3+ activity, as loss of pyp3+ function does not appear to lead to Cut divisions (Millar et al. 1992). In a simpler model stf1.1 may allow the formation of p34cdc2/cyclin B complexes at the SPB that are not subject to inhibition by Wee1/Mik1 kinases.
Potential interactions between Cut12 and MPF
Genetic studies in fission yeast have shown that the requirement for Cdc25 function can be altered by mutation of the cdc2+ gene and its cyclin B-type partner encoded by the cdc13+ gene. Like cut12.G71V, a gain-of-function mutation in cdc2+ termed cdc2.3w can bypass the requirement for Cdc25 activity at the G2-to-M transition (Carr et al. 1989; Gould et al. 1990; MacNeill and Nurse 1993), whereas loss-of-function mutations in cdc13+ are, like cut12.1, synthetically lethal with cdc25.22 (Bueno and Russell 1993). The genetic interaction between cdc25.22 and both loss- and gain-of-function alleles in cut12+ are consistent with Cut12 acting as a regulator or substrate of the p34cdc2/cyclin B kinase. Both p34cdc2 and Cdc13 have been localized to the SPB (Alfa et al. 1990), and mutations in the cdc2+ and cdc13+ genes have been identified that can specifically inhibit chromosome segregation but not other events of mitosis such as chromosome condensation and septation (Labib et al. 1995; Berry and Gould 1996; Gould and Feoktistova 1996). cdc2.Y15T and cdc13.A381V are perhaps particularly relevant in this regard, as these mutant proteins may be unable to promote spindle formation because of a defective interaction with a regulatory subunit that is specifically required for this process (Berry and Gould 1996; Gould and Feoktistova 1996). It has also been suggested that a higher threshold level of p34cdc2/cyclin B kinase activity may be required for mitotic spindle formation than for chromosome condensation and septation, and that mutants such as cdc2.Y15T may be unable to attain this level of activity (Gould and Feoktistova 1996). Therefore, Cut12 may be a limiting substrate normally phosphorylated only when the p34cdc2/cyclin B activity reaches the level required for bipolar spindle formation. The cut12.G71V mutation lies between the closely apposed amino-terminal MAP kinase and p34cdc2 consensus sites, and Cut12.G71V may conceivably mimic an activated form of Cut12 and allow progression through mitosis in the presence of a lower level of p34cdc2/cyclin B kinase activity. Loss-of-function mutants in cut12+ undergo chromosome condensation and septation without forming a bipolar spindle. A similar phenotype to cut12.1 is seen in mutants of A. nidulans that enter mitosis in the absence of NIMA function, a known mitotic substrate of p34cdc2/cyclin B kinase (Osmani et al. 1991a; Ye et al. 1995). Whereas the nimA5 mutant normally arrests in G2 with duplicated SPBs and high p34cdc2/cyclin B kinase activity, the bimE7 nimA5 double mutant escapes this G2 arrest and attempts an aberrant mitosis with defective SPBs (Oakley and Morris 1983; Osmani et al. 1991a,b). Thus, loss-of-function mutations in downstream effectors of p34cdc2 function may uncouple the normally coordinated events of mitosis such as spindle formation and chromosome condensation and septation.
Whatever the precise mechanism by which stf1.1 bypasses the requirement for Cdc25, the demonstration that an essential component of the SPB can influence the requirement for activators of p34cdc2/cyclin B highlights the importance of events at the spindle pole to cell cycle progression in general.
Materials and methods
Cell culture and strains
Cell culture and maintenance was carried out according to Moreno et al. (1991). All Cut12 localization experiments, and the analysis of spindle formation defects in cut12.1, cut12.1 cdc25.22, and stf1.1 cdc25.22 mutants, were carried out on cells cultured in rich YES medium. The cut12+ deletion and overexpression analyses, plus all cell length measurements, were carried out on cells grown in Edinburgh minimal medium (EMMG) that is equivalent to EMM2 (Moreno et al. 1991) but substitutes 5 grams/liter glutamic acid for 5 grams/liter ammonium acetate as a nitrogen source. Synchronous cultures were generated by size selection of small G2 cells on a lactose gradient as described previously (Hagan and Yanagida 1997). The strains used in this paper are shown in Table 1.
Table 1.
Strains used in this study
| Strain
|
Genotype
|
Origin
|
|---|---|---|
| IH310 | leu1.32/leu1.32 ade6.M210/ade6.M216 ura4.d18/ura4.d18 h−/h+ | lab stock |
| IH365 | leu1.32 ura4.d18 h− | lab stock |
| IH566 | cdc25.22 leu1.32 ura4.d18 h− | lab stock |
| IH571 | cut12.M33GFP:ura4+ leu1.32 ura4.d18 h− | this study |
| IH572 | cut12.dl/cut12+ leu1.32/leu1.32 ade6.M210/ade6.M216 ura4.d18/ura4.d18 h−/h+ | this study |
| IH596 | cut12.1 leu1.32 ura4.d18 h− | this study |
| IH666 | stf1.1 leu1.32 h+ | Hudson et al. (1990) |
| IH667 | stf1.1 cdc25.22 leu1.32 ura4.d18 h90 | Hudson et al. (1990) |
| IH741 | stf1.1 cdc25.22 leu1.32 ura4.d18 h− | Hudson et al. (1990) |
| IH744 | cut12.M33Pk:ura4+ leu1.32 ura4.d18 h− | this study |
| IH751 | cdc25.22 cut12.M33Pk:ura4+ leu1.32 ura4.d18 h− | this study |
| IH970 | cdc25.22 leu1.32 ura4.d18 pREP81–cut12.G71V h− | this study |
| IH972 | cdc25.22 leu1.32 ura4.d18 pREP81–cut12+ h− | this study |
| IH1080 | cdc25.22 leu1.32 ura4.d18 pREP81 h− | this study |
| IH1110 | cut12.d1 ade6 leu1.32 pREP81–cut12+ h− | this study |
| IH1120 | stf1.1 leu1.32 ura4.d18 h− | this study |
| IH1225 | leu1.32 ura4.d18 pREP1 h− | this study |
| IH1226 | leu1.32 ura4.d18 pREP1–cut12+ h− | this study |
| IH1227 | cut12.1/cut12+ leu1.32/leu1.32 ade6.M210/ade6.M216 ura4.d18/ura4.d18 h−/h+ | this study |
Isolation and sequencing of the cut12+ gene
Standard DNA manipulations were carried out according to Sambrook et al. (1989). The cut12+ gene was isolated by complementation of the temperature-sensitive mutant. Fourteen transformants carrying five complementing plasmids were isolated from screens of two genomic S. pombe libraries (the generous gift of Dr. A. Carr; Barbet et al. 1993). These plasmids had an overlapping region of ∼2.8 kb, including the minimum complementing 2.0-kb EcoRV–PvuII fragment. Integration of a plasmid containing a portion of the cloned DNA linked to the ura4+ gene by homologous recombination showed that the cloned region contained the cut12+ gene, as no recombination was observed between the cut12.1 and ura4+ in >1100 spores (Nurse 1975). A 1.0-kb HindIII–EcoRV and a 2.0-kb HindIII fragment that together completely covered this minimum complementing region were cloned into the Bluescript plasmid to generate the pSCUT12-1 and pSCUT12-2 plasmids, respectively, and nested deletions made by use of exonuclease III. DNA sequencing was performed on both strands by the manual dideoxy method with T7 DNA polymerase. The presence of the predicted 42-bp intron was confirmed by sequencing a clone isolated by PCR amplification from a pREP1 based cDNA library (a gift from Dr. C. Norbury, John Radcliffe Hospital, Institute of Molecular Medicine, ICRF, Molecular Oncology Laboratory, Oxford, UK) by use of primers (5′-TTCAGAGATCCGATAGAATGTCAG-3′) and (5′-CCTTCGTCTTCTCCTTTCCTCTAATC-3′). The cut12.1 mutant version of the cut12+ gene was isolated by PCR amplification with two primers (5′-CGACTAATACGGCCTTTTACAAGCC-3′) and (5′-CATGCAGAACAATTCAGAGATCCG-3′). PCR products were sequenced directly by a manual T7 DNA polymerase procedure. Where a mutation was identified, that region of the gene was sequenced again from four independent PCR reactions.
Construction of the cut12.d1 deletion
A 4-kb genomic Pst1–KpnI fragment from the shortest cut12.1 complementing genomic clone was cloned into Pst1–KpnI–digested pUC19 to generate the plasmid pDCUT12-1. A 1.6-kb Ppu10I fragment containing all of the cut12+ coding sequence, with the exception of the first 10 amino acids, was removed from pDCUT12-1 and replaced with a NotI linker (GCGGCCGC) to generate pDCUT12-2. A 1.8-kb AccIII fragment containing the ura4+ gene (Barbet et al. 1993) was inserted into the linker to generate pDCUT12-3. A 4-kb fragment containing the cut12+ gene deleted by the ura4+ gene was purified from pDCUT12-3 and used to transform a diploid (h+/h− leu1-32/leu1-32 ura4-d18/ura4-d18 ade6-M210/ade6-M216). Stable Ura+ diploids that had the desired replacement determined by genomic Southern hybridization were selected. Probing with a 2.0-kb HindIII fragment containing the 3′ region of the cut12+ gene revealed a 3.7-kb BglII fragment in the deletion diploid in addition to the 1.7- and 2.0-kb bands observed in wild type.
Construction of a full-length cut12+ cDNA for overexpression studies
pREP1 and pREP81 were used to express cut12+ under the control of the thiamine repressible nmt1+ promoter (Basi et al. 1993; Maundrell 1993). For the construction of the NdeI site (CATATG) at the initiator codon, the plasmid pSCUT12-1 was digested with NsiI. Two oligonucelotides (5′-ATATGTCAGAAACTTTGAACACTCCGCCAACC-3′) and (5′-TAGGTTGGCGGAGTGTTCAAAGTTTCTGACA-3′) were allowed to anneal to generate a linker that was inserted at the NsiI site, thereby generating a clone bearing the 5′ region of cut12+ gene with two copies of the cut12+ start codon and the first 10 amino acids, in which the original start codon was followed by the intron, and the newly introduced second start codon had an NdeI site at the ATG. The original NdeI site at nucleotides 29–34 of the cut12+ coding sequence was also removed by this procedure. The plasmid was designated pSCUT12-1/M1NdeI. A BglII–EcoRV fragment from pSCUT12-1/M1NdeI was subcloned back into the 4-kb genomic cut12+ clone. This construct was then digested with NdeI and PvuII, to yield a 2.2-kb fragment bearing the full length cut12+ cDNA and ∼300 bp 3′ of the gene. The NdeI–PvuII fragment was then inserted into NdeI–SmaI–digested pREP plasmids.
Construction of an integrated GFP-tagged version of the cut12+ gene
The GFP tag was inserted at an NdeI site created at methionine 33 of the cut12+ sequence. The pSCUT12-1 plasmid was used as a template for site directed mutagenesis by use of the primer 5′-GCCTGTTACACATATGTCCTCTTAC-3′, and the resulting mutant clone (pSCUT12–1/M33NdeI) was sequenced entirely between the initiator codon and the HindIII site to confirm that no errors had been introduced during the mutagenesis procedure. The GFP cDNA as an NdeI fragment (R. Craven, D. Griffiths, K. Sheldrick, R. Randall, I. Hagan, and A. Carr, in prep.) was then inserted into this position to give pSCUT12-1/M33GFP. To create the plasmid for integrative transformation of cut12+-GFP into the genome, the HindIII–EcoRV fragment was then cloned into HindIII–EcoRV–digested Bluescript with ura4+ inserted at the NotI site to form pINTCUT12-1/M33GFP.
Construction of an integrated Pk epitope-tagged version of the cut12+ gene
For epitope tagging the cut12+ gene, we used a pREP42 plasmid (pREP42-PkN) bearing three copies of the Pk epitope tag (amino acid sequence IPNPLLGLD; R. Craven, D. Griffiths, K. Sheldrick, R. Randall, I. Hagan, and A. Carr, in prep.), inserted as an NdeI cassette at the multicloning site. The cassette also bears an internal NcoI site following the second copy of the tag; hence, it is possible to link NcoI-digested genes to the Pk tag bearing pREP42 plasmid, and then re-excise the gene along with two copies of the Pk tag by NdeI digest. An NcoI site was inserted at M33 of the cut12+ sequence by site-directed mutagenesis on the pSCUT12-1 plasmid by use of the primer 5′-GCCTGTTACAACCATGGCCTCTTAC-3′, and the resulting clone (pSCUT12–1/M33NcoI) sequenced between the initiator codon and the HindIII site. A BglII–EcoRV fragment was subcloned back into the 4-kb genomic cut12+ clone of pDCUT12-1 to form pDCUT12–1/M33NcoI. This construct was then digested with NcoI and PvuII, which yields a 2.2-kb fragment bearing a truncated form of the cut12+ gene encoding amino acids 33–548, along with ∼300 bp 3′ of the gene, which was inserted into pREP42-PkN. The regeneration of the full length cut12+ gene from the truncated version in pREP42-PkN vector was complicated by the NdeI site that lies immediately upstream of the EcoRV site in pDCUT12-1. To circumvent this problem, a 1.6-kb NsiI fragment that lacks the internal NdeI site was excised from pDCUT12–1/M33NdeI and cloned into the Bluescript plasmid to give pDCUT12-4. The 5′ region of the tagged truncated cut12+ gene was then isolated from pREP42-PkN by NdeI–BglII digest, and the 0.5-kb fragment produced ligated to NdeI–BglII–digested pDCUT12-4. The NsiI fragment from the resulting plasmid was then cloned back into pDCUT12-1, thereby generating a plasmid bearing the full length cut12+ gene with two copies of the Pk epitope tag inserted at methionine 33, termed pDCUT12–1/M33Pk. The HindIII–EcoRV fragment from this plasmid was then cloned into HindIII–EcoRV–digested Bluescript with ura4+ inserted at the NotI site to create the plasmid for integrative transformation of Pk-tagged cut12+ into the genome, termed pINTCUT12-1/M33Pk.
Isolation of the stf1.1 mutant locus by PCR and cloning PCR products
The stf1.1 mutant version of the cut12+ gene was isolated by PCR amplification according to the protocol described for cut12.1. To clone the stf1.1 mutant gene, a 0.5-kb NsiI–BglII fragment was purified from the PCR product and ligated to NsiI–BglII–digested pSCUT12-1/M1NdeI. The resulting clone was manually sequenced to confirm the presence of the stf1.1 mutation in codon 71. To recreate full-length clones from this mutated construct, a BglII–EcoRV fragment was subcloned back into the 4-kb genomic cut12+ clone, thereby regenerating a full-length stf1.1 mutant version of cut12+. This construct was then digested with NdeI and PvuII, to yield a 2.2-kb fragment bearing the full-length cut12.G71V cDNA and ∼300 bp 3′ of the gene, which was cloned into NdeI–SmaI–digested pREP81.
Construction of GST–Cut12 fusion protein and antiserum production
A full-length cut12+ with NdeI at M33 was digested with NdeI and PvuII and cloned into the plasmid pGEX4T-3. This construct was transformed into the Escherichia coli strain BRL21 for the expression of GST–Cut12 fusion protein. Bacterially produced protein was eluted from a polyacrylamide gel by electrophoresis at 100 mÅ and dialysed against phosphate buffered saline. One hundred fifty micrograms of the resulting insoluble suspension of protein was mixed with complete Freund’s adjuvant for the first injection, and incomplete Freund’s adjuvant for two subsequent boosts. Antisera and affinity-purified sera were produced as described in Harlow and Lane (1988). Western blot detection was carried out with peroxidase conjugated anti-rabbit secondary antibody.
Fluorescence and immunofluorescence microscopy
For immunofluorescence microscopy, the procedures described by Hagan and Hyams (1988) were followed. The affinity purified anti-Sad1 antibody AP9.2 (Hagan and Yanagida 1995), anti-tubulin antibody TAT1 (Woods et al. 1989), and anti-Pk tag antibody 336 (Hanke et al. 1992) have been described previously. To improve microtubule preservation in cells double labeled with anti-tubulin and anti-Cut12, cells were mixed with an equal volume of prewarmed YES media plus 2.4 m sorbitol for 5 min prior to fixation in 4% paraformaldehyde. For the observation of GFP–Cut12 protein, cells were cultured in YES media, washed once in phosphate-buffered saline, and dried onto coverslips. GFP fusions were observed by use of Zeiss FITC set 10.
Electron microscopy
For electron microscopic localization of Cut12 and Pk antigens, samples were prepared by a modification of the methods of Ding et al. (1997). Briefly, cells were collected on a 0.45 μm millipore filter by vacuum filtration and were frozen in a Balzer’s high pressure freezer (Balzers, Lichtenstein). Frozen samples were freeze-substituted on 0.1% gluteraldehyde in acetone at −90°C, warmed to −50°C, and embedded in Lowicryl HM20. Immunostaining was carried out as described previously (Demeter et al. 1995), by use of antibodies to Cut12 and Pk antigens followed by secondary antibodies conjugated to 10 and 5 nm of colloidal gold, respectively. Serial sections were imaged in a Philips CM10 electron microscope, negatives were digitized, and computer models were generated using the IMOD software package (Kremer et al. 1996).
Acknowledgments
We thank Paul Nurse for providing S. pombe strains, Keith Gull and Rick Randall for the TAT1 and 336 anti-Pk antibodies, respectively, and Tony Carr and Chris Norbury for providing the S. pombe genomic and cDNA libraries, respectively, and David Mastronardre for images of immunoelectron microscopic localization of Cut12. We also thank Tony Carr, Paul Clarke, Dick McIntosh, Paul Nurse, Viesturs Simanis, and Marc Sohrmann for stimulating discussions and critical reading of the manuscript. This work was supported by grants from the Cancer Research Campaign to I.H., National Institutes of Health grants GM33787 and RR00592 in support of M.M., and a grant from the British Council to the UK Japan Cell Cycle Group to R.B. Imaging facilities in Manchester were also supported by a grant from the Wellcome trust.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Iain.Hagan@man.ac.uk; FAX 44 161 275 5082.
References
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Bailly E, Doree M, Nurse P, Bornens M. p34cdc2 is located both in nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: Association with a detergent resistant compartment and with the centrosome. J Cell Sci. 1992;101:529–545. doi: 10.1242/jcs.101.3.529. [DOI] [PubMed] [Google Scholar]
- Balczon R. The centrosome in animal cells and its functional homologs in plant and yeast cells. Int Rev Cytol. 1996;169:25–82. doi: 10.1016/s0074-7696(08)61984-1. [DOI] [PubMed] [Google Scholar]
- Barbet N, Muriel WJ, Carr AM. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992;114:59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real time visualization of cell cycle dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Berry LD, Gould KL. Novel alleles of cdc13 and cdc2 isolated as suppressors of mitotic catastrophe in Schizosaccharomyces pombe. Mol Gen Genet. 1996;251:635–646. doi: 10.1007/BF02174112. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg E. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Broek D, Bartlett R, Crawford K, Nurse P. p34cdc2 is involved in establishing the dependence of S phase upon completion of the previous mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Buendia B, Draetta G, Karsenti E. Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: Role of cyclin A-associated protein kinase. J Cell Biol. 1992;116:1431–1442. doi: 10.1083/jcb.116.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A, Russell P. Two fission yeast B-type cyclins Cig2 and Cdc13 have different functions in mitosis. Mol Cell Biol. 1993;13:2286–2297. doi: 10.1128/mcb.13.4.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AM, MacNeill SA, Hayles J, Nurse P. Molecular cloning and sequence analysis of mutant alleles of the fission yeast cdc2 kinase gene: Implications for cdc2 protein structure and function. Mol & Gen Genet. 1989;218:41–49. doi: 10.1007/BF00330563. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Tang Z, Dunphy WG. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Demeter J, Morphew M, Sazer S. A mutation in RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc Natl Acad Sci. 1995;92:1436–1440. doi: 10.1073/pnas.92.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Haese GJ, Walworth N, Carr AM, Gould KL. The Wee1 protein kinase regulates T14 phosphorylation of fission yeast cdc2. Mol Biol Cell. 1995;6:371–385. doi: 10.1091/mbc.6.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, McDonald KL, McIntosh JR. Three dimensional reconstruction and analysis of mitotic spindles from the yeast Schizosaccharomyces pombe. J Cell Biol. 1993;120:141–151. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew M, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun B, Draetta G, Young P, Beach D. Fission yeast Cdc25 is a cell cycle regulated protein. Biochem Biophys Res Comm. 1990;167:301–309. doi: 10.1016/0006-291x(90)91765-k. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The Cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979;279:428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Glover DM, Ohkura H, Tavares A. Polo kinase: The choreographer of the mitotic stage? J Cell Biol. 1996;135:1681–1684. doi: 10.1083/jcb.135.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–42. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Tonks NK, Nurse P. Complementation of the mitotic activator, p80cdc25, by a human tyrosine phosphatase. Science. 1990;250:1573–1576. doi: 10.1126/science.1703321. [DOI] [PubMed] [Google Scholar]
- Gould K, Feoktistova A. Characterization of novel mutations at the Schizosaccharomyces pombe cdc2 regulatory phosphorylation site, tyrosine 15. Mol Biol Cell. 1996;7:1573–1586. doi: 10.1091/mbc.7.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- ————— The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Evidence for cell cycle specific, spindle pole body mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1997;110:1851–1866. doi: 10.1242/jcs.110.16.1851. [DOI] [PubMed] [Google Scholar]
- Hanke T, Szawlowski P, Randall RE. Construction of solid matrix-antibody-antigen complexes containing simian immunodeficiency virus p27 using tag-specific monoclonal antibody and tag linked antigen. J Gen Virol. 1992;73:653–660. doi: 10.1099/0022-1317-73-3-653. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2–mitotic cyclin B complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self amplification of MPF at mitosis. EMBO J. 1993;12:53–60. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley B, Tanaka K, Yanagida M. The fission yeast gamma tubulin is essential for mitosis and is localized to microtubule organizing centres. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Hudson D, Young P. Sequence of the Schizosaccharomyces pombe gtp1+ gene and identification of a novel family of putative GTP binding proteins. Gene. 1993;125:191–193. doi: 10.1016/0378-1119(93)90327-y. [DOI] [PubMed] [Google Scholar]
- Hudson JD, Feilotter H, Young PG. stf1; non-wee mutations epistatic to cdc25 in the fission yeast Schizosaccharomyces pombe. Genetics. 1990;126:309–315. doi: 10.1093/genetics/126.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JD, Feilotter H, Lingner C, Rowley R, Young PG. stf1: A new suppressor of the mitotic control gene cdc25 in Schizosaccharomyces pombe. Cold Spring Harbor Symp Quant Biol. 1991;56:599–604. doi: 10.1101/sqb.1991.056.01.068. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Phosphorylation and activation of the Xenopus cdc25 phosphatase in the absence of cdc2 and cdk2 kinase activity. Mol Biol Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt A, Schliwa M. Molecular components of the centrosome. Trends Cell Biol. 1993;3:118–128. doi: 10.1016/0962-8924(93)90174-y. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self assembly: From microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL, Gonzalez-Kuyvenhoven M, Penkala JE. cdc25 is one of the MPM-2 antigens involved in the activation of maturation promoting factor. Mol Biol Cell. 1994;5:135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- ————— Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–150. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- ————— Purification and molecular cloning of Plx1, a cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Labib K, Craven RA, Crawford K, Nurse P. Dominant mutants identify new roles of p34cdc2 in mitosis. EMBO J. 1995;14:2155–2165. doi: 10.1002/j.1460-2075.1995.tb07209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Enoch T, Piwnica-Worms H. mik1 encodes a tyrosine kinase that phosphorylates p34cdc2 on tyrosine 15. J Biol Chem. 1994;269:30530–30537. [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. Mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Lupas A, v-Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1102–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- MacNeill SA, Nurse P. Genetic analysis of human p34cdc2 function in fission yeast. Mol & Gen Genet. 1993;236:415–426. doi: 10.1007/BF00280381. [DOI] [PubMed] [Google Scholar]
- Maldonado-Codina G, Glover DM. Cyclins A and B associate with chromatin and the polar regions of spindles, respectively, and do not undergo complete degradation at anaphase in syncytial Drosophila embryos. J Cell Biol. 1992;116:967–976. doi: 10.1083/jcb.116.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Sevik M, Cande WZ. In vitro microtubule nucleating activity of spindle pole bodies in fission yeast Schizosaccharomyces pombe: Cell cycle dependent activation in Xenopus cell free extracts. J Cell Biol. 1992;117:1055–1066. doi: 10.1083/jcb.117.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. Thiamine repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JBA, Lenaers G, Russell P. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 1992;11:4933–4941. doi: 10.1002/j.1460-2075.1992.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Nurse P, Russell P. Regulation of mitosis by cyclic accumulation of p80cdc25 mitotic inducer. Nature. 1990;344:549–552. doi: 10.1038/344549a0. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. An introduction to the molecular genetic analysis in the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of Xenopus wee1-like kinase. Mol Biol Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. Cellular substrates of p34cdc2 and its companion cyclin dependent kinases. Trends Cell Biol. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- ————— Universal control mechanisms regulating onset of M-phase. Nature. 1990;344:547–551. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Morris NR. A mutation in Aspergillus nidulans that blocks the transition from interphase to metaphase. J Cell Biol. 1983;96:1155–1158. doi: 10.1083/jcb.96.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes & Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- Ohta K, Shiina N, Okumura E, Hisinaga SI, Kishimoto T, Endo S, Gotoh Y, Nishida E, Sakai H. Microtubule nucleating activity of centrosomes in cell free extracts from Xenopus eggs: Involvement of phosphorylation and accumulation of pericentriolar material. J Cell Sci. 1993;104:125–137. doi: 10.1242/jcs.104.1.125. [DOI] [PubMed] [Google Scholar]
- Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle regulated protein kinases is required to initiate mitosis in A. nidulans. Cell. 1991a;67:283–291. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- Osmani AH, O’Donnell K, Pu RT, Osmani SA. Activation of the NIMA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the BIME checkpoint. EMBO J. 1991b;10:2669–2679. doi: 10.1002/j.1460-2075.1991.tb07810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Walter SA, Young PG, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor wee1 by the nim1/cdr1 kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- Pichova A, Kohlwein SD, Yamamoto M. New arrays of cytoplasmic microtubules in the fission yeast Schizosaccharomyces pombe. Protoplasma. 1995;188:252–262. [Google Scholar]
- Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockwinse SM, Krockmalnic G, Doxsey SJ, Nickerson J, Lian JB, van Wijen AJ, Stein JL, Stein GS, Penman S. Cell cycle independent interaction of cdc2 with the centrosome, which is associated with the nuclear matrix intermediate filament scaffold. Proc Natl Acad Sci. 1997;94:3022–3027. doi: 10.1073/pnas.94.7.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- ————— Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987a;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- ————— The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987b;49:569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sohrmann M, Schmidt S, Hagan I, Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum inducing protein kinase Cdc7p. Genes & Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U, Labbe JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Doree M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by a human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kanbe T. Mitosis in the fission yeast Schizosaccharomyces pombe as revealed by freeze substitution electron microscopy. J Cell Sci. 1986;80:253–268. doi: 10.1242/jcs.80.1.253. [DOI] [PubMed] [Google Scholar]
- Tang Z, Coleman TR, Dunphy WG. Two distinct mechanisms for negative regulation of the wee1 protein kinase. EMBO J. 1993;12:3427–3436. doi: 10.1002/j.1460-2075.1993.tb06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Labbe JC, Doree M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell free extracts of Xenopus eggs. Nature. 1990;343:233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Verde F, Dogterom M, Stelzer E, Karsenti E, Leibler S. Control of microtubule dynamics and length by cyclin A and cyclin B dependent protein kinases in Xenopus egg extracts. J Cell Biol. 1992;118:1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human Wee1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- Ye XS, Xu G, Pu PT, Fincher RR, McGuire SL, Osmani AH, Osmani SA. The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: Coordination of two mitosis promoting kinases. EMBO J. 1995;14:986–994. doi: 10.1002/j.1460-2075.1995.tb07079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XS, Fincher RR, Tang A, O’Donnell K, Osmani SA. Two S phase checkpoint systems, one involving the function of both BIME and Tyr15 phosphorylation of p34cdc2/cyclin B, inhibit NIMA and prevent premature mitosis. EMBO J. 1996;15:3599–3610. [PMC free article] [PubMed] [Google Scholar]