Abstract
Background
Depressive symptoms are elevated in adolescents who experienced significant malnutrition early in life. Early malnutrition can also have a significant impact on cognitive functioning, presumably due to the adverse impact of the malnutrition on the very young brain. In the context of a developmental cascade model, we tested the hypothesis that the association between early malnutrition and adolescent depressive symptoms is mediated by the cognitive impairment that ensues from the malnutrition.
Method
We evaluated Barbadian youth (N=57) hospitalized for moderate to severe protein-energy malnutrition in the first year of life and healthy controls (N=60) longitudinally. The primary hypothesis was tested by multiple regression models.
Results
After adjusting for covariates, early malnutrition predicted both cognitive functioning in childhood (IQ, p<0.001; attention problems, p<.01; Common Entrance Examination, p<0.01) and adolescent depressive symptoms (p<0.05). Childhood cognitive functioning mediated the association between early malnutrition and depressive symptoms in adolescence (p<0.001). Maternal depressive symptoms were a significant but independent predictor of adolescent depressive symptoms (p<0.05).
Conclusions
Cognitive compromise in childhood accounts indirectly for elevated depressive symptoms in previously malnourished adolescents, consistent with a developmental cascade model. The direct link between malnutrition and depressive symptoms in adolescence is small.
Keywords: Malnutrition, depression, cognition, academic achievement, socioeconomic status
Severe malnutrition early in life has well known neurodevelopmental sequelae, with potentially lifelong functional consequences. Adverse clinical outcomes can involve cognition, behavior and affect1,2.
Pre-clinical studies have established an impact of pre- and post-natal malnutrition on neurochemistry and brain structure, affecting glial and dendritic development in a regionally specific fashion 3,4, 5. Prefrontal cortical and hippocampal structures are especially vulnerable, with permanent changes detectable into adulthood, even in animals rehabilitated after the neonatal exposure 6, 7. The potential for regionally specific impact of malnutrition on the prefrontal cortex and hippocampus in the developing brain is of particular interest because of their pivotal role in human learning and memory 8,9 as well as emerging links to depressive disorders 10, 11.
The Barbados Nutrition Study (BNS) is a now 40-year longitudinal study that has evaluated children who had been hospitalized for moderate to severe malnutrition in the first year of life and a comparison group from the same classrooms and social milieu12. Because the malnourished children were successfully rehabilitated nutritionally and followed until 12 years of age to assure adequate nutrition and normal growth and development, the episode was restricted to the first year of life, without ongoing undernutrition 13. Participants were evaluated three times in a 7-year period between 1977 and 1984, spanning grammar school age to late adolescence. Data are currently being collected on these same individuals, now entering their fifth decade.
At the first evaluation, when the children were 5 to 11 years of age, the previously malnourished group exhibited lower IQ, more attention problems and poorer grades in school12, 14, 15. Performance on the Common Entrance Examination (CEE), a standard academic achievement test administered to all Barbadian children at 11 years of age to determine high school placement, was also lower in the previously malnourished children, referable largely to their lower IQ and problems with attention and self-regulation as measured in the primary grades by a teacher questionnaire designed for the study 16.
Maternal depression can also adversely affect child development, including cognition and mood17 and may in fact contribute to the initial episode of malnutrition18, 19. Significantly, in the BNS, mothers of previously malnourished children reported more depressive symptoms when the children were school age and adolescent. These symptoms were associated with negative child outcomes, including poorer school grades and teacher reported hygiene.
These previously malnourished children themselves also reported more symptoms of depression than their adequately nourished peers as adolescents. Although maternal depressive symptoms predicted adolescent depressive symptoms, their effect was independent of the effects of malnutrition and standard of living 20. Depressive symptoms in adolescence have similarly been reported in other long-term follow-up studies of children malnourished early in life21.
Developmental psychopathologists have advanced the core concept of the developmental cascade. According to Masten and colleagues, a developmental cascade occurs when “functioning in one domain of adaptive behavior spills over to influence functioning in other domains in a lasting way (p. 735).” 22 Thus, importantly, a deviation or early insult having a direct effect on one functional domain may indirectly impair functioning in other domains, ultimately affecting development and adaptive success far more broadly. The relevance of this developmental framework to the problem of early nutrition has been previously discussed by Wachs, who observed that “Models based exclusively on nutrition and CNS pathways do not provide sufficient explanations of how nutritional deficiencies translate into maternal and child mental health problems (p. 937S).”23 Thus, although some neurodevelopmental consequences are likely the direct result of the nutritional insult to the immature brain, a developmental perspective argues that such consequences are not necessarily direct sequelae, but may be epiphenomena arising indirectly in the course of the child’s developmental adaptation to and interaction with the environment.
Along these lines, it is well established that children with learning impairment are vulnerable to internalizing symptoms as they become aware of their struggles. Ackerman et al.24, for example, found that poor readers from disadvantaged environments exhibited an increase in internalizing symptoms between the 3rd and 5th grades as a consequence of their reading problems. These symptoms were thought to be triggered by normative developmental advances in self-awareness as the children approached adolescence and became more cognizant of their struggles. In the present context, academic struggles resulting from cognitive compromise related to the malnutrition could lead to depressive symptoms in the setting of academic and other adaptive challenges, potentially mediating the relationship between malnutrition and the depressive symptoms detected in adolescence.
The goal of the present report is to integrate findings from the BNS within the framework of a developmental cascade, extending from infancy, when the episode of malnutrition and thus the original presumptive neurological insult occurred, to adolescence, when the elevated level of depressive symptoms was observed. The primary hypothesis is that the association between early malnutrition and adolescent depressive symptoms is mediated by the cognitive impairment that ensues from the malnutrition.
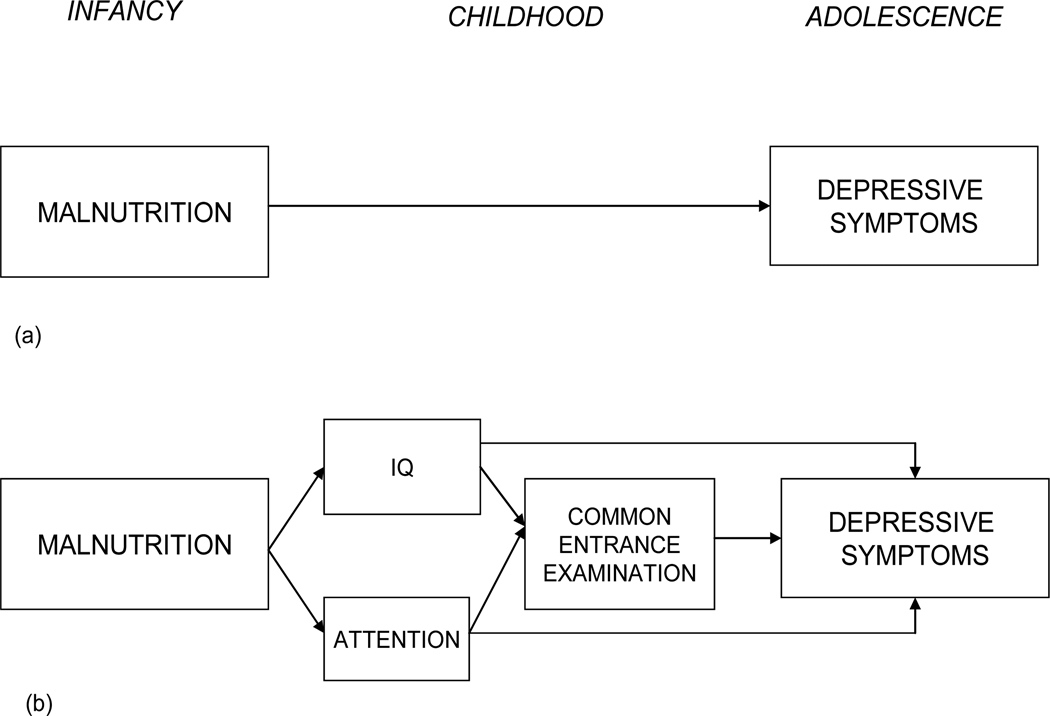
Figure 1a shows the simple, direct effect model; that is, early malnutrition leads directly to depressive symptoms in adolescence. Figure 1b shows the more elaborated developmental model to be evaluated. Here, the episode of early malnutrition has a presumed direct neurological impact, manifest in lowered IQ and increased attention problems. These cognitive sequelae serve to impair school achievement, measured by performance on the CEE. Poor school functioning in childhood then leads to depressive symptoms in adolescence. In the model, academic performance is included as a proximal mediator between the cognitive impairment and depressive symptoms because it is presumably the more salient experiential indicator of the impairment for the child. The model also includes a potential direct effect of early malnutrition on depressive symptoms since the cognitive symptoms may not fully mediate the association. Because of the known association between maternal and child depression, and also because of the known associations of both maternal depression and socioeconomic conditions with malnutrition in our sample, we controlled for the household standard of living in all analyses and include maternal depressive symptoms as a predictor in the final model.
Figure 1.
(a) Simple model showing early malnutrition leads to depressive symptoms in adolescence. (b). Hypothetical model showing that malnutrition affects IQ and school behavior (attention) which in turn affects performance on the CEE which then affects depressive symptoms in adolescence. Model also provides for direct effect of malnutrition on adolescent depressive symptoms as well as direct effects of IQ and attention on depressive symptoms.
Method
Site
The study was conducted in Barbados, a Caribbean country whose current population is approximately 260,000. Whereas moderate-severe cases of infant malnutrition were of significant concern when the participants were born in the late 1960’s, it is now virtually eliminated The longitudinal data evaluated in this study were collected between 1978, when the children were between 5 and 11 years of age, and 1984, when they were between 11 and 18 years of age. These archival data are relevant because the sample is currently being reevaluated at approximately 40 years of age.
Design and Participants
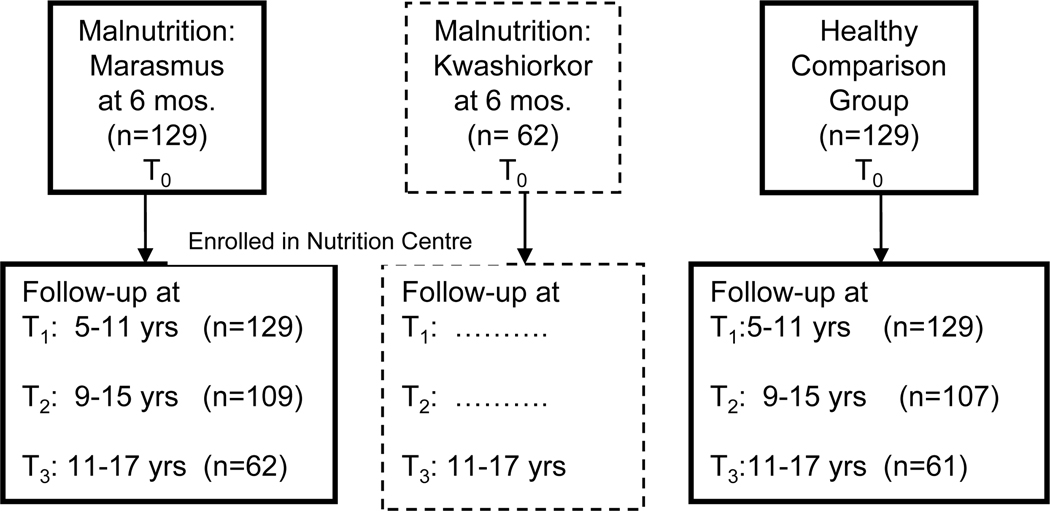
The study design is depicted in Figure 2. In 1977 (T1), all school-aged children participating in the National Nutrition Centre program and hospitalized in their first year of life with a diagnosis of Grade II or III (Gomez scale) protein-energy malnutrition (marasmus) between 1967 and 1972 were invited to participate in the study (n=129). Three healthy children, matched for sex, age (within 3 months) and handedness, were selected from the same classrooms as each index child. One was ultimately selected for the comparison group based on availability of birth and early childhood records and adequate documentation of good health and growth (n=129). Inclusion criteria for both groups were normal birth weight, absence of pre- or post-natal complications, and no known neurological deficits. These 258 children were followed up in 1983 (T2). In 1985 (T3), an additional group of children who had been hospitalized for kwashiorkor during the same period (N=62) was recruited for comparison purposes. One hundred twenty-three (marasmus, N=61; healthy comparison, N=62) of the original 258 children were selected for evaluation at that time because they were the best matches for age, sex and grade to the kwashiorkor group. Thus only 123 of the original 258 were available for longitudinal analyses.
Figure 2.
Study design showing 3 groups of participants in the Barbados Nutrition Study and age ranges at each time point. Kwashiorkor group (dotted line) was not included in the present study because longitudinal data were not available. N is reduced at T3 for Marasmus and Healthy Comparison groups because they were selected as controls for the 62 children in the Kwashiorkor group.
The present report is based on these 123 children. Of these, 116 (Marasmus, N=56; Controls, N=60) completed the self-report mood scale that is the focus of this study, They constitute the sample for the present report. Groups were comparable in mean age and sex distribution (Table 1).
Table 1.
Means, standard deviations and t-tests for model variables by malnutrition groups.
|
Protein Energy Malnutrition (N=56) |
Control (N=60) |
|||||
|---|---|---|---|---|---|---|
| % Male | 55.4 | 58.3 | ||||
| Mean | SD | Mean | SD | t | p | |
| Age in Years (T3) | 14.96 | 1.49 | 15.00 | 1.58 | 0.14 | n.s. |
| Child Depressive Symptomsab (T3) | 0.30 | 1.01 | −0.33 | 0.93 | 3.48 | 0.001 |
| Maternal Depressive Symptomsab (T3) | 0.16 | 0.85 | −0.13 | 0.95 | 1.72 | 0.10 |
| Standard of Livinga (T1) | −0.90 | 0.89 | −0.25 | 0.77 | 4.21 | <0.0001 |
| Standard of Livinga (T3) | 0.08 | 0.80 | 0.61 | 0.64 | 4.02 | <0.0001 |
| Full Scale IQ (T1) | 92.54 | 13.86 | 104.92 | 10.54 | 5.44 | <0.0001 |
| Teacher Rated Attention Symptomsab (T1) | 0.33 | 0.96 | −0.36 | 0.81 | 4.19 | <0.0001 |
| Common Entrance Examination (11 years) | 184.10 | 29.27 | 210.42 | 22.13 | 5.14 | <0.0001 |
Mean=0, s.d. = 1
Higher value indicates more symptoms
Informed consent was provided by all families who participated in the study, under Protocol E1962, approved by the Boston University Medical Center Institutional Review Board, and by the Ethics Committee of the Barbados Ministry of Health. Current oversight is provided by the Judge Baker Children’s Center Human Research Review Committee (Assurance No. FWA 00001811).
Measures
Measures of IQ and teacher rated attention (Time 1), the Common Entrance Examination (CEE, a standard achievement test administered to all students at 11 years), depressive symptoms and standard of living (both at Time 3) were extracted from the data base. Data from Time 2 were not used for this analysis. Importantly, because the study was initiated in 1977, the well developed instruments in common use today to measure some key constructs (i.e., attention, depression) were not yet available. Measures were derived from a series of questionnaires constructed for the study. Items were derived from instruments that existed at the time and modified as appropriate for the Barbadian setting. Factor analytic techniques were used to derive reliable and valid scales. Detailed descriptions of the derivation of these measures and their psychometric properties are referenced in the descriptions. Although instruments that might have been preferable on a psychometric basis did become available during the lifetime of the study, the original questionnaires were retained to provide for longitudinal analyses and also because of their relevance to the cultural setting. These study-specific measures allow for valid comparison and prediction among study participants, but they cannot be referenced to external or contemporary norms for interpretation of clinical severity or classification of disorders.
The measures used to test the model and the time point from which they were extracted were as follows:
Wechsler Intelligence Scale for Children25. (Time 1)
This standard IQ test was modified for Barbadian school children, with minor alteration of items to include local exemplars familiar to the children.
Teacher Behavior Questionnaire, School Functioning Scale (Time 1)
This scale was derived from a 30-item teacher questionnaire modeled after one used by Richardson et al.26. Teachers were blind to the nutritional history of the children. The first principal component of the factor analysis appeared to represent attention27; hence scores have a mean of 0 and standard deviation of 1.
Common Entrance Examination (CEE, 11-Plus Examination)
This test was administered annually to all 11-year old Barbadian children to classify them for the purpose of assigning seats in local secondary schools. It could thus have a major impact on life prospects. The test, which was modified annually, consisted of 140 items in English and 80 in mathematics during the years of the study.
Barbados Ecology Questionnaire, Standard of Living Scale (Times 1 and 3)
A Socioeconomic Status and Ecology Questionnaire20 was used to assess conditions in the home, as well as educational level and employment history of the parents. The 50-item questionnaire was developed specifically for use in Barbados and was adapted from a scale used in a similar population in Jamaica26. Factor analysis, based on data combined for all participants across all three time points, identified a first principal component (Armor theta = 0.86) that appeared to represent household standard of living. This factor score (mean=0, SD=1) was used as the indicator of socioeconomic status.
Youth and Maternal Mood Scale, Depressive Symptoms (Time 3)
Youth depressive symptoms were measured by the Minnesota General Adjustment and Morale Scale28. Factor analysis of the items identified a first unrotated principal component (Armor theta = 0.64), accounting for 13% of the total variance, that appeared to reflect depressive symptoms, especially hopelessness20. Scores had a mean of 0 and standard deviation of 1.
Maternal depressive symptoms were assessed by the same questionnaire, but the data were submitted to a separate factor analysis29. Again, the first principal component appeared to represent depressive symptoms (Armor theta =0.72). It also showed high concurrent validity with the Zung Depression and Anxiety Scales in an independent sample of healthy Barbadian women18. Factor scores (mean = 0, SD=1) were used in the analyses.
Statistical Methods
Pearson correlations were computed between the malnutrition variable and the other variables in the model. In particular, to demonstrate mediation it was important that the mediating variables (IQ, attention, CEE) be significantly correlated (p<0.05) with both malnutrition and adolescent depressive symptoms.
A series of multiple regression analyses were then implemented to evaluate potential mediating effects of the cognitive variables in the relationship of malnutrition to adolescent depressive symptoms. This sequence of analyses is equivalent to path analysis, but preferable because it provides more detail about change in specific coefficients in the context of different models. The first set of analyses evaluated malnutrition status as a predictor of IQ and attention. The second evaluated malnutrition status as a predictor of performance on the CEE with and without adjusting for IQ and attention. The third set evaluated malnutrition as a predictor of depressive symptoms with and without adjusting for CEE, the primary hypothesized mediator. The final set of analyses added maternal depression to the model, to determine whether it further mediated association between malnutrition and adolescent depressive symptoms. All analyses were conducted in SAS Version 9.1.3.
Preliminary analyses indicated that age and sex were not predictors in the first two sets of models, and so they were dropped. For the third set of analyses, however, age (but not sex) was a significant predictor and so it was included in further models that predicted depressive symptoms. Mediation effects were characterized by determining the degree to which the direct effect (of malnutrition) was diminished with the hypothesized mediating variables in the model and tested according to the method suggested by Preacher and Hayes30 for evaluating multiple mediator models.
Results
Table 1 shows means and standard deviations for the variables included in the models, including the developmental time point at which they were measured. Simple correlations between malnutrition group and the other model variables were highly significant, as expected (Standard of Living, r=−0.366, p<.0001; IQ, r=−0.453, p<.0001; Attention, r=−0.364, p<.0001; CEE, r=−0.428, p<.0001; Adolescent Depressive Symptoms, r=−0.27, p<.01). Importantly as well, Adolescent Depressive Symptoms was significantly correlated with all 3 hypothesized mediating variables (IQ, r=−0.29, p <.01; Attention, r=.22, p<.05; CEE, r=−0.32, p<.001). Details of the results of the sequence of regression analyses are summarized in Table 2; individual analyses are referenced alphabetically on the Table and in the text.
Table 2.
Regression models showing predictive relationships displaying standardized beta coefficients and model total R2.
| Dependent Variable | Predictors | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1: Malnutrition as predictor of IQ and Attention Adjusted for Effects of Standard of Living¶ | |||||||||
|
(a) IQ |
Malnutrition | Standard of Living (T1) | |||||||
| β | −0.374*** | 0.216+ | 0.246 | ||||||
|
(b) Attention |
Malnutrition |
Standard of Living (T1) |
|||||||
| β | 0.363** | −0.003 | 0.133 | ||||||
| Step 2: Malnutrition as Predictor of CEE with and without adjusting for IQ and Attention¶ | |||||||||
| (c) CEE | Malnutrition | Standard of Living (T1) | |||||||
| β | −0.315** | −0.309** | 0.266 | ||||||
| (d) CEE | Malnutrition | Standard of Living (T1) | IQ | Attn | |||||
| β | −0.038 | 0.221* | 0.401*** | −0.346*** | 0.582 | ||||
| Step 3: Malnutrition as Predictor of Depressive Symptoms with and without Adjusting for Age and Cognitive Predictors (IQ, Attention, CEE)§ | |||||||||
| (e) Depressive Symptoms | Malnutrition | Age | Standard of Living (T3) | ||||||
| β | 0.295* | −0.322** | −0.029 | 0.203 | |||||
| (f) Depressive Symptoms | Malnutrition | Age | Standard of Living (T3) | IQ | Attn | ||||
| β | 0.10 | −0.306*** | 0.032 | −0.311* | 0.218+ | 0.360 | |||
| (g) Depressive Symptoms | Malnutrition | Age | Standard of Living (T3) | IQ | Attn | CEE | |||
| β | 0.08 | −.306*** | 0.043 | −.216+ | 0.151 | −.208 | 0.380 | ||
| Step 4: Malnutrition as Predictor of Depressive Symptoms with Maternal Depressive Symptoms Included in Model§ | |||||||||
| (h) Depressive symptoms | Malnutrition | Age | Standard of Living (T3) | Maternal Depression | |||||
| β | 0.270* | −0.275* | 0.011 | 0.261* | 0.265 | ||||
| (i) Depressive symptoms | Malnutrition | Age | Standard of Living (T3) | IQ | Attn | CEE | Maternal Depression | ||
| β | 0.07 | −.269** | 0.070 | −.245+ | .127 | −.169 | 0.220* | 0.42 | |
Age and sex dropped from model because betas approach 0.
Age included in model
p<.05
p <.01
p<.001
p<.0001
Attn=Attention; CEE=Common Entrance Examination
Step 1
These analyses replicated our prior finding that malnutrition significantly predicted both (a) IQ and (b) teacher observed attention problems12, 15, after adjusting for standard of living. Standard of living predicted IQ (p<.05) but not teacher-rated attention problems, suggesting that the latter may more directly reflect the biological effect of the malnutrition.
Step 2
As expected, malnutrition status predicted CEE scores, and IQ and attention problems mediated this association. The standardized coefficient for Malnutrition without IQ and attention in the model was −0.315 (c), but dropped to −0.038 with these variables in the model (d), confirming that these early cognitive indicators account almost entirely for nutrition group differences in CEE achievement. Standard of living is a significant additional predictor of CEE, even with these powerful predictors in the model, including IQ which itself is predicted by standard of living. Thus, standard of living plays a meaningful role in academic achievement as measured by the CEE, but does not mediate the association between malnutrition and CEE.
Step 3
These analyses address the primary focus of this report, that is, the extent to which the cognitive impairment associated with malnutrition mediates its association with depressive symptoms in adolescence. As reported previously20, the severity of depressive symptoms is predicted by both malnutrition status and age; depressive symptoms decline with age (e). We next introduced IQ and attention to the model (f), as a result of which the coefficient associated with malnutrition was substantially reduced, from 0.30 to 0.10. Addition of CEE to the model (g) resulted in a small but additional reduction, to 0.08.
Step 4
As Table 1 indicates, depressive symptoms were higher at T1 in mothers of previously malnourished children, although the probability level is in the marginal range. Thus, the final model addressed the contribution of maternal depressive symptoms to the developmental pathway. These analyses indicated that maternal depressive symptoms were indeed a significant predictor of adolescent depressive symptoms, but reduced the effect of malnutrition only slightly (compare h to e). Furthermore, addition of maternal depressive symptoms to model g (see model i) reduces the malnutrition coefficient minimally (from 0.08 to 0.07). Thus, maternal depressive symptoms do not appear to play a significant role in the pathway from malnutrition to adolescent depressive symptoms.
The primary hypothesis, that the cognitive variables (IQ, attention and CEE) mediate the association between infant malnutrition and adolescent depressive symptoms, was tested within the context of this final model (i). The test of mediation30 was highly significant (z=3.6, p<.001).
Summary
Cognitive functioning significantly mediates the path from early malnutrition to adolescent depressive symptoms, even after adjusting for social contextual variables. The direct effect from early malnutrition to adolescent depression is attenuated by approximately two-thirds. Importantly, maternal depressive symptoms independently predict, but do not mediate the relationship between infant malnutrition and adolescent depressive symptoms.
Discussion
This study integrates data from a unique longitudinal sample of Barbadian children who experienced a well documented episode of moderate to severe malnutrition in the first year of life and were subsequently rehabilitated, with adequate health and nutritional status documented until at least age 12. Consistent with the study hypothesis, the increased prevalence of depressive symptoms in the previously malnourished adolescents was mediated by their cognitive and academic compromise, which was presumably a manifestation of their altered brain development. The findings conform to a developmental cascade, whereby a biological insult that primarily affects neural development can become more widely expressed in the context of environmental transactions, in the course of which the child’s cognitive functioning is impaired; that impairment can then cascade to affect another domain, affect. The depressive symptoms are thus, in large part, an epiphenomenon of the presumably more direct impact of the biological insult, which subsequently affects cognition, including school achievement, and hence mood. Their depressed mood could, in turn, have other adverse effects as these individuals become adult, compounding the effects of the original insult, which occurred in the first year of life, and its associated cognitive impairment.
Although the discrete impact of childhood malnutrition can be difficult to detect because it is so typically embedded in a context of poverty, we controlled for standard of living using a contextually relevant indicator that clearly discriminated families of children who had experienced malnutrition from healthy controls, even though both groups were recruited from the same classrooms, neighborhoods, and general social milieu. Standard of living importantly predicted both IQ and CEE scores, but did not further predict depressive symptoms. Thus, its association with depressive symptoms also appears to be mediated by its more direct impact on cognitive development and hence academic functioning.
Further analyses confirmed that the impact of malnutrition on adolescent depressive symptoms via its cognitive consequences and the impact of the maternal depression, which is also associated with malnutrition, on adolescent depressive symptoms are separate and additive20. It does not appear that the child’s episode of malnutrition resulted in maternal depression which then led to adolescent depressive symptoms. Rather, it appears that the malnutrition and maternal depression were co-morbid; indeed, our findings are consistent with reports that pre-existing maternal depression contributes to infantile malnutrition18,19
The magnitude of the association between early malnutrition and later depressive symptoms is weaker than its association with the cognitive variables. This may reflect psychometric issues; a more reliable measure of depression, such as those currently available, might have demonstrated a more robust association. Yet given that the depressive symptoms appear to be secondary, at least in part, to the cognitive sequelae of the malnutrition, a more attenuated association is plausible. Moreover, the association between early malnutrition and adolescent depressive symptoms is not reduced to zero with all the cognitive variables in the model. Thus, biological vulnerability may account to a limited extent for the association between infant malnutrition and adolescent depressive symptoms. In this regard, an altered neural response to stress in prenatally malnourished animals is suggestive 31. Potential affective sequelae of the malnutrition evolving during the preschool period32 may also contribute to depressive symptoms in adolescence or compromise cognitive development. Since maternal depression is more prevalent in mothers of malnourished infants, it could promote the evolution of these sequelae.
The longer-term impact of this developmental trajectory is of considerable interest. Our data indicate, consistent with the developmental psychopathology literature 33 and other studies of longer-term effects of early nutritional compromise 34, that depressive symptoms generally decrease with age. Moreover, life-span studies suggest that the affective consequences of school struggles are moderated once affected individuals can find appropriate vocational and psychosocial niches as young adults 35. The Barbados Nutrition Study is currently obtaining detailed data on the psychiatric, neuropsychological, adaptive and physical functioning in midlife of the individuals who are the subject of the present report. These data will provide a more comprehensive life-span perspective on the significance of the findings reported here.
This study has important limitations, the most obvious of which is the measures used to assess key variables, most of which were developed specifically for this study decades ago. Similarly, the archival nature of the data, collected more than twenty-five years ago, is a potential limitation. In addition, since depressive symptoms were not measured in these adolescents when they were children, we cannot know for certain that the depression and cognitive impairment are not manifestations of a common underlying process or that early social-emotional compromise did not contribute to the cognitive impairment or the observed depressive symptoms. Nevertheless, these data provide a rare opportunity to chronicle the lifespan implications of a discrete episode of moderate to severe malnutrition early in life for later development, particularly as its interacts with environmental influences that may serve as risk or protective factors. Because the BNS remains active, these archival findings thus will be highly relevant to interpretation of the more contemporary data on adult functioning.
The primary significance of these developmental cascades lies in their relevance to intervention. Childhood malnutrition and undernutrition unfortunately remains a significant problem, afflicting an estimated 200 million young children worldwide32, most commonly in economically underdeveloped countries but also in impoverished segments of more developed countries. Our data suggest that intervention may be advantageously focused not only on adequate nutrition for all young children, but also on arresting potential developmental cascades by actively supporting subsequent educational and adaptive success. Collectively, these data will provide a broad perspective on the life-long burdens of early malnutrition on its victims and their families, as well as insight into potential protective influences.
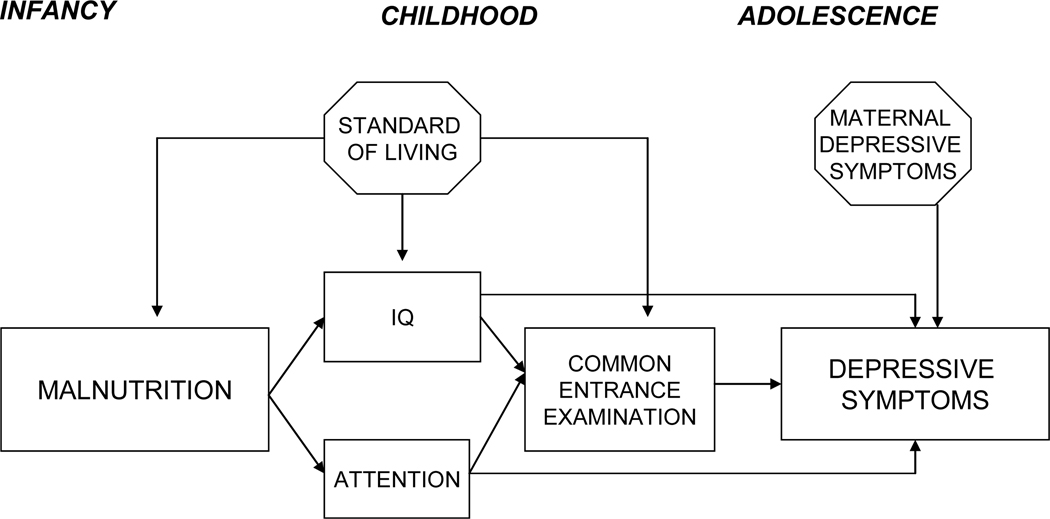
Figure 3.
Final model showing that the cognitive variables (IQ, attention and Common Entrance Examination) mediate the developmental pathway from early malnutrition to adolescent depressive symptoms. Standard of living is included in the model, having direct effects on malnutrition and cognitive measures but not depressive symptoms. Maternal depression has a direct effect on adolescent depressive symptoms but does not mediate path from infant malnutrition to adolescent depressive symptoms.
Acknowledgements
The authors would like to acknowledge the late Sir Frank C. Ramsey, former Director of the Barbados Nutrition Center and Co-Principal Investigator of the Barbados Nutrition Study, who followed these children for most of their lives and whose efforts resulted in the elimination of malnutrition from Barbados, and Marjorie Bowen, Mrs. Jean Ramsey, Community Health Sisters, and Victor Forde, Psychologist, who collected the data included in this report. We express our deepest gratitude to the parents and children (now parents themselves) who participated in and contributed to this research over their lifetimes.
This research was conducted in cooperation with the Ministries of Health and Education of Barbados. Support was provided by a grant (to JRG) from the National Institute of Mental Health (R01 MH065877).
Contributor Information
Deborah P. Waber, Children’s Hospital Boston, Boston, Department of Psychiatry, Massachusetts.
David Eaglesfield, Judge Baker Children’s Center, Boston, Massachusetts
Garrett M. Fitzmaurice, Lab for Biostatisticis, McLean Hospital, Belmont, Massachusetts
Cyralene Bryce, Barbados Nutrition Study, St. Michael Barbados
Robert H. Harrison, Boston, Massachusetts
Janina R. Galler, Judge Baker Children’s Center, Boston, Massachusetts Barbados Nutrition Study, St. Michael, Barbados.
References
- 1.Galler JR. Behavioral consequences of malnutrition in early life. In: Galler JR, editor. Nutrition and Behavior. New York: Plenum Press; 1984. pp. 63–117. [Google Scholar]
- 2.Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. The Journal Of Nutrition. 1995;125:2233S–2238s. doi: 10.1093/jn/125.suppl_8.2233S. [DOI] [PubMed] [Google Scholar]
- 3.Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neuroscience & Biobehavioral Reviews. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 4.Benitez-Bribiesca L, De la Rosa-Alvarez I, Mansilla-Olivares A. Dendritic spine pathology in infants with severe protein-calorie malnutrition. Pediatrics. 1999;104:e21–e21. doi: 10.1542/peds.104.2.e21. [DOI] [PubMed] [Google Scholar]
- 5.Feoli AM, Leite MC, Tramontina AC, et al. Developmental changes in content of glial marker proteins in rats exposed to protein malnutrition. Brain Research. 2008;1187:33–41. doi: 10.1016/j.brainres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Lister JP, Blatt GJ, DeBassio WA, et al. Effect of Prenatal Protein Malnutrition on Numbers of Neurons in the Principal Cell Layers of the Adult Rat Hippocampal Formation. Hippocampus. 2005;15:393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- 7.Mokler DJ, Torres OI, Galler JR, et al. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Research. 2007;1148:226–233. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Current Opinion in Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 11.Koenigs M, Huey ED, Calamia M, et al. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galler JR, Ramsey F, Solimano G, et al. The influence of early malnutrition on subsequent behavioral development: I. Degree of Impairment in Intellectual Performance. Journal of the American Academy of Child & Adolescent Psychiatry. 1983;22:8–15. doi: 10.1097/00004583-198301000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Galler JR, Ramsey F, Salt P, et al. Long term effects of early kwashiorkor compared with marasmus. I. Physical growth adn sexual maturaiton. Journal of Pediatric Gastroenterology and Nutrition. 1987;6:841–846. doi: 10.1097/00005176-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Galler JR, Ramsey F, Solimano G. The influence of early malnutrition on subsequent behavioral development III. Learning Disabilities as a sequel to malnutrition. Pediatric Research. 1984;18:309–313. doi: 10.1203/00006450-198404000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Galler JR, Ramsey F, Solimano G, et al. The influence of early malnutrition on subsequent behavioral development: II. Classroom behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 1983;22:16–22. doi: 10.1097/00004583-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Galler JR, Ramsey F, Morley DS, et al. The long-term effectso f early kwashiorkor compared with marasmus. IV. Performance on the National High School Entrance Examination. Pediatric Research. 1990;28:235–239. doi: 10.1203/00006450-199009000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Hammen C, Brennan PA. Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Archives Of General Psychiatry. 2003;60:253–258. doi: 10.1001/archpsyc.60.3.253. [DOI] [PubMed] [Google Scholar]
- 18.Galler JR, Harrison RH, Biggs MA, et al. Maternal moods predict breastfeeding in Barbados. Journal of Developmental and Behavioral Pediatrics. 1999;20:80–87. doi: 10.1097/00004703-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Rahman A, Iqbal Z, Bunn J, et al. Impact of maternal depression on infant nutritional status and illness. Archives Of General Psychiatry. 2004;61:946–952. doi: 10.1001/archpsyc.61.9.946. [DOI] [PubMed] [Google Scholar]
- 20.Galler JR, Bryce CP, Waber D, et al. Early childhood malnutrition predicts depressive symptoms at ages 11–17. Journal of Child Psychology and Psychiatry. 51:789–798. doi: 10.1111/j.1469-7610.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozoff B, Jimenez E, Hagen J, et al. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51–E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 22.Masten AS, Roisman GI, Long JD, et al. Developmental Cascades: Linking Academic Achievement and Externalizing and Internalizing Symptoms Over 20 Years. Developmental Psychology. 2005;41:733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- 23.Wachs TD. Multiple influences on children's nutritional deficiencies: a systems perspective. Physiology & Behavior. 2008;94:48–60. doi: 10.1016/j.physbeh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman BP, Izard CE, Kobak R, et al. Relation between reading problems and internalizing behavior in school for preadolescent children from economically disadvantaged families. Child Development. 2007;78:581–596. doi: 10.1111/j.1467-8624.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children. New York: Psychological Corporation; 1949. [Google Scholar]
- 26.Richardson S, Birch H, Grabie E, et al. The behavior of children in school who were severely malnourished in the first two years of life. Journal of Health and Social Behavior. 1972;13:276–285. [PubMed] [Google Scholar]
- 27.Galler JR, Ramsey F. A follow-up study of the influence of early malnutrition on development: Behavior at home and at school. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28:254–261. doi: 10.1097/00004583-198903000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Rundquist EA, Sletto RF. Personality in the Depression - A Study in the Measurement of Attitudes. Minneapolis: University of Minnesota Press; 1936. [Google Scholar]
- 29.Galler JR, Waber D, Bryce C, et al. A Longitudinal Study of Depressive Symptoms in Mothers of Children and Adolescents with Histories of Early Malnutrition in Barbados. Nutritional Neurosciences. In press. [Google Scholar]
- 30.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavioral Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 31.Rosene DL, Lister JP, Schwagerl AL, et al. Prenatal protein malnutrition in rats alters the c-Fos response of neurons in the anterior cingulate and medial prefrontal region to behavioral stress. Nutritional Neuroscience. 2004;7:281–289. doi: 10.1080/10284150400015573. [DOI] [PubMed] [Google Scholar]
- 32.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natsuaki MN, Biehl MC, Xiaojia G. Trajectories of Depressed Mood From Early Adolescence to Young Adulthood: The Effects of Pubertal Timing and Adolescent Dating. Journal of Research on Adolescence (Blackwell Publishing Limited) 2009;19:47–74. [Google Scholar]
- 34.Corapci F, Calatroni A, Kaciroti N, et al. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. Journal Of Pediatric Psychology. 35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raskind MH, Goldberg RJ, Higgins EL, et al. Patterns of Change and Predictors of Success in Individuals With Learning Disabilities: Results. Learning Disabilities Research & Practice (Lawrence Erlbaum) 1999;14:35. [Google Scholar]