Abstract
OBJECTIVE
To ascertain progression and regression of cervical dysplasia in HIV-infected women in Soweto.
DESIGN
Prospective cohort
METHODS
Women attending an HIV wellness clinic were offered cervical smears as part of care; smears were assessed using the Bethesda system. Those with high grade lesions or worse were referred for colposcopy. Progression analyses included women with at least two smears ≥5.5 months apart. Hazard ratios (HR) were used to ascertain predictors of progression.
RESULTS
2,325 women had a baseline smear; their median age and CD4 count was 32 yrs and 312 cells/μl respectively; 17% were taking highly active antiretroviral therapy (HAART); 62%, 20% and 14% had normal, low grade squamous intraepithelial lesions (LSIL) or high grade squamous intraepithelial lesions (HSIL), respectively. Of those with baseline normal or LSIL smears, 1,074 had another smear; progression from normal to LSIL was 9.6/100py (95% CI 8.3-11.1) and progression from normal or LSIL to HSIL was 4.6/100py (95% CI 3.9-5.5). Of 225 women with LSIL at baseline and ≥1 subsequent smear ≥11.5 months later, 44.0% regressed to normal (21.2/100py (95% CI 17.5-25.7)). Multivariate models suggested risk for progression in women with CD4 count <200; HAART reduced the risk of progression (aHR 0.72 [0.52-0.99]).
CONCLUSION
HIV-infected women have high rates of prevalent and incident HSIL and LSIL with relatively low risk of regression to normal from LSIL. HAART appears to protect against progression. Our findings suggest cervical screening intervals should be less than 10 years - irrespective of age in women with CD4 counts under 500 cells/mm3.
Keywords: antiretroviral therapy, cohort, squamous intraepithelial lesion, CD4, cervical cancer, HPV
BACKGROUND
Squamous carcinoma of the uterine cervix is the second leading cancer in women worldwide and the leading cause of cancer death in women living in many developing settings[1]. Of the 500 000 cases and 275 000 deaths from squamous carcinoma worldwide in 2002, over 80% occurred in developing settings [2]. Squamous cervical cancer is initiated by infection with the Human Papillomavirus (HPV) [3,4,5], and generally progresses in stages from low grade squamous intraepithelial lesions (LSIL) to high grade squamous intraepithelial lesions (HSIL). HSIL may then progress to invasive squamous carcinoma. The risk of progression from LSIL to HSIL and invasive carcinoma is low, with a small minority (~11%) of untreated LSIL women eventually progressing to HSIL and invasive carcinoma [6] over several decades. This long premalignant phase allows for preventive interventions: screening with curative excision of the affected zone of the cervix for cases shown to have progressed to HSIL.
Most longitudinal data on the natural history of HPV infection and progression to malignancy has been derived from HIV-seronegative women in developed settings. In HIV infected women however, higher rates of HPV infection [7,8,9,10], more rapid and more frequent progression [11], with poorer treatment outcomes [11,12,13] compared to HIV-seronegative have been reported. South African women have very high rates of invasive cervical cancer; in 1997 Black South Africa women had a lifetime risk of 1 in 23 for invasive cervical cancer [14].
South Africa has the highest burden of HIV-infected people, globally and women in developing settings such as South Africa bear a higher burden of HIV infection than men; in young South African women HIV seroprevalence is double that of men [15]. Moreover, as HIV-infected women increasingly access antiretroviral treatment (ART) programmes in large numbers –over 920000 people had been initiated on HAART by May 2010 in South Africa alone [16]– a larger proportion will survive for many years.
Data on progression and regression of premalignant lesions in HIV-infected women is increasingly important to include their needs in planning of cervical screening and treatment programs, and to estimate potential benefits of novel cervical cancer prevention methods. We report the prevalence and rates of both regression and progression of premalignant cervical lesions in an operational cohort of HIV-infected women in Soweto, South Africa.
METHODS
An operational cohort of treatment naïve HIV-infected men and women, 18 years and older was initially established in 2003 in Soweto to rapidly transition patients onto antiretrovirals (ARVs) once they became widely available a year later. Methods have been described previously [17,18]. The cohort continues to enroll new participants. In brief, all participants receive a package of care provided by primary care nurses, supported by a doctor, that includes symptomatic screening for TB and further investigation of those with TB symptoms, symptomatic screening for sexually transmitted infections (STIs) with syndromic management for those with symptoms, and treatment for common HIV-related ailments with referral to the local hospital for more severe illness. Female contraceptive methods are dispensed by nurses as part of the consultation. CD4 counts are scheduled semi-annually and those eligible for ARVs - according to South African guidelines - are referred to ART treatment clinics. [19]. Women in the cohort are offered annual cervical smears. Those who provide written informed consent have a baseline questionnaire administered to collect socio-demographic, clinical and behavioral data and again at visits scheduled six monthly. This cohort has active follow-up, triggered by non-attendance at the clinic for longer than 6 months and escalates from telephone contact to letters posted to home addresses and then a home visit.
Conventional Pap smears are performed, assessed at the National Health Laboratory Service (NHLS) Cytopathology Division in Johannesburg which is accredited by the South African National Accreditation System (SANAS) using the 2001 Bethesda reporting system [20]. The cytology report of women found to have LSIL or atypical squamous cells of uncertain significance (ASCUS) includes a recommendation to have a repeat smear in 6-12 months; those with atypical squamous cells - cannot exclude a high grade lesion (ASCH), HSIL or a more severe diagnosis recommends that the woman be referred for colposcopy. Women in our cohort are referred to the colposcopy clinic at the adjacent hospital for further curative treatment [21]. Laboratory reports of women whose smear is unable to be evaluated either due to inadequate endocervical or ectocervical cell sampling, or due to obscuring blood or inflammation includes a recommendation for a repeat smear either immediately or, following treatment for infection.
Smear readers were not blinded to previous smear results but every smear was verified by a second reader as per standard screening protocols in this laboratory. The study was approved by the Ethics Committee of the University of the Witwatersrand, and analysis of anonymized data by the Johns Hopkins University Institutional Review Board.
Study Definitions
Progression was defined either as a subsequent smear with a cytological diagnosis of ASCH, HSIL or worse in women who had a previous normal or LSIL smear with an interval between smears of >5.5 months, or smears that were normal at baseline and progressed to LSIL over an interval of >5.5 months. Regression was defined as an initial LSIL smear, followed by a subsequent normal cervical smear. For the purposes of this study, sufficient time had to elapse between smears to allow regression to occur; the interval between smears for our analysis of regression was therefore >11.5 months. A sensitivity analysis was also performed using all women with baseline LSIL diagnoses who had at least one subsequent smear irrespective of its timing.
For 16 women diagnosed with ASCUS at baseline, their subsequent smear was used instead of their baseline smear in the longitudinal analysis of both regression and progression. Women with ASCUS at baseline who did not have another smear or who had only subsequent ASCUS smears were not included in the longitudinal regression/progression analysis (n=57). Women with cytologically normal baseline smears and then only ASCUS subsequent smears were excluded from the progression analysis (n=12); similarly, women with LSIL baseline smears and then only ASCUS subsequent smears were excluded from the progression and regression analyses (n=4).
Statistical Issues
We compared baseline characteristics of women who did and did not receive cervical smears using chi square equality of median tests or equality of proportions tests as appropriate. Logistic regression was performed to assess associations with prevalent pre-malignant lesions. For regression and progression analyses, both cumulative percentages of women who progressed/regressed and the incidence rate using person time of progression/regression are reported. Cox proportional hazards models were used to assess associations with progression and regression. The final analysis of risk factors for incidence of progression was further restricted from 1,074 to 950 women who had CD4 counts available within 6 months of their baseline cervical smear. CD4 count at time of baseline cervical smear was used in the final analysis; sensitivity analyses using time-varying CD4 counts were also performed. Variables were selected to be included in regression models based on prior literature and biological plausibility. Weight was not included in the final multivariate model as it was biologically the least plausible of the variables explored, highly correlated with CD4 cell count and was not shown to be associated with the outcome in the univariate model. Weight was stratified into tertiles for convenience as it was not expected to have a linear relationship with the model outcomes.
RESULTS
There were 2,475 women in this operational cohort of whom 2,325 (94%) had at least one analyzable cervical smear between August 2003 and May 2009. The 150 women not screened had a statistically significantly lower prevalence of STI symptoms, and a higher BMI on entry into the cohort than those women who had at least one cervical smear (Table 1); other characteristics however, were similar between the two groups. Out of the 4,425 cervical smears included in the study sample, 39 smears were rejected as unsatisfactory (0.9%). For those participants with subsequent satisfactory cervical smears, this smear was used in the analysis. Twenty-two individuals with rejected smears did not have a subsequent satisfactory smear.
Table 1.
Baseline characteristics of 2,475 wellness cohort women recruited between August 2003 and May 2009 and including both women who had at least one cervical smear and those who did not have a smear on record.
| Women without a cervical smear result [95% CI or IQR] (n=150)1 | Women with an interpretable cervical smear result [95% CI or IQR] (2,325) | p-value2 | |
|---|---|---|---|
| Median age (yrs) | 32.8 [29.1 – 38.1] | 31.7 [27.5 – 36.8] | 0.060 |
| Median CD4 count (cells/mm3) | 356 [215 – 474] | 312 [168 – 486] | 0.123 |
| Median weight (kg) | 65.5 [56.7 – 79.4] | 63.4 [54.9 – 74.3] | 0.105 |
| Proportion ever smokers (%) | 20.1 [14.2 – 27.1] | 15.9 [14.4 – 17.4] | 0.126 |
| Proportion with STI symptoms at baseline (%) | 11.0 [5.9 – 16.0] | 20.8 [19.1 – 22.5] 3 | 0.004 |
| Median monthly income (ZAR) | 1,200 [420 – 2,150] | 1,000 [590 – 1,900] | 0.074 |
| BMI (kg/m2) | 26.5 [22.5 – 31.0] | 25.0 [21.5 – 29.5] 4 | 0.041 |
| Median number of sexual partners in the last 6 months | 1 [1 – 1] | 1 [1 – 1] 5 | 0.444 |
| Proportion using hormonal contraception at first visit (%) | 30.1 [21.6 – 38.5] | 32.7 [30.5 – 35.0] 6 | 0.560 |
Twelve of the 150 women with missing cervical data had a cervical smear taken but it was un-interpretable and a repeat smear was not done;
Differences between groups assessed through equality of medians tests (χ2) or equality of proportions as appropriate;
the denominator for this calculation is 2,435;
the denominator for this calculation is 1,749;
the denominator for this calculation is 850;
the denominator for this calculation is 1,765
STI – sexually transmitted infection; HH – household; BMI – body mass index; IQR – interquartile range CI- Confidence intervals. ZAR- South African Rands
In women who had at least one cervical smear, a median of 7 days (IQR 0-188) elapsed between their first study visit and the smear being taken. At baseline cervical smear, 94.3% of the participants were not yet receiving-HAART. One hundred and fifty-two women were on HAART at the time of their first cervical smear and in follow up, another 457 women were initiated on HAART. The median follow-up time while receiving HAART was 24.3 months (IQR 14.3-33.4).
Prevalence of premalignant cervical lesions
Thirty-eight percent (38%) of women had a prevalent premalignant uterine cervical lesion diagnosed cytologically at their first smear (Table 2). The median age and CD4 count of women with premalignant lesions was 31.9 (IQR 27.7-36.4) and 254 cells/μL (IQR 136-401) respectively compared to those with a baseline normal smear whose median age was 32.3 (IQR 28.2-37.6) years (p=0.252) and median CD4 count was 351 cells/μL (IQR 192-530) (p=0.000). Ninety-one percent of participants with a baseline cervical smear result were also syndromically screened for symptoms of other sexually transmitted infections within 5.5 months of their baseline smear. Overall, 18.6% of these women had symptoms of an STIs within 5.5 months of their baseline cervical smear. Amongst women with normal, LSIL and HSIL/ASCH diagnoses at baseline smear, STI symptoms were present in 17.2%, 21.0% and 18.2% respectively. Multiple logistic regression with LSIL as the outcome suggested a 13% decrease in odds of prevalent LSIL at baseline for every 100 CD4 cells/μL increase (OR 0.87, 95% CI 0.83-0.92) and a decrease in the odds of prevalent LSIL of 10% for every five additional years of age (OR 0.90, 95% CI 0.84-0.98). Smoking, weight tertile and presence of STI symptoms at baseline were not associated with prevalent LSIL.
Table 2.
Prevalence normal smears and of pre-malignant lesions of the cervix in HIV-infected women in Soweto at their baseline cervical smear (n=2,325)
| n | Prevalence (%) [95% CI] | Median age (Yrs) [IQR] | Median CD4 count (cells/mm3) [IQR] | |
|---|---|---|---|---|
| Normal Smears | 1,439 | 61.9 [59.9 – 63.9] | 32.3 [28.2 – 37.6] | 351 [191 – 531] |
| Pre-malignant Lesions (ALL) | 886 | 38.1 [36.1 – 40.1] | 31.9 [27.7 – 36.4] | 254 [136 – 401] |
| ASCUS | 73 | 3.1 [2.4 – 3.8] | 29.3 [25.4 – 34.7] | 265 [175 – 504] |
| LSIL | 474 | 20.4 [18.7 – 22.0] | 31.6 [27.1 – 36.0] | 273 [144 – 406] |
| ASCH | 23 | 1.0 [0.6 – 1.4] | 33.9 [27.1 – 37.0] | 263 [155 – 463] |
| HSIL | 315 | 13.5 [12.1 – 14.9] | 32.9 [29.2 – 37.3] | 229 [125 – 374] |
| AGUS | 1 | 0.0 [0 – 0.1] | 26.5 [ -- ] | 115 [ -- ] |
| Cancer | 0 | -- | -- | -- |
ASCUS – atypical cell of uncertain significance; LSIL – low grade intraepithelial lesion, ASCH – atypical cells cannot exclude HSIL; HSIL – high grade squamous intraepithelial lesion; AGUS – atypical glandular cells of uncertain significance.
In a separate multivariate logistic regression model assessing predictors of prevalent HSIL at baseline, higher CD4 count was also associated with decreased adjusted odds of prevalent HSIL (OR 0.82, 95% CI 0.77-0.87 per increment of 100 CD4 cells/μL). However, age was not associated with prevalent HSIL (OR 1.04, 95% CI 0.96-1.13 per 5 year increase); but being in the highest tertile of weight decreased the adjusted odds of prevalent HSIL (OR 0.71; 95% CI 0.51-0.97).
Progression and regression of cervical premalignant lesions
In the prospective analysis, 1,193 (51.3%) women had at least one smear, > 5.5 months after their baseline smear. Women who had only one smear during this time period were more likely to have a baseline intra-epithelial lesion (45% v 33% [p=0.000]) and more likely to be lost to follow up or have died (p=0.000) than women with two or more smears. However, they did not differ by CD4 count (p=0.202), age (p=0.290) or highly active antiretroviral treatment (HAART) status at time of their first smear (p=0.320). One hundred and nineteen women who had more than one smear at least 5.5 months apart but with a baseline ASCH, HSIL or AGUS smear were excluded from the progression/regression analyses. Thus, 1,074 women with baseline normal or LSIL smears and subsequent smears which were either normal, LSIL, ASCH, HSIL or invasive malignancy were included in the prospective analyses. The median follow-up time from first smear to last smear in women with two or more cervical smears, and whose baseline smear was normal or LSIL, was 2.5 years (IQR 1.7-3.4).
Overall, 10.5% (95% CI: 8.7-12.4) of women with multiple smears and a baseline of normal or LSIL smear progressed to HSIL during follow-up, and in person years of follow up, progression to LSIL and HSIL amongst those with baseline normal smears was 9.6/100 person years (py) (95% CI 8.3-11.1) and 3.3/100 py (95% CI 2.6-4.2), respectively (Table 3). Of 832 women with a baseline normal smear, 69.6% (95%CI 66.5 – 72.7) had a subsequent normal smear, 21.8% (95% CI 18.9-24.6) progressed to LSIL, 7.3% (95% CI 5.6%-9.1%) progressed to HSIL, and 1.3% (95% CI 0.5-2.1) to ASCH. In 242 women with baseline LSIL, the proportion whose subsequent smear at least 5.5. months later was LSIL, ASC-H or HSIL was 45.5% [95%CI 39.1 – 51.8], 0.8% [0.0 – 2.0], and 21.5% [95%CI 16.3 – 26.7], respectively and incidence in these women of HSIL was 9.8/100 py (95% CI 7.5-12.7). Overall, the combined progression to ASCH or HSIL from a baseline normal or LSIL smear was 4.6/100 py (95% CI 3.9-5.5).
Table 3.
Progression in events per 100 person years in women from Soweto who had at least 2 interpretable cervical smears August 2003 – May 2009.
| Subsequent Smear1 | ||||
|---|---|---|---|---|
| Incident LSIL per 100 PY [95% CI] | Incident HSIL or ASCH per 100 PY [95% CI] | Any Progression (LSIL, HSIL, ASCH) per 100 PY [95% CI] | ||
| Baseline Smear | Normal (n=832) | 9.6 [8.3 – 11.1] | 3.3 [2.6 – 4.2]2 | 12.3 [10.9 – 13.9] |
| LSIL (n=242) | NA | 9.8 [7.5 – 12.7]3 | NA | |
Intervals between smears at least 5. 5 months apart;
61 cases were diagnosed as HSIL and 11 as ASCH;
52 cases were diagnosed as HSIL and 2 as ASCH
LSIL – low grade squamous intraepithelial lesion; HSIL – high grade squamous intraepithelial lesion; ASCH – atypical squamous cells cannot exclude HSIL
In those women with smears at least 11.5 months apart and whose initial smear was LSIL (n=225), incidence of regression to a subsequent normal smear was 21.2/100 py [95%CI 17.5 – 25.7]. Seventeen of the participants with a baseline LSIL diagnosis had only one subsequent smear which was taken before the full 11.5 months had elapsed. These smears ranged in time from 144-349 days after the baseline LSIL smear. In a sensitivity analysis using all 242 participants with a baseline LSIL smear and at least one subsequent smear, regardless of elapsed time between the first and last smear, the incidence of regression was 20.7/100 py [95%CI 17.1-25.1].
Associations with progression and regression
Women with baseline normal or LSIL smears contributed 2,353 person-years in the analysis of time-to-progression. In the univariate models assessing risk factors for any progression from baseline normal or baseline LSIL, only lower CD4 count was statistically significantly (p<0.05) associated with progression (Table 4). Multivariate model results, which assess the effect of CD4 count at time of baseline smear on the risk of progression, controlling for category of age, baseline smear result (normal vs. LSIL), smoking history (ever vs. never) and time-varying HAART status showed that baseline CD4 strata remained strongly associated with progression and followed a dose-response relationship across CD4 categories. The risk of progression was increased two-fold in those with CD4 counts <200 cells/μL vs. >500 cells/μL (aHR 1.96; 95% CI 1.33-2.88); likewise, adjusted hazard ratios for CD4 strata 200-350 and 351-500 as compared to >500 were 1.90 (95% CI 1.32-2.73) and aHR 1.65 (95% CI 1.14-2.39) respectively. This relationship was maintained in a sensitivity analysis using time-varying CD4 count in a subset of the cohort who had CD4 counts within 6 months of every cervical smear (n=632) (results not shown). HAART was also independently and statistically significantly associated with a decrease of 28% in risk of progression (aHR 0.72; 95% CI 0.52-0.99) in adjusted analysis. No other measured variables were associated with progression in the adjusted model. Kaplan-Meier curves suggest a threshold effect of baseline CD4 count <500 cells/μL on time to progression (Figure 1).
Table 4.
Progression rates and Univariate and Multivariate Hazard Ratios for Progression (n=950)
| Progression events /100py [95% CI] | Univariate Hazard Ratio [95% CI] | Multivariate Hazards Ratio [95% CI] | |
|---|---|---|---|
| Age in years | |||
| 18-25 | 13.6 [10.3 – 17.9] | REF | REF |
| 26-35 | 12.1 [10.3 – 14.2] | 0.86 [0.62 – 1.18] | 0.86 [0.62 – 1.18] |
| 36-45 | 9.4 [7.2 – 12.3] | 0.68 [0.46 – 1.00] | 0.68 [0.46 – 1.01] |
| >45 | 12.1 [7.3 – 20.0] | 0.89 [0.50 – 1.58] | 0.83 [0.46 – 1.49] |
| Baseline CD4 Count | |||
| <200 | 12.8 [10.3 – 16.0] | 1.54 [1.09 – 2.19]* | 1.96 [1.33 – 2.88]*** |
| 200-350 | 13.2 [10.5 – 16.4] | 1.69 [1.19 – 2.42]** | 1.90 [1.32 – 2.73]*** |
| 351-500 | 12.9 [10.2 – 16.4] | 1.62 [1.12 – 2.34]** | 1.65 [1.14 – 2.39]** |
| >500 | 8.1 [6.1 – 10.7] | REF | REF |
| Baseline Smear Result | |||
| Normal | 12.3 [10.9 – 14.1] | REF | REF |
| LSIL | 9.2 [6.9 – 12.3] | 0.79 [0.57 – 1.09] | 0.73 [0.53 – 1.02] |
| HAART Status | |||
| On HAART | 11.4 [8.9 – 14.5] | 0.86 [0.65 – 1.13] | 0.72 [0.52 – 0.99]* |
| Not on HAART | 11.8 [10.3 – 13.5] | REF | REF |
| Smoking Exposure | |||
| Ever Smoker | 13.4 [10.2 – 17.8] | 1.16 [0.85 – 1.58] | 1.13 [0.83 – 1.54] |
| Never Smoker | 11.4 [10.0 – 12.9] | REF | REF |
| Baseline Weight (kg) | |||
| Bottom Tertile (34.0 – 59.0 kgs) | 12.2 [9.9 – 15.0] | REF | -- |
| Middle Tertile (59.1 – 72.3 kgs) | 12.0 [9.8 – 14.7] | 1.07 [0.80 – 1.42] | -- |
| Upper Tertile (72.4 – 153.0 kgs) | 11.0 [8.9 – 13.4] | 1.03 [0.77 – 1.37] | -- |
p≤0.05;
p≤0.01;
p≤0.001
LSIL – low grade squamous intraepithelial lesion; HAART – highly active antiretroviral therapy
Figure 1.
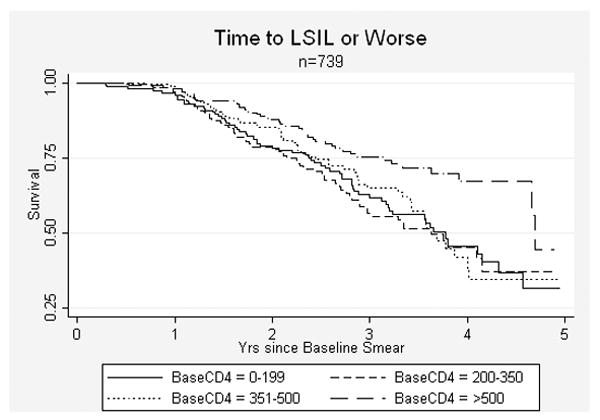
Kaplan-Meier Survival Curves for Time-to-diagnosis of low grade intraepithelial lesion (LSIL) or worse in women with baseline normal smears stratified by CD4 count at time of baseline cervical smear.
Amongst women with multiple smears separated by at least 11.5 months whose baseline smear was LSIL and who had a CD4 count within six months of the first smear (n=198), the incidence of regression to a normal smear was 20.5/100 py (95% CI 16.7-25.3). In the univariate and final adjusted Cox proportional hazards models, none of the CD4 count strata were strongly or statistically significantly associated with regression to normal. After adjusting for CD4 count at time of baseline smear, time-varying HAART status, smoking history, and age strata, only being in the highest age category (>45) as compared to the reference group of women of 18-25 years was associated with regression to normal (aHR 6.71; 95% CI 2.08-21.67). There was, however, a general trend across the age strata suggesting that older age is associated with higher rates of regression. This trend held across sensitivity analyses, including time-varying CD4 count and age as a continuous variable.
DISCUSSION
This large cohort of HIV-infected women followed prospectively shows extremely high rates of intra-epithelial cervical lesions– both prevalence (38.1%) and progression (11.7/100 person-years). Cumulatively, over one quarter of women with a baseline normal or LSIL lesion progressed to a high grade cervical lesion during follow-up, but lesions of fewer than half of women with baseline LSIL regressed. An important finding was that none of the cervical smears showed cytological features of carcinoma.
Similar high rates of prevalent squamous intra-epithelial lesions in HIV-infected women at a relatively young age have been reported from South Africa [7,22]. In Cape Town, prevalence rates of 35% and 13% for LSIL and HSIL respectively were reported at baseline in a cohort of HIV infected women, 68% of whom had high risk HPV subtypes. Over 36 months, 17% of the women progressed to incident LSIL compared to 21.8% in our study, also, fewer progressed from LSIL to HSIL (4% v 21.5%) but none received HAART. A cross-sectional study from Johannesburg reports increasing risk of cervical lesions with decreasing CD4 counts, and HPV types 16 (41.7%) and HPV 56 (22.2%) were most frequently detected in women with HSIL [22].
We noted higher rates of regression from LSIL to normal with increasing age. The natural history of low grade cervical lesions in the general population is that they are more frequent in younger women in the first decade after sexual debut, but most regress spontaneously over time leaving behind a small proportion of lesions likely to progress [23,24,25]. Thus, LSIL in an older woman would be expected to be a more persistent lesion. The protective effect of age on persistence/progression in this HIV infected cohort is unexplained. We found, like others in developed settings [26,27,28] that LSIL persistence was a predictor of progression. Although this is the first report that we are aware of describing the effect of HAART on progression and regression of premalignant lesions in a high HIV prevalence setting, our ability to draw inferences on the benefits of HAART is limited because HAART initiation is indicated by a CD4 count of less than 200 cells/μl. However, in our primary analysis including baseline CD4 count as well as in a sensitivity analysis using time-varying CD4 counts as a covariate in the multivariate model of progression, HAART independently protected against progression. Indeed, we were able to show a protective effect of HAART despite short durations on antiretroviral therapy. Conflicting findings on the effect of HAART on risk of progression in HIV-infected women have been reported [26,29,30] and studies with longer follow-up on HAART are warranted, especially in resource limited settings. Inferences able to be drawn from our data are limited by the relatively few women with third and fourth cervical smears. However, as far as we are aware, this is the most comprehensive longitudinal report of cytological progression and regression of a large number of HIV-infected women followed prospectively in a high HIV, high HPV prevalence setting. Moreover, we do not have a comparative group of HIV-seronegative women. Smear readers were not blinded to prior smear results; we posit that this may have resulted in spuriously higher rates of premalignant lesions than if readers were blinded. However, each smear was verified by a second screener, and all previous smears with a higher/lower grade diagnosis to the current were then re-reviewed by a senior technologist. We believe that these measures countered this potential source of bias. Finally, we did not collect data on whether women had a cervical smear before they came to our study. Guidelines for a South African National Cervical Smear Screening Programme were established in 2000 but implementation has been poor. We therefore think that over-diagnosis bias does not apply.
A multicenter South African study [31] reported prevalence of abnormal smears to be far lower than that reported by ourselves; 2.4% LSIL and 1.8% HSIL. Although HIV serostatus of participants was not reported on, at the time of the study, antenatal seroprevalence in South Africa is estimated at 24.5%. [32].
Our study suggests that shorter screening intervals than the currently implemented 10-yearly in South Africa are warranted across all ages of adult HIV-infected women whose CD4<500 cells/mm3. However, annual screening of HIV-infected women currently being discussed in South Africa may not be cost-effective in resource constrained settings considering the high rates of prevalent LSIL and the absence of squamous carcinoma detected in our study. This combined with the long duration between LSIL and invasive cancer [7,8,9,10] suggests that intervals longer than a year between smears may be appropriate in this population - especially as more women are treated with HAART; LSILs can take 1-2 years to clear in an immune competent women [33] and clearance is likely prolonged in immune compromised women. Annual screening may, therefore, use scarce screening and colposcopic resources for the diagnosis of transient cervical abnormalities destined to resolve with time. A screening interval triaged by CD4 count, however, may be of value considering the dose response relationship we observed with progression. Moreover, our data suggests that women with persistent LSIL may benefit from more aggressive treatment – in line with colposcopic treatment data from Soweto [21]. We hope that our data will assist in studies assessing these questions and inform the design of randomized trials - both of treatment and prevention of premalignant and malignant cervical lesions in women – irrespective of their HIV serostatus.
Acknowledgments
The HIV-infected women who allowed us to use their data. Primary health care nurses Ms Thembi Dlamini, Ms Cynthia Mokaba and Ms Mamolefe Pudutsoane, took most of the cervical smears. The colposcopy clinic at Chris Hani Baragwanath Hospital for treating patients referred to them. Mr Yudesh Baliram for data management, and ANOVA Health Institute for grant administration.
Funding Sources: Patient care and cervical smears are funded by the President’s Emergency Plan for AIDS Relief (PEPFAR), through the US Agency for International Development (USAID) (674-A-00-05-00003-00). TO and TM received research training funded by Fogarty International Center grants for HIV-related malignancies (D43TW000010-21S1) and TB (U2RTW007370/3), respectively. NM is partially funded from NIH grant R01HL090312. The opinions expressed herein do not necessarily reflect those of the funders.
Footnotes
CONTRIBUTIONS TO, NM and SS conceived the study and wrote the paper, SS did statistical analyses assisted by CH and JG. NM set up the cohort with GG and JM. NT and LM oversaw patient care and data collection and TM managed, merged and extracted data from databases.
None of the authors report a conflict of interest.
An earlier analysis of this data was presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI) in Montreal, February 2009.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. IARC CancerBase No. 5 version 2.0. Lyon: IARC Press; 2004. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, Tafur L, Izarzugaza I, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 5.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- 7.Denny L, Boa R, Williamson AL, Allan B, Hardie D, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol. 2008;111:1380–1387. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- 8.Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 9.Minkoff HL, Eisenberger-Matityahu D, Feldman J, Burk R, Clarke L. Prevalence and incidence of gynecologic disorders among women infected with human immunodeficiency virus. Am J Obstet Gynecol. 1999;180:824–836. doi: 10.1016/s0002-9378(99)70653-8. [DOI] [PubMed] [Google Scholar]
- 10.Cubie HA, Seagar AL, Beattie GJ, Monaghan S, Williams AR. A longitudinal study of HPV detection and cervical pathology in HIV infected women. Sex Transm Infect. 2000;76:257–261. doi: 10.1136/sti.76.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright TC, Jr, Ellerbrock TV, Chiasson MA, Van Devanter N, Sun XW. Cervical intraepithelial neoplasia in women infected with human immunodeficiency virus: prevalence, risk factors, and validity of Papanicolaou smears. New York Cervical Disease Study. Obstet Gynecol. 1994;84:591–597. [PubMed] [Google Scholar]
- 12.Adam Y, van Gelderen CJ, de Bruyn G, McIntyre JA, Turton DA, et al. Predictors of persistent cytologic abnormalities after treatment of cervical intraepithelial neoplasia in Soweto, South Africa: a cohort study in a HIV high prevalence population. BMC Cancer. 2008;8:211. doi: 10.1186/1471-2407-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos MC, Pizarro De Lorenzo BH, Michelin MA, Murta EF. High-grade cervical intraepithelial neoplasia, human papillomavirus and factors connected with recurrence following surgical treatment. Clin Exp Obstet Gynecol. 2008;35:242–247. [PubMed] [Google Scholar]
- 14.Mqoqi N, Kellet P, Madhoo J, Sitas F. Incidence of histologically diagnosed cancer in South Africa, 1996-1997. Johannesburg: National Cancer Registry of South Africa, National Health Laboratory Service; 2003. [Google Scholar]
- 15.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? HSRC Press; Cape Town: 2009. [Google Scholar]
- 16.Anonymous. South Africa and HIV/AIDS. Setting a better example. The Economist. 2010;395:41. [Google Scholar]
- 17.Lurie M, Pronyk P, de Moor E, Heyer A, de Bruyn G, et al. Sexual behavior and reproductive health among HIV-infected patients in urban and rural South Africa. J Acquir Immune Defic Syndr. 2008;47:484–493. doi: 10.1097/QAI.0b013e3181648de8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub JE, Pronyk P, Mohapi L, Tshabangu N, Moshabela M, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: A prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Operational plan for comprehensive HIV and AIDS care, management and treatment for South Africa. Department of Health; Republic of South Africa Pretoria: 2003. [Google Scholar]
- 20.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2000;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 21.Adam Y, van Gelderen CJ, Newell K. ‘Look and Lletz’--a Chris Hani Baragwanath Hospital experience. S Afr Med J. 2008;98:119–122. [PubMed] [Google Scholar]
- 22.Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control. 2010;21:433–443. doi: 10.1007/s10552-009-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol. 1996;175:1099–1104. doi: 10.1016/s0002-9378(96)70011-x. [DOI] [PubMed] [Google Scholar]
- 24.Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–258. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 25.Miller AB. Natural history of cervical human papillomavirus infections. Lancet. 2001;357:1816. doi: 10.1016/S0140-6736(00)05008-X. [DOI] [PubMed] [Google Scholar]
- 26.Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15:2157–2164. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 27.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190:37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Heard I, Schmitz V, Costagliola D, Orth G, Kazatchkine MD. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS. 1998;12:1459–1464. doi: 10.1097/00002030-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Paramsothy P, Jamieson DJ, Heilig CM, Schuman PC, Klein RS, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstet Gynecol. 2009;113:26–31. doi: 10.1097/AOG.0b013e31819225cb. [DOI] [PubMed] [Google Scholar]
- 31.Fonn S, Bloch B, Mabina M, Carpenter S, Cronje H, et al. Prevalence of pre-cancerous lesions and cervical cancer in South Africa--a multicentre study. S Afr Med J. 2002;92:148–156. [PubMed] [Google Scholar]
- 32.The National HIV and syphilis antenatal sero-prevalence survey in South Africa 2007. Department of Health Republic of South Africa; Pretoria: 2008. [Google Scholar]
- 33.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]


