Abstract
Xamoterol, a partial β1-adrenergic receptor agonist, has been reported to impair the retrieval of hippocampus-dependent spatial reference memory in rats. In contrast, xamoterol restores memory retrieval in gene-targeted mice lacking norepinephrine (NE) and in a transgenic mouse model of Down syndrome in which NE levels are reduced. Restoration of retrieval by xamoterol in these two models complements the observation that NE and β1 signaling are required for hippocampus-dependent retrieval of contextual and spatial reference memory in wild-type mice and rats. Additional evidence indicates that cAMP-mediated PKA and Epac signaling are required for the retrieval of hippocampus-dependent memory. As a result, we hypothesized that xamoterol has effects in addition to the stimulation of β1 receptors that, at higher doses, act to counter the effects of β1 signaling. Here we report that xamoterol-induced disruption of memory retrieval depends on β2-adrenergic receptor signaling. Interestingly, the impairment of memory retrieval by xamoterol is blocked by pretreatment with pertussis toxin, an uncoupling agent for Gi/o signaling, suggesting that β2 signaling opposes β1 signaling during memory retrieval at the level of G protein and cAMP signaling. Finally, similar to the time-dependent roles for NE, β1, and cAMP signaling in hippocampus-dependent memory retrieval, xamoterol only impairs retrieval for several days after training, indicating that its effects are also limited by the age of the memory. We conclude that the disruption of memory retrieval by xamoterol is mediated by Gi/o-coupled β2 signaling, which opposes the Gs-coupled β1 signaling that is transiently required for hippocampus-dependent emotional memory retrieval.
Among the neural systems that underlie episodic memory, the hippocampus is central. Bilateral damage to the hippocampus eliminates the ability to form new episodic memories, as well as impairs the ability to recall recent but not remote episodic memories (McClelland et al. 1995; Squire et al. 2001). Intense, psychologically traumatic experiences can lead to psychiatric problems such as post-traumatic stress disorder (PTSD). Dysregulation of episodic memory is one of the cardinal symptoms in PTSD patients, who often suffer from recurrent, intrusive memories of traumatic events (Rubin et al. 2008). Frequent retrieval of such memories can be debilitating due to the fear and anxiety they invoke.
For this and other reasons, the mechanisms underlying the consolidation, retrieval and, reconsolidation of episodic memory are of great interest. Many studies have contributed to our understanding of the molecular and cellular signaling pathways important for episodic memory consolidation (Kandel 2001; Morris et al. 2003; Tonegawa et al. 2003; Abel and Nguyen 2008). In addition, some studies have begun to illuminate signaling pathways required for the retrieval of episodic memory (Szapiro et al. 2002; Martin and Clark 2007). As a result, it has been suggested that the consolidation and retrieval of hippocampus-dependent memory have common neurobiologic substrates and signal transduction pathways. As an example, nearly four decades of research across species illustrate that agents that acutely elevate cAMP (cyclic 3′,5′-adenosine monophosphate) usually enhance memory consolidation, whereas agents that block cAMP signaling impair consolidation (Kandel 2001; Abel and Nguyen 2008).
Recent evidence suggests that cAMP signaling is also involved in hippocampus-dependent memory retrieval. Bilateral infusion of a cAMP antagonist into the dorsal hippocampus (DH) of rats 10 min before testing inhibitory avoidance 1 d after training reduces step-down latency (Barros et al. 2000). Further, signaling by norepinephrine (NE) through the β1-adrenergic receptor is required for the retrieval of an intermediate phase (from 2 h to ∼4 d after training) of hippocampus-dependent memory (Murchison et al. 2004). Mice with targeted disruption of the gene for the β1 receptor (β1 KO) display deficits in contextual fear, and systemic administration of the β1 antagonist betaxolol produces retrieval deficits in wild-type mice and rats. These effects are reproduced by infusion of the β1-selective antagonist atenolol into the DH.
β1 receptors couple to the adenylyl cyclase stimulatory G protein, Gs, and their activation elevates cAMP levels in the DH (Fowler and O'Donnell 1988). cAMP antagonists impair retrieval when infused into the DH shortly before testing contextual fear, and DH infusion of cAMP agonists rescues retrieval in gene-targeted mice lacking NE (Ouyang et al. 2008). Further, activation of at least two targets of cAMP signaling, protein kinase A (PKA) and the exchange protein activated by cAMP (Epac), is also required for hippocampus-dependent memory retrieval. Finally, transgenic mice expressing an invertebrate, Gs-coupled octopamine receptor exhibit both octopamine-dependent increases in DH cAMP and enhanced contextual fear memory retrieval (Isiegas et al. 2008).
In contrast to the above, it has been suggested that stimulation of β1 receptors impairs hippocampus-dependent memory retrieval. In rats, DH infusion of the β1-selective antagonist atenolol blocks the impairing effects of a glucocorticoid receptor agonist on the retrieval of spatial reference memory (Roozendaal et al. 2004). Similar to a glucocorticoid agonist, injection of 3 or 10 mg/kg of the β1-selective agonist xamoterol decreases spatial reference memory retrieval. The authors conclude that the impairment of memory retrieval by glucocorticoids and xamoterol involves the activation of β1-coupled cAMP signaling in the hippocampus. This observation is surprising because administration of xamoterol at 1–3 mg/kg fully rescues contextual memory retrieval in gene-targeted NE-deficient mice, suggesting that β1 signaling is sufficient for the role of NE in retrieval (Murchison et al. 2004).
The goal of this study was to resolve the seemingly conflicting observations that β1 signaling is required for hippocampus-dependent memory retrieval and is also responsible for the impairment of retrieval. Of note, somewhat higher doses of xamoterol were needed to impair retrieval in wild-type rats as compared to those needed to rescue retrieval in mutant mice. Differences in dosing requirements could be due to differences between species, genetic manipulation, or mechanism of action. To address this, we first confirmed that xamoterol impairs memory retrieval in wild-type mice, making a difference between species unlikely. As a result, we hypothesized that the pharmacologic characterization of xamoterol-induced retrieval impairment was incomplete and that another adrenergic receptor might mediate this effect. We considered the β2 receptor as a potential target of xamoterol because this receptor is most closely related to the β1 receptor pharmacologically, and roles for β2 signaling in hippocampus-dependent memory retrieval have not been described. Of potential relevance, β1 and β2 signaling in the prefrontal cortex have opposite effects on working memory (Ramos et al. 2005, 2008).
In this study, animals were tested in both Pavlovian and instrumental fear conditioning paradigms. Xamoterol and other pharmacologic reagents previously untested in these paradigms were administered to wild-type mice, as well as to mice genetically modified to lack either β1- or β2-adrenergic receptors. Our results provide substantial and unexpected evidence that signaling by Gi/o-coupled β2-adrenergic receptors in the hippocampus mediates xamoterol-induced impairment of memory retrieval.
Results
Xamoterol impairs hippocampus-dependent memory retrieval
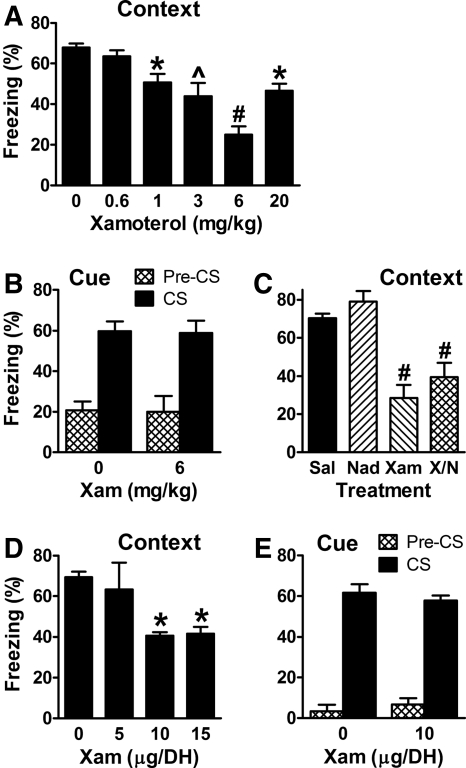
To confirm the impairing effects of xamoterol on hippocampus-dependent memory retrieval, mice were trained using either Pavlovian or instrumental fear conditioning. When 1–6 mg/kg of xamoterol was administered 30 min prior to testing, wild-type (WT) mice showed reduced contextual freezing and shorter avoidance latencies 1 d after training (Figs. 1A, 4A below). Because xamoterol-induced impairment of contextual freezing could be due to effects on memory or to nonspecific effects on fear or freezing, xamoterol (6 mg/kg) was also administered 30 min prior to testing cued fear 1 d after training. Xamoterol did not affect cued fear expression, indicating that it does not alter fear or freezing per se (Fig. 1B).
Figure 1.
Xamoterol impairs hippocampus-dependent memory. (A) Mice were fear conditioned and 1 d later vehicle or xamoterol was administered subcutaneously 30 min before testing contextual fear. Xamoterol caused a U-shaped, dose-dependent reduction in freezing that was maximal at 6 mg/kg. There were 5 mice per group except at 0 and 3 (11 and 10). F(5,35) = 10.1 and P < 0.0001 for the main effect of dose. (B) Mice were fear conditioned and 1 d later vehicle or xamoterol (Xam) was administered subcutaneously 30 min before testing cued fear. Xamoterol did not affect freezing before (Pre-CS) or during the tone used for training (conditioned stimulus = CS), indicating that xamoterol does not alter fear or freezing per se. There were 8 and 5 mice per group for vehicle and xamoterol. F(1,24) = 48 and P < 0.0001 for the main effect of tone; the main effect of drug and the interaction were not significant. (C) Xamoterol (6 mg/kg) impaired freezing when combined (X/N) with 3 mg/kg of the peripheral β receptor antagonist nadolol (Nad), indicating that xamoterol acts centrally to impair retrieval. F(1,28) = 48 and P < 0.0001 for the main effect of xamoterol; the main effect of nadolol (P = 0.1) and the interaction were not significant, although there was a trend for nadolol to increase freezing slightly in both groups. (D) Bilateral cannulae targeting the DH were implanted in mice. One week later, the mice were fear conditioned, and on the following day they were tested for contextual fear. DH infusions were performed 15 min before testing, with xamoterol causing impairment at 10 and 15 µg/DH. There were 5 and 6 mice per group for 0 and 10 and 5 and 15 µg/DH groups, respectively. F(3,18) = 3.65 and P = 0.032 for the main effect of dose. (E) Mice were fear conditioned, and 1 d later vehicle or xamoterol was infused into the DH 15 min before testing cued fear. Xamoterol did not affect freezing before or during the CS, indicating that DH infusion also does not alter fear or freezing. There were 5 mice per group. F(1,16) = 269 and P < 0.0001 for the main effect of tone. (* = P < 0.05, ∧ = P < 0.01, and # = P < 0.001 for post-hoc comparisons.)
Xamoterol could affect memory via receptors located in the periphery or the CNS (Gold 2004). To distinguish between these possibilities, the peripheral nonselective β receptor antagonist nadolol (Goldaniga et al. 1980; Woods and Robinson 1981) was administered with or without xamoterol. Nadolol impairs contextual fear memory retrieval when administered centrally but not peripherally (Murchison et al. 2004). Nadolol did not prevent the impairment induced by xamoterol (Fig. 1C), suggesting that xamoterol acts centrally to impair retrieval. To determine if xamoterol acts specifically in the hippocampus, cannulae were implanted in mice so that bilateral DH infusions could be performed. One week after surgery, cannulated mice were fear conditioned. One day later, either vehicle or xamoterol was infused 15 min before testing. Xamoterol decreased contextual freezing with a maximum effect at 10 µg (Fig. 1D). As a control, xamoterol (10 µg) was infused into the DH 15 min prior to testing cued fear 1 d after training. Xamoterol did not affect cued fear expression, indicating that it does not alter fear or freezing when delivered directly into the DH (Fig. 1E).
Although motivation and performance effects were excluded above, it remained possible that xamoterol affected contextual fear memory by impairing either retrieval itself or expression of the retrieved memory. To distinguish between these, extinction of the contextual fear memory was examined 1 d after the initial retrieval test. If xamoterol impairs retrieval 1 d after training, then testing memory again the next day (2 d after training) without further treatment should demonstrate normal contextual fear (i.e., minimal extinction). Alternatively, if xamoterol impairs expression but not retrieval, then it is predicted that testing memory again the next day should demonstrate reduced (i.e., extinguished) contextual fear. In performing similar studies examining the role of NE in retrieval and extinction, we demonstrated that it is necessary to retrieve but not express contextual fear memory for extinction to proceed (Ouyang and Thomas 2005). When vehicle was administered shortly before testing on the day after training, freezing was high, as expected (Fig. 2). When these mice were tested again the next day (no injection), freezing was significantly lower. In contrast, when xamoterol was administered shortly before testing on the day after training, freezing was low, as expected. When these mice were tested again the next day (no injection), freezing was significantly higher compared to the first day of testing and compared to the vehicle-injected mice on the second day of testing. The results are consistent with the idea that xamoterol impairs memory retrieval rather than memory expression.
Figure 2.

Retrieval of hippocampus-dependent memory is impaired by xamoterol. Vehicle (Veh) or xamoterol (Xam, 6 mg/kg) was injected 30 min before testing 1 d after training. As expected, xamoterol impaired contextual fear memory relative to vehicle. The mice were tested again the next day without injection. Mice treated with vehicle on day 1 exhibited significantly lower freezing on day 2 compared to day 1, consistent with the extinction of contextual fear. Mice treated with xamoterol on day 1 exhibited significantly higher freezing on day 2 compared to day 1 and compared to vehicle-treated mice on day 2, indicating that extinction was minimal. Because it is not necessary to express contextual fear to obtain extinction (Ouyang and Thomas 2005), the results suggest that xamoterol impairs retrieval rather than the expression of contextual fear. There were 5 mice for Veh and 9 mice for Xam. F(1,26) = 25 and P < 0.0001 for the interaction of treatment and day; the main effects of treatment and day were not significant. (* = P < 0.05, ∧ = P < 0.01, and # = P < 0.001 for post-hoc comparisons.)
Pharmacologic evidence that xamoterol disrupts retrieval through β2- and not β1-adrenergic signaling
The above data indicate that 0.6 mg/kg xamoterol does not impair contextual memory retrieval (Fig. 1A). In comparison, 0.3 mg/kg xamoterol induces significant rescue of contextual memory retrieval in NE-deficient Dbh−/− mice (Murchison et al. 2004). This difference could be the result of a potentially greater sensitivity to β1-adrenergic agonists in Dbh−/− mice. Alternatively, the impairment of retrieval in wild-type mice could be due to effects of xamoterol that are independent of β1 signaling. In this regard, the most closely related receptor to β1 is the β2-adrenergic receptor. To test the idea that higher doses of xamoterol might stimulate β2 in addition to β1 receptors, a highly selective antagonist of β2 receptors (ICI 118,551) was employed (Tsuchihashi et al. 1990). In agreement with previous results (Murchison et al. 2004), 1 mg/kg ICI 118,551 had no effect on contextual memory retrieval 1 d after training (Fig. 3A). However, co-injection of the β2 antagonist with xamoterol (6 mg/kg) blocked the impairment of contextual fear in a dose-dependent manner, with 1 mg/kg ICI producing full rescue of retrieval (Fig. 3A). To determine whether ICI nonspecifically elevates contextual freezing when freezing is relatively low, mice were conditioned using a less intense shock (0.35 mA), and vehicle or ICI was administered 30 min prior to testing. Contextual fear did not differ between the groups, indicating that ICI does not enhance fear or freezing per se (Fig. 3B).
Figure 3.
Impairment of retrieval by xamoterol is prevented by a β2 but not a β1 antagonist. (A) Co-injection of xamoterol (Xam, 6 mg/kg) with the β2 antagonist ICI 118,551 (ICI) blocked xamoterol-induced impairment of contextual fear, with 1 mg/kg ICI completely preventing disruption of retrieval. ICI (1 mg/kg) alone had no effect on retrieval. There were 5 mice per group except at 0.3 mg/kg ICI (6). F(5,25) = 9.4 and P < 0.0001 for the main effect of treatment. (B) ICI does not enhance low levels of contextual fear in the absence of xamoterol (P = 0.9). There were 5 and 4 mice for vehicle and xamoterol. Animals were trained with lower shock intensity (0.35 mA) to approximate xamoterol-impaired freezing. (C) Xamoterol (6 mg/kg) reduced retention latencies for inhibitory avoidance relative to vehicle. Co-administration of xamoterol with ICI (1 mg/kg) prevented xamoterol-induced disruption of avoidance, while co-injection of xamoterol with the β1 antagonist betaxolol (Bet, 1 mg/kg) failed to prevent disruption of retrieval. Neither Bet nor ICI altered avoidance when administered alone. There were 24, 22, 12, 15, 7, and 6 mice per group from left to right. F(5,80) = 5.14 and P = 0.0004 for the main effect of treatment. (* = P < 0.05, ∧ = P < 0.01, and # = P < 0.001 for post-hoc comparisons.)
In the above experiments, the use of contextual fear testing was limited by the fact that β1 antagonists impair contextual memory retrieval on their own. To directly compare the effects of β1 and β2 antagonists on the retrieval impairment induced by xamoterol, mice were subjected to instrumental fear conditioning. Similar to the results for contextual fear, 1 mg/kg ICI did not reduce retention latencies; however, it completely prevented the impairment in retrieval induced by xamoterol (Fig. 3C). Further, the highly selective β1 antagonist betaxolol (Tsuchihashi et al. 1990) at 1 mg/kg did not affect retention latencies when administered alone. In addition, betaxolol did not prevent xamoterol-induced disruption of inhibitory avoidance (Fig. 3C), even though 1 mg/kg betaxolol is a maximally effective dose for blocking the retrieval of contextual fear memory (Murchison et al. 2004).
Genetic evidence that xamoterol disrupts retrieval through β2- and not β1-adrenergic signaling
We sought to confirm the pharmacologic evidence suggesting that xamoterol is activating β2 and not β1 receptors to impair retrieval. Toward this goal, we examined the effects of xamoterol on retrieval in mice lacking either β1- or β2-adrenergic receptors. Instrumental fear conditioning was used because β1 KOs exhibit impaired contextual memory retrieval (Murchison et al. 2004). β1 KO and β2 KO mice displayed normal retention latencies when treated with saline, which allowed for the examination of drug affects (Fig. 4). In WT mice, xamoterol significantly reduced avoidance latencies at 1 and 6 mg/kg. Importantly, xamoterol did not reduce avoidance latencies in β2 KOs at any dose tested, indicating that β2 signaling is necessary for the impairing effects of xamoterol on retrieval. In contrast, xamoterol at 0.3 and 1 mg/kg impaired avoidance in β1 KOs, indicating that β1 signaling is not required for the disruption of retrieval by xamoterol. Interestingly, there was about a three-fold leftward shift in the U-shaped dose-response curve for xamoterol in the absence of β1 receptors, indicating that xamoterol more potently impaired retrieval in the absence of β1 receptors.
Figure 4.
Genetic evidence that β2 signaling mediates the impairment of memory retrieval by xamoterol. (A) Xamoterol at 1 or 6 mg/kg maximally disrupted avoidance in WT mice. There were 7, 7, 8, 8, 7, and 10 mice per group from left to right. F(5,41) = 3.3 and P = 0.014 for the main effect of treatment. (B) Xamoterol at 0.3 and 1 mg/kg maximally disrupted avoidance in β1 KO mice. There were 10, 9, 12, 10, and 24 mice per group from left to right. F(4,60) = 4.0 and P = 0.006 for the main effect of treatment. (C) Xamoterol did not impair avoidance at any dose when tested in β2 KO mice, indicating that β2 activation is required for xamoterol-induced disruption of memory retrieval. There were 9, 7, 9, 8, and 7 mice per group from left to right. F(4,35) = 0.41 and P = 0.8 for the main effect of treatment. (* = P < 0.05 for post-hoc comparison.)
Pertussis toxin blocks the impairing effects of xamoterol on retrieval
Canonical β-adrenergic receptor signaling is via coupling to and activation of the adenylyl cyclase-stimulatory G protein Gs. However, there is evidence that β2 receptors can also signal through Gi/o (Xiao et al. 1995; Daaka et al. 1997; Devic et al. 2001). Because cAMP signaling is necessary and sufficient for contextual memory retrieval mediated by NE and β1 receptors (Ouyang et al. 2008), we asked whether β2 signaling might oppose β1/Gs signaling in retrieval by activating Gi/o.
To test this idea, pertussis toxin (PTx) was employed because it uncouples Gi/o proteins from their receptors by ADP-ribosylation. Because PTx can take several days to be fully effective in the hippocampus in vivo (Goh and Pennefather 1989; Stratton et al. 1989), PTx was infused into the DH 1 d before fear conditioning to minimize potential effects on acquisition and consolidation. Mice were then tested 2 d after conditioning. Pretreatment with PTx did not affect retrieval in mice infused with vehicle 15 min before testing (Fig. 5). Strikingly, however, PTx pretreatment completely prevented the impairment of retrieval induced by xamoterol.
Figure 5.

Gi/o signaling is required for the impairment of retrieval by xamoterol. Bilateral cannulae targeting the DH were implanted in mice, and one week later the mice were infused with either saline (Sal) or pertussis toxin (PTx, 10 ng/DH). The next day, the mice were fear conditioned, and 2 d later the mice were infused with either vehicle or xamoterol (Xam, 10 µg/DH). Contextual fear was tested 15 min later. Pertussis toxin pretreatment blocked the impairing effect of xamoterol on retrieval, indicating that this effect is mediated by the Gi/o class of G proteins. There were 5 mice per group. F(1,18) = 6.5 and P = 0.021 for the main effect of pretreatment; F(1,18) = 10.1 and P = 0.006 for the main effect of treatment; and F(1,18) = 9.9 and P = 0.006 for the interaction. (∧ = P < 0.01 for post-hoc comparison.)
Xamoterol impairs the retrieval of intermediate-term memory
NE, β1, cAMP, and PKA signaling all have time-limited roles in hippocampus-dependent emotional memory retrieval (Murchison et al. 2004; Ouyang et al. 2008). NE-deficient mice display deficits in contextual fear 0.1–4 d but not 5+ d after training, and mice and rats treated with a β receptor antagonist show compromised contextual fear and spatial navigation over a similar time course. Therefore, we determined if β2 signaling induced by xamoterol might also have time-limited effects on retrieval. To examine short-term contextual memory, mice were injected systemically 30 min before testing and were tested at either 1 or 2 h after training. Xamoterol impaired retrieval at 2 h but not at 1 h after training (Fig. 6A). To examine longer-term contextual memory, mice were tested at either 1, 3, or 5 d after training. Contextual fear did not differ between days 1, 3, and 5 in vehicle-injected animals (Fig. 6B). However, the effects of xamoterol were time-sensitive, with animals displaying the greatest impairment 1 d after training, less impairment 3 d after training, and no impairment 5 d after training. Similar results for longer-term memory were obtained for inhibitory avoidance (Fig. 6C). The results indicate that xamoterol affects an intermediate phase of memory retrieval. They also indicate that xamoterol does not impair contextual information processing or the expression of instrumental fear via mechanisms unrelated to memory retrieval.
Figure 6.
Xamoterol impairs retrieval of an intermediate phase of hippocampus-dependent memory. (A) Xamoterol (6 mg/kg) or vehicle was administered 30 min prior to testing at 1 or 2 h after training (separate groups of mice for each time). Xamoterol impaired contextual fear at 2 h but not 1 h after training. There were 5 mice per group except vehicle at 1 h (8). F(1,21) = 9.65 and P = 0.006 for the interaction of treatment and time; the main effects of time and treatment were not significant. (B,C) Xamoterol (6 mg/kg) or vehicle was administered 30 min prior to testing at 1, 3, or 5 d after training (separate groups of mice for each test day). (B) Xamoterol impaired contextual fear at 1 and 3 but not 5 d after training. There were 5 mice per group except for vehicle on day 5 (7) and xamoterol on day 3 (10). F(1,35) = 44.1 and P < 0.0001 for the main effect of treatment; F(2,34) = 20.7 and P < 0.0001 for the main effect of day; F(2,34) = 18.8 and P < 0.0001 for the interaction. (C) Xamoterol impaired inhibitory avoidance at 1 but not 3 or 5 d after training. There were 22, 24, 7, 8, 9, and 7 mice per group going from left to right. F(1,43) = 4.1 and P = 0.05 for the main effect of treatment; F(2,42) = 4.13 and P = 0.024 for the main effect of day; F(2,42) = 4.9 and P = 0.013 for the interaction. (* = P < 0.05, ∧ = P < 0.01, and # = P < 0.001 for post-hoc comparisons.)
Discussion
A role for NE and β1-adrenergic signaling in hippocampus-dependent emotional memory retrieval is supported by multiple experimental outcomes. NE-deficient Dbh−/− mice exhibit impaired retrieval of contextual and spatial reference memory, and contextual memory retrieval is rescued in these mice by xamoterol (Thomas and Palmiter 1997; Murchison et al. 2004). Further, β1 KO mice exhibit impaired contextual memory retrieval, as do wild-type mice treated with β1 antagonists. Relevant to this, the locus coeruleus has been found to degenerate in Down syndrome (Mann et al. 1985; Marcyniuk et al. 1988). In a mouse model of Down syndrome (Ts65Dn), there is less CNS NE, fewer locus coeruleus adrenergic neurons, and more β receptor-positive neurons in the hippocampus, suggesting the presence of functional NE deficiency (Dierssen et al. 1997; Salehi et al. 2009). Similar to Dbh−/− mice, Ts65Dn mice display deficits in contextual fear memory retrieval but not in cued fear memory, and administration of a NE precursor or xamoterol reverses the impairment of memory retrieval in Ts65Dn mice by activating central β1 receptors (Salehi et al. 2009; Faizi et al. 2011).
The goal of this study was to resolve the conflicting ideas that β1 signaling is required for the retrieval of hippocampus-dependent emotional memory as outlined above and is also responsible for the impaired retrieval of such memories by xamoterol (Roozendaal et al. 2004). In Roozendaal et al., doses of 3 and 10 mg/kg were used to demonstrate retrieval impairment. In contrast, xamoterol rescues contextual memory retrieval in NE-deficient Dbh−/− mice at 0.3 and 1 mg/kg. The potency of xamoterol for β1 relative to β2 receptors is ∼10-fold in rat tissue ex vivo and at mouse adrenergic receptors in vitro (Malta et al. 1985; Beer et al. 1988; Murchison et al. 2004). As a result, we hypothesized that the higher doses of xamoterol activate β2 as well as β1 receptors and that the activation of β2 receptors impairs retrieval. In the current study, we found that xamoterol impairs memory retrieval in the presence of a β1 antagonist and in β1 KO mice (Figs. 3C, 4B), indicating that β1 signaling is not required for the impairment of retrieval by xamoterol. In contrast, we found that the impairing effects of xamoterol were absent both in the presence of a β2 antagonist and in β2 KO mice (Figs. 3, 4C). These results demonstrate that xamoterol impairs retrieval through the stimulation of β2 receptors. In support of a critical role for β2 receptors, we have also found that low doses of the β2 receptor agonists procaterol and zinterol impair memory retrieval in mice and rats (Schutsky et al. 2011). The results suggest that β2 receptors oppose the role of β1 receptors in retrieval and provide an explanation for why relatively high doses of xamoterol are required to observe impairment, i.e., because xamoterol stimulates both receptor subtypes. Consistent with this idea, xamoterol impaired memory retrieval for inhibitory avoidance at lower doses in β1 KO mice as compared to wild-type mice (Fig. 4).
Classically, β-adrenergic receptors couple to the adenylyl cyclase-stimulatory G protein Gs, and their activation increases cAMP levels. Given that β1 receptors and cAMP signaling in the hippocampus are required for hippocampus-dependent emotional memory retrieval (Murchison et al. 2004; Ouyang et al. 2008), it was not apparent how activation of β2 receptors in the hippocampus would impair retrieval. One possibility was that these two receptor subtypes are expressed on different cells or in distinct subcellular compartments, where increases in cAMP would have opposing effects on processes important for retrieval. However, a second possibility was suggested by recent studies examining signaling by β2 receptors. Evidence suggests that β2 receptors can couple to the pertussis toxin-sensitive, adenylyl cyclase-inhibitory G protein Gi and that such coupling can limit or prevent increases in cAMP in cardiac myocytes (Xiao et al. 1995; Daaka et al. 1997; Devic et al. 2001). In support of the relevance for such a mechanism in the brain for memory retrieval, uncoupling Gi/o proteins from their receptors by PTx pretreatment of the DH blocked the impairing effect of xamoterol (Fig. 5) and the β2 agonist zinterol (Schutsky et al. 2011).
Based on the above, the simplest explanation for the impairing effects of xamoterol is that they oppose the increase in cAMP that is necessary for retrieval. This idea is consistent with the observation that xamoterol was more potent at impairing memory retrieval in β1 KO mice as compared to wild-type mice (Fig. 4). In other words, when both receptors are present, higher levels of β2 stimulation are required to offset the stimulation of β1 receptors. Highlighting the contrasting roles of these two receptors, β2 signaling in the heart acts to protect cardiomyocytes from excessive β1 stimulation that can lead to apoptosis and heart failure if unchecked (Rockman et al. 2002; Zheng et al. 2005). Further, stimulating β2 receptors in the prefrontal cortex can enhance working memory, while stimulating β1 receptors impairs working memory (Ramos et al. 2005, 2008). Other mnemonic roles for β2 signaling that have been recently described include reconsolidation and stress-induced reinstatement of conditioned place preference for cocaine (Bernardi et al. 2009; Mantsch et al. 2010).
Xamoterol exhibited U-shaped dose-response curves for retrieval impairment when administered systemically to wild-type and to β1 KO mice (Figs. 1A, 4). The reason why impairment dissipates with higher doses is not clear. The U-shaped dose-response data from β1 KO mice rule out stimulation of β1 receptors as an explanation. It is possible that higher levels of β2 stimulation result in more rapid desensitization of β2 signaling, or that the recruitment of additional signaling pathways by β2 receptors mitigates signaling induced by lower levels of stimulation. Alternatively, xamoterol may stimulate other receptors (adrenergic or non-adrenergic) at the highest doses that counteract the effects of β2 stimulation on retrieval.
Interestingly, our data demonstrate that the duration between acquisition and retrieval is an important factor for determining the extent of retrieval impairment (Fig. 6). The time course for the effects of xamoterol on retrieval was essentially identical to that for the requirement of NE, β1, cAMP, and PKA signaling in retrieval (Murchison et al. 2004; Ouyang et al. 2008), i.e., retrieval of an intermediate phase of memory is susceptible. Short-term (1 h) and longer-term (5 d) memory were not affected by xamoterol. The results are consistent with the idea that different phases of memory, likely defined by various consolidation processes, can have distinct requirements for their retrieval. Further, the lack of impairment at 1 h and at 5 d indicates that xamoterol does not impair non-mnemonic emotional or sensorimotor processes. With respect to mnemonic function, the effect of xamoterol is not due to a rapid reversal of consolidation or a rapid facilitation of extinction during testing, because the contextual fear memory was expressed at high levels the following day in the absence of further treatment (Fig. 2).
A potentially important aspect of β-adrenergic receptor physiology is that NE, the adrenergic neurotransmitter in the DH, is an order of magnitude more potent in activating β1 receptors than β2 receptors (Zhang et al. 2004). As a result, it seems likely that moderately arousing conditions would facilitate hippocampus-dependent emotional memory retrieval through the release of NE and activation of β1 receptors. Extending this idea, strongly arousing conditions typical of “stress” would impair memory retrieval through the release of additional NE and also glucocorticoids that together would activate β2 receptors. Other studies have demonstrated that glucocorticoids impair hippocampus-dependent emotional memory retrieval (de Quervain et al. 1998, 2000). Studies from our lab indicate that this effect of glucocorticoids is mediated by activation of the β2 receptor and that NE and glucocorticoids synergize to activate this receptor at doses that are ineffective when present individually (Schutsky et al. 2011). Because strongly arousing conditions enhance memory consolidation, this could facilitate a temporary shift away from processes favoring retrieval toward those favoring consolidation.
Materials and Methods
Subjects
Wild-type, β1 KO, and β2 KO mice were on a hybrid 129/Sv x C57BL/6 background and were derived by mating either heterozygotes or homozygotes (Rohrer et al. 1996; Chruscinski et al. 1999). Gender and parental genotype did not affect the results, so data were combined. Genotype was determined by PCR. Mice were maintained on ad lib food and water and a 12-h light:dark cycle, with lights on beginning at 07:00. Animals were housed in relatively small, quiet rooms for 3–4 wk before studies began to minimize the stress associated with caretaking and colony management during the light phase. Mice were 3–6-mo old when tested. Studies were performed during the light phase, with most experiments taking place between 08:00–15:00. Studies were in accordance with NIH guidelines and had the approval of the IACUC at the University of Pennsylvania.
Pavlovian fear conditioning
Adjacent to the training room, animals were placed in pairs into opaque plastic holding buckets (∼12 cm diameter) with bedding and covers for 30–60 min before being manipulated further. Animals were given two 3-min handling sessions over 2 d in the training room. Saline was injected at the end of handling each day. Training consisted of placing the animal in the conditioning apparatus (ENV-010MC with ENV-414S, Med Associates) for 2 min, after which an 84 dB, 4.5 kHz tone was activated for 30 sec. Two seconds before the end of the tone, a 2-sec, 1 mA footshock was delivered. The animal was returned to its home cage 30 sec after shock, and the apparatus was cleaned with Versa-Clean (Fisher). Contextual fear was tested for 5 min in the conditioning apparatus in the absence of the tone. Cued fear was tested in a separate cohort using a Plexiglas cylinder (21-cm-diameter, 24 cm tall) with green wire grid floor and vertical green and white wall stripes 240° around. The cylinder was located in a different area of the training room and was cleaned with lemon-scented Ajax after training each animal. After 2 min, the training tone was turned on for 3 min. Percent freezing was estimated by scoring the presence or absence of nonrespiratory movement every 5 sec. Tests were conducted 1 d after training except where indicated.
Instrumental fear conditioning
Animals were handled as described above. Training consisted of placing a rodent in the lighted chamber of the apparatus used for Pavlovian conditioning and timing its latency to enter (except for the tail) the dark chamber. Once the animal entered the dark chamber, the retractable partition separating the two chambers was lowered, and a shock was delivered for 2 sec (0.30–0.35 mA). After waiting 15 sec, rodents were returned to their home cage, and the apparatus was cleaned. Animals that did not enter the dark chamber after 100 sec during conditioning were excluded (<4% of mice, independent of genotype). Testing was identical to training except that no shock was delivered, and the partition remained up. Latencies to enter the dark chamber were recorded. If an animal did not enter the dark chamber within 10 min, it was returned to its cage and assigned a latency of 10 min. Assigned latencies of 10 min ranged from half of the mice in some groups to no mice in other groups. Tests were conducted 1 d after training except where indicated.
Drugs
Nadolol (Sigma), betaxolol HCl, ICI 118,551 HCl, and/or xamoterol hemifumarate (Tocris) were dissolved in vehicle (0.9% saline containing 0.1 mg/mL ascorbic acid, pH 7.4, Sigma) and administered subcutaneously 30 min before testing because the behavioral effects of xamoterol are maximal with this time interval. For DH infusion, xamoterol and pertussis toxin (Tocris) were dissolved in the same vehicle. Xamoterol was infused 15 min before testing because vehicle infusion at this time does not impair retrieval. Nadolol was used at 3 mg/kg because at 1 mg/kg it mimics ICI (1 mg/kg) in the near complete blockade of peripheral β2 receptor-mediated effects in mice (Criscuoli and Subissi 1988).
Dorsal hippocampus infusion
A double guide cannula (C235 system, Plastics One) was implanted under pentobarbital anesthesia (72.5 mg/kg ip) using a stereotax (SAS75/EM40M, Cartesian Research). The guide was placed −1.7 mm AP and 1.5 mm bilateral for DH infusions. The guide projected 1.5 mm below the base, and the dummy cannula extended 0.5 mm below the guide. One week after surgery, bilateral infusions were made into conscious mice while gently holding the nape of the neck. The dual injection cannula extended 0.9 mm below the guide. Infusions were 1 µL/side at 0.4 µL/min, and the injection cannula was left in place for 30 sec before the mouse was returned to its home cage. Because studies indicate that the effects of PTx are best evaluated 3 d after infusion (Goh and Pennefather 1989; Stratton et al. 1989), PTx was infused 3 d before testing. To minimize potential effects of PTx on conditioning, training was administered the day after PTx infusion, and testing was performed 2 d after training. A pilot study indicated that 1 ng and 10 ng PTx (comparable to or lower than what has been used by others) were similarly effective. Infusion location was assessed by infusing 1 µL of 1% methylene blue after behavioral testing. Methylene blue was observed in the center of the DH in all cases, with spread reaching each hippocampal subfield but not outside the DH, except for some in the cannula track, as previously shown (Murchison et al. 2004). Thus, all animals were included in the current study unless blockage of the injection cannula was observed immediately after drug infusion.
Statistics
Data were analyzed with Statistica 9.1 (StatSoft) using one- or two-way ANOVA with alpha = 0.05. The Bartlett χ2-test was employed to analyze homogeneity of variances. Duncan's range test was employed for post-hoc analysis. Data are presented as mean ± standard error. For all figures, * indicates P < 0.05, ∧ indicates P < 0.01, and # indicates P < 0.001. Comparisons marked as significant are to the reference group except where indicated.
Acknowledgments
We thank Brian Kobilka (Stanford University, CA) for providing stock for the β receptor KO mouse lines. This work was supported by NIH grant 5R01MH063352 and DOD grant PT075099 to S.A.T, and NIH grant 5T32GM008076 to Randall Pittman (University of Pennsylvania, Philadelphia, PA).
References
- Abel T, Nguyen PV 2008. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res 169: 97–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros DM, Izquierdo LA, Mello e Souza T, Ardenghi PG, Pereira P, Medina JH, Izquierdo I 2000. Molecular signaling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res 114: 183–192 [DOI] [PubMed] [Google Scholar]
- Beer M, Richardson A, Poat J, Iversen LL, Stahl SM 1988. In vitro selectivity of agonists and antagonists for β 1- and β 2-adrenoceptor subtypes in rat brain. Biochem Pharmacol 37: 1145–1151 [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM 2009. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala β2- and α1-adrenergic antagonists. Learn Mem 16: 777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK 1999. Targeted disruption of the β2 adrenergic receptor gene. J Biol Chem 274: 16694–16700 [DOI] [PubMed] [Google Scholar]
- Criscuoli M, Subissi A 1988. Catecholamines released from the adrenal medulla exert a compensatory, protective effect at β 2-adrenoceptors against Paf-induced death in mice. Br J Pharmacol 93: 132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ 1997. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88–91 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394: 787–790 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C 2000. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 3: 313–314 [DOI] [PubMed] [Google Scholar]
- Devic E, Xiang Y, Gould D, Kobilka B 2001. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from β(1) and β(2) adrenoceptor knockout mice. Mol Pharmacol 60: 577–583 [PubMed] [Google Scholar]
- Dierssen M, Vallina IF, Baamonde C, Garcia-Calatayud S, Lumbreras MA, Florez J 1997. Alterations of central noradrenergic transmission in Ts65Dn mouse, a model for Down syndrome. Brain Res 749: 238–244 [DOI] [PubMed] [Google Scholar]
- Faizi M, Bader PL, Tun C, Encarnacion A, Kleschevnikov A, Belichenko P, Saw N, Priestley M, Tsien RW, Mobley WC, et al. 2011. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: Activation of β(1)-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol Dis 43: 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JC, O'Donnell JM 1988. Antagonism of the responses to isoproterenol in the rat hippocampal slice with subtype-selective antagonists. Eur J Pharmacol 153: 105–110 [DOI] [PubMed] [Google Scholar]
- Goh JW, Pennefather PS 1989. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science 244: 980–983 [DOI] [PubMed] [Google Scholar]
- Gold PE 2004. Coordination of multiple memory systems. Neurobiol Learn Mem 82: 230–242 [DOI] [PubMed] [Google Scholar]
- Goldaniga G, Montesanti L, Pianezzola E, Valzelli G 1980. Pharmacokinetics and metabolism of a new β-adrenergic blocking agent, the 1, ter-butyl-amino-3-(1,2,3,4-tetrahydro-1,4-ethano-8-hydroxy-5-naphthoxy)-2-propanol (K 5407). Eur J Drug Metab Pharmacokinet 5: 9–20 [DOI] [PubMed] [Google Scholar]
- Isiegas C, McDonough C, Huang T, Havekes R, Fabian S, Wu LJ, Xu H, Zhao MG, Kim JI, Lee YS, et al. 2008. A novel conditional genetic system reveals that increasing neuronal cAMP enhances memory and retrieval. J Neurosci 28: 6220–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER 2001. The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030–1038 [DOI] [PubMed] [Google Scholar]
- Malta E, Mian MA, Raper C 1985. The in vitro pharmacology of xamoterol (ICI 118,587). Br J Pharmacol 85: 179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Marcyniuk B, Ravindra CR 1985. Pathological evidence for neurotransmitter deficits in Down's syndrome of middle age. J Ment Defic Res 29: 125–135 [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H 2010. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: Role for β-2 adrenergic receptors. Neuropsychopharmacology 35: 2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcyniuk B, Mann DM, Yates PO, Ravindra CR 1988. Topography of nerve cell loss from the locus caeruleus in middle aged persons with Down's syndrome. J Neurol Sci 83: 15–24 [DOI] [PubMed] [Google Scholar]
- Martin SJ, Clark RE 2007. The rodent hippocampus and spatial memory: From synapses to systems. Cell Mol Life Sci 64: 401–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC 1995. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457 [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C 2003. Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci 358: 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA 2004. A distinct role for norepinephrine in memory retrieval. Cell 117: 131–143 [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA 2005. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci 102: 9347–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA 2008. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci 105: 11993–11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF 2005. The β-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry 58: 894–900 [DOI] [PubMed] [Google Scholar]
- Ramos BP, Colgan LA, Nou E, Arnsten AF 2008. β2 adrenergic agonist, clenbuterol, enhances working memory performance in aging animals. Neurobiol Aging 29: 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ 2002. Seven-transmembrane-spanning receptors and heart function. Nature 415: 206–212 [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr, Barsh GS, Bernstein D, Kobilka BK 1996. Targeted disruption of the mouse β1-adrenergic receptor gene: Developmental and cardiovascular effects. Proc Natl Acad Sci 93: 7375–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL 2004. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci 24: 8161–8169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK 2008. A memory-based model of post-traumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychol Rev 115: 985–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Faizi M, Colas D, Valletta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner SL, Aisen P, Shamloo M, et al. 2009. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med 1: 7–17 [DOI] [PubMed] [Google Scholar]
- Schutsky KS, Ouyang M, Castelino CB, Zhang L, Thomas SA 2011. Stress and glucocorticoids impair memory retrieval via β2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ 2001. Retrograde amnesia. Hippocampus 11: 50–55 [DOI] [PubMed] [Google Scholar]
- Stratton KR, Cole AJ, Pritchett J, Eccles CU, Worley PF, Baraban JM 1989. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res 494: 359–364 [DOI] [PubMed] [Google Scholar]
- Szapiro G, Galante JM, Barros DM, Levi de Stein M, Vianna MR, Izquierdo LA, Izquierdo I, Medina JH 2002. Molecular mechanisms of memory retrieval. Neurochem Res 27: 1491–1498 [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD 1997. Disruption of the dopamine β-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci 111: 579–589 [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Nakazawa K, Wilson MA 2003. Genetic neuroscience of mammalian learning and memory. Philos Trans R Soc Lond B Biol Sci 358: 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi H, Nakashima Y, Kinami J, Nagatomo T 1990. Characteristics of 125I-iodocyanopindolol binding to β-adrenergic and serotonin-1B receptors of rat brain: Selectivity of β-adrenergic agents. Jpn J Pharmacol 52: 195–200 [DOI] [PubMed] [Google Scholar]
- Woods PB, Robinson ML 1981. An investigation of the comparative liposolubilities of β-adrenoceptor blocking agents. J Pharm Pharmacol 33: 172–173 [DOI] [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG 1995. Functional coupling of the β 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol 47: 322–329 [PubMed] [Google Scholar]
- Zhang WP, Ouyang M, Thomas SA 2004. Potency of catecholamines and other L-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology 47: 438–449 [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhu W, Han Q, Xiao RP 2005. Emerging concepts and therapeutic implications of β-adrenergic receptor subtype signaling. Pharmacol Ther 108: 257–268 [DOI] [PubMed] [Google Scholar]