Abstract
Background
Recently, use of advanced imaging modalities, such as MRI, has increased dramatically. One novel but still evolving use for MRI is in the diagnosis and clinical staging of newly diagnosed breast cancer patients. Compared with mammography, MRI is more sensitive, but less specific, and far more expensive. The purpose of this study is to examine the prevalence and predictors of MRI use for clinical staging in older women with newly diagnosed breast cancer.
Materials and Methods
SEER-Medicare data were used to identify incident breast cancer cases between 2003 and 2005. Outpatient Medicare claims data were queried for receipt of breast MRI. Multivariate logistic regression analyses were performed to examine associations between receiving MRI and patient demographics, clinical characteristics, and SEER region.
Results
A total of 46,824 patients with breast cancer met inclusion criteria. MRI use increased from 3.9% of women diagnosed in 2003 to 10.1% of women diagnosed in 2005. In the bivariate analyses race, urban/rural location, SEER region, poverty level, education level, stage, surgery type, and tumor size were all significantly associated with receipt of MRI. In the multivariate analysis, those who were younger, white, living in more metropolitan areas, and living in wealthier areas were more likely to receive MRI. There was substantial variability in odds of MRI among different SEER regions.
Conclusions
Breast MRI for patients with newly diagnosed breast cancer in the SEER-Medicare population is increasingly common. Ongoing examination of the dissemination of technology is critical to understanding current practice patterns and to the development and implementation of future guidelines.
Keywords: breast, breast neoplasm, breast cancer, magnetic resonance imaging, MRI, neoplasm staging, dissemination of technology, preoperative care
INTRODUCTION
Breast cancer is the most common cancer and the second leading cause of cancer death among women in the United States [1]. Mammography and ultrasound are the standard imaging modalities used to evaluate breast disease. In recent years, magnetic resonance imaging (MRI) has emerged as a potential adjunct to conventional imaging for selection of optimal local therapy in women with known breast cancer. MRI is more sensitive than is conventional mammography for identifying cancer in certain groups, and has been reported to improve staging of disease in others [2, 3]. Multiple single institution studies suggest that MRI has been rapidly adopted for use in treatment selection and assisting with surgical planning [4–6].
However, considerable uncertainty remains about the downstream clinical benefits of routine use of breast MRI in women with a new cancer diagnosis [7]. This lack of clarity about the appropriate use of breast MRI in the general population is a quality of care problem, as defined by the Institute of Medicine (IOM) in 2001 [8]. For women at average or low risk, MRI may be overused, resulting in increased cost of care and risk of over-treatment associated with false positive findings. Previous studies have demonstrated that uncertainty surrounding the utility of a test or treatment is associated with increased variation in its use [9].
Although case series suggest that breast MRI utilization is increasing, the actual frequency of utilization in the population at large remains unknown. Such utilization data are important for both clinicians and policy makers amidst increasing pressures to optimize both cost and outcomes. This study examines the use of breast MRI in the Medicare population, including clinical and sociodemographic factors associated with its use. To our knowledge, this is the first study to investigate this issue in a population-based sample. The goal of this study is to inform the development and implementation of guidelines for risk-appropriate use of breast MRI.
MATERIALS AND METHODS
Dataset and Study Cohort
This study used the Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked database. The SEER-Medicare database is a collaboration between the SEER registries, the National Cancer Institute (NCI), and the Center for Medicare and Medicaid Services (CMS) to capture Medicare beneficiaries with an incident cancer diagnosis who live in a SEER registry area. SEER cases of such patients are then linked to the patients’ Medicare administrative claims. It is estimated that 97% of the incident cancers of Medicare beneficiaries in SEER registry areas are captured in the data [10, 11]. This combination of clinical information with administrative claims allows for longitudinal analysis of oncologic care and health care resource utilization. As of 2000, the database included 17 population-based registry areas, comprising 26% of the US population [12].
Patients with stage 0–IV breast cancer diagnosed between January 1, 2003 and December 31, 2005 were identified in the database. Patients were excluded if they were male; their diagnosis occurred at the time of death or autopsy; or they were <65- y-old at diagnosis. To ensure complete claims, the sample was then limited to women enrolled in both Medicare both Parts A and B for 3 mo prior to and 12 mo after diagnosis, and not enrolled in a “Medicare Advantage” plan during this same time period.
Analysis
Outpatient insurance claims during the study period were queried to identify receipt of breast magnetic resonance imaging (MRI). Breast MRI was captured in outpatient Medicare files using specific American Medical Association Current Procedural Terminology (CPT) or HCPCS codes (76093-94, 77058-59, C8903-C8908). Inpatient MRIs are variably captured in Medicare claims, and breast MRI would rarely be employed preoperatively on a hospitalized patient; therefore we excluded MRIs performed in the inpatient setting. Definitive surgical management was obtained from SEER records, with additional surgical information obtained from Medicare claims when possible.
Two categories of variables were examined as factors associated with breast MRI use; sociodemographic (age, race, year of diagnosis, SEER region, poverty level, urban/rural residence, and level of education) and clinical (stage, surgery type, and tumor size) characteristics (Table 1). Because we did not have access to patient-level socioeconomic variables, area-based data were used to measure these factors. Although these variables are not merely proxies for individual-level data, they have been shown to be predictive of health outcomes and are widely used as a measure of socioeconomic status [13]. Area-based education level was measured using year 2000 National Census Tract data documenting percent of census tract inhabitants with high-school level education only. Census tract data were also used to ascertain area-level poverty estimates by examining the percent of inhabitants living at or below the poverty line. Rural-Urban Continuum Codes for each county (developed by the United States Department of Agriculture), are used by SEER to define areas that are more or less metropolitan. These variables were used for area-level rural/urban information. American Joint Committee on Cancer, 6th edition, stage information and tumor size (cm) were included as clinical variables. All data for patient-level, area-level, and clinical independent variables came solely from SEER records.
TABLE 1.
Subject Demographics and Percent of Patients Receiving Breast MRI
| Variable | Total n = 46,824 | Received MRI n = 3241 (% of total)* |
|---|---|---|
| Age (mean) | 76.4 | 73.1 (6.9) |
| Year | ||
| 2003 | 15,729 | 607 (3.9) |
| 2004 | 15,777 | 1094 (6.9) |
| 2005 | 15,318 | 1540 (10.1) |
| Race | ||
| White | 39,672 | 2832 (7.1) |
| Black | 3383 | 133 (3.9) |
| Hispanic | 1836 | 152 (8.3) |
| Asian/Pacific Isl. | 1589 | 92 (5.8) |
| Other/unknown | 344 | 32 (9.3) |
| SEER urban/rural | ||
| Big metro | 26,835 | 2289 (8.5) |
| Metro | 13,320 | 732 (5.5) |
| Urban | 2744 | 109 (4.0) |
| Less urban | 3259 | 95 (2.9) |
| Rural | 666 | 16 (2.4) |
| Poverty level† (n = 43,958) | ||
| 1st Quartile ($$$) | 10,973 | 1048 (9.6) |
| 2nd Quartile | 11,002 | 856 (7.8) |
| 3rd Quartile | 10,985 | 712 (6.5) |
| 4th Quartile ($) | 10,998 | 436 (4.0) |
| Education level† (43,962) | ||
| 1st Quartile (highest) | 10,876 | 1182 (10.9) |
| 2nd Quartile | 11,043 | 798 (7.2) |
| 3rd Quartile | 11,070 | 655 (5.9) |
| 4th Quartile (lowest) | 10,973 | 418 (3.8) |
| Stage | ||
| 0/in situ | 7,694 | 496 (6.5) |
| I | 19,019 | 1318 (6.9) |
| II | 11,556 | 887 (7.7) |
| III | 3361 | 295 (7.2) |
| IV | 2137 | 82 (3.8) |
| Unknown | 3057 | 163 (6.9) |
| Surgery type | ||
| None | 4,036 | 184 (4.6) |
| Breast conservation | 26,005 | 1901 (7.3) |
| Mastectomy | 16,444 | 1139 (6.9) |
| Unknown/missing | 339 | 17 (5.0) |
| Tumor size | ||
| 0/micro | 3133 | 151 (4.8) |
| 0–2 | 26,495 | 1886 (7.1) |
| 2–5 | 11,173 | 775 (6.9) |
| >5 | 2724 | 221 (8.1) |
| Unknown | 3299 | 208 (7.1) |
[Source: SEER-Medicare data 2003–2005].
Determined by χ2 test for categorical and student’s t-test for continuous variables (P < 0.01 for all analyses).
Grouped in quartiles by author.
The main outcome of interest was presence or absence of breast MRI. Bivariate analysis was performed using χ2 statistical analysis for categorical variables and Student’s t-test for continuous variables. The independent continuous variables of age, education level, and poverty level were consolidated into categorical groups (quartiles) for the bivariate and multivariate analyses. Because of the large overall sample size, we knew a priori that even small differences in characteristics would achieve statistical significance. Our choice of variables for the multivariate models was based on both clinical significance and independent variables’ statistical significance in bivariate analysis. Therefore, for the bivariate analysis we set the level of significance to P < 0.01. Multivariate analysis was done using logistic regression modeling with a P value <0.05 indicating statistical significance in the final model. Analysis was done using STATA ver.11 and results were reported as percentages, means (except in one case where median was more appropriate), and odds ratios. The study was reviewed by the institutional review board of the University of North Carolina and exempted from a full review.
RESULTS
The database includes 87,433 women who received a diagnosis of breast cancer between January 1, 2003 and December 31, 2005. Of these, 37,073 were excluded because they were either enrolled in a Medicare Advantage plan, or did not meet continuous enrollment criteria. An additional 3534 were excluded because they were <65 y the time of diagnosis. The number of incident breast cancer cases remained stable over the study period (Table 1). The mean age of the women at diagnosis was 76.4 y (range 65–107). Additional baseline characteristics of the study population are presented in Table 1.
Of the 46,824 included cases, 3241 (6.9%) were identified as having undergone at least one breast MRI. The use of breast MRI increased over time, from 3.9% of patients diagnosed in 2003 to 10.1% of patients diagnosed in 2005 (P < 0.01) (Fig. 1). Breast MRI was performed a median of 31 d after the date of diagnosis (range 44 d before to 395 d after). Women in the MRI group were slightly younger than those in the non-MRI group, (mean age 73.1 y versus 76.4 y, P < 0.01). All bivariate analyses reached statistical significance at the level of P < 0.01, and these data are presented in Table 1. In the bivariate analyses, those diagnosed most recently, younger women, white and Hispanic women, women living in areas of higher income, and women living in areas with a greater proportion of high school graduates were most likely to receive breast MRI.
FIG. 1.
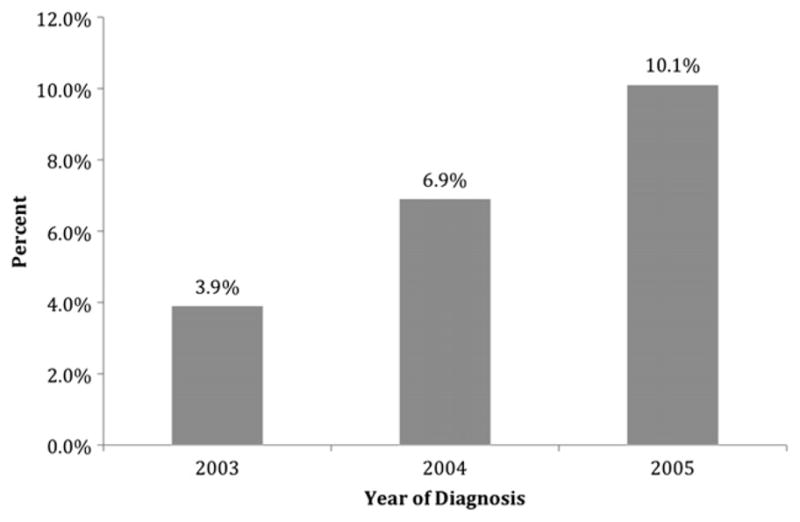
Percent of incident breast cancer cases receiving MRI by year.
A large proportion of study patients lived in either “big metro” (population > 1 million) or “metro” (metro areas with population < 1 million) areas as defined by RUCCs (57.3% and 28.5%, respectively), with certain SEER regions more heavily represented in the study population than others. MRI use was associated with geographic variation: women living in more metropolitan areas were more likely to receive MRI than were women living in non-metropolitan areas, and different SEER regions showed marked differences in overall utilization rate of MRI. The percentage of women receiving MRI ranged from 0.85% in Hawaii to 18.0% in New Mexico (Fig. 2). For bivariate and multivariate analyses, San Francisco was used as the referent group because its prevalence of MRI was closest to the mean for all regions.
FIG. 2.
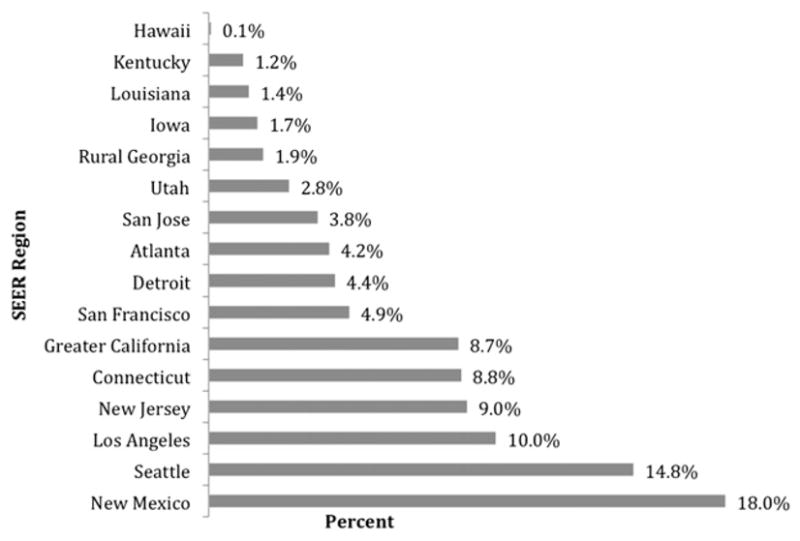
Percent of incident breast cancer cases receiving breast MRI by SEER region.
Ascertaining the association of MRI use with stage of disease and tumor size is somewhat problematic because we do not know whether coding for these variables was based on pre-MRI clinical findings, or influenced by MRI, operative, or postoperative pathologic findings. Still, we did examine the bivariate relationship between MRI use and stage of disease and tumor size (Table 1). Although the differences were statistically significant, they were too small to be clinically meaningful. We preliminarily examined other clinical features captured in the SEER data that might differ between the MRI and non-MRI groups and found no clinically important differences between the two groups in tumor histology or receptor status. Because of this, and the fact that including them did not change the overall findings of our adjusted model, we did not include these clinical variables in our final multivariate model.
Similarly, although not the focus of our study, we do report surgical procedure performed. The average time from diagnosis to first surgical intervention was 39 d (SD = 35). MRI was performed in 3057 women who underwent cancer directed surgery, 184 who did not undergo surgery, and 17 with missing data. Overall bivariate analysis of MRI and surgery type was statisticially significant (Table 1). Bivariate analysis of MRI and subsequent surgery type was done using only those patients who underwent MRI prior to breast conservation therapy (BCT) or mastectomy (n = 41,992). There was no clinically significant difference between receipt of MRI and subsequent surgery performed, with 38.8% of women undergoing mastectomy in the non-MRI group and 40.8% in the MRI group (P = 0.04).
As in the bivariate analysis, women from wealthier areas were more likely to receive MRI than were women from impoverished areas. White patients had higher odds of receiving MRI than black patients in both bivariate and multivariate analysis. Although in bivariate analysis Hispanic women were slightly more likely to receive MRI, they were less likely to receive MRI after incorporating other variables into the model, likely reflecting the effect of the relationship between geography and ethnicity. However, the differences seen in this group, as well as the findings in the “other” race category, are limited by sample size and do not reach statitstical significance in multivariate analysis. Asian women were slightly less likely to receive MRI, and this difference reached statistical signifiance in both bivariate and multivariate analysis.
In contrast to the minimal effect of clinical variables on receipt of MRI in our analysis, the effect sizes for the influence of age, year of diagnosis, and SEER region on the likelihood of MRI were large (Table 2). Younger patients were most likely to receive MRI. Those diagnosed in 2005 were almost three times more likely to receive MRI than were those diagnosed in 2003 (OR 2.88). Adjusted odds ratios among SEER regions ranged from 0.30 in Hawaii to 9.98 in New Mexico. Poverty level and education level were correlated in the data (r = 0.29, P < 0.001). To eliminate problems of collinearity, we included only poverty level in the multivariate analysis.
TABLE 2.
Unadjusted and Adjusted Odds of Breast MRI
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Age | ||
| 65–69 | 1.0 | 1.0 |
| 70–74 | 0.74 (0.68–0.81) | 0.72 (0.65–0.79) |
| 75–79 | 0.51 (0.46–0.56) | 0.49 (0.44–0.54) |
| 80–84 | 0.32 (0.28–0.36) | 0.29 (0.26–0.33) |
| 85–90 | 0.18 (0.15–0.22) | 0.17 (0.14–0.21) |
| >90 | 0.08 (0.05–0.13) | 0.08 (0.05–0.12) |
| Year | ||
| 2003 | 1.0 | 1.0 |
| 2004 | 1.86 (1.68–2.06) | 1.91 (1.71–2.12) |
| 2005 | 2.78 (2.53–3.07) | 2.88 (2.60–−3.19) |
| Race | ||
| White | 1.0 | 1.0 |
| Black | 0.53 (0.45–0.64) | 0.75 (0.61–0.91) |
| Hispanic | 1.17 (0.99–1.39) | 0.84 (0.69–1.01) |
| Asian/Pacific Isl. | 0.80 (0.65–0.99) | 0.71 (0.56–0.89) |
| Other/unknown | 1.33 (0.92–1.92) | 1.10 (0.75–1.65) |
| SEER urban/rural | ||
| Big metro | 1.0 | 1.0 |
| Metro | 0.62 (0.57–0.68) | 0.62 (0.55–0.69) |
| Urban | 0.44 (0.36–0.54) | 0.41 (0.32–0.51) |
| Less urban | 0.32 (0.26–0.40) | 0.54 (0.42–0.70) |
| Rural | 0.26 (0.16–0.43) | 0.76 (0.44–1.30) |
| Poverty level | ||
| 1st Quartile | 1.0 | 1.0 |
| 2nd Quartile | 0.78 (0.71–0.86) | 0.83 (0.75–0.92) |
| 3rd Quartile | 0.64 (0.58–0.71) | 0.72 (0.65–0.80) |
| 4th Quartile | 0.39 (0.35–0.44) | 0.47 (0.41–0.53) |
[Source: SEER-Medicare data 2003–2005].
Adjusted for age, year of dx, race, urban/rural, poverty level, and SEER region using logit model.
DISCUSSION
Early evidence, including a recent randomized controlled trial (Comparative Effectiveness of MRI in Breast Cancer, COMICE) [14], suggests routine use of breast MRI in newly diagnosed breast cancer patients may not improve short-term clinical outcomes such as reoperation rate, mastectomy rate, or local recurrence rate [4, 5, 14–16]. The likelihood that use of MRI will improve breast cancer survival is even smaller given the already low rates of local recurrence. Yet, multiple single-institution studies document increasing utilization of this technology [4–6, 17–19]. The goal of our study was to provide the first population-based assessment of breast MRI in breast cancer patients to determine an approximation of true utilization rates. We also examined clinical and sociodemographic variables associated with breast MRI use. Our data confirm previously reported single-institution findings, with an increase in utilization of breast MRI among incident cases from 2003 (3.9%) to 2005 (10.1%).
The overall rate of breast MRI utilization in this study was lower than in the previously cited single-institution studies for several potential reasons. First, the study time period represents the early adoption period for breast MRI technology. Second, MRI use would be expected to be lower in the Medicare population, as most of the commonly proposed indications are for younger women. Published reports may also be more likely to arise from institutions with familiarity with the procedure. MRI has been touted as superior to mammography for women with dense breast tissue who are typically younger [20], meaning that, in our population of women ages 65 y and older, we would anticipate extremely low rates of breast MRI utilization.
Several studies demonstrate racial disparities in the receipt of recommended oncologic treatment in women with breast cancer [21]. In our multivariate model, we too found differences in the odds of receiving breast MRI by race, although some of this difference was explained by other factors in the multivariate analysis. While this study was not designed to focus on disparities or clinical outcomes, it does suggest that novel technologies, such as breast MRI, may be disparately adopted in different patient populations. The effects of this varied adoption may result in different outcomes for those who do and do not receive novel technologies. But if the use of a technology does not confer benefit, the harms of its disparate use are not straightforward. The degree to which sociodemographic factors contribute to disparate clinical practices itself varies across geographic region, adding an additional layer of complexity. For breast MRI, we may eventually conclude that those who did not receive the novel technology in fact received more appropriate care. Tracking whether disparities diminish or increase over time, as well as the consequences of disparities for outcomes, will be important.
Given the observed clinical similarities between those who received MRI and those who did not, this study demonstrates two different kinds of variability: differential adoption and geographic variation. The ways in which technology is differentially adopted are well studied in a body of literature beginning several decades ago. Variables such as the medical/training culture, institutional characteristics, specific attributes of the technology, and the political and economic climate of the time have all been shown to influence adoption of medical technologies and, specifically, MRI technology [22]. While much of the literature addressing MRI focuses on acquisition of scanners, it is reasonable to suggest that these other variables may also contribute to variation in adoption of breast MRI techniques, especially in the early stages of adoption.
Analyses of Medicare data have frequently noted geographic variation in care [23, 24]. The etiology has generally been determined to be multifactorial and/or largely unexplained. We similarly documented considerable differences in MRI utilization among different SEER regions (Fig. 2). Although SEER region may not be the optimal unit of analysis for geographic variation, the substantial differences we identified even at this level warrant further exploration of the potential driving factors for geographic variation in adoption of this technology.
First and foremost is the desire to deliver high quality care to our patients. Breast MRI has the potential to be a valuable tool in some situations, and the enthusiasm to expand its application is understandable. However, we must carefully consider both the benefits and harms of adopting any new technology while its utility remains unclear. Other influences on the rapid uptake of MRI include the availability of in-office or free standing imaging centers and the potential financial incentives for self-referral. The most comprehensive trial to address the question of spending was the COMICE trial [14]. This trial did not find a statistically significant difference in the overall cost of care between those who were randomized to MRI versus those who received no MRI. However, the study was conducted in the UK and may not be generalizable to the US, where the structure of health care financing is entirely different and procedure-driven. Still others attribute imaging overuse, and to some extent, variation in practice, to factors such as defensive medicine, increasing patient demand for more studies, and lack of accepted guidelines. Guidelines for the use of MRI in average risk women are still evolving, and differ among issuing groups [25, 26]. As researchers continue to monitor this technology, we should consider measuring these factors and assessing their correlation with utilization.
Our study has several limitations. SEER-Medicare data are not entirely nationally representative. Elderly persons residing in a SEER region are more likely to be non-white, of higher socioeconomic status, and more urban than are those living in non-SEER regions [10]. Findings from the data represent the older population, and are unlikely to be generalizable to the younger population of breast cancer patients, since there are more potential indications for breast MRI in younger women. It is also possible that elderly patients with extensive medical comorbidities might be less likely to receive MRI, however, we feel this is unlikely to account for substantial variation in our population. Specific comorbidities, such as having a pacemaker, preclude MRI utilization, and this is a potential limitation of our study. Also, the significant time lag for available SEER-Medicare data precludes analysis of more recent trends. We expect that more current data will reflect even higher utilization rates, further strengthening our hypothesis that MRI use is increasing despite conflicting evidence regarding its utility.
Administrative claims are intended for billing, and must be interpreted with caution, as they do not always accurately and completely represent clinical findings. For example, those services not covered by Medicare, such as treatments delivered as part of a clinical trial, are not represented in the claims data. Although commonly used to measure socioeconomic status, area-based data are not representative of any one individual living in that area. This must be considered when making inferences about sociodemographic disparities. Finally, the majority of analysis of geographic variation in clinical practice, outcomes, and utilization of resources is done at the health service area (HSA), hospital referral region (HRR) level, or even at the institutional level, and, ideally, our analysis would have followed suit. However, the low overall prevalence of breast MRI in many SEER regions made analysis at a geographic unit smaller than that of the SEER region unreliable. In light of this limitation, we were unable to conduct a full exploration of the sources of regional variation in adoption of this technology.
Summary
The standard for appropriate use of breast MRI in women with newly diagnosed breast cancer is not yet established. In order to encourage efficient and appropriate use of this technology, evidence-based criteria for breast MRI utilization need to be developed. Our findings contribute to efforts to understand practice patterns in light of available evidence that does not support routine use of breast MRI. This study demonstrates that even in the older population, advanced imaging modalities are being adopted rapidly, variably, and not necessarily targeted appropriately, as demonstrated by marked differences in utilization based on geographic region, race, and area socioeconomic status—factors that should not be related to clinical appropriateness. This is important for both policy makers and clinicians. It is our hope that this type of population-level analysis will, when combined with primary data collected from trials and observational studies, encourage more appropriate targeting of advanced testing to those patients most likely to benefit.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute, and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
This research was partially supported by a National Service Research Award Post-Doctoral Traineeship from the Agency for Health Care Research and Quality sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, grant no. 5-T-32 HS000032-21.
This research was partially supported by NCI 5R01CA124402, Implementing Systemic Interventions to Close the Discovery-Delivery Gap (purchase of data; initial data management).
References
- 1.CDC. [Accessed November 1, 2010];Breast Cancer Trends. Available at: http://www.cdc.gov/cancer/breast/statistics/trends.htm.
- 2.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Messersmith H, Causer P, et al. Systematic review: Using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148:671. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180. doi: 10.1016/j.jamcollsurg.2009.04.010. quiz 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang N, Schiller DE, Crystal P, et al. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: Its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol. 2009;16:3000. doi: 10.1245/s10434-009-0607-1. [DOI] [PubMed] [Google Scholar]
- 6.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow M. Magnetic resonance imaging for screening, diagnosis, and eligibility for breast-conserving surgery: Promises and pitfalls. Surg Oncol Clin N Am. 2010;19:475. doi: 10.1016/j.soc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8.America IOM(COQOHCI) Crossing the quality chasm: A new health system for the 21st century. National Academies Press; 2001. [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Sharp SM, Finlayson SR, et al. Variation profiles of common surgical procedures. Surgery. 1998;124:917. [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;6:2343. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed Jun 22, 2010];SEER-Medicare Linked Database. Available at: http://healthservices.cancer.gov/seermedicare/
- 13.Krieger N, Chen JT, Waterman PD, et al. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: A comparison of area-based socioeconomic measures–The Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet. 2010;375:563. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 15.Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 16.Pengel KE, Loo CE, Teertstra HJ, et al. The impact of preoperative MRI on breast-conserving surgery of invasive cancer: A comparative cohort study. Breast Cancer Res Treat. 2009;116:161. doi: 10.1007/s10549-008-0182-3. [DOI] [PubMed] [Google Scholar]
- 17.Dang CM, Zaghiyan K, Karlan SR, et al. Increased use of MRI for breast cancer surveillance and staging is not associated with increased rate of mastectomy. Am Surg. 2009;75:937. [PubMed] [Google Scholar]
- 18.Hollingsworth AB, Stough RG, O’Dell CA, et al. Breast magnetic resonance imaging for preoperative locoregional staging. Am J Surg. 2008;196:389. doi: 10.1016/j.amjsurg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191:332. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 20.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic Resonance Imaging of the Breast Prior to Biopsy. JAMA. 2004;292:2735. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 21.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 22.Hillman AL, Schwartz JS. The adoption and diffusion of CT and MRI in the United States. A comparative analysis. Med Care. 1985;23:1283. doi: 10.1097/00005650-198511000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Skinner J, Bynum J, et al. Regional variations in diagnostic practices. N Engl J Med. 2010;363:45. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34:1351. [PMC free article] [PubMed] [Google Scholar]
- 25.Saslow D, Boetes C, Burke W, et al. Group ACSBCA. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 26. [Accessed Dec 17, 2010];American College of Radiology Guidelines. Available at: http://www.acr.org/secondarymainmenucategories/quality_safety/guidelines/breast/mri_breast.aspx.


