Abstract
Climate has a significant impact on malaria incidence and we have predicted that forecast climate changes might cause some modifications to the present global distribution of malaria close to its present boundaries. However, it is quite another matter to attribute recent resurgences of malaria in the highlands of East Africa to climate change. Analyses of malaria time-series at such sites have shown that malaria incidence has increased in the absence of co-varying changes in climate. We find the widespread increase in resistance of the malaria parasite to drugs and the decrease in vector control activities to be more likely driving forces behind the malaria resurgence.
Plasmodium falciparum malaria takes a life in Africa every 30 seconds [1], and this rate is increasing [2]. Strong evidence has accumulated that the world has warmed by ~0.6°C over the past century [3], with a range of ecological consequences [4]. Many have concluded that the reported malaria increases and the re-emergence of other vector-borne diseases are likely to be the result of these climatic changes [5-10]. On the basis of the sensitivity of vector-borne diseases to climate [11], and using projections of incomplete biological models [12,13], widespread increases in global malaria burden have also been predicted [14-16]. However, there has been considerable dissent by specialists in malaria epidemiology concerning both the cause of recent malaria resurgences [17-20] and to what such climate changes might augur [21,22]. Although reports of malaria increases are not confined to high-altitude locations [2], this debate has become focused on the highland regions of Africa [23] because malaria is limited by low temperatures at such elevations [24]; hence, these locations are considered sensitive sentinels to temperature increases. Furthermore, it is often assumed that highland populations are immunologically naive and therefore at particular risk from malaria resurgences [25], although this assumption about the immunity status of highland populations has been questioned [20].
Evidence for possible causes of malaria resurgences
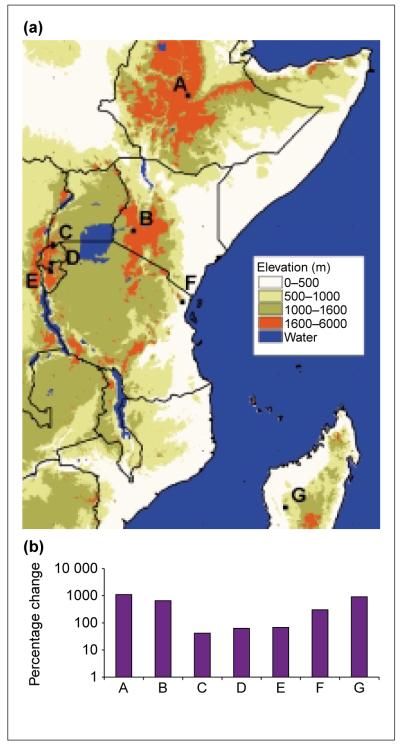
Since the early 1980s, there have been massive percentage increases in P. falciparum burden at African highland locations (Fig. 1). Possible causes are discussed below.
Fig. 1.
(a) A digital elevation model showing reported malaria resurgence in high altitude regions of East Africa: Debre Zeit, Ethiopia (A) (A.N. Tulu, PhD thesis, University of London, 1996); Kericho, Kenya (B) [30,31]; Kabale, Uganda (C) [49,50]; Gikonko, Rwanda (D) [34]; Muhanga, Burundi (E) [51]; Amani, Tanzania (F) [32,33]; and Analaroa, Madagascar (G) [52]. The highland areas surrounding A–E are extensive and the sites are not spatially isolated ‘highland’ islands. F and G have been included so as to be comprehensive regarding cited reports of malaria resurgences in highland areas. (b) Percentage change of incidence of Plasmodium falciparum malaria (1980–2000). Data are for the longest interval that cases were reported during this period. Where possible, a five-year average annual incidence was taken at the temporal extremes of the data.
Meteorological changes
Although the world appears to have warmed over the past 100 years [3], malaria does not respond to approximated global averages, and the bulk of this warming trend (and precipitation increases) has occurred in the northern and, to a lesser extent, southern temperate and polar latitudes [3,4]. When climatic changes were investigated in the highlands of equatorial Africa [19], the Augmented Dickey Fuller (ADF) test revealed no evidence for significant trends in climate at four highland sites (Kericho, Kabale, Gikonko and Muhanga) contemporary with the malaria resurgences. This was true for mean, minimum and maximum temperatures, as well as for rainfall, vapour pressure and combinations of meteorological variables necessary for malaria transmission [24]. In essence, climate and climate variability had not changed, so could not have caused the malaria resurgences.
Identical ADF analyses have been extended to test the significance of any trends in the meteorological variables at Debre Zeit, Amani and Analaroa (Table 1). In common with the other East African sites [19], Amani revealed no trends in any of the meteorological variables over any time period. By contrast, Debre Zeit and Analaroa exhibited significant warming trends during 1970–1995. Interestingly, these two sites lie at the latitudinal edge of the East African highlands and suffered the greatest increases in malaria (Fig. 1), but even here the malaria resurgence cannot be attributed with any certainty solely to climate change because of concomitant changes in other factors detailed below.
Table 1. Trend of monthly meteorological variables at three sites in the highlands of East Africa, 1970–1995a.
| Debre Zeit, central Ethiopia (8.75 N, 38.99 E; 1490–2200 m) b | ||||||||
| p | ADF | β | t | p-value | τ α | Q | Sig. Q | |
| Temp min. (°C) | 1 | − 6.73 ° | 0.0133 | 2.43 | 0.0156 | 0.0035 | 22.5240 | 0.9611 |
| Temp mean (°C) | 2 | −5.02 | 0.0108 | 2.57 | 0.0106 | 0.1532 | 28.9200 | 0.7929 |
| Temp max. (°C) | 1 | −8.20 | 0.0202 | 3.17 | 0.0017 | 0.1179 | 33.4282 | 0.5915 |
| Rainfall (mm) | 2 | −11.13 | 0.0603 | 0.23 | 0.8209 | −0.0645 | 37.6854 | 0.3921 |
| Vapour pressure (hPa) | 1 | −6.52 | 0.0102 | 2.44 | 0.0155 | 0.0029 | 24.6356 | 0.9240 |
| Amani, north–east Tanzania (5.10 S, 38.63 E, 600–1000 m) b | ||||||||
| p | ADF | β | t | p–value | τ α | Q | Sig. Q | |
| Temp min. (°C) | 4 | −3.51 | 0.0032 | 0.99 | 0.3223 | −0.1605 | 44.0236 | 0.1684 |
| Temp mean (°C) | 2 | −5.95 | 0.0031 | 1.01 | 0.3156 | −0.0712 | 49.0893 | 0.0716 |
| Temp max. (°C) | 1 | −8.41 | −0.0012 | −0.28 | 0.7782 | 0.0024 | 56.8429 | 0.0149 |
| Rainfall (mm) | 6 | −5.21 | 0.3319 | 0.65 | 0.5141 | 0.1973 | 50.8691 | 0.0513 |
| Vapour pressure (hPa) | 4 | −3.59 | 0.0499 | 1.01 | 0.3159 | −0.1575 | 42.8075 | 0.2021 |
| Analaroa, central plateau Madagascar (18.85 S, 45.49 E, 1500 m) b | ||||||||
| p | ADF | β | t | p–value | τ α | Q | Sig. Q | |
| Temp min. (°C) | 2 | −7.22 | 0.0058 | 1.47 | 0.1433 | −0.1166 | 61.0848 | 0.0056 |
| Temp mean (°C) | 1 | −8.31 | 0.0087 | 2.49 | 0.0133 | −0.0215 | 67.5492 | 0.0011 |
| Temp max. (°°C) | 6 | −4.63 | 0.0121 | 2.77 | 0.0060 | −0.0226 | 42.6762 | 0.2060 |
| Rainfall (mm) | 1 | −13.31 | 0.0262 | 0.09 | 0.9288 | −0.1257 | 46.7825 | 0.1077 |
| Vapour pressure (hPa) | 2 | −7.18 | 0.0092 | 1.67 | 0.0961 | −0.1478 | 56.0451 | 0.0177 |
Methodological and statistical details for these tests follow the methods outlined in Ref. [19]. p is the number of lagged differenced dependent variables selected. ADF is the Augmented Dickey Fuller t test for γ = 0. The 5% critical value is −3.45. Exact p-values are not available for the ADF and τα statistics. The distribution of the t-statistic for the slope parameter β has the standard t distribution under the assumption that γ < 0. τα is the t-statistic for the intercept term in the autoregression without a linear time trend. This is the appropriate test for a trend if γ = 0. Its 5% critical value is 2.54. The Q statistic is a Portmanteau test for general serial correlation and is distributed as Chi-square. The significance is shown in the final column (Sig. Q). Figures in bold denote significance at the 5% level.
Information in brackets corresponds to the geo-location and elevation range information details for each site.
Drug resistance
Chloroquine (CQ) resistance, first reported in East Africa in 1978 among non-immune tourists [26], has now been verified in all tropical African countries [27], where this drug commonly remains the first-line treatment for malaria [28]. Although there is a lack of systematic longitudinal evidence for the onset of resistance and its evolution in populations, there is quantitative information that, during the past two decades, CQ resistance has spread geographically, increased in prevalence within its range, and intensified in its severity of clinical failure (from predominantly RI-type to RII- and RIII-type responses [27,29]). Among the sites we have highlighted, CQ resistance has been identified as an important factor in the malaria resurgence on the Kericho tea estates [30], where climate, the environment, the human population and its structure, health care provision and malaria control measures are known not to have changed [31]. Likewise, the malaria resurgence in the Usambara mountains has been linked to the rise in antimalarial drug resistance [32], rather than previously postulated local climatic changes in Amani [33]. At Gikonko, climate was originally asserted to have caused increases in malaria incidence [34], although the significance of the trends in the temperature variables was not tested, and the case for drug resistance was dismissed on the basis of two local estimates made just one year apart (1986 and 1987) [35], even though malaria resurgence was documented throughout Rwanda. The suggestion that climate change was responsible for epidemics across the highlands of Burundi was supported by only four measures of the mean January temperature, between 1993 and 2001, from one location with no statistical analyses [36]. This was in the face of unequivocal evidence of the importance of CQ resistance in the region [37], whose continued use could have contributed to the widespread epidemic [38]. Only at Debre Zeit does the emergence of CQ resistance sometime after 1992 [39] preclude its being a factor in the malaria increase.
Vector control
Many countries in Africa at one time maintained effective vector-control services. Quantitative indicators of declines in such services are rarely published; however, by chance, massive decreases in previously successful vector-control efforts have been documented at the two sites mentioned above where climate has also changed in the past two decades.
In the Debre Zeit sector, the amount of DDT applied by indoor residual spraying for malaria control decreased from an average of 26 000 kg per annum (1965–1979) to only 4000 kg per annum (1980–1993), coincident with the period of most marked malaria resurgence [39]. The number of houses sprayed and the proportion of the population protected also decreased by a similar magnitude [39].
The history of malaria in the highlands of Madagascar has also been comprehensively described [40]. In 1878, a severe epidemic swept the Madagascan highlands following the extensive development of rice irrigation, and the disease soon became endemic. In 1949, a malaria eradication programme was introduced using DDT spraying in combination with malaria treatment centres. By 1960, the incidence of malaria was massively reduced and spraying was terminated in all but three foci. In 1975, spraying in these foci was also stopped and the treatment centers were closed four years later. Malaria gradually increased over the next decade, culminating in a severe epidemic in 1988. Since 1993, DDT spraying has been re-introduced and has successfully and continually reduced malaria to less than 10% of 1988 levels. Although there have been small, yet significant, increases in temperature (Table 1), the stepwise changes in malaria incidence follow the variation in control activities much more closely.
Health service provision
Little quantitative information exists on the state of public and private health service provision in most sub-Saharan countries, but in East Africa the population served by each hospital bed increased considerably between 1980 and 1990 [41] in all countries except Rwanda, where there was a marginal decrease (Fig. 2a). The situation at specific sites cannot be inferred from these national data, but they do provide evidence of a regional decline in per caput health service provision coincident with many of the malaria resurgences.
Fig. 2.
(a) The percentage change in population per hospital bed using data for 1980 and for 1990 (the only times available). These data were abstracted from the World Bank Africa Database on CD-ROM [41]. (b) The percentage change in normalized difference vegetation index (NDVI) derived from the Pathfinder AVHRR Land dataset [53]; the 1981–1985 average is compared with the 1996–2000 average for each site. (c) The percentage change in the human population – total (open bars) and urban (solid bars) – between 1980 and 2000, also abstracted from the World Bank Africa Database on CD-ROM [41].
Land-use change
Land cover and land use has a significant impact on malaria transmission [42]. The normalized difference vegetation index (NDVI) is a measure of the amount of photosynthetically active vegetation, and is thus a proxy for land cover [43]. Many studies have reported positive associations between the NDVI and malaria incidence [44,45]. Interestingly, during the period of malaria resurgence, most sites have greened by a small amount (<8%), suggesting that conditions might have become marginally more conducive to malaria (Fig. 2b). The exceptions are at Debre Zeit and Analaroa, where decreased NDVI is consistent with local temperature increases. These NDVI data are at too coarse a spatial resolution (8 × 8 km) to indicate the exact nature of the land use changes and serve only to document the overall change in vegetation coverage at each of the sites.
Population growth and urbanization
In each of the East African countries investigated, populations have increased by more than 50% since 1980 (Fig. 2c). Without significant improvements in health care provision (Fig. 2a), malaria will have become harder to treat and control, simply because the susceptible reservoir of humans has expanded. However, the urban proportion has more than doubled since 1980 so that, by the year 2000, 26% of the 65 million East Africans were urban dwellers. This is estimated to increase to 44% by 2030 because virtually all of the population doubling of the region during this time will be concentrated in urban areas [46]. The reduction in malaria risk with increased urbanization is well known [47]. At 159 sites across Africa where annual entomological inoculation rates had been recorded, people in rural areas received on average 146 P. falciparum-infected bites per annum, compared with 14 for urban residents [42]. In contrast to the high profile of future climate scenarios, the impact of urbanization trends on the future malaria burden has received too little attention.
The most parsimonious explanation
The evidence that climate change is the most significant factor in recent malaria resurgences in the highlands of East Africa is at best equivocal, at worst unfounded. At five of the seven sites (Kericho, Kabale, Gikonko, Muhanga and Amani), climate has not changed significantly. At the two other sites, where malaria up-surges were most marked (Debre Zeit and Analaroa), temperatures have shown significant long-term increases; however, the collapse of vector control at these sites shows equally good, if not better, matches to changes in malaria incidence, especially at Analaroa where malaria declined again rapidly on the resumption of house spraying. The role of the spread and intensification of antimalarial drug resistance, particularly to CQ, is also particularly persuasive because it explains both increased transmission (increased number of drug-suppressed infections producing gametocytes) and increased severity of the disease (treatment failure owing to lack of affordable, effective drugs). However, it should be emphasized that such changes are occurring against a site-specific background of decreases in vector control, decreasing per caput health service provision, a greening of the landscape and massive increase in population.
Policy considerations
The threat from climate change is not a top priority for African nations faced with a contemporary resurgence in malaria. Our hope is that the research reviewed here might help focus attention on the real and immediate causes of these malaria resurgences, rather than fuel further speculation on the future epidemiological impacts of climate change. Although predictions of the impact of forecast climate surfaces [e.g. the high scenario from the HadCM2 experiment described on the Intergovernmental Panel on Climate Change website (http://ipcc-ddc.cru.uea.ac.uk)] do show parts of the highlands of East Africa and Ethiopia becoming newly suitable for malaria by 2050 [22], reversing, or even delaying, such global climate change will not address the problems faced by Africans at risk from malaria today. By comparison, addressing the need for effective and affordable drug treatments [48] for a disease that is both preventable and treatable offers a direct route by which the international community can act now to save lives.
Acknowledgements
S.I.H. is supported as an Advanced Training Fellow by the Wellcome Trust (#056642) and is affiliated to the Kenya Medical Research Institute/Wellcome Trust Collaborative Programme, PO Box 43640, Nairobi, Kenya. S.E.R. is a Natural Environment Research Council (UK) Senior Research Fellow (#341). J.C. is supported by the Dept for International Development, UK. G.D.S. is supported by the US Army Medical Research and Materiel Command, Ft Detrick, MA, USA. The opinions and assertions contained herein are private views of the authors and are not to be construed as official or as reflecting the views of the US Department of Defense. R.W.S. is a Senior Wellcome Trust Fellow (#058992) and acknowledges the support of the Kenya Medical Research Institute. He is also affiliated to the Centre for Tropical Medicine, Nuffield Dept of Clinical Medicine, University of Oxford, John Radcliffe Hospital, Oxford, UK.
Contributor Information
Simon I. Hay, Dept of Zoology, University of Oxford, South Parks Road, Oxford, UK OX1 3PS.
David J. Rogers, Dept of Zoology, University of Oxford, South Parks Road, Oxford, UK OX1 3PS
Sarah E. Randolph, Dept of Zoology, University of Oxford, South Parks Road, Oxford, UK OX1 3PS
David I. Stern, Dept of Economics, Rensselaer Polytechnic Institute, Troy, NY 12810, USA
Jonathan Cox, Dept of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, Keppel Street, London, UK WC1E 7HT.
G. Dennis Shanks, United States Army Medical Component – Armed Forces Research Institute of Medical Sciences, 315/6 Rajvithi Road, Bangkok 10400, Thailand.
Robert W. Snow, Kenya Medical Research Institute/Wellcome Trust Collaborative Programme, PO Box 43640, Nairobi, Kenya
References
- 1.Bremen JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 2.Snow RW, et al. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 3.Houghton JT, et al. Climate Change 2001: The Scientific Basis – Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2001. [Google Scholar]
- 4.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 5.Patz JA, et al. Global climate change and emerging infectious diseases. J. Am. Med. Assoc. 1996;275:217–223. [PubMed] [Google Scholar]
- 6.McMichael AJ, et al. Climate Change and Human Health. World Health Organization; 1996. Effects on biological disease agents. [Google Scholar]
- 7.Epstein PR, et al. Biological and physical signs of climate change: focus on mosquito-borne diseases. Bull. Am. Meteorol. Soc. 1998;79:409–417. [Google Scholar]
- 8.Lindsay SW, Martens WJM. Malaria in the African highlands: past, present and future. Bull. WHO. 1998;76:33–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Martens P. How will climate change affect human health? Am. Sci. 1999;87:534–541. [Google Scholar]
- 10.Kovats RS, et al. Early effects of climate change: do they include changes in vector-borne disease? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1057–1068. doi: 10.1098/rstb2001.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers DJ, Packer MJ. Vector-borne diseases, models, and global change. Lancet. 1993;342:1282–1284. doi: 10.1016/0140-6736(93)92367-3. [DOI] [PubMed] [Google Scholar]
- 12.Martin PH, Lefebvre MG. Malaria and climate – sensitivity of malaria potential transmission to climate. Ambio. 1995;24:200–207. [Google Scholar]
- 13.Martens P, et al. Climate change and future populations at risk of malaria. Global Environ. Change. 1999;9:S89–S107. [Google Scholar]
- 14.Watson RT, McMichael AJ. Global climate change – the latest assessment: does global warming warrant a health warning? Glob. Change Hum. Health. 2001;2:64–75. [Google Scholar]
- 15.McCarthy JJ, et al. Climate Change 2001: Impacts, Adaptation, and Vulnerability – Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2001. [Google Scholar]
- 16.McMichael AJ. Human Frontiers, Environments and Disease. Past Patterns, Uncertain Futures. Cambridge University Press; 2001. Global environmental change: overstepping limits; pp. 283–317. [Google Scholar]
- 17.Mouchet J, et al. Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors. J. Am. Mosq. Control Assoc. 1998;14:121–130. [PubMed] [Google Scholar]
- 18.Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109:141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay SI, et al. The clinical epidemiology of malaria in the highlands of Western Kenya. Emerg. Infect. Dis. 2002;8:543–548. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council . Under the Weather: Climate, Ecosystems, and Infectious Disease. National Academy Press; 2001. Analytical approaches to studying climate/disease linkages; pp. 59–79. [Google Scholar]
- 22.Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- 23.Patz JA, Lindsay SW. New challenges, new tools: the impact of climate change on infectious diseases. Curr. Opin. Microbiol. 1999;2:445–451. doi: 10.1016/s1369-5274(99)80078-2. [DOI] [PubMed] [Google Scholar]
- 24.Garnham PCC. The incidence of malaria at high altitudes. J. Nat. Mal. Soc. 1948;7:275–284. [PubMed] [Google Scholar]
- 25.Patz JA, Reisen WK. Immunology, climate change and vector-borne diseases. Trends Immunol. 2001;22:171–172. doi: 10.1016/s1471-4906(01)01867-1. [DOI] [PubMed] [Google Scholar]
- 26.Fogh S, et al. Chloroquine-resistant Plasmodium falciparum malaria in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1979;73:228–229. doi: 10.1016/0035-9203(79)90220-7. [DOI] [PubMed] [Google Scholar]
- 27.Trape JF. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001;64:12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 28.Trape JF, et al. Combating malaria in Africa. Trends Parasitol. 2002;18:224–230. doi: 10.1016/s1471-4922(02)02249-3. [DOI] [PubMed] [Google Scholar]
- 29.Anonymous . Chemotherapy of Malaria and Resistance to Antimalarials. World Health Organization; 1973. [PubMed] [Google Scholar]
- 30.Shanks GD, et al. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans. R. Soc. Trop. Med. Hyg. 2000;94:253–255. doi: 10.1016/s0035-9203(00)90310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay SI, et al. Etiology of interepidemic periods of mosquito-borne disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9335–9339. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bødker R, et al. Resurgence of malaria in the Usambara mountains, Tanzania, an epidemic of drug-resistant parasites. Glob. Change Hum. Health. 2000;1:134–153. [Google Scholar]
- 33.Matola YG, et al. The changed pattern of malaria endemicity and transmission at Amani in the eastern Usambara Mountains, north-eastern Tanzania. J. Trop. Med. Hyg. 1987;90:127–134. [PubMed] [Google Scholar]
- 34.Loevinsohn ME. Climatic warming and increased malaria incidence in Rwanda. Lancet. 1994;343:714–718. doi: 10.1016/s0140-6736(94)91586-5. [DOI] [PubMed] [Google Scholar]
- 35.Bugilimfura L, et al. Efficacité de la chloroquine, de l’amodiaquine et de l’association pyriméthamine-sulfadoxine pour le traitement du paludisme à Gikonko. Rev. Méd. Rwandaise. 1988;20:93–96. [Google Scholar]
- 36.Bonora S, et al. Rising temperature and the malaria epidemic in Burundi. Trends Parasitol. 2001;17:572–573. doi: 10.1016/s1471-4922(01)02095-5. [DOI] [PubMed] [Google Scholar]
- 37.Di Perri G, et al. Response of uncomplicated falciparum malaria to oral chloroquine and quinine in Burundi highlands. Acta Trop. 1998;70:25–33. doi: 10.1016/s0001-706x(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 38.Etchegorry MG, et al. Malaria epidemic in Burundi. Lancet. 2001;357:1046–1047. doi: 10.1016/s0140-6736(05)71623-8. [DOI] [PubMed] [Google Scholar]
- 39.Tulu AN, et al. Failure of chloroquine treatment for malaria in the highlands of Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1996;90:556–557. doi: 10.1016/s0035-9203(96)90322-3. [DOI] [PubMed] [Google Scholar]
- 40.Mouchet J. L’origine des épidémies de paludisme sur les Plateaux de Madagascar et les montagnes d’Afrique de L’est et du Sud. Bull. Soc. Pathol. Exot. 1998;91:64–66. [PubMed] [Google Scholar]
- 41.The International Bank for Reconstruction and Development/The World Bank . World Bank Africa Database on CD-ROM: Users’Manual. The International Bank for Reconstruction and Development/The World Bank; 2001. pp. 1–10. [Google Scholar]
- 42.Hay SI, et al. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker CJ, et al. African land-cover classification using satellite data. Science. 1985;227:369–375. doi: 10.1126/science.227.4685.369. [DOI] [PubMed] [Google Scholar]
- 44.Hay SI, et al. From predicting mosquito habitat to malaria seasons using remotely sensed data: practice, problems and perspectives. Parasitol. Today. 1998;14:306–313. doi: 10.1016/s0169-4758(98)01285-x. [DOI] [PubMed] [Google Scholar]
- 45.Hay SI, et al. Earth observation, geographic information systems and plasmodium falciparum malaria in sub-Saharan Africa. Adv. Parasitol. 2000;47:173–215. doi: 10.1016/s0065-308x(00)47009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.United Nations Population Division . World Population Prospects. The 1999 Revision. United Nations; 2000. Analysis of urban and rural population at the regional level; pp. 36–52. [Google Scholar]
- 47.Lines J, et al. Trends, priorities and policy directions in the control of vector-borne diseases in urban environments. Health Policy Plann. 1994;9:113–129. doi: 10.1093/heapol/9.2.113. [DOI] [PubMed] [Google Scholar]
- 48.Shretta R, et al. Using evidence to change antimalarial drug policy in Kenya. Trop. Med. Int. Health. 2000;5:755–764. doi: 10.1046/j.1365-3156.2000.00643.x. [DOI] [PubMed] [Google Scholar]
- 49.Kilian AHD, et al. Rainfall pattern, El Niño and malaria in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1999;93:22–23. doi: 10.1016/s0035-9203(99)90165-7. [DOI] [PubMed] [Google Scholar]
- 50.Lindblade KA, et al. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Niño. Trans. R. Soc. Trop. Med. Hyg. 1999;93:480–487. doi: 10.1016/s0035-9203(99)90344-9. [DOI] [PubMed] [Google Scholar]
- 51.Marimbu J, et al. Environment and malaria in Burundi. Apropos of a malaria epidemic in a non-endemic mountainous region. Bull. Soc. Path. Exotique. 1993;86:399–401. [PubMed] [Google Scholar]
- 52.Mouchet J, et al. La reconquête des Hautes Terres de Madagascar par le paludisme. Bull. Soc. Pathol. Exot. 1997;90:162–168. [PubMed] [Google Scholar]
- 53.Hay SI. An overview of remote sensing and geodesy for epidemiology and public health application. Adv. Parasitol. 2000;47:1–35. doi: 10.1016/s0065-308x(00)47005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]