Abstract
The attempts to develop new treatments for acute ischemic stroke have been fraught with costly and spectacularly disappointing failures. Repurposing of safe, older drugs provides a lower risk alternative. Vascular protection is a novel strategy for improving stroke outcome. Promising targets for vascular protection after stroke have been identified, and several of these targets can be approached with “repurposed” old drugs, including statins, angiotensin receptor blockers (ARBs), and minocycline.
We tested the vascular protection (ability to reduce hemorrhagic transformation) of three marketed drugs (candesartan, minocycline, and atorvastatin) in the experimental stroke model using three different rat strains [Wistar, spontaneously hypertensive rats (SHR) and type 2 diabetic Goto-Kakizaki (GK) rats]. All agents decreased the infarct size, improved the neurological outcome and decreased bleeding. Mechanisms identified include inhibition of MMP-9, activation of Akt, and increased expression of proangiogenic growth factors. Premorbid vascular damage (presence of either diabetes or hypertension) increased the likelihood of vascular injury after ischemia and reperfusion and improved the response to vascular protection.
Keywords: Stroke, Drug repurposing, Hemorrhagic transformation
Introduction
Drug repurposing has emerged as an antidote to the sluggish drug development pipeline [1-3], where marketed drugs are exploited for their secondary activity. This strategy is particularly well-suited to the public sector, where off-patent agents can undergo high throughput screening for their ability to interact with identified molecular targets both in vitro and in vivo [1]. Since neuroprotection has failed for stroke therapy, we have focused on vascular protection with an eye toward better success. Promising targets for vascular protection after stroke have been identified and include the inhibition of endogenous mediators of vascular damage (superoxide, endothelin, matrix metalloproteases, cytokines and caspases) and the stimulation of endogenous protectors (nitric oxide, angiopoietin 1, vascular endothelial growth factor-VEGF and superoxide dismutases) [4]. Several of these targets can be approached with “repurposed” old drugs, including statins, angiotensin receptor blockers (ARBs) and minocycline [5].
The purpose of this investigation was to compare the vascular protective properties of these agents in experimental stroke both with and without premorbid vascular disease.
Methods
The Institutional Animal Care and Use Committee (IACUC) of the Augusta VA Medical Center approved the protocol. Male Wistar rats (n = 90) and spontaneously hypertensive rats (n = 45) from the Charles River Breeding Company (Wilmington, MA) and GK rats (n = 40) from Taconic Farms, Inc. (Germantown, NY) within a narrow range of body weight (270–305 g) were used. Data from previously published reports [6-11] and unpublished data (Minocycline IP) from our group were included.
Experimental Cerebral Ischemia
Anesthesia was performed by using 2% isoflurane via inhalation. Cerebral ischemia was induced using the intraluminal suture middle cerebral artery occlusion (MCAO) model [12]. Then 19–21 mm 3-0 surgical nylon filament was introduced from the external carotid artery lumen into the internal carotid artery to block the origin of the right MCA. The animals were kept under anesthesia for only 15 min for the surgical procedure. Temperature was constantly maintained at 36.5–37.0°C using a controlled heating system. The suture was removed after 3 h of occlusion, and the animals were returned to their cages. At reperfusion, animals received one of the following drugs or vehicle: atorvastatin (5 or 15 mg/kg) (Pfizer Inc., NY, NY), candesartan (0.1, 0.3 or 1 mg/kg) (Astra Zeneca), hydralazine 1 mg/kg, enalapril (either 5 or 10 mg/kg) or minocycline (45 mg/kg IP or 3 mg/kg IV with tissue plasminogen activator-tPA, 10 mg/kg, Sigma Chemical and Genentech, Inc.). In the case of atorvastatin and minocycline, a second dose was administered 12 h later.
Assessment of Infarct Volume and Hemoglobin Content
At 24 h after the onset of MCAO, anesthesia was performed with intramuscular ketamine 75 mg/kg and xylazine 13 mg/kg (cocktail); animals were then perfused with saline and killed, and their brains were removed. The brain tissue was sliced into seven 2 mm thick slices in the coronal plane and stained with 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma Chemical Co., St. Louis, MO) for 15–20 min. Images of the stained sections were taken. Using image analysis software (Zeiss-KS300, Oberkochen, Germany), infarction zones were measured, and infarct volume was calculated. The ischemic and non-ischemic hemispheres of the slices taken from the core of the infarct for the enzyme-linked immunosorbent assay (ELISA) were separated and processed using the non-ischemic side as a control. After homogenizing the slices in the core of the infarct and taking the supernatants, ELISA was performed to measure the hemoglobin in the brain tissue [13].
Conversion and Analysis
In order to convert percent of contralateral hemisphere to cubic millimeters for infarct size, and hemoglobin per gram of protein to hemoglobin per gram of tissue, we used data from the control group of the study of the effect of candesartan 1 mg/kg in temporary MCAO [8], where both measures were performed. In those animals, total volume of the ipsilateral hemisphere was 1,192 mm3, and there was 0.17 g protein/g tissue.
For each of the three strains of rats, the line describing the relationship between infarct size and hemoglobin excess was determined and compared. The slopes of the lines were compared using analysis of covariance testing for slope homogeneity using SAS 9.2.
Results
Wistar
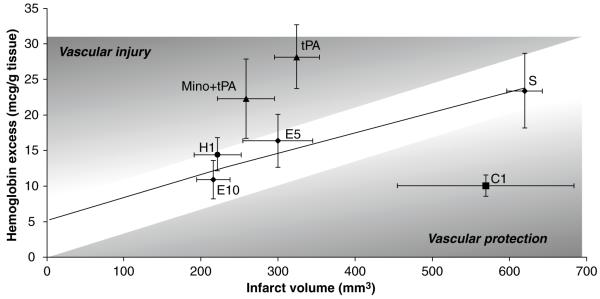
In animals treated with either saline or either of two doses of enalapril, the slope of the line describing the relationship between infarct size and degree of hemorrhagic transformation was significantly different from zero (p < 0.05) (Fig. 1). Hydralazine was similar to enalapril, but did not achieve statistical significance. Candesartan treatment resulted in significantly more vascular protection than neuroprotection.
Fig. 1.
Wistar rats. The trendline describes the relationship between infarct volume and hemorrhage formation in the saline- and enalapril-treated animals. Candesartan resulted in more vascular protection, and minocycline provided vascular protection when combined with tPA. tPA = tissue plasminogen activator 10 mg/kg (n = 13); Mino + tPA = mino-cycline 3 mg/kg IV + tPA 10 mg/kg IV (n = 11); S = saline (n = 12); C1 = candesartan 1 mg/kg IV (n = 19); E5 = enalapril 5 mg/kg (n = 6); E10 = enalapril 10 mg/kg (n = 9); H1 = hydralazine 1 mg/kg (n = 8)
In animals treated with minocycline IP, neither neuroprotection nor vascular protection was demonstrated (not shown). Animals receiving tPA alone had more vascular injury; however, when minocycline 3 mg/kg IV was administered to animals receiving tPA, both neuro and vascular protection was demonstrated.
Diabetes
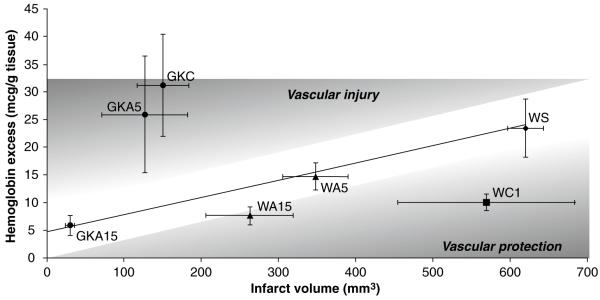
Diabetic (GK) animals had much higher vascular injury than Wistars when treated with saline. Atorvastatin significantly reduced neurovascular injury, and the line describing the relationship between infarct size and bleeding was significantly steeper in the GKs than in the Wistars (p = 0.0022). This suggests an improved response to vascular protection in these animals (Fig. 2).
Fig. 2.
Diabetic rats. The trendline describes the relationship between infarct volume and hemorrhage formation in the saline- and enalapril-treated Wistar animals (as in Fig. 1). Diabetic (GK) rats had a steeper slope than Wistar rats and treatment with atorvastatin (A) significantly reduced the injury in a dose-dependent manner. A15mg/kg IV showed the best vascular protection in both Wistars (W) and GKs. A treatment showed more vascular protection than neuroprotection in GK rats. GK C = GK control (n = 10); GK A5 = GK atorvastatin 5 mg/kg (n = 6); GK A15 = GK atorvastatin 15 mg/kg (n = 5); W S = Wistar saline (n = 12); W A5 = Wistar atorvastatin 5 mg/kg (n = 8); W A15 = Wistar atorvastatin 15 mg/kg (n = 8); W C1 = Wistar candesartan 1 mg/kg IV (n = 19)
SHRs
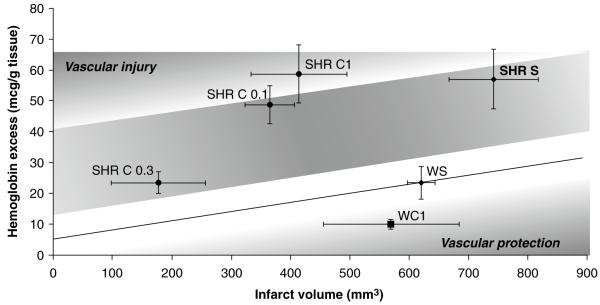
Previously hypertensive animals experienced the largest injuries in response to the 3 h of MCAO and reperfusion, with the vascular injury being predominant compared to Wistars (values above the curve describing Wistars). Again, these animals appeared to have increased sensitivity to candesartan when compared to Wistar rats, but none of the slopes of the raw data in the SHRs were significantly different from zero (Fig. 3).
Fig. 3.
Spontaneously hypertensive rats (SHRs). The trendline describes the relationship between infarct volume and hemorrhage formation in the saline- and enalapril-treated Wistar animals (as in Fig. 1). SHRs had predominant vascular injury compared to Wistars, but the relationship between infarct volume and hemorrhage was not significant in any of the four treatment groups. Candesartan 0.3 mg/kg showed the best neurovascular protection in SHR rats. SHR S = SHR saline (n = 16); SHR C1 = SHR candesartan 1 mg/kg IV (n = 11); SHR C 0.3 = SHR candesartan 0.3 mg/kg IV (n = 11); SHR C 0.1 = SHR candesartan 0.1 mg/kg IV (n = 7); W S = Wistar saline (n = 12); W C1 = Wistar candesartan 1 mg/kg IV (n = 19)
Discussion
The vascular injury that occurs during cerebral ischemia and leads to hemorrhagic transformation varies directly with infarct size. Neuroprotective agents reduce bleeding (vascular injury) as a result of decreasing infarct size. Vascular protective agents are particularly effective in reducing vascular injury and bleeding out of proportion to the reduction in infarct size. In the analysis presented, candesartan and atorvastatin were predominantly vascular protective, especially in animals with preexisting vascular damage (diabetes, hypertension). Minocycline demonstrated vascular protection when administered in combination with tPA. Acute vascular protection after cerebral ischemia can reduce excess risk caused by premorbid vascular injury and improve outcome. Vascular protection should be pursued, particularly in stroke patients with known hypertension and diabetes.
Acknowledgment
SCF has received grant support from Pfizer, Inc, VA Merit Review (BX000891) and NIH-NINDS (RO1 - NS063965)
Footnotes
Conflict of interest statement SCF is a consultant for Pfizer, Inc and Genentech, Inc. Candesartan was a gift from Astra Zeneca and tPA was a gift from Genentech, Inc.
Contributor Information
Weihua Guan, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Charlie Norwood VA Medical Center, Augusta, GA, USA.
Anna Kozak, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Charlie Norwood VA Medical Center, Augusta, GA, USA.
Susan C. Fagan, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Charlie Norwood VA Medical Center, Augusta, GA, USA and University of Georgia College of Pharmacy, Charlie Norwood VA Medical Center, HM-1220 Medical College of Georgia, 1120 15th St., Augusta, GA, USA
References
- 1.Bisson WH, Cheltsov AV, Bruey-Sedano N, Lin B, Chen J, Goldberger N, May LT, Christopoulos A, Dalton JT, Sexton PM, Zhang XK, Abagyan R. Discovery of antiandrogen activity of nonsteroidal scaffolds of marketed drugs. Proc Natl Acad Sci USA. 2007;104:11927–11932. doi: 10.1073/pnas.0609752104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boguski MS, Mandl KD, Sukhatme VP. Repurposing with a difference. Science. 2009;324:1394–1395. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]
- 3.Duenas-Gonzalez A, Garcia-Lopez P, Herrera LA, Medina-Franco JL, Gonzalez-Fierro A, Candelaria M. The prince and the pauper. A tale of anticancer targeted agents. Mol Cancer. 2008;7:82. doi: 10.1186/1476-4598-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagan SC, Hess DC, Pollock DM, Hohnadel EJ, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35(9):2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 5.Fagan SC, Hess DC, Machado LS, Hohnadel EJ, Pollock DM, Ergul A. Tactics for vascular protection after acute ischemic stroke. Pharmacotherapy. 2005;25(3):387–395. doi: 10.1592/phco.25.3.387.61592. [DOI] [PubMed] [Google Scholar]
- 6.Elewa HF, Kozak A, Johnson MH, Ergul A, Fagan SC. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J Hypertens. 2007;25:855–859. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 7.Elewa HF, Kozak A, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, Ergul A, Hess DC. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 9.Kozak A, El-Remessy AB, Ergul A, Machado LS, Elewa HF, Johnson MH, Abdelsaid M, Wiley DA, Fagan SC. Candesartan augments ischemia-induced proangiogenic state and results in sustained functional improvement after stroke. Stroke. 2009;40:1870. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozak W, Kozak A, Elewa HF, Johnson MH, Fagan SC. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther. 2008;326:773–782. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- 11.Machado LS, Sazanova I, Kozak A, Wiley DC, El-Remessy AB, Ergul A, Hess DC, Fagan SC. Minocycline and tissue plasminogen activator for stroke: assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 13.Hilali HM, Simpkins AN, Hill WD, Waller JL, Knight RA, Fagan SC. Single slice method for quantification of hemorrhagic transformation using direct ELISA. Neurol Res. 2004;26:93–98. doi: 10.1179/016164104773026606. [DOI] [PubMed] [Google Scholar]