Abstract
Background
Anemia is associated with morbidity and mortality and frequently leads to transfusion of erythrocytes. We sought to compare directly the effect of high inspired oxygen fraction vs. transfusion of erythrocytes on the anemia-induced increased heart rate (HR) in humans undergoing experimental acute isovolemic anemia.
Methods
We combined HR data from healthy subjects undergoing experimental isovolemic anemia in seven studies performed by our group. We examined HR changes associated with breathing 100% oxygen by non-rebreathing face mask vs. transfusion of erythrocytes at their nadir hemoglobin (Hb) concentration of 5 g/dL. Data were analyzed using a mixed-effects model.
Results
HR had an inverse linear relationship to hemoglobin concentration with a mean increase of 3.9 beats per minute per gram of Hb (beats/min/g Hb) decrease (95% confidence interval [CI], 3.7 – 4.1 beats/min/g Hb), P < 0.0001. Return of autologous erythrocytes significantly decreased HR by 5.3 beats/min/g Hb (95% CI, 3.8 – 6.8 beats/min/g Hb) increase, P < 0.0001. HR at nadir Hb of 5.6 g/dL (95% CI, 5.5 – 5.7 g/dL) when breathing air (91.4 beats/min; 95% CI, 87.6 – 95.2 beats/min) was reduced by breathing 100% oxygen (83.0 beats/min; 95% CI, 79.0 -87.0 beats/min), P < 0.0001. The HR at hemoglobin 5.6 g/dL when breathing oxygen was equivalent to the HR at Hb 8.9 g/dL when breathing air.
Conclusions
High arterial oxygen partial pressure reverses the heart rate response to anemia, probably owing to its usability, rather than its effect on total oxygen content. The benefit of high arterial oxygen partial pressure has significant potential clinical implications for the acute treatment of anemia and results of transfusion trials.
Introduction
Anemia is associated with many comorbidities, such as renal and cardiac disease, as well as with numerous adverse outcomes, including mortality. A comprehensive review of reported cases and series of untransfused Jehovah Witness patients found no mortality when the hemoglobin concentration exceeded 5 g/dL, but a 44% mortality when the hemoglobin concentration was less than 5 g/dL.1 Database analyses of surgical patients who refused transfusion found increased mortality with lower hemoglobin concentrations 2,3 and that the presence of cardiovascular disease substantially increased that risk.2 Two more recent retrospective analyses of large databases found that anemia was independently associated with adverse outcomes in surgical patients, even when controlling for comorbidities.4,5 The reasons for worse outcomes are not known, but likely include inadequate oxygenation of tissues, and stresses related to physiological compensation for anemia. Elevation of heart rate is an important compensatory mechanism for maintaining oxygen delivery during anemia.6 However, tachycardia has adverse consequences for patients with coronary artery disease. It is associated with myocardial ischemia in the post-operative period in those patients with or at risk for coronary artery disease,7 and post-operative ischemia is associated with adverse outcomes in those patients.8
Erythrocytes are often transfused to reduce or prevent cardiac ischemia associated with the tachycardia of anemia, but the benefits may not outweigh the risks.9 We have previously shown that heart rate increases linearly as hemoglobin decreases during isovolemic anemia in awake, unsedated humans,10 and that transfusion of erythrocytes or breathing oxygen reverses the signs and symptoms of acute anemia.11–13 There are few studies examining oxygen reversal of the effects of anemia, and none comparing a quantitative physiological effect of oxygen with that of erythrocyte transfusion. We report here the effect of transfusion, and that of oxygen, on reversing the increased heart rate due to anemia, using the data we have accumulated in several studies of acute isovolemic anemia in more than 100 awake, unmedicated healthy people.
Materials and Methods
We combined data from 6 published studies over 8 years during which we produced acute isovolemic anemia (Table 1). Data from some published studies were subsets of the studies in Table 1 and are not listed separately;14–16 however, all studied subjects are included in our analyses. All studies were performed with approval of the Institutional Review Board at the University of California, San Francisco and informed consent of every subject. All subjects were healthy volunteers except for 11 healthy patients about to undergo major orthopedic surgery. Subjects and patients were free of cardiovascular, pulmonary, renal, and hepatic disease as determined by history, physical exam and laboratory analyses.
Table 1.
Summary of Studies
| Publication | # Subjects | Gender (F/M) | Return | O2 | Slope (95%CI) |
|---|---|---|---|---|---|
| Weiskopf et al.6 | 32 | 16/16 | No | No | −4.48 (−4.17 to −4.79) |
| Hopf et al.34 | 14 | 10/4 | No | No | −3.79 (−3.30 to −4.27) |
| Weiskopf et al.35 | 11 | 5/17 | Yes | No | −3.80 (−3.25 to −4.35) |
| Weiskopf et al.12 | 30 | 20/10 | No | Yes | −3.36 (−2.99 to −3.74) |
| Weiskopf et al.36 | 8 | 5/3 | No | No | −3.61 (−2.76 to −4.46) |
| Weiskopf et al.13 | 14 | 10/4 | No | Yes | −3.74 (−2.99 to −4.50) |
| Weiskopf et al.11 | 9 | 6/3 | Yes | No | −3.92 (−3.41 to −4.42) |
| Totals | 129 | 72/57 | |||
Gender, M = male, F = female; Return refers to data available on re-transfusion of autologous blood; O2 refers to data taken on and off oxygen by non-rebreather; in some cases we report more subject than in the original manuscript because of incomplete dataset for the primary outcome
The method for producing isovolemic anemia has been previously reported.6 Briefly, whole blood was removed from non-anesthetized awake subjects or patients through an indwelling large-bore peripheral intravenous cannula into CPDA-1 collection bags (Baxter Healthcare Corporation, Deerfield, IL). We infused warmed 5% albumin or the subject's autologous platelet-rich plasma, or both, simultaneously through a peripheral intravenous cannula in the opposite arm, in sufficient quantities to maintain isovolemia.6 At least 10 minutes were allowed for removal of each unit of blood. Heart rate was recorded 5–10 minutes after removal of blood to assure physiological stabilization.
At least 2 units of packed erythrocytes were reinfused, at the end of each study. Remaining erythrocytes were infused slowly overnight, but without recording heart rate data. Two studies included a total of 36 subjects randomly allocated to breathe air or 100% oxygen through a non-rebreathing face mask at the nadir hemoglobin concentration. All available heart rate and hemoglobin data are included in the analyses reported here.
Statistics
We assessed the effects of breathing oxygen during anemia by examining the relationship between heart rate (HR) and hemoglobin (Hb) concentration when each subject breathed either air or oxygen (order randomly allocated), using a mixed-effects linear regression model. This is a repeated-measures model that allows each person to have an individual fitted line with its own slope and intercept. To examine whether the relationship between HR and hemoglobin was linear for the entire range of data, the linear model was applied to the data set using several upper cut-off values of hemoglobin. To test for linearity, a quadratic term (Hb2) was added to the model and tested for statistical significance. The effect of gender on the slope of the relationship was also examined in the model by testing the interaction term of gender and hemoglobin. The mixed-effects model was also performed separately for male and female individuals, and the 95% confidence limits for the slopes computed. A random effect for the different studies was also added in the model. Thus, the complete model included a repeated measures analysis, with random effects of study identity and of subjects nested within gender, and the interaction of gender and hemoglobin.
The basic mixed effects model was then expanded to include data from the re-infusion of autologous erythrocytes for those subjects for whom we had data both during dilution and return of autologous erythrocytes, or for whom we had data when they breathed oxygen in addition to room air at their nadir hemoglobin concentration. The heart rate at the nadir hemoglobin while subjects breathed air was compared by paired t-test with their heart rate when they breathed oxygen.
Data were analyzed using JMP 7.0 (SAS Institute, Cary, NC). Data are reported as mean (95% confidence intervals [CI]). P < 0.05 was considered statistically significant.
Results
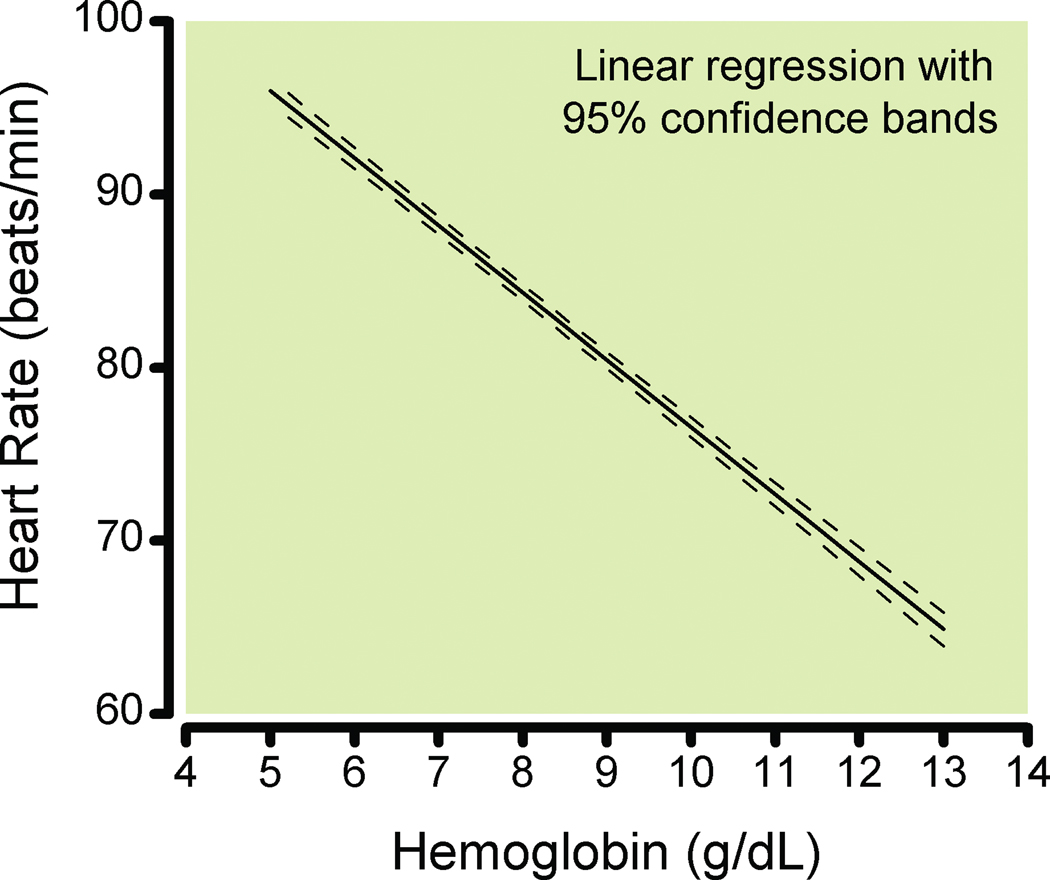
Data from 129 subjects (72 women and 57 men) were combined. HR had an inverse linear relationship to hemoglobin (Hb) concentration (P < 0.0001, Fig. 1), with an increase of 3.9 (95% CI, 3.7 – 4.1) beats per minute per gram hemoglobin concentration (beats/min/g Hb) decrease.
Figure 1.
Linear regression of heart rate and hemoglobin for 129 individuals during isovolemic hemodilution. Dashed lines show the 95% confidence bands derived from the mixed-effects (repeated measures) model.
Return of Erythrocytes
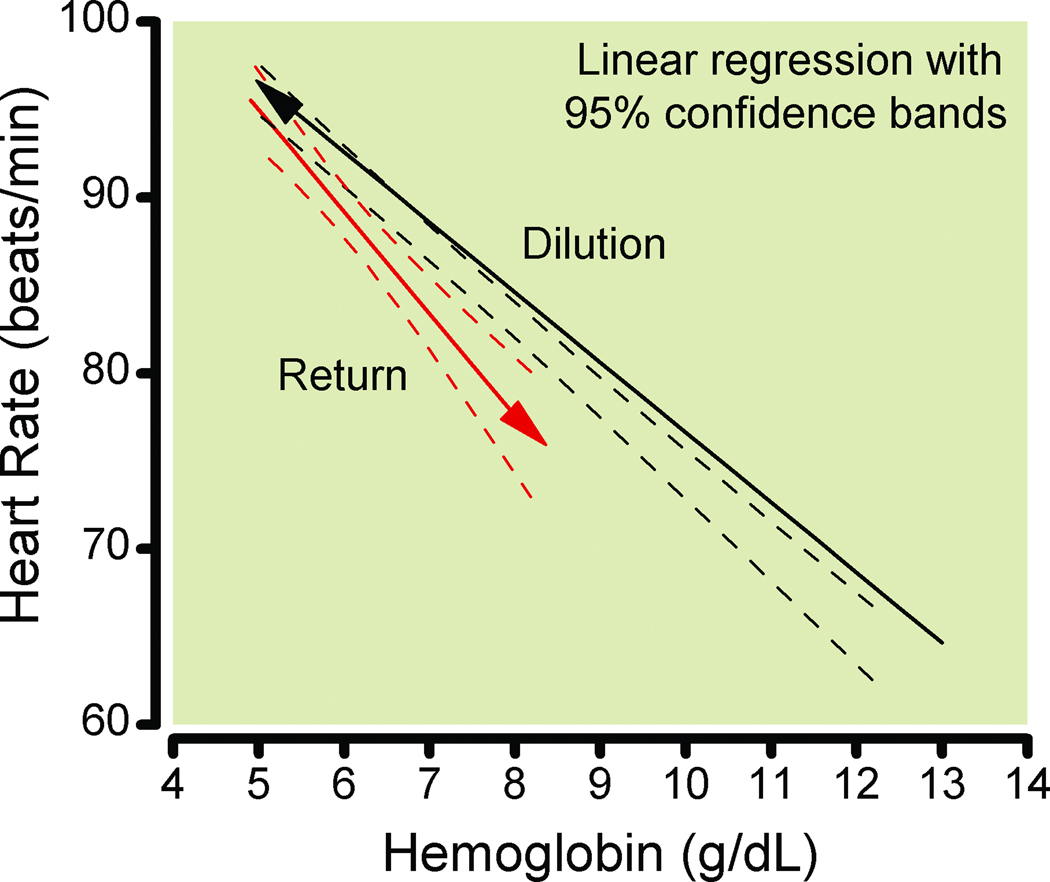
Data after transfusion of autologous erythrocytes were available for 61 subjects, of which 33 subjects had at least two data points during return of blood for both HR and hemoglobin. These included 9 subjects who were studied on two separate days comparing the effects fresh and stored blood. Data from the two days were not statistically different and were pooled.
HR differed significantly between hemodilution and return of erythrocytes, (P = 0.009). Return of autologous erythrocytes significantly decreased HR by 5.3 beats/min/g Hb increase (95% CI, 3.8–6.8 beats/min/g Hb) (P < 0.0001, Fig. 2). This slope was not different (P = 0.09) from the slope for these same 33 individuals during hemodilution, 4.0 beats/min/g Hb (95% CI, 3.6–4.4 beats/min/g Hb). (In the mixed-effects model, the “direction” (return vs. dilution) was statistically significant, but the interaction term between hemoglobin and direction was not statistically significant.)
Figure 2.
Linear regressions for the 33 individuals that had at least 2 hemoglobin values after transfusion. The solid line is the regression for these subjects during isovolemic hemodilution, with 95% confidence bands. The dashed line is the regression for the same individuals during transfusion of their autologous red blood cells, with 95% confidence bands. Heart rate during dilution differed significantly from return of erythrocytes, P = 0.009. Isovolemia was not maintained with return of erythrocytes.
Effect of Oxygen
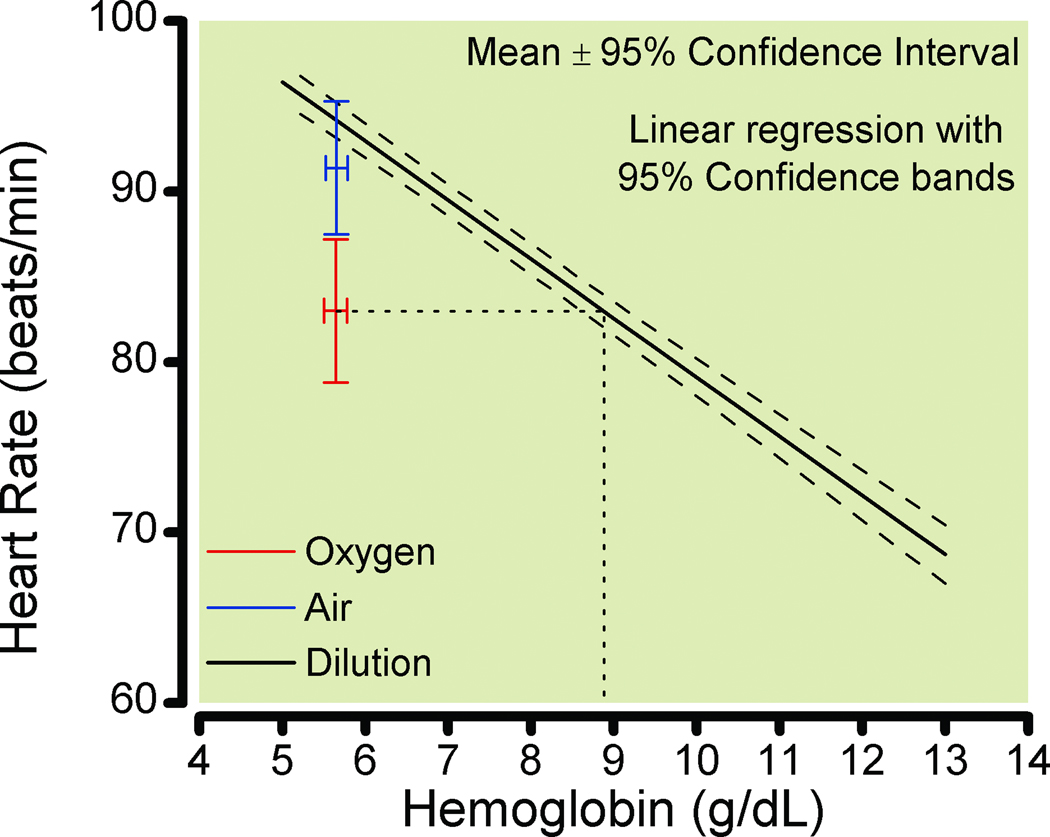
Thirty-six subjects (25 women and 11 men) had heart rate data at nadir hemoglobin concentrations when breathing both air and oxygen (doubled-blinded and order randomly allocated). The HR at nadir hemoglobin of 5.6 g/dL (95% CI, 5.5–5.7 g/dL) when breathing air was 91.4 beats/min (95% CI, 87.5 – 95.3 beats/min). This differed from the HR when breathing oxygen at the same hemoglobin concentration, 83.0 beats/min (95% CI, 78.8 -87.2 beats/min) (P < 0.0001). In 29 subjects with heart rate data at the nadir hemoglobin concentration (while breathing air) prior to beginning the randomized air or oxygen breathing, the heart rate was 96.3 beats/min (95% CI, 92.0 -100.5 beats/min), which was significantly different from corresponding air value, 92.3 beats/min (95% CI, 87.3 – 97.3 beats/min), P = 0.0063. The HR when breathing oxygen differed significantly from the 95% confidence limits of the regression during hemodilution (while breathing air) for these 36 subjects (effect of oxygen, P < 0.0001; Fig. 3). The HR when subjects breathed oxygen at hemoglobin 5.6 g/dL was approximately equivalent to that when breathing air at hemoglobin 8.9 g/dL (Fig 3). The effect of oxygen was confirmed in the complete mixed-effects regression model (P < 0.0001).
Figure 3.
Linear regression during isovolemic hemodilution for the 36 individuals with complete data when breathing oxygen and air (random assignment) at the nadir hemoglobin with 95% confidence bands. The circle indicates heart rate (mean ± 95% confidence intervals) breathing air; the diamond indicates heart rate breathing oxygen. The dashed line from the diamond projected to the dilution regression indicates the hemoglobin at a heart rate equivalent to that breathing oxygen at a hemoglobin concentration of 5.6 g/dL.
Linearity and Gender During Hemodilution
The relationship between heart rate and hemoglobin fit a linear model. A statistically significant quadratic term could only be found in male subjects when restricting the analysis to Hb ≤ 12 g/dL and Hb ≤ 13 g/dL. The linear relationship between heart rate and hemoglobin concentration differed significantly (P < 0.0001) between men and women. In a comparable range of hemoglobin values (4–12 g/dL), men increased HR 3.4 beats/min/g Hb (95% CI, 3.0 – 3.8 beats/min/g Hb) while women increased HR by 4.6 beats/min/g Hb (95% CI, 4.2 – 5.0 beats/min/g Hb).
Discussion
Our main finding is that breathing oxygen during severe anemia reduces heart rate by an amount equivalent to the augmentation of hemoglobin concentration by approximately 3 g/dL. In addition, (1) we have extended our previous finding of a linear relationship between heart rate and acute isovolemic anemia with heart rate increasing 4 beats/min/g Hb decrease; and (2) the relationship between heart rate and hemoglobin concentration is different between men and women.
The relationship between hemoglobin and heart rate was linear. Our previous analysis of this relationship10 was confirmed with this extended data set.
Women had a larger heart rate increase in response to decreasing hemoglobin concentration than men. The predicted heart rate difference of 6 beats/min at a hemoglobin concentration of 5 g/dL is the equivalent to a hemoglobin difference of about 1.5 g/dL. While this may not seem large, it is a difference that could impact choice of transfusion thresholds and the tolerance of profound anemia between men and women. We have shown previously that the oxygen delivery decrease during profound anemia occurs later in women than in men.6 The heart rate differences probably are an important component of this greater tolerance to profound anemia.
Reversal of the heart rate response by transfusion of erythrocytes was expected. We had found previously that there were no differences in the reversal of heart rate changes between fresh autologous blood and autologous blood stored for 21 days.11 In the present analysis, there were small but statistically significant differences between the heart rate during hemodilution and the reinfusion of erythrocytes. Our experimental method provides for very good control of isovolemia during dilution and we have shown that our physiological measurements are not confounded by change in cardiac preload.6 However, return of red blood cells was not accomplished with maintenance of isovolemia: the transfusion of 2 units of erythrocytes likely expanded blood volume by approximately 7%. Therefore, the heart rate changes may have been affected not only by the increase in hemoglobin concentration, but also by this relatively modest increase in blood volume, and thus, it is not unexpected that the heart rate changes during transfusion differed slightly and statistically significantly from changes during isovolemic dilution. The differences are not clinically significant, were detected only by the use of a very sensitive statistical analysis, and were likely due to slight augmentation of blood volume, and thus, preload.
A high inspired oxygen fraction (FiO2) substantially reduced the increased heart rate produced by anemia. This finding is not consistent with the conventional wisdom that dissolved oxygen does not deliver a substantial quantity of oxygen. We noted this effect of high FiO2 during earlier studies,12,13 and sought to quantify its physiological consequences here with greater accuracy, using data pooled from our several studies, and to compare the effect with that of transfusion. Ideally, we would have data for supplemental oxygen and transfusion in the same subjects; we do not. However, the amount of data available to us retrospectively was substantial, and analysis of the complete data set has produced valuable results. Our estimate that the heart rate changes from supplemental oxygen are equivalent to an increase of 3 g/dL of hemoglobin are based on comparing the HR while breathing oxygen to the HR during hemodilution while breathing air. Comparing the HR while breathing air (91.4 beats/min) to that while breathing oxygen (83.0 beats/min) at the nadir hemoglobin would produce an equivalent of 2 g/dL hemoglobin (figure 3). The HR when breathing air, while within the 95% confidence estimates in figure 3, appears slightly lower than expected. HR data for 29 subjects immediately prior to the randomized air or oxygen treatment showed a significantly higher heart rate of 96.0 beats/min. A decreased HR response, or a prolonged effect of oxygen, 17 may have occurred in those subjects who received oxygen first; however our inability to demonstrate an effect of the treatment order12 argues against these possibilities.
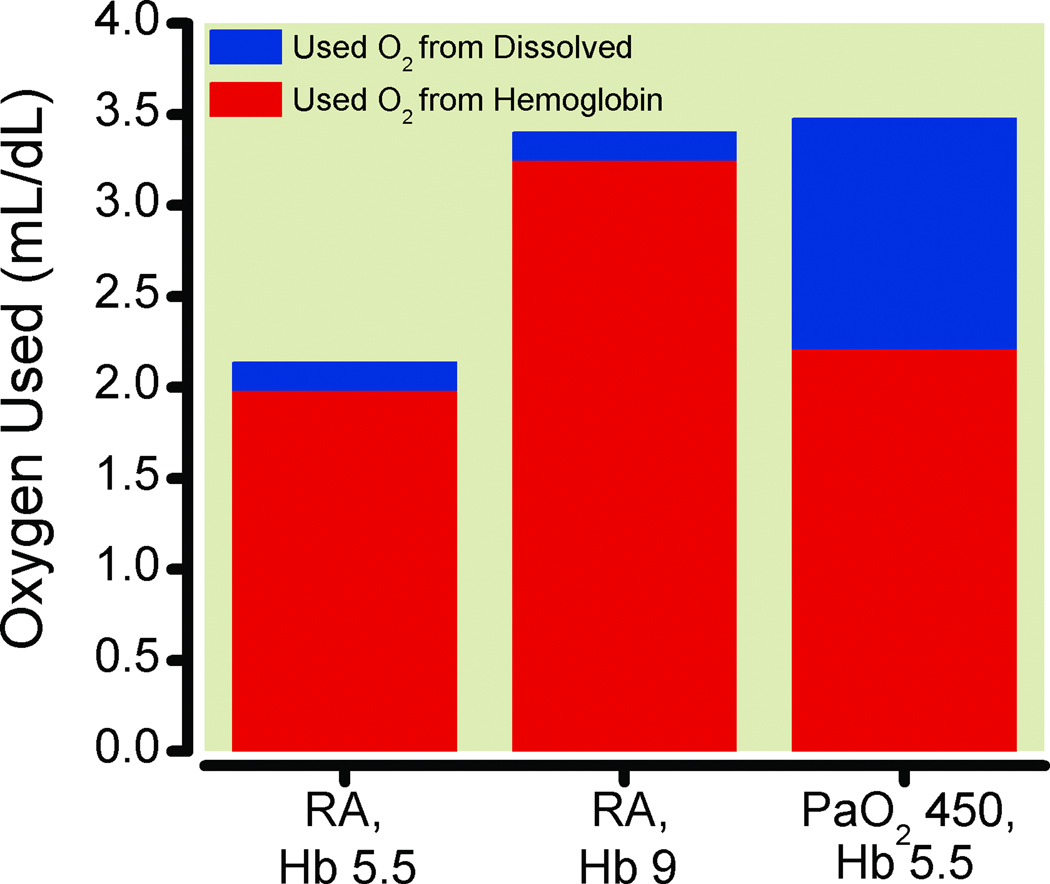
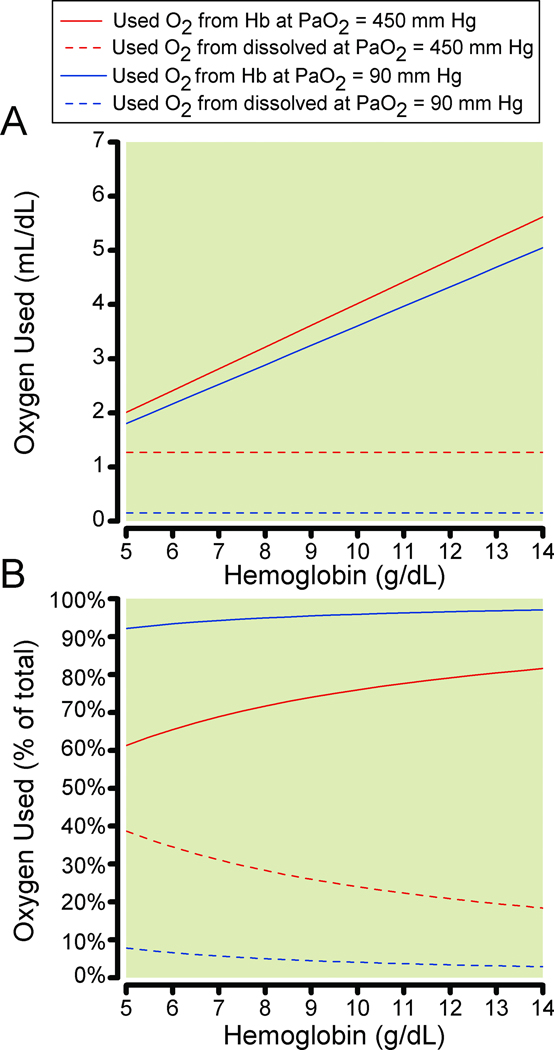
The small amount of oxygen dissolved in plasma (0.0031 ml O2/dL/mm Hg) at normal arterial oxygen partial pressure (PaO2) (approximately 0.3 mL/dL) is more substantial at high FiO2. However, mathematically, a PaO2 of 450 mmHg would be required to have an amount of dissolved oxygen equivalent to the 1.34 ml of oxygen carried by one gram of hemoglobin. This suggests that even high FiO2 would not contribute a sufficient amount of dissolved oxygen to lower the heart rate by more than 4 beats per minute in anemic subjects. With normoxia, even with anemia, only about 22% of the oxygen carried by hemoglobin is utilized when arterial blood at a PaO2 of 90 mmHg with an oxyhemoglobin saturation of 97% traverses to venous blood and a PvO2 of 40 mmHg with an oxyhemoglobin saturation of 75%. At normal PaO2, approximately 56% ([90–40]/90) of dissolved oxygen is utilized. However, the volume of dissolved oxygen used is only 0.16 mL/dL, while the volume of oxygen used of that carried by hemoglobin is 4.7 mL/dL. Increasing arterial PO2 by about 94 mmHg would add an amount of utilized oxygen from plasma (94• 0.0031 = 0.29 mL/dL) that would be equivalent to the amount of oxygen utilized from that carried by one gram of hemoglobin ([1.34 • (0.97 – 0.75) = 0.29]). Thus, increasing PaO2 to over 400 mmHg (augmenting PaO2 by 300 mmHg by increasing FiO2), as was accomplished in healthy volunteers in our studies,12 should theoretically produce the same reduction in heart rate as 3 grams of hemoglobin (Figure 4). This is consistent with our findings, where subjects breathing oxygen at a nadir hemoglobin of 5.6 g/dL had the same HR as was found during isovolemic hemodilution with these subjects breathing air at a hemoglobin concentration of approximately 8.9 g/dL (Fig. 3). The effect of dissolved oxygen and its higher usability is true as well at higher concentrations of hemoglobin, although the relative size of the impact is reduced (Fig. 5).
Figure 4.
Calculated data for the amount of oxygen (O2) used by tissues that came from hemoglobin and dissolved sources: A) at hemoglobin (Hb) 5.5 g/dL and a room air (RA) arterial oxygen partial pressure (PaO2) = 90 mm Hg (RA, Hb 5.5), B) at hemoglobin 9.0 g/dL and RA, PaO2 = 90 mm Hg (RA, Hb 9) and C) at hemoglobin 5.5 g/dL and PaO2 = 450 mm Hg (PaO2 450, Hb 5.5). Venous (tissue) PO2 was 40 mm Hg and venous oxygen saturation was 70% for the calculations.
Figure 5.
Calculated data for the oxygen (O2) used by tissues that came from hemoglobin and dissolved sources vs. hemoglobin concentration. Figure 5A shows the total oxygen used from hemoglobin at 1) room air (RA) arterial oxygen partial pressure (PaO2) = 90 mm Hg (solid gray line), 2) PaO2 = 450 mm Hg (solid black line) and from dissolved at 1) RA, PaO2 = 90 mm Hg (dashed gray line), 2) PaO2 = 450 mm Hg (dashed black line). Figure 5B shows the percentage of oxygen used from hemoglobin at 1) room air, PaO2 = 90 mm Hg (solid gray line), 2) PaO2 = 450 mm Hg (solid black line) and from dissolved at 1) RA, PaO2 = 90 mm Hg (dashed gray line), 2) PaO2 = 450 mm Hg (dashed black line).
Observing heart rate changes in response to higher FiO2 in patients would be difficult. Many other factors affect HR clinically, including surgical stimulation, opioids, inhaled anesthetic agents, beta-adrenergic antagonists, and patient co-morbidities. HR also may not increase in response to anemia during general anesthesia.18 Human volunteer studies such as ours are very robust, allowing repeated-measures analysis, and control of confounding factors. Despite these issues in patients, the physiological greater usability of dissolved oxygen would still be present. We would also emphasize that our model is of acute isovolemic anemia. Adequate cardiac output and tissue blood flow are necessary for the benefits of dissolved oxygen, which would not necessarily be present in the case of blood loss with significant hypovolemia.
This larger effect of increased utilization of dissolved oxygen at high FiO2 has potentially important clinical and therapeutic implications. For example, treatment of patients with symptomatic anemia with supplemental oxygen could be initiated while awaiting transfusion. In patients at risk for myocardial ischemia, short-term high FiO2 has no risks, whereas rapid transfusion can precipitate circulatory overload and consequent pulmonary edema.19,20 When erythrocyte transfusion is warranted in this group, it may be possible to proceed more slowly and safely with the administration of supplemental oxygen as a temporizing measure. High FiO2 is often already used for patients under general anesthesia, where anemia may occur due to surgical blood loss. Notably, the two editions of the American Society of Anesthesiologists Practice Guidelines for Blood Component Therapy did not been address this possibility.21,22 Additionally, supplemental oxygen has been shown to decrease heart rate following abdominal surgery.23
Treatment of anemia with oxygen has been shown to be effective in laboratory studies.24–26 Ventilation with 100% oxygen decreases critical hemoglobin concentration,26 reduces mortality,25 myocardial ischemia, and signs of myocardial ischemia24 in swine. Although these investigators recognized the greater relative contribution of dissolved oxygen toward the total amount of oxygen utilized at the lowest concentration of hemoglobin, these studies did not directly compare oxygen administration to transfusion.
Fontana et al. produced isovolemic hemodilution to a mean hemoglobin concentration of 3.0 g/dL, in children undergoing scoliosis surgery with an FiO2 of 1, without evidence of inadequate tissue oxygenation.27 Haque found no decrease in HR, but a decrease in cardiac output and stroke volume in patients with left ventricular failure given oxygen.28 The effect of oxygen on cardiac output was considered an adverse effect, apparently based on the misconception that dissolved oxygen could not contribute a clinically significant amount, despite the observed increases in mixed-venous PO2. Estimates of the efficacy of supplemental oxygen are still based on the effect on total oxygen content, not “usable” oxygen.
The substantial effect of increasing PaO2 on the heart rate response to anemia raises the possibility that this could be an important factor in clinical trials concerned with anemia and transfusion, in as much as a substantial increase in HR is associated with adverse cardiac outcomes in those with or at risk for cardiovascular disease,7,8 and that risk is mitigated by lessening the HR increase by use of beta-adrenergic antagonists.29 Investigators should acknowledge this effect of breathing high oxygen concentrations in their study design and data analysis.
The receptor or transduction mechanism for increasing heart rate in response to anemia is not known. Our data describe the relationship, but do not address the mechanism. We found previously that this reflex/response could not be eliminated with beta-adrenergic blockade, using substantial doses of esmolol in conscious humans,15 yet under general anesthesia, heart rate may not increase with anemia18. The importance of the carotid and aortic bodies in the physiological responses, including heart rate, to anemia has produced inconsistent results in laboratory studies.30–32 However, aortic chemoreceptor activation is not sufficient to explain the heart rate responses to anemia in humans, as humans lack active aortic chemoreceptors.33
Combining the data from several studies of similar volunteers allowed us to produce a substantial dataset, avoiding the necessity of performing repetitive studies that are physiologically challenging and invasive. We have extended our previous finding of a consistent linear increase in heart rate during anemia in unmedicated healthy volunteers and that return of subjects’ autologous erythrocytes reverses the HR response to anemia. Most importantly, we have shown that a high FiO2 reverses the HR response to anemia. This is consistent with greater usability of dissolved oxygen. The benefit of high PaO2 has potential clinical implications and its effect should also be considered in transfusion trial design and data analysis.
What we already know about this topic
Anemia is an independent predictor of increased morbidity in surgical patients and compensatory tachycardia that occurs during anemia may contribute to increased risk, particularly, in patients with cardiovascular disease
What this study tells us that is new
Healthy subjects breathing oxygen during severe anemia demonstrated decreases in heart rate by an amount equivalent to that of increasing hemoglobin concentration by approximately 3 g/dL
Supplemental oxygen could be a temporizing measure before transfusion of erythrocytes is initiated
Acknowledgments
Supported, in part, by a Public Health Service Award from the National Heart, Lung and Blood Institute, National Institutes of Health, Grant # 1 P50 HL54476, Bethesda, Maryland, United States. These studies were carried out, in part, in the General Clinical Research Center, Moffitt Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources, 5 MO1 RR-00079.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah's Witnesses. Transfusion. 1994;34:396–401. doi: 10.1046/j.1537-2995.1994.34594249050.x. [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, Noveck H, Strom BL. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: A single-center cohort study. Anesthesiology. 2009;110:574–581. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 5.Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 6.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 7.Mangano DT, Wong MG, London MJ, Tubau JF, Rapp JA. Perioperative myocardial ischemia in patients undergoing noncardiac surgery--II: Incidence and severity during the 1st week after surgery. The Study of Perioperative Ischemia (SPI) Research Group. J Am Coll Cardiol. 1991;17:851–857. doi: 10.1016/0735-1097(91)90864-6. [DOI] [PubMed] [Google Scholar]
- 8.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Weiskopf RB, Feiner J, Hopf H, Viele MK, Watson JJ, Lieberman J, Kelley S, Toy P. Heart rate increases linearly in response to acute isovolemic anemia. Transfusion. 2003;43:235–240. doi: 10.1046/j.1537-2995.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Weiskopf RB, Feiner J, Hopf HW, Viele MK, Watson JJ, Kramer JH, Ho R, Toy P. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96:871–877. doi: 10.1097/00000542-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf RB, Toy P, Hopf HW, Feiner J, Finlay HE, Takahashi M, Bostrom A, Songster C, Aminoff MJ. Acute isovolemic anemia impairs central processing as determined by P300 latency. Clin Neurophysiol. 2005;116:1028–1032. doi: 10.1016/j.clinph.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Leung JM, Weiskopf RB, Feiner J, Hopf HW, Kelley S, Viele M, Lieberman J, Watson J, Noorani M, Pastor D, Yeap H, Ho R, Toy P. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93:1004–1010. doi: 10.1097/00000542-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JA, Weiskopf RB, Kelley SD, Feiner J, Noorani M, Leung J, Toy P, Viele M. Critical oxygen delivery in conscious humans is less than 7.3 ml O2 × kg(−1) × min(−1) Anesthesiology. 2000;92:407–413. doi: 10.1097/00000542-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Toy P, Feiner J, Viele MK, Watson J, Yeap H, Weiskopf RB. Fatigue during acute isovolemic anemia in healthy, resting humans. Transfusion. 2000;40:457–460. doi: 10.1046/j.1537-2995.2000.40040457.x. [DOI] [PubMed] [Google Scholar]
- 17.Eggers GW, Paley HW, Leonard JJ, Warren JV. Hemodynamic responses to oxygen breathing in man. J Appl Physiol. 1962;17:75–79. [Google Scholar]
- 18.Ickx BE, Rigolet M, Van Der Linden PJ. Cardiovascular and metabolic response to acute normovolemic anemia. Effects of anesthesia. Anesthesiology. 2000;93:1011–1016. doi: 10.1097/00000542-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 20.Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243–244. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–747. [PubMed] [Google Scholar]
- 22.Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg-Adamsen S, Lie C, Bernhard A, Kehlet H, Rosenberg J. Effect of oxygen treatment on heart rate after abdominal surgery. Anesthesiology. 1999;90:380–384. doi: 10.1097/00000542-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kemming GI, Meisner FG, Meier J, Tillmanns J, Thein E, Eriskat J, Habler OP. Hyperoxic ventilation at the critical hematocrit: effects on myocardial perfusion and function. Acta Anaesthesiol Scand. 2004;48:951–959. doi: 10.1111/j.0001-5172.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 25.Meier J, Kemming GI, Kisch-Wedel H, Wolkhammer S, Habler OP. Hyperoxic ventilation reduces 6-hour mortality at the critical hemoglobin concentration. Anesthesiology. 2004;100:70–76. doi: 10.1097/00000542-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Pape A, Meier J, Kertscho H, Steche M, Laout M, Schwerdel F, Wedel M, Zwissler B, Habler O. Hyperoxic ventilation increases the tolerance of acute normovolemic anemia in anesthetized pigs. Crit Care Med. 2006;34:1475–1482. doi: 10.1097/01.CCM.0000215826.45839.36. [DOI] [PubMed] [Google Scholar]
- 27.Fontana JL, Welborn L, Mongan PD, Sturm P, Martin G, Bunger R. Oxygen consumption and cardiovascular function in children during profound intraoperative normovolemic hemodilution. Anesth Analg. 1995;80:219–225. doi: 10.1097/00000539-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27:353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 29.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–1720. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 30.Davies RO, Nishino T, Lahiri S. Sympathectomy does not alter the response of carotid chemoreceptors to hypoxemia during carboxyhemoglobinemia or anemia. Neurosci Lett. 1981;21:159–164. doi: 10.1016/0304-3940(81)90375-x. [DOI] [PubMed] [Google Scholar]
- 31.Hatcher JD, Chiu LK, Jennings DB. Anemia as a stimulus to aortic and carotid chemoreceptors in the cat. J Appl Physiol. 1978;44:696–702. doi: 10.1152/jappl.1978.44.5.696. [DOI] [PubMed] [Google Scholar]
- 32.Szlyk PC, King C, Jennings DB, Cain SM, Chapler CK. The role of aortic chemoreceptors during acute anemia. Can J Physiol Pharmacol. 1984;62:519–523. doi: 10.1139/y84-083. [DOI] [PubMed] [Google Scholar]
- 33.Swanson GD, Whipp BJ, Kaufman RD, Aqleh KA, Winter B, Bellville JW. Effect of hypercapnia on hypoxic ventilatory drive in carotid body-resected man. J Appl Physiol. 1978;45:871–877. doi: 10.1152/jappl.1978.45.6.971. [DOI] [PubMed] [Google Scholar]
- 34.Hopf HW, Viele M, Watson JJ, Feiner J, Weiskopf R, Hunt TK, Noorani M, Yeap H, Ho R, Toy P. Subcutaneous perfusion and oxygen during acute severe isovolemic hemodilution in healthy volunteers. Arch Surg. 2000;135:1443–1449. doi: 10.1001/archsurg.135.12.1443. [DOI] [PubMed] [Google Scholar]
- 35.Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, Watson JJ, Hopf HW, Toy P. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646–1652. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Weiskopf RB, Aminoff MJ, Hopf HW, Feiner J, Viele MK, Watson JJ, Ho R, Songster C, Toy P. Acute isovolemic anemia does not impair peripheral or central nerve conduction. Anesthesiology. 2003;99:546–551. doi: 10.1097/00000542-200309000-00008. [DOI] [PubMed] [Google Scholar]