Abstract
During in vivo development, articular cartilage is exposed to several different forms of stress. This study examined the effects of radial confinement and passive axial compression-induced vertical confinement, on the biomechanical, biochemical, and histological properties of self-assembled chondrocyte constructs. The self-assembled constructs, engineered without the use of an exogenous scaffold, exhibited significant increases in stiffness in the direction orthogonal to that of the confinement surface. With radial confinement, the significantly increased aggregate modulus was accompanied by increased collagen organization in the direction perpendicular to the articular surface, with no change in collagen or glycosaminoglycan (GAG) content. Additionally, radial confinement was most beneficial when applied before t=2 wks. With passive axial compression, the significantly increased Young’s modulus and ultimate tensile strength were accompanied by a significant increase in collagen production. This study is the first to demonstrate the beneficial effects of confinement on tissue engineered constructs in the direction orthogonal to that of the confinement surface.
INTRODUCTION
Cartilage degeneration, from injury or osteoarthritis, is a tremendous problem in current orthopaedic practice. Following injury or osteoarthritis, articular cartilage is unable to repair itself, resulting in a permanent defect or the formation of mechanically inferior fibrocartilage.1 Therefore, tissue engineering is a promising approach for the treatment of articular cartilage injuries, as this approach may eventually allow for the production of engineered tissue indistinguishable from native cartilage.
A chondrocyte self-assembling process for tissue engineering articular cartilage was recently developed2 that allowed constructs to reach 1/3 the stiffness of native cartilage. Additionally, the benefits of hydrostatic pressure stimulation on self-assembled constructs have been demonstrated,3 as intermittent hydrostatic pressure applied at 10 MPa and 1 Hz, for 4 hrs per day and 5 days per wk was shown to increase collagen production. The self-assembling process avoids many of the problems associated with scaffold use, namely concerns over stress shielding, biocompatibility and biodegradation.
Articular cartilage is exposed to a variety of forces in vivo including compression, shear, and hydrostatic pressure. Additionally, mechanical stimulation is vital for maintaining the integrity of the tissue, as articular cartilage demonstrates changes representative of a loss of function when immobilized.4, 5 Therefore, it is likely that some form of mechanical intervention will be required for further refinement of tissue engineering techniques. Although the precise signaling pathways involved in mechanotransduction have not been fully elucidated, several studies have shown promising results involving the use of mechanical stimulation including dynamic compression,6–12 shear,10, 13 and hydrostatic pressure.14–17
Although coupling mechanical stimulation with the self-assembling process for tissue engineering articular cartilage represents a promising solution for treatment of injuries, several questions remain concerning this approach. Aside from the studies showing beneficial effects of dynamic compression and hydrostatic pressure, studies comparing the effects of passive confinement on the anisotropy of articular cartilage are lacking. Additionally, studies involving the effects of mechanical intervention at different times are limited.18
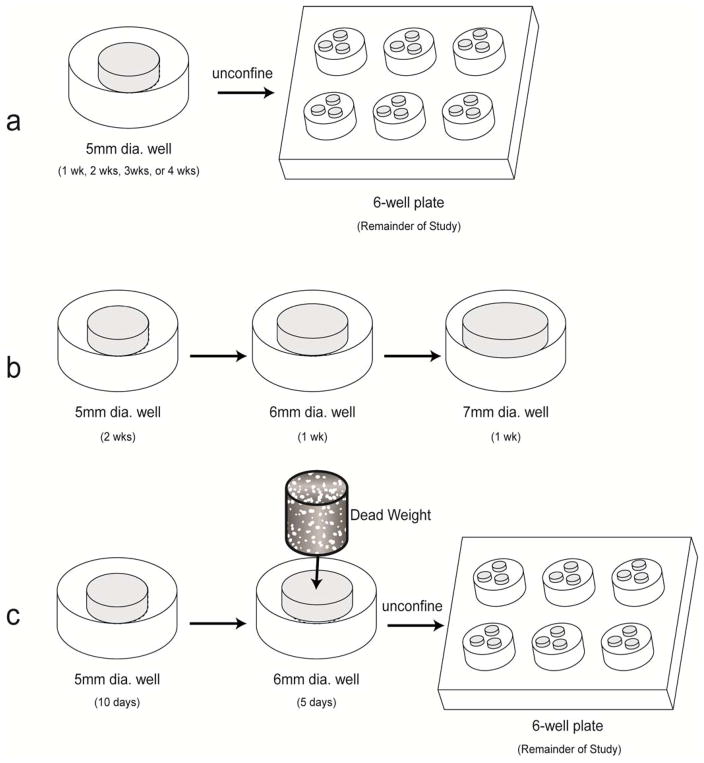
The purpose of this study was to examine the effects of construct confinement in different directions and at different times on construct mechanical properties. Radial confinement and passive axial compression-induced vertical confinement of self-assembled constructs were used. It was hypothesized that the application of confinement would enhance the mechanical properties of the constructs in the orthogonal direction. It was further hypothesized that confinement at different timepoints in construct development would have a significant effect on construct properties. To test these hypotheses, three experiments were performed (Fig. 1). First, self-assembled constructs were radially confined in agarose wells for 1, 2, 3, or 4 wks, after which the constructs were cultured unconfined for the remainder of the 4-wk study; the effects of confinement on compressive stiffness were investigated. Second, the constructs were cultured in the same wells used in the first experiment for 2 wks, after which they were transferred to incrementally larger wells for the 3rd and 4th wk of culture. Finally, the effects of vertical confinement, in the form of passive axial compression, on the tensile stiffness were examined.
Fig. 1.
Schematic diagram demonstrating each experimental design. (a) 1st Study: Radial Confinement of Self-Assembled Constructs. (b) 2nd Study: Maintenance of Radial Confinement of Self-Assembled Constructs. (c) 3rd Study: Passive Axial Compression of Self-Assembled Constructs.
METHODS
Chondrocyte Isolation and Seeding
Chondrocytes were isolated from the distal femur of wk-old male calves19–21 (Research 87 Inc.) less than 36 hrs after slaughter, with collagenase type 2 (Worthington) in the culture medium. The medium was DMEM with 4.5 g/L-glucose and L-glutamine, 100 nM dexamethasone, 1% fungizone, 1% Penicillin/Streptomycin, 1% ITS+, 50 μg/mL ascorbate-2-phosphate, 40 μg/mL L-proline, and 100 μg/mL sodium pyruvate (termed chemically-defined medium). Each leg came from a different animal and yielded roughly 150 million chondrocytes. To reduce variability among animals, cells from all legs were pooled together to yield a mixture of chondrocytes; a mixture of cells from 8 legs was used in the 1st study, while a mixture of cells from 6 legs was used in the 2nd and 3rd studies (see descriptions below). The pooled cells were counted on a hemocytometer, and viability was assessed using a trypan blue exclusion test. Viability was >99% for the pooled cells. Chondrocytes were frozen in culture medium supplemented with 20% FBS and 10% DMSO at −80°C for 2 wks to a month before use. After thawing, viability remained greater than 75%. A polysulfone die consisting of 5 mm dia. x 10 mm long cylindrical prongs was constructed to fit into 6 wells of a 48-well plate. Additional polysulfone die consisting of 6 mm dia. x 10 mm long cylindrical prongs and 7 mm dia. x 10 mm long cylindrical prongs were fabricated. To construct each agarose mold, sterile, molten 2% agarose was introduced into a well fitted with the polysulfone die. The agarose was allowed to gel at room temperature for 60 min. The agarose mold was then separated from the polysulfone die and submerged into two exchanges of culture medium to completely saturate the agarose well with culture medium by the time of cell seeding. To each agarose well, 5.5 × 106 cells were added in 150 μl of culture medium. The cells self-assembled within 24 hrs in the agarose wells and were maintained in the same well for a specified amount of time; t=0 was defined as 24 hrs after seeding.
1st Study: Radial Confinement of Self-Assembled Constructs
At t=1, 2, or 3 wks, self-assembled constructs (n=6) were removed from confinement in the 5 mm dia. agarose well, and placed in one well of a 6-well culture plate coated with 2% agarose (Fig. 1a). Each agarose-coated well contained 3–4 constructs, and 500 μl of medium per construct was changed daily (1.5–2 ml per well). At t=4 wks, all samples were tested for morphological, histological, biochemical, and biomechanical properties.
2nd Study: Maintenance of Radial Confinement of Self-Assembled Constructs
At t=2 wks, self-assembled constructs (n=5) were removed from confinement in the 5 mm dia. agarose wells, and transferred to 6 mm dia. agarose wells (Fig. 1b). At t=3 wks, these constructs were removed from confinement in the 6 mm dia. agarose wells, and transferred to 7 mm dia. agarose wells. A control consisted of constructs confined in 5 mm dia. agarose wells for 2 wks, and then maintained in agarose-coated wells, as described above. Each day, 500 μl of medium was changed. At t=4 wks, all samples were tested for morphological, histological, biochemical, and biomechanical properties.
3rd Study: Passive Axial Compression of Self-Assembled Constructs
At t=10 days, self-assembled constructs (n=5) were removed from confinement in 5 mm dia. agarose wells, and transferred to 6 mm dia. agarose wells. Vertical confinement, in the form of passive axial compression with a dead weight, was applied by placing a 5 mm dia. x 1 cm long, 1 g, porous, sintered steel cylinder on top of each construct in the 6 mm dia. wells (Fig. 1c). The dead weight corresponded to a stress of 0.5 kPa. At t=14 days, the porous cylinders were removed, and the constructs were transferred to agarose-coated wells for the remainder of the study. A control consisted of constructs cultured in 5 mm dia. agarose wells, then transferred to 6 mm dia. agarose wells at t=10 days, and finally maintained in agarose-coated wells for the remainder of the study.
Histology and Immunohistochemistry
Samples were frozen and sectioned at 14 μm. Safranin-O and fast green staining were used to examine GAG distribution.22, 23 Picrosirius red was used for qualitative examination of collagen content. Polarized light microscopy of picrosirius red-stained sections was used to examine the collagen organization of the constructs. Slides were also processed with immunohistochemistry (IHC) to test for the presence of collagen types I (COL1) and II (COL2) on a Biogenex (San Ramon, CA) i6000 autostainer. After fixing in chilled acetone, the slides were rinsed with IHC buffer (Biogenex), quenched of peroxidase activity with hydrogen peroxide/methanol, and blocked with horse serum (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). The slides were then incubated with either mouse anti-COL1 (Accurate Chemicals (Westbury, NY)) or mouse anti-COL2 (Chondrex (Redmond, WA) antibodies). The secondary antibody (mouse IgG, Vectastain ABC kit) was then applied, and color was developed using the Vectastain ABC reagent and DAB (Vector Laboratories).
Quantitative Biochemistry
Samples were digested with 125 μg/ml papain (Sigma) in 50 mM phosphate buffer (pH = 6.5) containing 2 mM N-acetyl cysteine (Sigma) and 2 mM EDTA (Sigma) at 65°C overnight. Total DNA content was measured by Picogreen® Cell Proliferation Assay Kit (Molecular Probes). Total sulfated GAG was then quantified using the Blyscan Glycosaminoglycan Assay kit (Biocolor), based on 1,9-dimethylmethylene blue binding.24, 25 After being hydrolyzed by 2 N NaOH for 20 min at 110°C, samples were assayed for total collagen content by a chloramine-T hydroxyproline assay.26
Indentation Testing
Samples were evaluated with an automated indentation apparatus.27 A step mass of 0.7 g (0.007 N) was applied with a 1 mm flat-ended, porous indenter tip, and the specimens were allowed to creep until equilibrium, as described elsewhere.2 Preliminary estimations of the Young’s modulus of the samples were obtained using the analytical solution for the axisymmetric Boussinesq problem with Papkovich potential functions.28, 29 The intrinsic mechanical properties of the samples were then determined using the linear biphasic theory.30
Tensile Testing
Samples were cut into 500 μm thickness and tested under incremental stress relaxation conditions, using a uniaxial materials testing machine (Instron 5565), as described elsewhere.31 Following preconditioning, specimens were exposed to incremental stress relaxation, whereby they were exposed to 15 min constant strain increments until failure. A maximum of 11 increments were allowed (4, 8, 12, 16, 20, 25, 35, 50, 70, 100 and 130% strain). A strain rate of 6 mm/min was employed between constant-strain increments. Stress vs. strain plots were constructed from the 1) peak stress and 2) relaxed stress corresponding to the strain at each increment. Material properties were then calculated from these stress-strain plots. The modulus was equal to the slope of the linear region of the curve, the tensile strength equal to the maximum stress, the maximum strain was the strain corresponding to the maximum stress, and the energy was equal to the area under the curve (trapezoid rule) from zero strain to maximum strain.
Statistical Analysis
All samples were assessed biochemically and biomechanically (n=5 or 6). A single factor ANOVA was used to analyze the samples, and Tukey’s post hoc test was used when warranted. Significance was defined as p < 0.05.
RESULTS
Gross Appearance and Histology
There were no differences in gross morphology between any of the groups. After 2 wks of culture, all constructs reached a diameter slightly below 6 mm. By 3 wks of culture, constructs reached a diameter slightly below 7 mm, and by 4 wks of culture, constructs reached a diameter approaching 7.5 mm. In the confinement study, there were no significant differences in thickness between the 2-wk confinement group and the 1, 3, or 4-wk confinement groups, with thicknesses of 1.05±0.05 mm, 1.02±0.06 mm, 1.07±0.17 mm, and 1.10±0.14 mm respectively. Likewise, in the passive axial compression study, there were no significant differences in thickness between treatment groups. The compressed group had a thickness of 0.73±0.09 mm and the control group had a thickness of 0.81±0.07 mm. Additionally, in the follow-up confinement study, there was no significant difference in thickness between the 2-wk confinement group and the group confined for 2 wks in 5 mm dia. wells, 1 wk in 6 mm dia. wells, and 1 wk in 7 mm dia. wells, with values of 0.58±0.09 and 0.51±0.04 respectively. At t=4 wks, all constructs stained positive for collagen throughout the thickness of the construct (Fig. 2b). Additionally, safranin-O staining for GAG was observed throughout the constructs (Fig. 2c). COL2 immunostaining was observed throughout the constructs, with no differences in production among the treatment groups (Fig. 2d). Based on IHC, there was no COL1 production for any constructs (Fig. 2e).
Fig. 2.
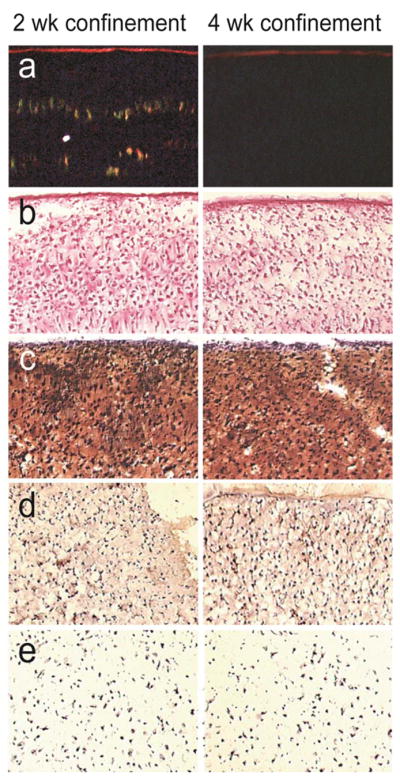
Histological images of 2-wk confined and 4-wk confined constructs at t=4 wks. 10× original magnification. (a) Polarized light microscopy images of picrosirius-red stained sections, with the construct surface at the top of each image. 2-wk confined group demonstrated extensive organization of collagen fibrils in direction perpendicular to articular surface. (b) Picrosirius-red stained sections corresponding to polarized light microscopy images. (c) Safranin-O stained sections. (d) Collagen 2 IHC sections. (e) Collagen 1 IHC sections.
Polarized Light Microscopy
Polarized light microscopy was used to assess the collagen organization of the constructs in the confinement study. The constructs that were confined in 5 mm dia. wells for 2 wks and then unconfined and cultured in agarose-coated wells for the remaining 2 wks exhibited small-fiber collagen organization in the direction perpendicular to the construct surface as well as larger-fiber collagen organization in the direction parallel to the construct surface (Fig. 2a). The alignment of the small collagen fibers resembled struts. Small-fiber collagen organization was minimally observed in the other treatment groups (Fig. 2a), namely confinement in 5 mm dia. wells for 1, 3, or 4 wks.
The increased collagen organization was not observed in the passive axial compression study, as the constructs to which a dead weight was applied did not show any small-fiber collagen organization.
Quantitative Biochemistry
There was no significant difference in WW/construct, DNA/construct, GAG/WW, and collagen/WW between the 2-wk confinement group and the 1, 3, or 4-wk confinement groups. The 2-wk confinement group had a WW of 39±4 mg, while the 1-wk, 3-wk, and 4-wk confinement groups had a WW of 44±3 mg, 37±4 mg, and 41±2 mg respectively. The 2-wk confinement group had a DNA/construct of 49±2 μg, while the 1-wk, 3-wk, and 4-wk confinement groups had a DNA/construct of 44±11 μg, 46±13 μg, and 47±5 μg respectively. The 2-wk confinement group had a GAG/WW of 0.061±0.009 mg/mg, while the 1-wk, 3-wk, and 4-wk confinement groups had a GAG/WW of 0.067±0.014 mg/mg, 0.055±0.003 mg/mg, and 0.050±0.004 mg/mg respectively. The 2-wk confinement group had a collagen/WW of 0.039±0.006 mg/mg, while the 1-wk, 3-wk, and 4-wk confinement groups had a collagen/WW of 0.032±0.005 mg/mg, 0.041±0.009 mg/mg, and 0.036±0.008 mg/mg respectively. Additionally, in the follow-up confinement study, there was no significant difference in WW/construct, DNA/construct, GAG/WW or collagen/WW between the 2-wk confinement group and the group confined for 2 wks in 5 mm dia. wells, 1 wk in 6 mm dia. wells, and 1 wk in 7 mm dia. wells. These groups had WW values of 16±1 mg and 14±2 mg, DNA/construct values of 31±2 μg and 31±4 μg, GAG/WW values of 0.074±0.008 mg/mg and 0.065±0.006 mg/mg, and collagen/WW values of 0.071±0.019 mg/mg and 0.088±0.011 mg/mg respectively. The collagen/WW for the passive axial compression group at 0.067±0.009 mg/mg was significantly higher than the unloaded control group, which had a collagen/WW of 0.044±0.008 mg/mg. There was no significant difference in GAG/WW between the passive axial compression group and the control, with values of 0.070±0.006 mg/mg and 0.064±0.007 mg/mg respectively. Finally, the passive axial compression group had WW values of 20±1 mg and 29±3 mg respectively; there was no difference in DNA/construct between the passive axial compression group and the control group, with values of 41±7 μg and 40±2 μg respectively.
Mechanical Evaluation
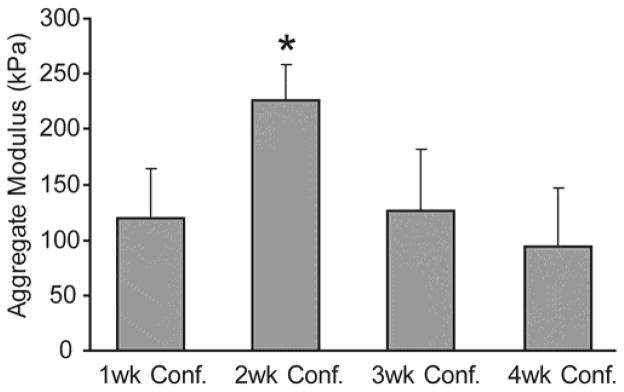
The aggregate modulus of the 2-wk confinement group reached 225±32 kPa, and was significantly higher than the aggregate moduli of the 1, 3, or 4-wk confinement groups, with values of 120±43 kPa, 126±56 kPa, and 94±52 kPa respectively (Fig. 3). In the follow-up confinement study, the aggregate modulus of the 2-wk confinement group at 214±110 kPa was insignificantly higher than that of the group confined for 2 wks in 5 mm dia. wells, 1 wk in 6 mm dia. wells, and 1 wk in 7 mm dia. wells, at 177±96 kPa.
Fig. 3.
Mechanical properties of constructs in radial confinement study. Constructs confined for 2 wks demonstrated significantly higher aggregate modulus than the other treatment groups. Columns and error bars represent means and standard deviations.
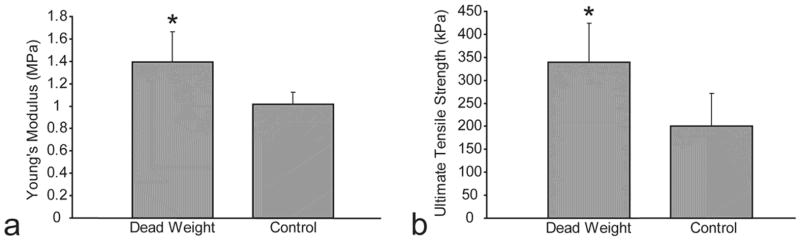
The tensile modulus of the passive axial compression group at 1.4±0.3 MPa was significantly higher than the tensile modulus of the control group at 1.0±0.1 MPa (Fig. 4a). Additionally, the ultimate tensile strength of the passive axial compression group at 339±86 kPa was significantly higher than the ultimate tensile strength of the control group at 200±71 kPa (Fig. 4b). However, there was no significant difference between the aggregate modulus of the passive axial compression group and control group, with values of 101±48 kPa and 111±52 kPa respectively.
Fig. 4.
Mechanical properties of constructs in passive axial compression study. (a) Passive axial compression group exhibited significantly higher Young’s modulus than control group. (b) Passive axial compression group exhibited significantly higher ultimate tensile strength than control group. Columns and error bars represent means and standard deviations.
DISCUSSION
This study was designed to assess the effects of radial confinement and, separately, to determine the effects of passive axial compression-induced vertical confinement, on the mechanical properties of 3-D self-assembled articular cartilage constructs over a 4-wk culture period. Confining constructs for 2 wks in 5 mm dia. agarose wells led to a significantly increased aggregate modulus. This increased compressive stiffness was accompanied by increased collagen organization without a change in GAG or collagen content. However, confinement in 5 mm dia. wells for 2 wks, followed by confinement in 6 mm dia. wells for 1 wk and confinement in 7 mm dia. wells for 1 wk did not enhance the compressive properties of the constructs, and trended towards a decrease in aggregate modulus. The application of a 0.01 N dead weight to the constructs, corresponding to 0.5 kPa of stress, resulted in significant increases in both tensile modulus and ultimate tensile strength, as well as total collagen per wet weight. These results, discussed further below, support our hypothesis, as changes in mechanical properties were identified in a direction orthogonal to the confinement surface in tissue-engineered articular chondrocyte constructs. Additionally, this study demonstrates further refinement and characterization of the self-assembling process.
Radial confinement in 5 mm dia. agarose wells for 2 wks led to approximately a 2-fold increase in aggregate modulus at t=4 wks, relative to confinement for 1, 3, or 4 wks. There was no difference in ECM content among the different confinement groups; however, increased collagen organization in the direction parallel to that of the compression testing and orthogonal to the confinement surface was observed only in the group confined for 2 wks. The organized collagen fibers appeared to form struts that may help to increase the compressive stiffness of the constructs. These results were unexpected as the collagen of articular cartilage is typically responsible for the tensile stiffness, while the GAG content is responsible for the compressive stiffness. However, these results agree with those found in previous studies,32, 33 that have demonstrated that total GAG and collagen content may not be an ideal indicator of mechanical properties; rather, ECM organization may play a significant role in predicting mechanical properties. A possible explanation is that the organization of the collagen fibers aids the proteoglycans in resisting compressive forces.
Confinement during the self-assembly process may lead to radial construct compression. We observed that at t=10 days of confinement, the constructs reached the wall of the 5 mm dia. wells. At t=2 wks of confinement, constructs slightly less than 6 mm dia. were unconfined from the 5 mm dia. wells. Therefore, we hypothesized that the constructs confined for 2 wks exhibited a higher aggregate modulus and increased collagen organization due to the effects of a low-magnitude radial compression, as well as contact with the agarose well. This radial compression would neither be constant strain nor constant stress, since the constructs continued to grow radially while confined, thus resulting in potentially increasing strain and increasing stress with construct growth. This could account for the results observed in the other three confinement groups, as the constructs confined for 1 wk did not contact the walls of the well and therefore may not have been radially compressed. Additionally, at t=3 wks of confinement, constructs slightly less than 7 mm dia. were unconfined from the 5 mm dia. wells, and at t=4 wks of confinement, constructs approaching 7.5 mm dia. were unconfined from the 5 mm dia. wells; therefore, they may have experienced higher magnitudes of radial compression, which negated the positive effects of the lower-magnitude radial compression.
Since the aforementioned radial confinement-induced stress could not be quantified, it is possible that the constructs merely were radially confined rather than radially compressed. Perhaps, confinement may have diminished the nutrient supply through the lateral surface, potentially becoming more detrimental over periods longer than 2 wks. However, the wells were constructed of agarose, with a 98% fluid phase to allow for adequate nutrient diffusion to the edges of the constructs. Additionally, confinement did not affect the cellularity of the constructs, as there was no difference in histological images and DNA/construct between the 2-wk confinement group and the other confinement groups.
In a follow-up to this study, we examined the temporal effects of the radial confinement of the 2-wk confinement group, and found that t=10–14 days was the most beneficial time for constructs to be confined by the agarose well. To maintain radial confinement similar to that experienced by the 2-wk confinement group during t=10–14 days for a longer period, constructs were confined in incrementally larger agarose wells, to mimic the radial growth of the constructs with time and approximately allow the constructs to contact the edge of the wells from t=1.5 wks to 4 wks. Constructs only confined for 2 wks in 5 mm dia. wells and unconfined for the duration of the study were used as controls. Interestingly, maintaining confinement for 4 wks caused the aggregate modulus to trend lower than the 2-wk confined control, from 214±110 kPa to 177±96 kPa, although there was no significant difference between these values. These results demonstrate that the application of radial confinement between 1 and 2 wks was more beneficial than the maintenance of a similar level of radial confinement through later time-points, which may actually be detrimental to the constructs. However, due to the constraints of the experimental setup used for the confinement studies, we were unable to apply radial confinement before approximately t=1.5 wks, so it is possible that applying radial confinement at even earlier timepoints may be more beneficial.
As described above, radial confinement resulted in changes in the compressive stiffness of constructs. Therefore, the effects of vertical confinement, in the form of a passive axial stress, on the tensile characteristics of the constructs were examined. The application of a dead weight from t=10–14 days increased the tensile properties of the constructs. To eliminate the effects of radial confinement, the control constructs were placed in incrementally larger agarose wells. At t=4 wks, the passive axial compression group demonstrated a 1.4-fold increase in Young’s modulus, as well as a 1.7-fold increase in ultimate tensile strength, relative to the control group, again confirming our hypothesis that the application of confinement to self-assembled articular cartilage constructs affects the mechanical properties in the direction orthogonal to the confinement surface. In this case, the increased tensile strength of the passive axial compression group was accounted for by a significantly higher value of collagen/WW for the passive axial compression group vs. the control group, with minimal small-fiber collagen organization for either group. Interestingly, vertical confinement led to different changes in the construct ECM than found in radial confinement. This suggests that there may be different mechanotransduction pathways for radial confinement and passive axial compression, and future studies should be performed to elucidate these potential differences. Finally, the application of a dead weight had no effect on the cellularity of the constructs, as there was no difference in histological images or DNA/construct between the passive axial compression group and the control group.
To our knowledge, this study is the first to provide evidence of the benefits of confinement on mechanical properties in the direction orthogonal to the confinement surface. It shows that an increased construct aggregate modulus can be accounted for by increased collagen organization in the direction orthogonal to the construct surface. Previous studies have also demonstrated a relationship between cartilage mechanical properties and collagen organization, as determined by polarized light microscopy. For example, Kiviranta et al.34 found that in bovine knee osteochondral plugs, there was a significant correlation between Poisson’s ratio and collagen organization, as assessed by quantitative polarized light microscopy. Additionally, Kelly et al.33 found that dynamic deformational loading of chondrocyte-seeded agarose hydrogels led to an increased bulk Young’s modulus with increased collagen organization in the radial direction relative to the free-swelling control. Although several other studies have examined collagen organization in cartilage explants, to our knowledge, this study is the only one to demonstrate a relationship between collagen organization and aggregate modulus in tissue engineered constructs.
Several prior studies have investigated the use of dynamic compression 9, 12, 13 and/or shear 10, 13 on the ECM of tissue-engineered cartilage constructs. These studies demonstrated 1.5–2.8-fold increases in GAG, and 1.4-fold increases in collagen with mechanical stimulation, which differ from the results of the radial confinement study, which demonstrated no change in ECM content, and the passive axial compression study, which demonstrated a 1.5-fold increase in collagen without an increase in GAG. Since the other studies all involved dynamic, rather than passive stimulation, it is possible that simultaneous GAG and collagen increases may only be observed under dynamic mechanical stimulation as a result of the increased nutrient diffusion to the construct in dynamic stimulation, as noted elsewhere.33 However, Waldman et al.12 found that dynamic compression of 5% amplitude at 1 Hz, for 400 cycles every other day for 1 wk, resulted in increased collagen with no change in GAG content, which matches the results of the passive axial compression study.
Although many studies have examined the relationship between mechanical stimulation and construct mechanical properties, to our knowledge, this is the first tissue engineering study to indicate the beneficial effects of passive confinement for a short term. This result indicates the possibility of an adaptive response to confinement that either results in increased collagen organization as seen in the radial confinement experiment, or increased matrix synthesis as seen in the passive axial compression experiment. Consistent with this finding, although using immature bovine cartilage explants, Boustany et al.35 found that static compression of <25% strain for 60 hours increased the biosynthetic rate of GAG and collagen production, although the mechanical properties of the explants were not examined.
Additional studies should be performed in the future to track construct development using electron microscopy in order to elucidate the mechanism leading to strut-like collagen organization, observed only in the 2-wk confined constructs. In addition, future studies should investigate the combination of confinement with other modalities of mechanical stimulation such as hydrostatic pressure and direct compression, as the combination may result in synergistic effects on construct mechanical properties. Finally, future work should examine the effects of the addition of growth factors to the culture medium both before and after the application of confinement, in order to look for a synergistic effect between the growth factors and confinement.
In summary, this study permitted the examination of the hypothesis that the mechanical properties of self-assembled articular cartilage constructs are influenced by the application of stress in a direction orthogonal to the confinement surface. This study furthers our prior work involving the self-assembling process, by indicating that the ECM of self-assembled constructs may be modulated by both radial and vertical confinement. Also, this study provides evidence to support early (<2 wks) application of confinement and passive axial compression, and demonstrates the benefit of low-magnitude passive stress application.
Acknowledgments
The authors acknowledge funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, grant no. R01 AR053286.
Contributor Information
Benjamin D. Elder, Department of Bioengineering, Rice University
Kyriacos A. Athanasiou, Department of Bioengineering, Rice University
References
- 1.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12(5):1337–44. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 4.Helminen HJ, Saamanen AM, Jurvelin J, et al. The effect of loading on articular cartilage. Duodecim. 1992;108(12):1097–107. [PubMed] [Google Scholar]
- 5.Palmoski MJ, Colyer RA, Brandt KD. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980;23(3):325–34. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- 6.Hunter CJ, Imler SM, Malaviya P, Nerem RM, Levenston ME. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials. 2002;23(4):1249–59. doi: 10.1016/s0142-9612(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 7.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 8.AufderHeide AC, Athanasiou KA. Mechanical stimulation toward tissue engineering of the knee meniscus. Ann Biomed Eng. 2004;32(8):1161–74. doi: 10.1114/b:abme.0000036652.31658.f3. [DOI] [PubMed] [Google Scholar]
- 9.Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 10.Stoddart MJ, Ettinger L, Hauselmann HJ. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng. 2006;95(6):1043–51. doi: 10.1002/bit.21052. [DOI] [PubMed] [Google Scholar]
- 11.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Hong J, Kandel RA. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. J Bone Joint Surg Am. 2003;85-A(Suppl 2):101–5. doi: 10.2106/00004623-200300002-00013. [DOI] [PubMed] [Google Scholar]
- 12.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Eng. 2004;10(9–10):1323–31. doi: 10.1089/ten.2004.10.1633. [DOI] [PubMed] [Google Scholar]
- 13.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21(4):590–6. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 14.Almarza AJ, Athanasiou KA. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 2006;12(5):1285–94. doi: 10.1089/ten.2006.12.1285. [DOI] [PubMed] [Google Scholar]
- 15.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006 doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 16.Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 17.Lammi MJ, Elo MA, Sironen RK, Karjalainen HM, Kaarniranta K, Helminen HJ. Hydrostatic pressure-induced changes in cellular protein synthesis. Biorheology. 2004;41(3–4):309–13. [PubMed] [Google Scholar]
- 18.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41(3–4):223–37. [PubMed] [Google Scholar]
- 19.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2006 doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 20.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 21.Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10(5–6):825–32. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Minakuchi K, Kaji S, Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22(3):309–15. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 24.Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6(4):297–305. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 25.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1):30–6. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 26.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 27.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–9. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 28.Sneddon I. The relaxation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 29.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5(5):541–51. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 30.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression. Theory and experiments. J Biomech Eng. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 31.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125(4):558–65. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 32.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11(12):879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39(8):1489–97. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Toyras J, Jurvelin JS. Collagen network primarily controls Poisson’s ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24(4):690–9. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 35.Boustany NN, Gray ML, Black AC, Hunziker EB. Time-dependent changes in the response of cartilage to static compression suggest interstitial pH is not the only signaling mechanism. J Orthop Res. 1995;13(5):740–50. doi: 10.1002/jor.1100130514. [DOI] [PubMed] [Google Scholar]