Abstract
Saquinavir (SQV) was the first human immuno-virus-1 (HIV-1) protease inhibitor approved by FDA. However, P-glycoprotein (P-gp), an efflux pump limits its oral and brain bioavailabilities. The objective of this study is to investigate whether prodrug modification of SQV to dipeptide prodrugs Valine-Valine-Saquinavir (Val-Val-SQV) and Glycine-Valine-Saquinavir (Gly-Val-SQV) targeting intestinal peptide transporter can enhance intestinal permeability of SQV by circumventing P-gp mediated efflux. Single pass intestinal perfusion experiments in rat jejunum were performed to calculate the absorption rate constant and intestinal permeability of SQV, Val-Val-SQV and Gly-Val-SQV. Equimolar concentration (25 μM) of SQV, Val-Val-SQV and Gly-Val-SQV were employed in the perfusion studies. Perfusion experiments were also carried out in the presence of cyclosporine (10 μM) and glycyl-sarcosine (20mM). Absorption rate constants in rat jejunum (ka) for SQV, Val-Val-SQV and Gly-Val-SQV were found to be 14.1±3.4 ×10−3, 65.8±4.3 ×10−3, and 25.6±5.7 ×10−3 min−1 respectively. Enhanced absorption of Val-Val-SQV and Gly-Val-SQV relative to SQV can be attributed to their translocation by the peptide transporter in the jejunum. Significant permeability enhancement of SQV across rat jejunum was observed in the presence of cyclosporine 10 μM (P-gp inhibitor). However, permeability of Val-Val-SQV was unchanged in the presence of cyclosporine suggesting lack of any interaction of the prodrug with efflux pump. Intestinal absorption of Val-Val-SQV was significantly inhibited in the presence of gly-sar indicating the involvement of peptide transporter in intestinal absorption. In conclusion, peptide transporter targeted prodrug modification of P-gp substrates could lead to shielding of these drug molecules from efflux pumps.
1. Introduction
Saquinavir (SQV) was the first HIV protease inhibitor (PI) approved by FDA for the management of AIDS (Kakuda et al., 1998). Pharmacokinetics studies have revealed that SQV possesses very low oral bioavailability in the range of 4–16% of administered dose (Dupre et al., 1995; Schapiro et al., 1996). Recent investigations have demonstrated that all protease inhibitor (e.g. ritonavir, saquinavir, amprenavir, nelfinavir) anti-HIV drugs are substrates for Pgp, a membrane bound efflux protein that contributes significantly to their poor oral absorption. Moreover, these drugs are also good substrates for both hepatic and gut CYP3A4 enzyme which further result in their high metabolism and low oral bioavailability (Alsenz et al., 1998; Kim et al., 1998; Polli et al., 1999; Lee et al., 2003).
P-gp is a 170kDa protein, member of ATP binding cassette (ABC) transporter family (Germann, 1996). P-gp is ubiquitously expressed on human tissues such as intestinal mucosa, brain capillary endothelial cells, biliary canaliculus, and kidney tubules (Thiebaut et al., 1987). Broad substrate specificity of P-gp is a major factor responsible for sub-therapeutic levels of various drugs in blood and tissues (Varma et al., 2003). A recent report suggests that presence of this efflux transporter on the brush border membrane of intestinal epithelium not only diminishes permeability of various therapeutic agents but also enhances the metabolism of these molecules by effluxing the drugs into the intestinal lumen or blood capillaries thereby increasing drug exposure to cellular as well as lumenal enzymes (Lown et al., 1997; Watkins, 1997; Ito et al., 1999).
Several reports speculate that low and variable oral bioavailability of SQV and other protease inhibitors is primarily caused by membrane bound efflux proteins like P-glycoprotein and multi drug resistance proteins (MRPs) and partly due to CYP3A4 mediated metabolism (Dupre et al., 1995; Fitzsimmons and Collins, 1997; Kim et al., 1998; Polli et al., 1999; Williams et al., 2002). Presence of P-gp on blood brain barrier further limits the entry of these drugs into the brain making it a sanctuary site for viral replication (Bachmeier et al., 2005). Several approaches have been taken in the past to enhance oral bioavailability and brain penetration of HIV protease inhibitors with variable degree of success (Polli et al., 1999; Edwards et al., 2002; Savolainen et al., 2002).
Besides efflux transporters, such as P-gp, a number of nutrient influx transporters are also expressed on the cellular membranes. These nutrient transporters are responsible for the influx of various nutrients and drugs into various epithelial (enterocytes) and endothelial cells (e.g. blood brain barrier) (Tsuji and Tamai, 1996; Bolger et al., 1998; Tamai and Tsuji, 2000; Lee et al., 2003). Recently, peptide transporters (PEPT1 and PEPT2) have gained lot of attention among drug delivery scientists. Intestinal peptide transporter especially PEPT1, has been a key target protein for prodrug design. Several prodrugs have already been designed such that the modified compounds become substrates of peptide transporters leading to enhanced absorption of these compounds across various physiological barriers (Rousselle et al., 2000; Rouquayrol et al., 2002; Rice et al., 2003; Anand et al., 2004). When a substrate binds to a nutrient transporter it triggers a configurational change in the transport protein as a result of which it is translocated across the membrane and released into the cell cytoplasm. During this process the substrate is not freely available in the inner leaflet of the cell membrane and may avoid recognition by P-gp as a substrate (Jain et al., 2005). Recently, we have reported that di-peptide conjugates of SQV circumvent P-gp mediated efflux which resulted in enhanced permeability across MDCK-MDR1 cells (Jain et al., 2005). Peptide transporters have been exploited in this study since these transporters are robust and high capacity transporters. Utilization of this transporter to ferry the dipeptide prodrugs of SQV across the physiological barriers may prove to be a highly effective strategy.
Single pass intestinal perfusion (SPIP) is a well established technique employed to study the intestinal absorption of drugs. Among the other techniques, SPIP provides an advantage of experimental control (e.g. permeant concentration, intestinal perfusion rate), intact intestinal blood supply and ability to determine regional absorption rates of drugs in intestinal segments. In this model, a known concentration of drug is perfused through a section of the jejunum with intact blood supply. Disappearance of drug from the perfusate is attributed to intestinal absorption (Cummins et al., 2003; Berggren et al., 2004). Anatomical and physiological parameters of the gastrointestinal tract dramatically affect the rate and extent of absorption of ingested compounds. Rats are used as an animal model in this study because of similarity in basic intestinal structures and physiology of rats and humans. It has been shown that rat Peff values can predict the quantitative amount of drug absorbed in vivo in humans very well (Fagerholm et al.,1996).
The objective of this study is to investigate whether transporter targeted prodrug derivatization of SQV, a P-gp substrate, can result in avoidance of P-gp mediated efflux and consequently can enhance SQV transport. To accomplish these goals two novel dipeptide prodrugs of SQV i.e., Val-Val-SQV, and Gly-Val-SQV, were synthesized in our laboratory. In this report, intestinal absorption kinetics of SQV and two dipeptide prodrugs were investigated employing a rat single pass intestinal perfusion technique (SPIP). In this model SQV and prodrugs were perfused through a section of the jejunum with the intact blood supply. Disappearance of SQV and its prodrugs from the perfusate can be attributed to intestinal absorption. P-gp inhibitor cyclosporine was added to the perfusate to delineate the role of P-gp in intestinal absorption of SQV and its prodrugs. The perfusion studies were also conducted in the presence of a PepT-1 inhibitor (gly-sar) to delineate the effect of peptide influx transporter in absorption kinetics of SQV prodrugs.
2. EXPERIMENTAL SECTION
2.1 Materials
Saquinavir mesylate was generously supplied by Hoffmann-La Roche. All the prodrugs used in the study were synthesized in our laboratory according to published procedure(Jain et al., 2005). Glycylsarcosine, cyclosporine and all other chemicals were obtained from Sigma-Aldrich (St.Louis, MO). All solvents were of HPLC grade and were obtained from Fisher Scientific Co. (Fair Lawn, NJ).
2.2. Perfusion Solution
The composition of the perfusate solution was NaCl (48mM), KCl (5.4mM), Na2HPO4 (28mM), NaH2PO4 (43mM), mannitol (35mM), polyethylene glycol (PEG 4000;1g/L) and D-glucose (10mM). The pH and osmolality were maintained at 6.5 and 290mOsm/L, respectively.
2.3. Adsorption and Stability of Drug/Prodrug
Briefly in this study, the solution of SQV and prodrug (25 μM) in distilled deionized water were incubated at 37 °C with the tubings for 2 hr. Samples were collected at appropriate time interval and analyzed by HPLC. No drug adsorption to the tube and glass surface was noted. Stability studies of SQV and its prodrugs (25 μM) were also conducted in intestinal perfusion buffer. SQV and prodrugs were incubated perfusion buffer for 2 hr at 37 °C. Samples were collected at predetermined time points and analyzed by HPLC to monitor any drug degradation.
2.4. Analytical Procedure
SQV, Val-Val-SQV and Gly-Val-SQV samples were analyzed by a reversed phase HPLC technique (Ucpinar and Stavchansky, 2003). The HPLC system comprised of a HP 1050 pump, Waters dual wavelength absorbance UV detector, and an Alcott auto sampler (model 718AL HPLC). A C(8) Luna column (250 × 4.6 mm; Phenomenex, Torrance, CA) was employed for the separation of analytes. Mobile phase composed of acetonitrile:water:triethylamine (55:44:1%; v/v/v) and the pH was adjusted to 6.5 with o- phosphoric acid. Flow rate was maintained at 0.8 mL/min and detection wavelength was set at 240 nm. Elution times for SQV, Gly-Val- SQV and for Val-Val- SQV were about 8, 6 and 12 minutes respectively.
2.5. Rat Single-pass Intestinal Perfusion Technique
Male Sprague Dawley rats (200–250 g) were utilized for all absorption studies. Animal studies were performed in accordance with a protocol approved by the University of Missouri-Kansas City IACUC. Animals were fasted for 14–20 hours (water ad libitum) prior to initiation of a perfusion study. The surgery for SPIP of the rat jejunum was performed as described in details elsewhere (Berggren et al., 2004). Briefly, rats were anesthetized and the abdomen was opened with a midline longitudinal incision. A jejunal segment of approximately 10 cm was measured and cannulated with plastic tubing (4mm o.d., inlet tube 40 cm, outlet tube 25cm). Care was taken to avoid injury to local circulatory system. Intestinal segment was rinsed with intestinal perfusate maintained at 37 °C for approximately 30 minutes until the outlet solution was visually clear. A bolus dose of 3–5 mL of drug solution was allowed to equilibrate with intestinal segment. Thereafter the jejunum segment was perfused at a constant flow rate (Qin) of 0.2 mL/min with a peristaltic pump (ismatec pump, Cole Parmer Instrument Co., IL.) Each perfusion experiment lasted for 120 minutes and samples were collected at an interval of every 15 minutes in a pre-weighed glass tubes. All the perfusate solutions collected were weighed and stored at −80 °C until analysis. At the end of the experiment the animal were euthanized with a cardiac injection of saturated potassium chloride solution. Finally, at the end of the experiment the intestine was removed and length of intestine was measured. The radius of the intestine was taken to be 0.18 cm (Issa et al., 2003).
2.6. Stability Studies
Stability studies were conducted at five pH values. Buffers, HCl (pH 1.4), phthalate (pH 3.4 and 5.4), phosphate (pH 7.2), and boric acid (pH 9.2), were prepared and ionic strength was adjusted to 0.1M. The buffer strength was also adjusted to 50 mM for all the buffers. Stock solutions of prodrugs (0.5–1 mg/mL) were prepared in methanol and used immediately for stability studies. Aliquots (9.8 mL) of the buffer were placed in screw capped vials and allowed to equilibrate at 37 °C. Prodrug stock solution (0.2 mL) was subsequently added to the buffer and vials were placed in a water bath maintained at 37 °C and rotated at 60 rpm. Samples (0.2 mL) were collected at appropriate time interval for up to 7 days. Samples were immediately stored at −80 °C until further analysis. All experiments were conducted at least in triplicate.
2.7. Intestinal and Liver homogenate studies
Male Sprague-Dawley rats were euthanized by a lethal injection of sodium pentobarbital through the tail vein (100 mg/kg). Intestinal segments and livers were isolated and stored at −80 °C. Tissues were homogenized in 10 mL of chilled isotonic phosphate buffered saline (IPBS) with a tissue homogenizer (Multipro variable speed homogenizer, DREMEL; Racine, WI) in an ice bath. Subsequently, the homogenates were centrifuged at 14,000g for 25 min at 4 °C to remove cellular debris, and the supernatant was used for hydrolysis studies. Suitable dilutions were made to achieve a final protein concentration of 1.0 mg/mL. Protein content was determined according to the method of Bradford (Bradford, 1976) with BioRad protein estimation kit. Supernatant was equilibrated at 37 °C for about 30 minutes prior to initiation of an experiment. Intestinal and liver homogenate studies were initiated by adding 0.5 mL of a 100 μM prodrug stock solution to 4.5 mL of tissue homogenate. The control consisted of 4.5 mL of IPBS instead of supernatant. Aliquots (50 μL) were withdrawn at appropriate time interval for up to 48 hr. Samples were immediately diluted with equal volumes of ice-cold acetonitrile:methanol (5:4) mixture to stop the enzymatic reaction and then stored at −80 °C until further analysis. Apparent first order rate constants were calculated and corrected for any chemical hydrolysis observed in the control.
2.8 Data Analysis
2.8.1. Calculation of Absorption Constant (ka)
Prodrug/drug concentrations obtained from the perfusate samples were corrected for changes in the water flux during each time interval. Density corrected gravimetric method was utilized for the calculation of net water flux across the incubated intestinal segment. The advantage of this method over the usage of non absorbable markers (like phenol red and 14C polyethylene glycols) is that it does not interfere with analytical method and have no radiation safety issues. The density of collected samples was determined by weighing the contents using an electronic weighing balance of a known volume of perfusate. Net water flux (NWF) was calculated by using Eq. (1)
| Eq. (1) |
Qin is the measured flow rate (mL/min) of entering intestinal perfusate, Qout is the measured flow (mL/min) of exiting intestinal perfusate for the specified time interval calculated from the actual intestinal perfusate density (g/mL), l is the length (cm) of intestinal segment perfused.
Absorption rate constant ka and Cout(corr) were calculated from the Eq. 2&3
| Eq. (2) |
and
| Eq. (3) |
and Cout(corr) is the water flux corrected concentration of the compound measured in the exiting perfusate at the specified time interval (45, 60, 75, 90, 105, 120 minutes); Cin denotes drug concentration of the drug measured in entering perfusate; Q is the perfusion rate (~ 0.2 mL/min); and V is the volume of perfused segment.
2.8.2. Drug Permeability Determination
The single pass intestinal perfusion is based on reaching steady state with respect to the diffusion of compound across intestine. Steady state is confirmed by plotting the ratio of the outlet to inlet concentrations (corrected for water transport) versus time. Permeability calculations across rat jejunum (Peff) were performed from intestinal perfusate samples collected over 45 to 120 minutes (steady state). Peff of SQV, Val-Val- SQV and Gly-Val-SQV was calculated from Eq. (4)
| Eq. (4) |
Where Qin is the flow rate (mL/min) of entering perfusate, Cout(corr) is the water flux corrected concentration of the permeant in the exiting perfusate, Cin is the concentration in entering perfusate, A is the surface area (cm2) of the intestinal segment perfused.
2.9. Statistical Analysis
All experiments are conducted at least in triplicate and results are expressed as mean ± S.E.M/S.D. Statistical comparison of mean values were performed with one-way analysis of variance (ANOVA) or Student t test (Graph Pad INSTAT, version 3.1). *P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Drug Adsorption and Stability Studies
These studies were carried out to ensure that the loss of drug during SPIP is due to absorption only and not due to other losses (e.g. nonspecific binding to the tubing or chemical degradation). No loss of SQV, Val-Val-SQV and Gly-Val-SQV was observed during the perfusion of the drug solution through the tubings. Both SQV and prodrugs were also found to be stable in perfusion buffer as well as intestinal perfusate at 37 °C for 2 hr (data not shown).
3.2. Aqueous Stability
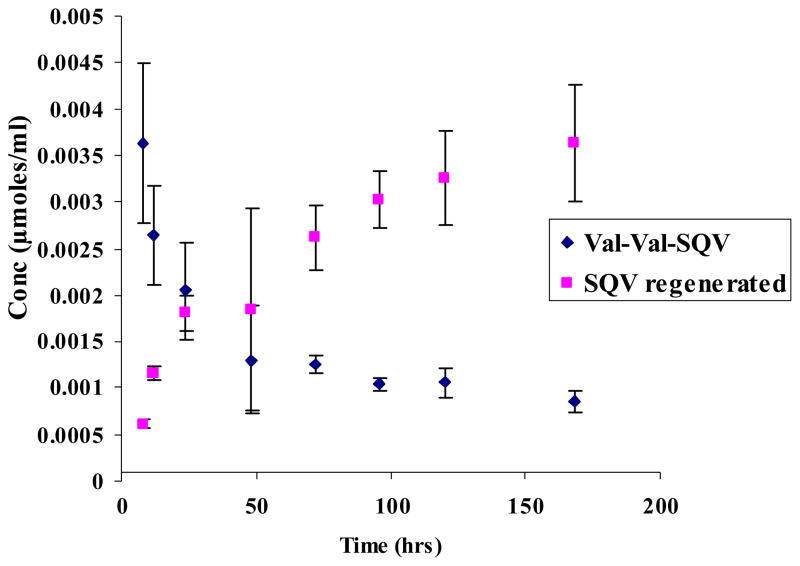
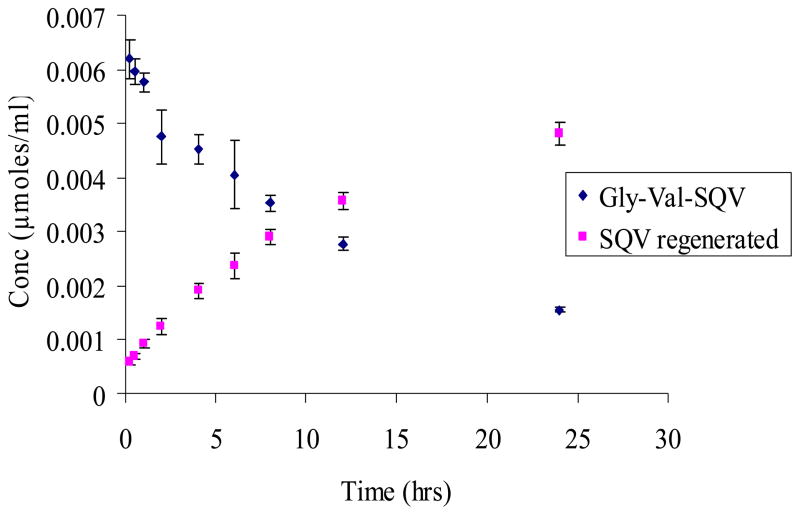
Degradation rate constants for SQV prodrugs are summarized in Table 1. Stability of Val-Val-SQV and Gly-Val-SQV was examined within a pH range of 1.4–9.2. Apparent first order degradation kinetics was observed for all the prodrugs and ester hydrolysis was the predominant degradation mechanism (Figs 2&3). SQV prodrugs exhibited similar pH rate profiles within the pH range studied with Val-Val-SQV being more stable than Gly-Val-SQV. No appreciable degradation of prodrugs was noticed even at pH 1.4 for 7 days at 37 °C. Increased susceptibility to hydrolysis was observed as the pH was raised toward the alkaline range.
Table 1.
Half-lives (hr) and degradation rate constant (kobs) of SQV prodrugs as a Function of pH. Phthalate (pH 3.4 and 5.4), phosphate (7.2), and borate (pH 9.2) buffers (50 mM) were employed. Hydrochloric acid was used to prepare the pH 1.4 solution. Ionic strength adjusted to 0.1 M with KCl. Studies were conducted at 37°C. Values reported are mean ± SD (n=3).
| Val-Val-SQV | Gly-Val-SQV | |||
|---|---|---|---|---|
| pH | kobs (hr−1) × 104 | t1/2 (hr) | kobs (hr−1) | t1/2 (hr) |
| 1.4 | ND | ND | ND | ND |
| 3.4 | 23.7 ±3.5 | 297 ± 48 | 31.7 ± 0.5 | 218 ± 3.4 |
| 5.4 | 82.5 ± 4.6 | 84.2 ± 4.7 | 292 ± 6.4 | 23 ± 0.5 |
| 7.2 | 117 ± 27 | 61.8 ± 11.2 | 586 ± 13.9 | 11.8 ± 0.2 |
| 9.2 | 127 ± 33 | 56.9 ± 12.8 | 976 ± 230.6 | 7.4 ± 2.0 |
ND: No degradation detected.
Figure 2.
Regeneration of SQV from Val-Val-SQV after chemical hydrolysis at pH 7.2. Values are expressed as mean ± S.D. (n=3).
Figure 3.
Regeneration of SQV from Gly-Val-SQV after chemical hydrolysis at pH 7.2. Values are expressed as mean ± S.D. (n=3).
3.3 Intestine and Liver Homogenate Studies
The first order rate constant and half-lives for dipeptide prodrugs of SQV in intestinal and liver homogenate have been summarized in Table 2. The dipeptide ester prodrugs are first converted to amino acid prodrug and then further degraded to the active parent drug, SQV. The major pathways of enzymatic hydrolysis appeared to be cleavage of the peptide and ester bond. There was no significant difference in the half-lives of Val-Val-SQV and Gly-Val-SQV in liver and intestinal homogenates.
Table 2.
Half-lives (hr) and degradation rate constant (kobs)of SQV prodrugs in intestinal and liver homogenate (1 mg/mL). Studies were conducted at 37 °C. Values reported are mean ± SD (n=3).
| Val-Val-SQV | Gly-Val-SQV | |||
|---|---|---|---|---|
| kobs (hr−1) × 102 | t1/2 (hr) | kobs (hr−1) × 102 | t1/2 (hr) | |
| Intestine | 2.3 ± 0.3 | 29.3 ± 4.2 | 2.8 ±0.3 | 24.2 ± 1.1 |
| Liver | 26.2 ± 2.7 | 2.7 ± 0.3 | 31.2 ± 2.6 | 2.2 ± 0.2 |
3.4 Rat Single Pass Intestinal Perfusion of SQV and its Prodrugs
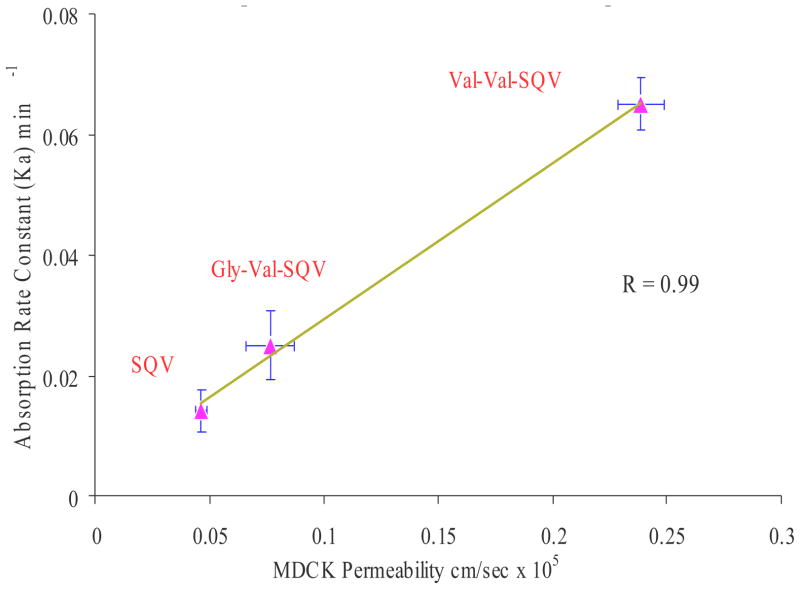
Single pass intestinal perfusion experiments in rat jejunum were undertaken to determine absorption rate constant and intestinal permeability of SQV, Val-Val-SQV and Gly-Val-SQV. Equimolar concentrations (25 μM) of SQV, Val-Val-SQV and Gly-Val-SQV were prepared for perfusion studies. Rank order of rat jejunum absorption rate constants (ka) and intestinal permeability for SQV prodrugs were Val-Val-SQV > Gly-Val-SQV > SQV (Table 3). Enhanced absorption of Val-Val-SQV and Gly-Val-SQV may be attributed to peptide transporter mediated translocation and to possible circumvention of P-gp mediated efflux. Val-Val-SQV exhibited highest absorption rate constant and intestinal permeabilities in comparison to SQV and Gly-Val-SQV (Table 3). This result clearly shows that Val-Val-SQV is a more effective prodrug than Gly-Val-SQV. Also a good correlation (R2 =0.99) was established between permeability across MDCKII-MDR1 cells in apical to basolateral direction (AP-BL) and rat intestinal absorption constant (Fig-4).
Table 3.
Absorption rate constant ka and Permeabilities of SQV, Val-Val-SQV and Gly-Val-SQV across rat jejunum. Values are expressed as mean ± S.E
| Compound | Rat Intestinal Absorption Rate Constant Ka (min−1) × 103 | Rat Intestinal Permeability cm sec−1 × 10 5 |
|---|---|---|
| SQV | 14.1 ± 3.4 | 2.3 ± 0.6 |
| Val-Val-SQV | 65.8 ± 4.3 * | 12.9 ± 1.7 * |
| Gly-Val-SQV | 25.6 ± 5.7 | 4.2 ± 1.0 |
| SQV+Cyclosporine | 31.3 ± 5.6 * | 5.4 ± 0.9 * |
| Val-Val-SQV+Cyclosporine | 69.4 ± 18.5 | 12.8 ± 3.8 |
| Val-Val-SQV+ Gly-sar | 25.6 ± 1.6 † | 4.1 ± 0.3† |
p<0.05 as compared to SQV
p<0.05 as compared to Val-Val-SQV
Figure 4.
Correlation between MDCKII-MDR1 cell permeability in apical to basolateral direction (AP-BL) and rat intestinal absorption constant.
3.5 Rat Single Pass Intestinal Perfusion of SQV and Val-Val-SQV in presence of Cyclosporine
Intestinal perfusion of Val-Val-SQV and SQV (25 μM) was also conducted in the presence P-gp inhibitor cyclosporine (10 μM). Rat jejunal absorption rate constant (ka) and intestinal permeability for SQV was significantly increased in the presence P-gp inhibitor cyclosporine (Table 3). Two fold increment in intestinal permeability of SQV in the presence of cyclosporine is due to inhibition of P-gp for which SQV is a very good substrate. No permeability enhancement of Val-Val-SQV was observed in the presence of cyclosporine indicating that conjugated peptide prodrugs do not interact with P-gp and thereby not effluxed by this pump (Table 3).
3.6 Rat Single Pass Intestinal Perfusion of Val-Val-SQV in presence of Gly-Sar
This study was conducted to delineate the role of peptide transporter in the intestinal translocation of Val-Val-SQV. Intestinal perfusion of Val-Val-SQV was conducted in the presence of inhibitory concentration of Gly-sar (20mM). The rat jejunum absorption rate constant (ka) for Val-Val-SQV decreased significantly in the presence of gly-sar suggesting a definite role of peptide transporter in the translocation of Val-Val-SQV (Table 3). Rat intestinal permeability of Val-Val-SQV in the presence and absence of gly-sar was 4.1 ± 0.3 ×10−5 and 12.9 ± 1.7 ×10−5 cm sec−1 respectively. Significant reduction in Val-Val-SQV permeability across rat jejunum in the presence of gly-sar clearly indicates that peptide transporter is involved in the translocation and permeability enhancement of Val-Val-SQV.
4. DISCUSSION
In recent years, considerable works has been focused on enhancing oral bioavailability and brain penetration of HIV protease inhibitors. All the protease inhibitors are good substrate of efflux proteins like P-glycoprotein (P-gp) and multi-drug resistance associated proteins (MRPs). Several rationale drug designing strategies have been employed in the past to circumvent P-gp mediated efflux (Raub, 2006). However P-gp mediated drug efflux still remains a major barrier for oral drug absorption.
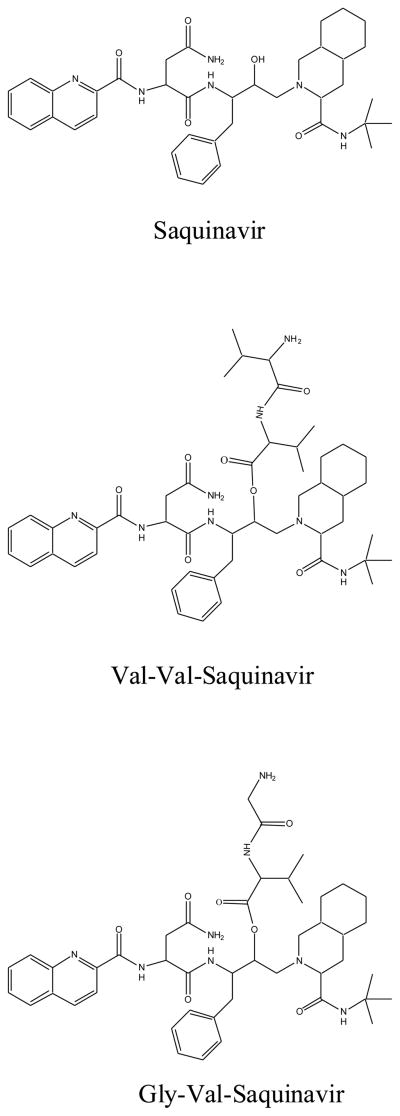
In this study, novel di-peptide conjugates of protease inhibitor SQV, Val-Val-SQV and Gly-Val-SQV (Fig. 1) targeting intestinal peptide transporter were synthesized in our laboratory (Jain et al., 2005) and evaluated for their ability to enhance absorption of SQV. An ideal prodrug should exhibit good chemical stability and must be enzymatically converted to parent drug following transport across cell membrane. Aqueous stability data indicate that the prodrugs are more stable in acidic pH as compared to alkaline pH (Table 1). Both prodrugs Val-Val-SQV and Gly-Val-SQV showed apparent first order degradation kinetics and ester hydrolysis was the predominant degradation mechanism observed for both prodrugs (Fig. 2&3). Upon hydrolysis, both prodrugs exhibited ester bond cleavage generating parent drug SQV. Val-Val-SQV is found to be more stable as compared to Gly-Val-SQV at all the pH ranges studied. Increased stability of Val-Val-SQV as compared to Gly-Val-SQV can be attributed possibly to steric hindrance caused by the terminal valine group of Val-Val-SQV towards hydroxyl ion attack on the ester bond. Steric hindrance prevents nucleophilic attack on Val-Val-SQV and renders it more stable compared to Gly-Val-SQV. Stability studies in liver and intestinal cell homogenate were also carried out to evaluate the regeneration kinetics of the parent drug from the dipeptide ester prodrug. Both prodrugs (Val-Val-SQV and Gly-Val-SQV) hydrolyzed to regenerate the active parent drug, SQV. No significant differences in the half-lives of Val-Val-SQV and Gly-Val-SQV in liver and intestinal homogenates were noted, demonstrating similar susceptibility of the prodrugs to the hydrolyzing enzymes (Table 2). Long half-lives of prodrugs in intestinal homogenate demonstrate their utility in targeting nutrient transporter expressed on other tissues like brain and testes. Biochemical stability of Val-Val-SQV and Gly-Val-SQV in enterocytes can also prevent its efflux back into intestinal lumen. In the basic SPIP experiment, the compound of interest is monitored in the perfusate only, not in the blood. Loss of compound as determined by the difference between the inlet and outlet concentrations is attributed to the absorption. Our preliminary stability studies in buffer and intestinal homogenate (<90% at the end of 2hr) suggests that the loss in outlet concentration is not due to metabolism of prodrugs during perfusion (Table 1&2).
Figure 1.
Structures of dipeptide ester prodrugs of saquinavir.
Rat jejunal single pass intestinal perfusion experiments were performed to estimate the absorption rate constant and intestinal permeability of SQV, Val-Val-SQV and Gly-Val-SQV (Table 3). Val-Val-SQV exhibits five fold increase in absorption rate constant and intestinal permeability relative to SQV. Gly-Val-SQV also showed two fold increase in absorption rate constant and intestinal permeability. Such permeability enhancement of the prodrugs can be attributed to their differential affinity toward the peptide transporter and P-glycoprotein. These results were consistent with our previous published result (Jain et al., 2005). A good correlation (R2 =0.99) was observed between permeability across MDCKII-MDR1 cells (Jain et al., 2005) in apical to basolateral direction (AP-BL) and rat intestinal absorption rate constant (Fig-4).
Intestinal perfusion with equimolar concentrations of Val-Val-SQV and SQV was carried out in presence of P-gp inhibitor cyclosporine (10 μM). Rat intestinal permeability of SQV was significantly enhanced in the presence of P-gp inhibitor cyclosporine (Table 3). Two fold increment in intestinal permeability of SQV in the presence of cyclosporine could be due to inhibition of P-gp for which SQV is a very good substrate. No permeability enhancement of Val-Val-SQV was observed in the presence of cyclosporine, supporting our hypothesis that modified prodrug is no longer a substrate for P-gp. Finally, intestinal perfusion of Val-Val-SQV was carried out in the presence of inhibitory concentration of gly-sar. A significant inhibition in the intestinal permeability of Val-Val-SQV in the presence of gly-sar indicates that Val-Val-SQV in translocated by peptide transporter (Table 3). Several earlier reports indicated that valine ester prodrugs are substrates of peptide transporters e.g valine esters of ganciclovir (Sugawara et al., 2000) and acyclovir (Balimane et al., 1998). High transport capacity, broad substrate specificity and dense expression of peptide transporter in the intestine and other biological barriers render the peptide transporter an ideal membrane protein for drug targeting. Recently it has been reported that intestinal metabolism plays a significant role in metabolism of SQV (Lee et al., 2003). Thus peptide prodrug derivatization of SQV is a viable strategy to bypass P-gp mediated efflux and to completely shut down repeated exposure to intestinal CYP3A mediated metabolism. Saquinavir is also known to be a substrate for several MRP’s which also play an important role in intestinal permeability and metabolism of SQV (Williams et al., 2002). The role of MRP mediated efflux in the disposition of SQV is under investigation in our laboratory.
In conclusion, we have demonstrated that peptide prodrug modification of SQV to Val-Val-SQV and Gly-Val-SQV can lead to an increased permeability across rat jejunum. Such increased permeability can be attributed partially to the peptide transporter mediated influx of these agents and partially to circumvention of their P-gp mediated efflux. Thus transporter targeted prodrug modification of P-gp substrates could lead to shielding of these drug molecules from efflux pump. This approach has high clinical significance as it will aid in enhancing intestinal absorption and oral bioavailability of poorly absorbed PIs and other P-gp substrates.
Acknowledgments
This work was supported by National Institute of Health Grant GM 64320-03.
Keywords
- SQV
saquinavir
- P-gp
P-glycoprotein
- SPIP
single pass intestinal perfusion
- ka
intestinal absorption rate constant
- Peff
effective intestinal permeability across rat jejunum
- NWF
net water flux
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsenz J, Steffen H, Alex R. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm Res. 1998;15:423–428. doi: 10.1023/a:1011924314899. [DOI] [PubMed] [Google Scholar]
- Anand BS, Katragadda S, Mitra AK. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: intestinal absorption and liver metabolism. J Pharmacol Exp Ther. 2004;311:659–667. doi: 10.1124/jpet.104.069997. [DOI] [PubMed] [Google Scholar]
- Bachmeier CJ, Spitzenberger TJ, Elmquist WF, Miller DW. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood-brain barrier. Pharm Res. 2005;22:1259–1268. doi: 10.1007/s11095-005-5271-y. [DOI] [PubMed] [Google Scholar]
- Balimane PV, Tamai I, Guo A, Nakanishi T, Kitada H, Leibach FH, Tsuji A, Sinko PJ. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998;250:246–251. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- Berggren S, Hoogstraate J, Fagerholm U, Lennernas H. Characterization of jejunal absorption and apical efflux of ropivacaine, lidocaine and bupivacaine in the rat using in situ and in vitro absorption models. Eur J Pharm Sci. 2004;21:553–560. doi: 10.1016/j.ejps.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bolger MB, Haworth IS, Yeung AK, Ann D, von Grafenstein H, Hamm-Alvarez S, Okamoto CT, Kim KJ, Basu SK, Wu S, Lee VH. Structure, function, and molecular modeling approaches to the study of the intestinal dipeptide transporter PepT1. J Pharm Sci. 1998;87:1286–1291. doi: 10.1021/js980090u. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cummins CL, Salphati L, Reid MJ, Benet LZ. In vivo modulation of intestinal CYP3A metabolism by P-glycoprotein: studies using the rat single-pass intestinal perfusion model. J Pharmacol Exp Ther. 2003;305:306–314. doi: 10.1124/jpet.102.044719. [DOI] [PubMed] [Google Scholar]
- Dupre J, Behme MT, Hramiak IM, McFarlane P, Williamson MP, Zabel P, McDonald TJ. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes. 1995;44:626–630. doi: 10.2337/diab.44.6.626. [DOI] [PubMed] [Google Scholar]
- Edwards JE, Brouwer KR, McNamara PJ. GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob Agents Chemother. 2002;46:2284–2286. doi: 10.1128/AAC.46.7.2284-2286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm U, Johansson M, Lennernas H. Comparison between permeability coefficients in rat and human jejunum. Pharm Res. 1996;13:1336–42. doi: 10.1023/a:1016065715308. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25:256–266. [PubMed] [Google Scholar]
- Germann UA. P-glycoprotein--a mediator of multidrug resistance in tumour cells. Eur J Cancer. 1996;32A:927–944. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Issa C, Gupta P, Bansal AK. Implications of density correction in gravimetric method for water flux determination using rat single-pass intestinal perfusion technique: a technical note. AAPS PharmSciTech. 2003;4:E16. doi: 10.1208/pt040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kusuhara H, Sugiyama Y. Effects of intestinal CYP3A4 and P-glycoprotein on oral drug absorption--theoretical approach. Pharm Res. 1999;16:225–231. doi: 10.1023/a:1018872207437. [DOI] [PubMed] [Google Scholar]
- Jain R, Majumdar S, Nashed Y, Pal D, Mitra AK. Circumventing P-glycoprotein-mediated cellular efflux of quinidine by prodrug derivatization. Mol Pharm. 2004;1:290–299. doi: 10.1021/mp049952s. [DOI] [PubMed] [Google Scholar]
- Jain R, Agarwal S, Majumdar S, Zhu X, Pal D, Mitra AK. Evasion of P-gp mediated cellular efflux and permeability enhancement of HIV-protease inhibitor saquinavir by prodrug modification. Int J Pharm. 2005;303:8–19. doi: 10.1016/j.ijpharm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Kakuda TN, Struble KA, Piscitelli SC. Protease inhibitors for the treatment of human immunodeficiency virus infection. Am J Health Syst Pharm. 1998;55:233–254. doi: 10.1093/ajhp/55.3.233. [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Mower R, Hollenbeck T, Castelo J, Johnson N, Gordon P, Sinko PJ, Holme K, Lee YH. Modulation of nonspecific binding in ultrafiltration protein binding studies. Pharm Res. 2003;20:1015–1021. doi: 10.1023/a:1024406221962. [DOI] [PubMed] [Google Scholar]
- Lown KS, Mayo RR, Leichtman AB, Hsiao HL, Turgeon DK, Schmiedlin-Ren P, Brown MB, Guo W, Rossi SJ, Benet LZ, Watkins PB. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR, Woolley JL. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res. 1999;16:1206–1212. doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm. 2006;3:3–25. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- Rice A, Michaelis ML, Georg G, Liu Y, Turunen B, Audus KL. Overcoming the blood-brain barrier to taxane delivery for neurodegenerative diseases and brain tumors. J Mol Neurosci. 2003;20:339–343. doi: 10.1385/JMN:20:3:339. [DOI] [PubMed] [Google Scholar]
- Rouquayrol M, Gaucher B, Roche D, Greiner J, Vierling P. Transepithelial transport of prodrugs of the HIV protease inhibitors saquinavir, indinavir, and nelfinavir across Caco-2 cell monolayers. Pharm Res. 2002;19:1704–1712. doi: 10.1023/a:1020913631309. [DOI] [PubMed] [Google Scholar]
- Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57:679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Edwards JE, Morgan ME, McNamara PJ, Anderson BD. Effects of a P-glycoprotein inhibitor on brain and plasma concentrations of anti-human immunodeficiency virus drugs administered in combination in rats. Drug Metab Dispos. 2002;30:479–482. doi: 10.1124/dmd.30.5.479. [DOI] [PubMed] [Google Scholar]
- Schapiro JM, Winters MA, Stewart F, Efron B, Norris J, Kozal MJ, Merigan TC. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89:781–789. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13:963–977. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- Ucpinar SD, Stavchansky S. Quantitative determination of saquinavir from Caco-2 cell monolayers by HPLC-UV. High performance liquid chromatography. Biomed Chromatogr. 2003;17:21–25. doi: 10.1002/bmc.205. [DOI] [PubMed] [Google Scholar]
- Varma MV, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- Williams GC, Liu A, Knipp G, Sinko PJ. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) Antimicrob Agents Chemother. 2002;46:3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]