Abstract
Alzheimer’s disease (AD) is the major form of age-related dementia and is characterized by progressive cognitive impairment, the accumulation of extracellular amyloid β-peptide (Aβ), and intracellular hyperphosphorylated tau aggregates in affected brain regions. Tau hyperphosphorylation and accumulation in neurofibrillary tangles is strongly correlated with cognitive deficits, and is apparently a critical event in the dementia process because mutations in tau can cause a tangle-only form of dementia called frontotemporal lobe dementia. Among kinases that phosphorylate tau, glycogen synthase kinase 3β (GSK3β) is strongly implicated in AD pathogenesis. In the present study, we established an ELISA to screen for agents that inhibit GSK3β activity and found that the flavonoid morin effectively inhibited GSK3β activity and blocked GSK3β-induced tau phosphorylation in vitro. In addition, morin attenuated Aβ-induced tau phosphorylation and protected human neuroblastoma cells against Aβ cytotoxicity. Furthermore, treatment of 3×Tg-AD mice with morin resulted in reductions in tau hyperphosphorylation and paired helical filament-like immunoreactivity in hippocampal neurons. Morin is a novel inhibitor of GSK3β that can reduce tau pathology in vivo and may have potential as a therapeutic agent in tauopathies.
Keywords: Alzheimer’s disease, GSK3β, Tau hyperphosphorylation, Morin
Introduction
Tauopathies are neurodegenerative disorders characterized by the abnormal intracellular accumulation of filamentous aggregates of hyperphosphorylated tau protein; prominent examples include Alzheimer’s disease (AD), frontotemporal dementia (FTD) and many cases of Parkinson’s disease (Stoothoff and Johnson, 2005; Yancopoulou and Spillantini, 2003). Tau proteins are microtubule-associated proteins that are abundantly expressed in neurons of peripheral and central nervous systems, primarily in axons wherein they stabilize microtubules (Pittman et al., 2006). The tau phosphorylation state influences its ability to bind and stabilize microtubules, with hyperphosphorylation resulting in tau detachment and self-aggregation and microtubule depolymerization (Alonso et al., 2001; Alonso et al., 1996; Alonso et al., 1994; Luk et al., 2009). Tau hyperphosphorylation and aggregation is sufficient to cause dementia as demonstrated by individuals with FTD, an inherited form of ‘tau only’ dementia (van Swieten and Spillantini, 2007). In AD, the numbers of tau tangle-bearing neurons in vulnerable brain regions such as the hippocampus and frontal cortex have been correlated with cognitive impairment in some studies (Dournaud et al., 1995; Nagy et al., 1995). The cause of tau pathology in AD is not fully understood, but is believed to result from increased oxidative stress (Mattson et al., 1997; Melov et al., 2007; Steinberg et al., 2004), perturbed cellular calcium homeostasis (Hartigan and Johnson, 1999; Li et al., 2005; Mattson, 1990) and energy deficits (Clodfelder-Miller et al., 2006). Altered proteolytic processing of the amyloid precursor protein (APP) and accumulation of extracellular amyloid β-peptide (Aβ) may be important antecedents to tauopathy and neuronal degeneration in AD (Mattson, 2004). Aggregation of hyperphosphorylated tau protein and deposition of Aβ are the two hallmark lesions of AD (Grundke-Iqbal et al., 1986; Hooper et al., 2008; Iqbal and Grundke-Iqbal, 2008; Kar et al., 2004).
Glycogen synthase kinase 3β (GSK3β) was originally identified as a regulator of glycogen metabolism and is now known to regulate a diverse range of biological functions (Lee and Kim, 2007). GSK3β is expressed at high levels in neurons in the brain where it plays important roles in regulating structural and metabolic plasticity; two well-studied substrates of GSK3β in neurons are the transcription factor β-catenin and tau (Soutar et al., 2010). There is accumulating evidence that GSK3β plays a role in the hyperphosphorylation of tau and the associated memory impairment in AD (Hooper et al., 2008). GSK3β expression is up-regulated in the hippocampus and post-synaptosomal supernatants from AD brain (Blalock et al., 2004; Pei et al., 1997) and in circulating peripheral lymphocytes in both AD and in mild cognitive impairment (Hye et al., 2005). In addition, GSK3β transgenic mice display tau hyperphosphorylation and neurodegeneration (Lucas et al., 2001), while chronic lithium (GSK3β inhibitor) treatment prevents tau hyperphosphorylation and neurofibrillary tangle formation in double transgenic mice overexpressing mutant tau (Noble et al., 2005). In addition, a maleimide derivative that inhibits GSK3β exhibited neuroprotective effects in cultured primary hippocampal neurons (Cross et al., 2001). Based upon its role in tau hyperphosphorylation and neuronal degeneration in experimental models, GSK3β has emerged as a therapeutic target for intervention in tauopathies including AD.
In the present study, we established an enzyme-linked immunosorbent assay (ELISA) to identify GSK3β small-molecule inhibitors among a panel of natural polyphenols. One such polyphenol called morin inhibited GSK3β activity, prevented tau hyperphosphorylation and protected cultured human neural cells against Aβ-induced cell death. Moreover, morin effectively reduced tau hyperphosphorylation in the 3×Tg-AD mouse model (Oddo et al., 2003b).
Materials and methods
Chemicals
Morin (2′,3,4′,5,7-pentahydroxyflavone), lithium chloride (LiCl) and 2-[4,5-dimethyl-2-ly]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Phytochemical libraries were provided by Prof. Hae Young Chung at Pusan University. Synthetic Aβ1–42 was obtained from American Peptide Company (CA, USA). 2′, 7′ – dichlorofluoresceindiacetate (DCF-DA), Hoechst 33342, and propidium iodide (PI) were purchased from Invitrogen (Carlsbad, CA, USA).
Expression and purification of recombinant enzymes and substrates
To develop an ELISA to screen GSK3β inhibitors from a panel of phytochemicals, we generated recombinant creatine kinase-1ε, GSK3β and β-catenin proteins. For active enzymes, full-length CK1ε (residues 1–319) and the GSK3β catalytic domains (residues 27–293) were cloned from a 17-day-old mouse embryo cDNA library (Clontech) and expressed in Escherichia coli (E. coli) and Bac-to-Bac baculovirus expression system (Life Technology), respectively (Cegielska et al., 1998; Dajani et al., 2001). For a substrate, the N-terminal region (residues 1–133) of β-catenin was expressed in E. coli BL21-codon Plus-RIL cells (Stratagene) as a GST-fusion protein with a TEV protease cleavage site (pGex-TEV-β-catenin). Cells were grown in Luria-Bertani medium at 7°C to an OD of 0.6–0.8, induced with 0.5 mM IPTG and grown for another 4 hours at 30°C. After pelleting and resuspension in 20 mM ethanolamine (pH 9.5), 150 mM NaCl, 2 mM β-mercaptoethanol and 20 mM glutathione (GSH), the cells were lysed in a French pressure cell. Subsequently, the GST tag was cleaved using recombinant TEV protease and was removed by repeated HiTrapQ anion-exchange chromatography.
ELISA Screening for GSK3β inhibitors
The N-terminal region of β-catenin was phosphorylated by incubation with the catalytic domain of CK1ε (0.28 mg/ml) at 37°C for 20 minutes in 50 mM Tris buffer (pH 8.0) containing 10 mM MgCl2, 10 mM 2-mercaptoethanol, and 1 mM ATP. Primed GST-tagged β-catenin (0.2 mg/ml) by CK1ε was then added to the GSH-coated 96-well plate (Thermo Fisher Scientific, Inc.) and incubated at room temperature (RT) for 30 minutes. Then GSK3β and phytochemicals in 5X buffer (250 mM Tris, 50 mM MgCl2 and 50 mM β-mercaptoethanol, pH 8.0) with 1 mM ATP were added and incubated at RT for 30 minutes. After the wash with washing buffer (20 mM Tris, 150 mM NaCl, 2 mM β-mercaptoethanol, pH 8.0), primary antibody against phospho-β-catenin (S33/37/T41) (1:4000; Cell signaling Technology, Inc.) was added and incubated at RT for 1 hour. Next, peroxidase-conjugated secondary antibody (anti-Rabbit:31460(#), 1:20000; Thermo Fisher Scientific, Inc.) was added for 1 hour, followed by incubation with 50 μl/well 3,3′,5,5′-tetramethylbenzidine (TMB) solution (TMB solution; Calbiochem, La Jolla, CA). After incubation of the TMB solution at 37°C for 10 minutes, H2SO4 was added for 5 minutes to stop the reaction.
Preparation of Aβ1–42
The oligomeric Aβ1–42 was prepared as described previously (Dahlgren et al., 2002). Aβ1–42 peptide was dissolved in 1,1,1,3,3,3-Hexafluoro-2-propanal (HFIP) to a final concentration of 1 mmol/L. The peptide was dried in a vacuum dryer and the oligomeric Aβ1–42 peptide was stored at −80°C until use. In our experiments, the peptide was dissolved in culture medium to a final concentration of 10 μM, and the peptide was incubated at 4°C for 24 hours.
Cell culture and cell viability assay
Human neuroblastoma SH-SY5Y cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in plastic culture dishes in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 2 mM glutamine in a humidified atmosphere of 5% CO2/95% air at 37°C. For the cell viability experiments, cells were plated in 24-well plates and incubated in RPMI 1640 medium containing 1% FBS to suppress cell proliferation. Twenty-four hours after plating, the cells were pretreated with morin (1 μM, 10 μM) or LiCl (100 μM) for 6 hours and then treated with 10 μM Aβ for 24 hours. Treatments were administered by direct dilution into the culture medium, and an equivalent volume of vehicle was added to the control cultures. At the end of the experimental treatment period, 150 μl of 0.5 mg/ml MTT in PBS was added to each well. The plate was incubated at 37°C for 4 hours and then the MTT solution was removed. Cells were dissolved in solubilization solution (DMSO:ethanol, 1:1), and the formazan dye product was quantified in an ELISA microplate reader at an absorbance of 560 nm.
Nuclear staining with Hoechst 33342 and PI
Cell death measurements were performed using a fluorometric method under a fluorescence microscope, as described previously (Wrede et al., 2002). The plasma membrane of all cells is permeable to Hoechst 33342 irrespective of whether they are damaged, and it emits blue fluorescence after the dye binds to the nucleus. However, the polar nuclear stain PI can only penetrate cells with damaged membranes. Cells were seeded in 60-mm cultured dishes and allowed to attach for 24 hours. Cells were pretreated with or without morin for 6 hours, and then 10 μM Aβ1–42 was applied for 24 hours. Hoechst 33342 and PI were added for 10 minutes at final concentrations of 10 μM and 50 μM, respectively. Images were acquired using a Nikon ECLIPSE TE 2000-U microscope (Nikon, Tokyo).
Assessment of oxidative stress
Briefly, 24 hours after seeding in a 96-well plate neuroblastoma cells were pretreated for 6 hours with 1 or 10 μM morin, and then 25 μM DCFDA was added to cell culture medium for 30 minutes. After 30 minutes, Aβ was added to each well. Changes in fluorescence intensity were measured at 0, 10, 20, 30, 40, 50 and 60 minutes after Aβ treatment with a fluorescence plate reader (GLOMAX, Wisconsin, USA), with excitation and emission wavelengths set at 485 and 530 nm, respectively.
Western blot analysis
After the experimental treatment, the cells and tissue homogenates were solubilized in SDS-polyacrylamide gel electrophoresis sample buffer, and the protein concentration in each sample was determined using a Bio-Rad protein assay kit with bovine serum albumin as the standard. Total protein equivalents for each sample (50 μg protein per lane) were then separated in 10% SDS-polyacrylamide gels and electrophoretically transferred to Immobilon-PSQ transfer membrane (Millipore Corp., Bedford, MA, USA). The membrane was immediately placed into blocking solution (5% nonfat milk) at room temperature for 30 minutes. The membrane was incubated with diluted primary antibody for phospho-tau (pSpS;Ser199/202, Rabbit; 1:1000, Invitrogen), phospho-tau (pTau;Ser396, Mouse; 1:1000, Cell signaling Technology, Inc.), or β-actin (Mouse; 1:1000, Sigma Chemicals Co.) in TBS-T buffer (Tris-HCl based buffer with 0.2% Tween 20, pH 7.5) at 4°C overnight. After washing four times for 10 minutes each, the membrane was incubated with secondary polyclonal anti-rabbit antibody or monoclonal anti-mouse antibody (1:10,000, Sigma Chemicals Co.) in TBS-T buffer at room temperature for 1 hour. Horseradish-conjugated secondary antibody labeling was detected by enhanced chemiluminescence (ECL) and exposure to radiographic film. Pre-stained blue markers were used for molecular weight determination.
Animals and drug administration
The 3×Tg-AD mice were originally derived by transfecting transgenes encoding human APPswe and tauP301L into single-cell embryos harvested from homozygous mutant PS1M146V knock-in mice. The 3×Tg-AD mice progressively develop age-dependent amyloid plaques, neurofibrillary tangles and cognitive impairment in AD-relevant brain regions. The mice also display synaptic dysfunction including long-term potentiation (LTP) deficits (Desai et al., 2009; Oddo et al., 2003a). The mice used in the present study were backcrossed to C57BL/6 mice for 8 generations. Eight-month-old male 3×Tg-AD mice and age-matched C57/BL6 wild-type (WT) mice were used in the current study. All mice were maintained under temperature- and light-controlled conditions (20–23°C, 12-hour light/12-hour dark cycle) and provided food and water ad libitum. Mice were randomly divided into four groups of 4 or 5 animals. Morin was administrated once daily intraperitoneally (i.p.) at 10 mg/kg body weight for 7 days. Mice in the control group received an injection of 5% ethanol in PBS (vehicle). The institutional animal care committee of Pusan National University approved the experimental protocol (#PNU-2010-000163).
Tissue preparation
For the histological study, mice were anesthetized and perfused intracardially with 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). After fixative perfusion, the brains were removed from the cranium, placed in the same fixation solution at 4°C overnight and transferred to a 30% sucrose solution. The cryoprotected brains were sectioned serially at 40-μm thickness in the coronal plane using a freezing microtome (MICROM, Germany), and the brain sections were collected in Dulbecco’s phosphate-buffered saline (DPBS) solution containing 0.1% sodium azide and stored at 4°C. Each section that contained the hippocampal formation was saved.
Immunohistochemistry (IHC)
Sections were treated with 0.6% H2O2 in Tris-buffered saline (TBS; pH 7.5) to block endogenous peroxidase. The sections were blocked in TBS/0.1% Triton X-100/3% horse serum (TBS-TS) for 30 minutes, and incubated with either anti-phospho-tau (mouse monoclonal; 1:500, Cell signaling) or anti-HT7 (mouse monoclonal; 1:500, Thermo) antibodies in TBS-TS at 4°C overnight. The sections were incubated with biotinylated secondary goat anti-mouse IgG antibodies (1:400, Vector Laboratories) at room temperature for 3 hours, followed by incubation with ABC solution (Vectastain ABC reagent Elite Kit, Vector Laboratories, Burlingame, CA, USA) at room temperature for 1 hour. After diaminobenzidine (DAB) solution was added to the sections for 5 minutes, images were acquired using a Nikon ECLIPSE TE 2000-U microscope (Nikon, Tokyo). Cells in every sixth section that included the hippocampus were counted. The reference region consisted of the CA1 neurons in the hippocampus. All cell counts were performed by the same blinded investigator (EG).
Double-Labeling Immunostaining
For double-labeling IHC, the sections were blocked with 3% normal goat serum (Gibco, Grand Island, NY, USA) and incubated with primary antibodies against the following proteins: the neuronal marker NeuN (mouse monoclonal; 1:500, Chemicon, CO, ASO); the astrocyte marker GFAP (rabbit polyclonal; 1:500, Sigma); the human tau marker HT7 (mouse monoclonal; 1:1000, Thermo); and the microglia marker Iba-1 (rabbit polyclonal; 1:500, Wako) at 4°C overnight. The brain sections were then washed with TBS and incubated for 3 hours in presence of anti-mouse IgG labeled with Alexa Fluor-488 and anti-rabbit IgG labeled with Alexa Fluor-568. Images were acquired using a FV10i FLUOVIEW Confocal Microscope (Olympus, Tokyo).
Nissl (Cresyl Violet) Staining
For Nissl staining, the sections were mounted on slides and dried overnight. Brain sections were hydrated in diminishing ethanol solutions stained with cresyl violet, then dehydrated in increasing ethanol solutions and extracted with xylene. Sections were mounted with permanent mounting medium (Fisher Scientific, Fair Lawn, NJ) and cover-slipped. Images were acquired using a Nikon ECLIPSE TE 2000-U microscope (Nikon, Tokyo).
Statistical analysis
The statistical significance of the differences between control and morin-treated groups was determined by analysis of variance (ANOVA) with Fisher’s protected least significant difference (PLSD) procedure. Values of p<0.05 were considered statistically significant. Analyses were performed using Statview software ®.
Results
ELISA development to screen GSK3β inhibitors
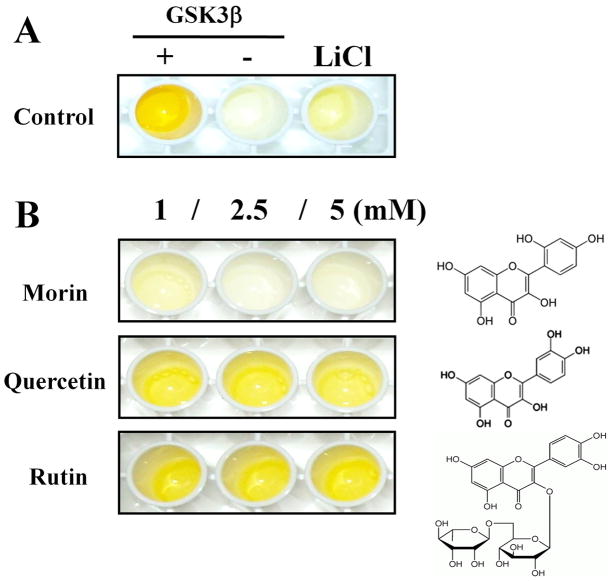
In order to screen GSK3β inhibitors in a phytochemical library, we established the ELISA to detect the enzymatic activity of GSK3β by using β-catenin as a substrate. The phosphorylated GST-tagged β-catenin primed by CKI was incubated in GSH-coated well plates and active GSK3β and phytochemicals (1, 2.5, 5 mM) were added to each well. Plates were incubated with primary antibody against phospho-β-catenin overnight and then peroxidase-conjugated secondary antibody. Enzyme activities were measured by the colorization of TMB solution and stopped by addition of H2SO4. We confirmed that the phospho-β-catenin antibody selectively recognized β-catenin only after phosphorylation by GSK3β and that this GSK3β activity was effectively blocked by 50 mM LiCl (Fig. 1A). We identified 3 phytochemicals in the flavonoid library that were effective inhibitors of GSK3β activity (data not shown), but morin showed the strongest inhibition in the GSK3β activity assay (Fig. 1B). Several flavonoids with structural similarity to morin, including quercetin and rutin, did not inhibit GSK3β activity (Fig. 1B).
Fig. 1.
Characterization of an ELISA to screen GSK3β inhibitors using β-catenin as a substrate.
(A) Adding GSK3β significantly increased β-catenin phosphorylation, and LiCl (50 mM) effectively blocked GSK3β-induced phosphorylation. (B) Among flavonoids tested, we found that morin decreased the levels of phosphorylated β-catenin, while flavonoids with similar structures had no effect on GSK3β-induced phosphorylation.
Morin inhibits GSK3β-induced tau phosphorylation ex vivo
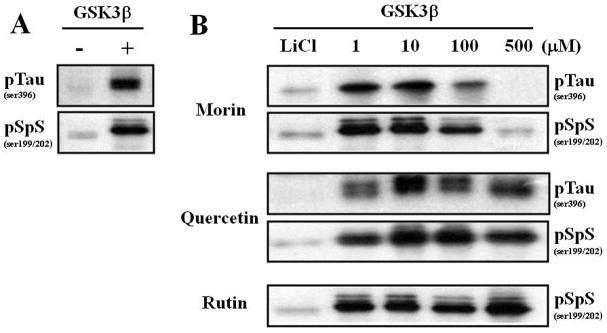
Since our ELISA screen is based on the phosphorylation of β-catenin by GSK3β, we next determined whether morin inhibited GSK3β-mediated tau phosphorylation. The GSK3β-inhibitory action of morin was tested ex vivo using brain tissue. We tested GSK3β-induced tau phosphorylation from 3 different brain tissues (hippocampus, cortex and cerebellum), and we found that hippocampal tau proteins were more quickly phosphorylated by GSK3β compared to tau from cerebellum, and this phosphorylation was maintained longer than that of tau from cortex (Supplemental Fig. 1). GSK3β dramatically increased the phosphorylation of hippocampal tau in 30 min (Fig. 2A). Morin significantly attenuated GSK3β-induced tau phosphorylation in a dose-dependent manner, whereas the negative controls quercetin and rutin failed to modulate tau phosphorylation (Fig. 2B). Note that GSK3β-induced tau phosphorylation was also effectively blocked by 50 mM LiCl (Fig. 2B). Therefore, we were able to confirm the inhibitory action of morin on GSK3β activity and GSK3β-induced tau phosphorylation. In addition, we have confirmed ex vivo experiments in the presence of phosphatase inhibitors (50 mM NaF, 5 mM β-glycophosphate, and 1 mM sodium vanadate) to show that morin decreased hyperphosphorylation of tau by inhibiting GSK-3β in the hippocampal homogenate (Supplemental Fig. 2).
Fig. 2.
Morin inhibits GSK3β activation-induced tau hyperphosphorylation in hippocampal homogenate.
GSK3β-inhibitory effect of morin was evaluated by tau phosphorylation inhibition. Hippocampal tissue homogenate in 5X buffer with 1 mM ATP was pre-incubated with phytochemicals on ice for 30 minutes and then GSK3β was added and incubated at 37°C in a water bath for 30 minutes. Tau hyperphosphorylation (pTauser396, pSpSser199/202) was determined by immunoblot analysis. Note the molecular band shift due to increased phosphorylation. (A) GSK3β significantly increased tau hyperphosphorylation compared to homogenates incubated in the absence of GSK3β. (B) LiCl (50 mM), a well-known GSK3β inhibitor, decreased the tau hyperphosphorylation induced by GSK3β. Morin effectively decreased the GSK3β-mediated tau hyperphosphorylation in a concentration-dependent manner. In contrast, quercetin and rutin did not inhibit GSK3β-induced tau hyperphosphorylation.
Morin decreases Aβ-induced cell death and tau phosphorylation in human neuroblastoma cells
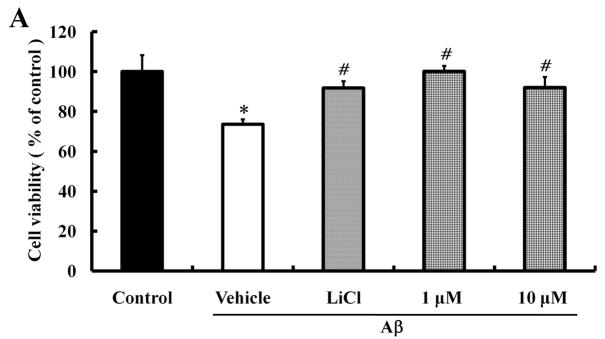
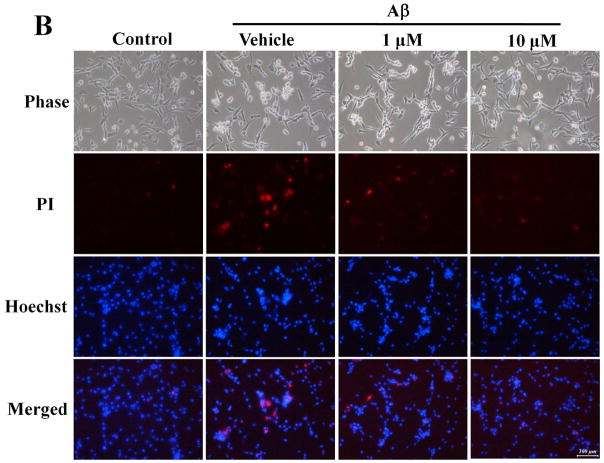
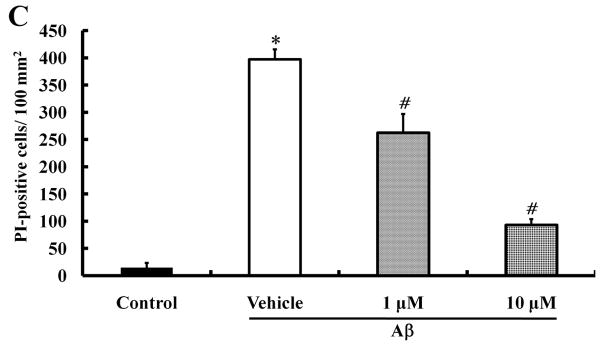
Several studies have shown that Aβ can cause cell death (Hoi et al., 2010; Kwon et al., 2010; Song et al., 2008). To determine whether morin can protect neural cells against Aβ-induced death, a cell viability test was performed in human neuroblastoma SH-SY5Y cells. Cells were seeded and cultured for 24 hours; the cells were then pretreated with different concentrations of morin for 6 hours and then incubated with 10 μM Aβ1–42 in 1% FBS medium for 24 hours. We also examined the protective effect of the GSK3β inhibitor LiCl (100 μM) against Aβ-induced neurotoxicity. MTT analysis showed that Aβ significantly decreased cell viability, and both morin and LiCl effectively reduced Aβ-induced cell loss (Fig. 3A). Aβ-induced cell death was also assessed by staining the cells with the DNA-binding dye Hoechst 33342 and the cell-impermeant dye PI. Cells were pretreated with morin (1 μM and 10 μM) for 6 hours, followed by treatment with Aβ for 24 hours. Aβ treatment resulted in an increase in the number of PI-stained cells, suggesting that Aβ caused cell death. There were significantly fewer PI-stained cells following Aβ treatment in cells pretreated with 1 μM or 10 μM morin compared to cells not pretreated with morin (Fig. 3B and C).
Fig. 3.
Protective effects of morin against Aβ-induced neurotoxicity and tau hyperphosphorylation in human neuroblastoma cells.
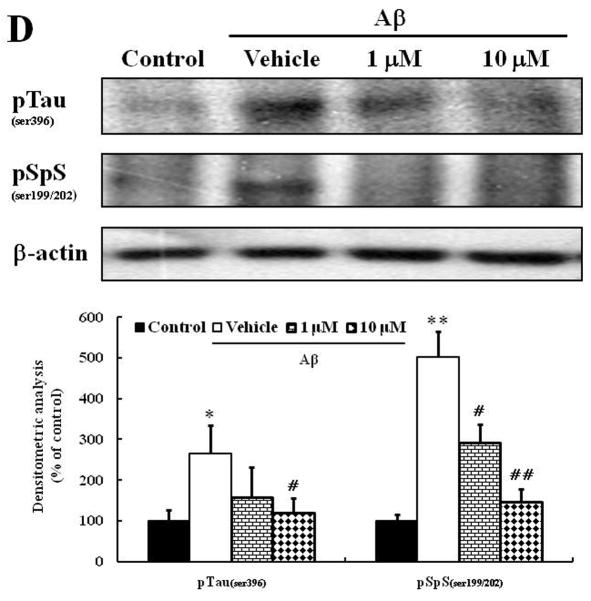
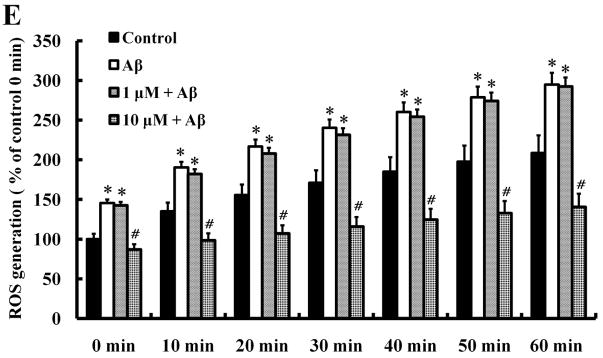
We investigated whether morin prevents Aβ-induced cell death and tau hyperphosphorylation in SH-SY5Y human neuroblastoma cells. (A) Cells were pretreated with morin (1 μM or 10 μM) and LiCl (100 μM) for 6 hours, followed by incubation with 10 μM Aβ for 24 hours. Cell viability was determined using the MTT assay. Pretreatment with morin and LiCl significantly increased cell viability in Aβ-treated cells. (B) We confirmed that morin protected against Aβ-induced cell death using Hoechst 33342 and PI staining. (C) Quantitative analysis of the number of PI-stained SH-SY5Y cells. The values are reported as the mean ± S.E.M (n=4). *p<0.01 compared to control. #p<0.01 compared to Aβ-treated culture. Scale bar = 100 μm. (D) Whole cell extracts from cells pretreated with morin for 6 hours, followed by treatment with 10 μM Aβ for 24 hours, were subjected to immunoblot analysis using antibodies against phosphorylated tau protein (pTauser396, pSpSser199/202). Level of β-actin was used as protein loading control. The blots were quantified by the densitometric analysis. (E) Total ROS were evaluated by DCF-DA method. Exposure to Aβ (10 μM) elevated ROS levels in the cells and morin treatment resulted in a significant attenuation of Aβ-induced ROS production. Values are the mean ± S.E.M (n=8). * p<0.01 compared to control. # p<0.01 compared to Aβ-treated culture (ANOVA with Fisher’s PLSD procedure).
It has been reported that Aβ1–42 induces tau phosphorylation in human neuroblastoma cells and rat hippocampal neurons (Busciglio et al., 1995; Ferrari et al., 2003; Sun et al., 2008; Zhang et al., 2009). Therefore, in order to evaluate whether the neuroprotective effect of morin is related with tau-phosphorylation, we examined the levels of phospho-tau protein in cultured human neuroblastoma cells treated with Aβ. We considered using primary rodent hippocampal neurons, but they did not exhibit nearly as robust tau hyperphosphorylation compared to the human neuroblastoma cells, presumably because the tau sequence is different in rodents. Aβ significantly increased phosphorylation levels of tau protein, and morin effectively inhibited tau hyperphosphorylation, supporting the neuroprotective action of morin (Fig. 3D).
Most flavonoids have an antioxidant activity (Hurst et al., 2009; Kim et al., 2005; Kim et al.,; Lambert and Elias 2010). As expected, Aβ significantly increased ROS production in a time-dependent manner. Interestingly, 1 μM morin was not able to prevent Aβ-induced ROS while 10 μM morin effectively blocked the ROS generation (Fig. 3E). These results suggest that the neuroprotective action of the low concentration morin (1 μM) may be mediated probably by suppression of tau hyperphosphorylation, rather than by a direct antioxidant action of morin. LiCl exhibited no antioxidant activity in our DCF assay (data not shown).
Morin treatment decreases tau hyperphosphorylation and HT-7 in the hippocampus of 3×Tg-AD mice
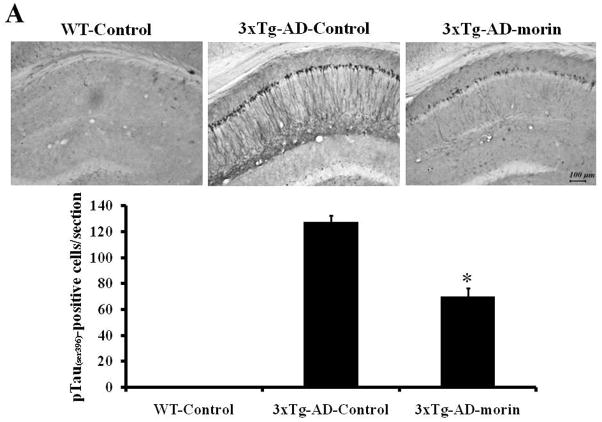
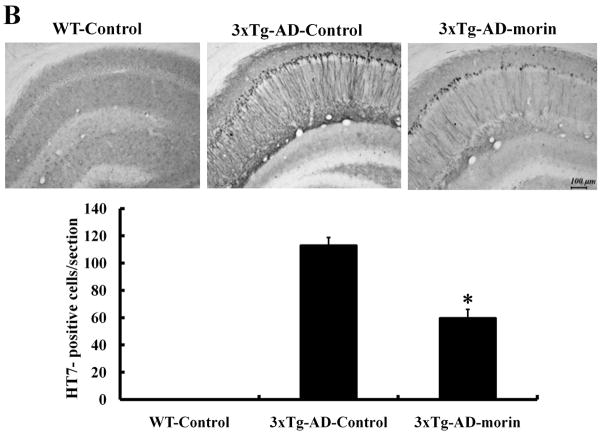
Previous studies have shown that 3×Tg-AD mice develop both plaque and tangle pathologies as they age (Oddo et al., 2003b). To verify the effect of morin on the tau hyperphosphorylation in vivo, we administered morin (10 mg/kg, i.p. daily for 7 days) to 8-month-old 3×Tg-AD mice. Immunostaining of brain sections revealed numerous phospho-tau (ser396, PHF13)-stained CA1 neurons in vehicle-treated 3×Tg-AD mice. In contrast, there was a marked reduction in phospho-tau immunoreactivity in CA1 neurons of morin-treated 3×Tg-AD mice (Fig. 4A). We also observed sparse phospho-tau-stained cells in the cortex and amygdala, and morin decreased levels of phospho-tau in these brain regions (data not shown). We also immunostained brain sections with antibody HT7, which recognizes all human tau (Kobayashi et al., 2003). HT7 immunostaining was not observed in wild-type mice, and the majority of immunostained cells were confined to the CA1 region of hippocampus in 3×Tg-AD mice. HT7 staining was significantly decreased in 3×Tg-AD mice treated with morin compared to that in mice treated with vehicle (Fig. 4B).
Fig. 4.
Levels of tau hyperphosphorylation and HT-7 immunoreactivity were alleviated by morin treatment in the hippocampus of 3×Tg-AD mice.
(A and B) Accumulation of tau phosphorylation and HT-7 in hippocampal neurons of vehicle- and morin-treated 3×Tg-AD mice were evaluated by immunohistochemistry using antibodies pTauser396 (A) and HT7 (B). Scale bar = 100 μm. Phospho-tau- and HT7-stained neurons were observed in CA1 region of 3×Tg-AD mice. However, morin treatment decreased the numbers of phospho-tau- and HT7-stained CA1 neurons. No staining was detected in WT mice. Value are the mean ± S.E.M (n=4). * p<0.01 compared to 3×Tg-AD-Control (ANOVA with Fisher’s PLSD procedure).
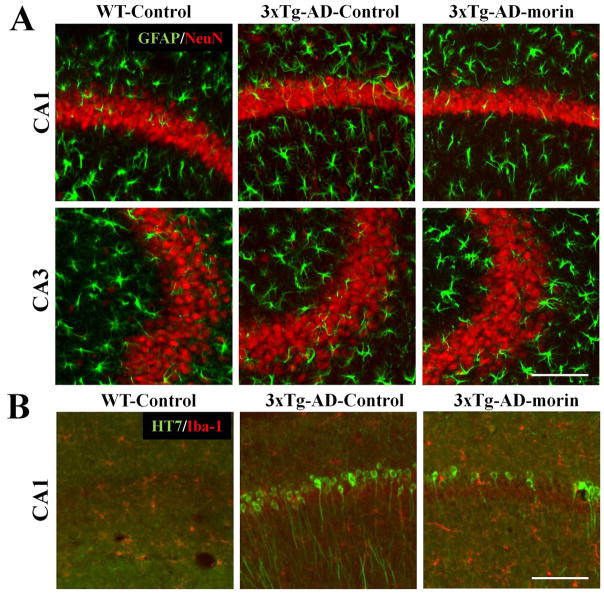
Tau hyperphosphorylation occurs early and prior to neuronal damage and glial reactivity in 3×Tg-AD
In order to evaluate neuronal loss and glial cell activation in 3×Tg-AD mice, double-label immunohistochemistry was performed using antibodies against the neuronal marker NeuN in combination with the astrocyte marker GFAP or the marker of activated microglia Iba-1. There was no significant difference in NeuN, GFAP or Iba1 staining in the hippocampus of 3×Tg-AD mice compared to wild-type mice (Fig. 5A). However, the modulatory effect of morin on tau hyperphosphorylation was reconfirmed (Fig. 5B). In addition, Nissl staining revealed no apparent differences in the neuronal density or shape of the hippocampus and cortex in 3×Tg-AD mice compared to wild-type mice (data not shown). These data suggest that, while there is tau hyperphosphorylation in 8-month-old 3×Tg-AD mice, there is no apparent glial hyperactivation or neuronal loss associated with the tau pathology. Nonetheless, we clearly observed tau hyperphosphorylation in the hippocampus and morin effectively reduced the tau hyperphosphorylation.
Fig. 5.
Early tau hyperphosphorylation is not associated with neuronal loss or glial hyperreactivity in 3×Tg-AD mice.
(A) To evaluate the neuronal loss/damage in 3×Tg-AD mice, double-label immunohistochemistry was performed with primary antibodies against a post-mitotic neuron marker NeuN (red) and the astrocyte marker GFAP (green). There was no sign of neuronal death and/or loss, or astroglial activation in the hippocampus. (B) Furthermore, microglia Iba-1 staining (red) showed no neuroinflammatory response in the hippocampus of 8 month-old 3×Tg-AD mice. However, we were able to confirm that morin significantly decreased HT7-stained CA1 neurons (green).
Discussion
Because AD is characterized by the abnormal accumulation of Aβ plaques, most studies have targeted β-secretase (BACE) and γ-secretase to modulate sequential proteolytic cleavage of APP so as to reduce Aβ production (Arbel et al., 2005; Evin and Kenche, 2007; Jo et al., 2010; Nunan and Small, 2000; Vassar et al., 1999). Unfortunately, in a recent major clinical trial, a γ-secretase inhibitor exacerbated the cognitive decline in AD patients (Extance), likely because of off-target actions of γ-secretase in the Notch signaling pathway (Wang et al., 2004). In the present study, we focused on a tau-based AD therapeutic strategy by targeting tau phosphorylation by GSK3β, a kinase previously reported to phosphorylate tau on the same amino acids that are hyperphosphorylated in tauopathies (Avila et al., 2010; Hooper et al., 2008)
We developed a novel ELISA system for GSK3β activity and used it to identify morin as a chemical inhibitor of GSK3β that blocks GSK3β-mediated tau hyperphosphorylation and decreases Aβ-induced cell death and tau phosphorylation. Morin is a naturally occurring flavonoid that occurs in several different plants including old fustic (Macluratinctoria), Osage orange (Maclurapomifera), common guava (Psidiumguajava), onion and apple. Morin exhibits various biological actions in non-neuronal cells including antioxidant, anti-inflammatory and anti-cancer effects and cytoprotection (Subash and Subramanian, 2008; Subash and Subramanian, 2009). Interestingly, it was reported that polyphenols including morin have potent anti-amyloidogenic effects by inhibiting the formation of Aβ fibrils (Ono et al., 2003). Moreover, some natural flavonoids can inhibit BACE to reduce the levels of secreted Aβ in primary neurons (Shimmyo et al., 2008). In contrast to morin (2′,3,4′,5,7-pentahydroxyflavone), the structurally related flavonoid quercetin (3,3′,4′,5,7-pentahydroxyflavone) had no effect on GSK3β activity and tau hyperphosphorylation.
Morin protected neurons against tau hyperphosphorylation induced by Aβ. The mechanism(s) by which Aβ promotes tau hyperphosphorylation are not understood fully, but are believed to involve oxidative stress (Mattson et al., 1997), perturbed cellular Ca2+ homeostasis (Mattson, 2004), and the activation of kinases and/or inhibition of phosphatases (Alvarez et al., 2002; Esposito et al., 2006; Stoothoff and Johnson, 2005). We found that morin prevented Aβ-induced tau hyperphosphorylation in human neuroblastoma cells. Because previous studies have shown that other flavonoids (epicatechin and Purple sweet potato anthocyanins) can suppress Aβ-induced oxidative stress in cultured cells (Kim et al., 2005; Martin et al., 2010; Ye et al., 2010), it is possible that reduced oxidative stress may contribute to the neuroprotective action of morin. However, we found that 1 μM morin did not prevent Aβ-induced oxidative stress but did inhibit Aβ-induced tau hyperphosphorylation and cell death. The latter result is consistent with a key role for the inhibition of GSK3β in the neuroprotective mechanism of action of morin. In addition, we found that 100–500 μM morin, which were necessary to efficiently block the exogenously added GSK3β-induced tau hyperphosphorylation in hippocampal homogenates, were cytotoxic to human neuroblastoma cells (data not shown). However, in the experiments of cell viability and generation of ROS, lower concentration of morin (1–10 μM) and LiCl (100 μM) were sufficient to inhibit endogenous GSK3β without affecting cell viability.
We found that treatment with morin ameliorated tau hyperphosphorylation in 3×Tg-AD mice, an animal model of AD. Morin-treated 3×Tg-AD mice exhibited significantly fewer phospho-tau immunoreactive neurons compared to vehicle-treated 3×Tg-AD mice. The 3×Tg-AD mice express mutant forms of tau and APP that cause early-onset FTD and AD, respectively. The tau hyperphosphorylation in hippocampal CA1 neurons and some cortical neurons occurs relatively early in the disease process in the 3×Tg-AD mice, concomitant with early accumulation of soluble Aβ oligomers and prior to cognitive impairment (Oddo et al., 2003a; Oddo et al., 2003b). It is possible that GSK3β acts both upstream and downstream of Aβ production/aggregation to increase tau hyperphosphorylation. We observed neuron-associated Aβ immunoreactivity in the hippocampus of 3×Tg-AD mice that was lessened in morin-treated 3×Tg-AD mice, suggesting that morin inhibits Aβ production and/or enhances its clearance (Supplemental Fig. 3). This is consistent with previous studies in which GSK3β inhibitors reduced Aβ production (Phiel et al., 2003; Sereno et al., 2009). On the other hand, perturbed cellular Ca2+ homeostasis occurs early in the disease process in 3×Tg-AD mice because of a pathogenic effect of mutant presenilin-1 that results in excessive elevation of intracellular Ca2+ levels (Goussakov et al., 2010; Guo et al., 1999). Data suggest that, by activating calcineurin, Ca2+ can activate GSK3β (Kim et al., 2009), consistent with a role for Ca2+ in GSK3β-mediated tau hyperphosphorylation in 3×Tg-AD- mice (Elliott et al., 1993; Mattson, 1990).
Collectively, our findings suggest that morin is an effective inhibitor of GSK3β-mediated tau hyperphosphorylation in cell culture and in vivo in a mouse model of AD. Further medicinal chemistry and preclinical development will be required to establish the molecular mechanism of the interaction of morin with GSK3β, and to determine whether this and related flavonoids ameliorate the cognitive deficits in animal models of AD and other tauopathies.
Supplementary Material
Acknowledgments
This research was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A084225). This work was also supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 20090083538).
Abbreviations used
- AD
Alzheimer’s disease
- GSK3β
Glycogen synthase kinase 3β
- Aβ
β-amyloid
- MTT
3-[4, 5-dimethylthiazol-2-yl]-2, 5diphenyl-tetrazolium bromide
- PI
Propidium iodide
- ELISA
Enzyme-linked immunosorbent assay
- LiCl
Lithium chloride
References
- Alonso A, et al. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–8. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AC, et al. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–7. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso AC, et al. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:5562–6. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez G, et al. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002;4:153–65. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- Arbel M, et al. Inhibition of amyloid precursor protein processing by beta-secretase through site-directed antibodies. Proc Natl Acad Sci U S A. 2005;102:7718–23. doi: 10.1073/pnas.0502427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, et al. Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother. 10:703–10. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- Blalock EM, et al. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–8. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, et al. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–88. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Cegielska A, et al. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J Biol Chem. 1998;273:1357–64. doi: 10.1074/jbc.273.3.1357. [DOI] [PubMed] [Google Scholar]
- Clodfelder-Miller BJ, et al. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–5. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, et al. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–53. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Dajani R, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–32. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Desai MK, et al. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57:54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dournaud P, et al. Differential correlation between neurochemical deficits, neuropathology, and cognitive status in Alzheimer’s disease. Neurobiol Aging. 1995;16:817–23. doi: 10.1016/0197-4580(95)00086-t. [DOI] [PubMed] [Google Scholar]
- Elliott EM, et al. Corticosterone exacerbates kainate-induced alterations in hippocampal tau immunoreactivity and spectrin proteolysis in vivo. J Neurochem. 1993;61:57–67. doi: 10.1111/j.1471-4159.1993.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Esposito G, et al. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006;84:253–8. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Evin G, Kenche VB. BACE inhibitors as potential therapeutics for Alzheimer’s disease. Recent Pat CNS Drug Discov. 2007;2:188–99. doi: 10.2174/157488907782411783. [DOI] [PubMed] [Google Scholar]
- Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 9:749–51. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- Ferrari A, et al. beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J Biol Chem. 2003;278:40162–8. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Goussakov I, et al. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci. 30:12128–37. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, et al. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem. 1999;72:1019–29. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem. 1999;274:21395–401. doi: 10.1074/jbc.274.30.21395. [DOI] [PubMed] [Google Scholar]
- Hoi CP, et al. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother Res. 24:1538–42. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- Hooper C, et al. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–9. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst WJ, et al. Stability of cocoa antioxidants and flavan-3-ols over time. J Agric Food Chem. 2009;57:9547–50. doi: 10.1021/jf901457s. [DOI] [PubMed] [Google Scholar]
- Hye A, et al. Glycogen synthase kinase-3 is increased in white cells early in Alzheimer’s disease. Neurosci Lett. 2005;373:1–4. doi: 10.1016/j.neulet.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12:38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DG, et al. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer’s disease. Neurobiol Aging. 31:917–25. doi: 10.1016/j.neurobiolaging.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, et al. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci. 2004;29:427–41. [PMC free article] [PubMed] [Google Scholar]
- Kim H, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–41. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- Kim MK, et al. EGCG and quercetin protected INS-1 cells in oxidative stress via different mechanisms. Front Biosci (Elite Ed) 2:810–7. doi: 10.2741/e142. [DOI] [PubMed] [Google Scholar]
- Kim Y, et al. Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J Neurochem. 2009;111:344–54. doi: 10.1111/j.1471-4159.2009.06318.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, et al. Association of phosphorylation site of tau protein with neuronal apoptosis in Alzheimer’s disease. J Neurol Sci. 2003;208:17–24. doi: 10.1016/s0022-510x(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, et al. Melatonin Potentiates the Neuroprotective Properties of Resveratrol Against Beta-Amyloid-Induced Neurodegeneration by Modulating AMP-Activated Protein Kinase Pathways. J Clin Neurol. 6:127–37. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S49–57. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Li F, et al. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288:E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, et al. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. Embo J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk C, et al. Development of a sensitive ELISA for quantification of three- and four-repeat tau isoforms in tauopathies. J Neurosci Methods. 2009;180:34–42. doi: 10.1016/j.jneumeth.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Martin MA, et al. Protection of human HepG2 cells against oxidative stress by the flavonoid epicatechin. Phytother Res. 24:503–9. doi: 10.1002/ptr.2961. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–17. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, et al. Estrogens stabilize mitochondrial function and protect neural cells against the pro-apoptotic action of mutant presenilin-1. Neuroreport. 1997;8:3817–21. doi: 10.1097/00001756-199712010-00031. [DOI] [PubMed] [Google Scholar]
- Melov S, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, et al. Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: correlations using three sets of neuropathological criteria. Dementia. 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- Noble W, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–5. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- Oddo S, et al. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003a;24:1063–70. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ono K, et al. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87:172–81. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Pei JJ, et al. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–8. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, et al. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–9. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Pittman AM, et al. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15(Spec No 2):R188–95. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- Sereno L, et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–67. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Shimmyo Y, et al. Flavonols and flavones as BACE-1 inhibitors: structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim Biophys Acta. 2008;1780:819–25. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Song MS, et al. Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci. 2008;28:1989–2002. doi: 10.1111/j.1460-9568.2008.06498.x. [DOI] [PubMed] [Google Scholar]
- Soutar MP, et al. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. J Neurochem. 115:974–83. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- Steinberg JG, et al. Depressed fatigue-induced oxidative stress in chronic hypoxemic humans and rats. Respir Physiol Neurobiol. 2004;141:179–89. doi: 10.1016/j.resp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–97. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Subash S, Subramanian P. Effect of morin on the levels of circulatory liver markers and redox status in experimental chronic hyperammonaemic rats. Singapore Med J. 2008;49:650–5. [PubMed] [Google Scholar]
- Subash S, Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem. 2009;327:153–61. doi: 10.1007/s11010-009-0053-1. [DOI] [PubMed] [Google Scholar]
- Sun ZK, et al. Protective effects of erythropoietin on tau phosphorylation induced by beta-amyloid. J Neurosci Res. 2008;86:3018–27. doi: 10.1002/jnr.21745. [DOI] [PubMed] [Google Scholar]
- van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007;17:63–73. doi: 10.1111/j.1750-3639.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Presenilins and gamma-secretase inhibitors affect intracellular trafficking and cell surface localization of the gamma-secretase complex components. J Biol Chem. 2004;279:40560–6. doi: 10.1074/jbc.M404345200. [DOI] [PubMed] [Google Scholar]
- Wrede CE, et al. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277:49676–84. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- Yancopoulou D, Spillantini MG. Tau protein in familial and sporadic diseases. Neuromolecular Med. 2003;4:37–48. doi: 10.1385/NMM:4:1-2:37. [DOI] [PubMed] [Google Scholar]
- Ye J, et al. Effect of purple sweet potato anthocyanins on beta-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem Res. 35:357–65. doi: 10.1007/s11064-009-0063-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, et al. Indirubin-3′-monoxime inhibits beta-amyloid-induced neurotoxicity in neuroblastoma SH-SY5Y cells. Neurosci Lett. 2009;450:142–6. doi: 10.1016/j.neulet.2008.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.