Abstract
Objective
Type IIB procollagen is characteristic of cartilage, comprising 50% of the extracellular matrix. The NH2-propeptide of type IIB collagen, PIIBNP, can kill tumor cells via binding to integrins αVβ3 and αVβ5. As osteoclasts rely on αVβ3 integrins for function in bone erosion, we sought to determine whether PIIBNP could inhibit osteoclast function.
Methods
We undertook in vitro and in vivo experiments to evaluate both osteoblast and osteoclast function in the presence of recombinant PIIBNP. Adhesion of osteoclasts to PIIBNP was analyzed by staining of attached cells with crystal violet. PIIBNP-induced cell death was evaluated by cell counting Trypan Blue stained cells. The mechanism of cell death was evaluated by DNA fragmentation, TUNEL staining and western blotting to detect cleaved caspases. To determine the role of αVβ3 integrin, osteoclasts were pretreated with αV or β3 integrin specific siRNA before the treatment with PIIBNP. To explore PIIBNP function in vivo, a lipopolysaccharide-induced mouse calvaria lysis model was employed.
Results
Osteoclasts adhered to PIIBNP via an RGD-mediated mechanism. When osteoclasts were plated on extracellular matrix proteins, PIIBNP induced apoptosis of osteoclasts via caspase 3/8 activation. Osteoblasts and macrophages were not killed. Reduction of αV or β3 integrin levels on osteoclasts by siRNA reduced cell death in a dose-dependent manner. In vivo, PIIBNP could inhibit bone resorption.
Conclusion
We conclude that PIIBNP can inhibit osteoclast survival and bone resorption via signal transduction through the αVβ3 integrins. Because of this property and the cell specificity, we propose that PIIBNP may play a role in vivo in protecting cartilage from osteoclast invasion and also could be a new therapeutic strategy for decreasing bone loss.
Keywords: Osteoclast, Integrin, Type IIB collagen NH2-propeptide, Bone resorption, Apoptosis
Introduction
200 million people worldwide suffer from diseases that result in bone loss (1). Osteoporosis causes low bone mass and increases bone fragility that leads to osteoporotic fracture, largely a problem of elderly women (2). Bone loss is due to the action of osteoclasts, cells of hematopoietic origin with the unique capacity to resorb bone (3). Osteoclasts play a major role in several common skeletal diseases including osteoporosis (4), inflammatory osteolysis (5) and bone destruction due to bone metastasis (6). In all these circumstances, increased number and activity of osteoclasts leads to an abnormally high bone resorption rate.
Integrins are heterodimeric integral membrane glycoproteins consisting of various combinations of alpha and beta subunits that are expressed on the cell surface and bind to extracellular ligands. In addition to cell/matrix attachment, integrins play the role of extracellular receptors leading to intracellular signal transduction (7, 8). The integrin αVβ3, known as the vitronectin receptor, is highly expressed on osteoclasts and plays a critical role in many aspects of osteoclast biology (9, 10, 11). Osteoclasts also express other integrins at lower levels, such as the collagen/laminin receptor α2β1 and the vitronectin/fibronectin receptor αvβ1 (12). Many integrins bind to an Arg-Gly-Asp (RGD) consensus sequence found in a variety of extra cellular matrix (ECM) proteins (13). Bone contains a variety of RGD-containing proteins including thrombospondin, fibronectin, vitronectin, osteopontin and bone sialoprotein (14, 15, 16, 17, 18) and the interaction of osteoclast integrins, particularly αVβ3, with the RGD domain in bone matrix proteins plays an important role in bone resorption (19, 20).
In cartilage and the cartilaginous growth plate, type IIB collagen is the most abundant cartilage protein representing 50% of the protein and 80–95% of the total collagen content (21). Unique to this collagen, are the presence of adjacent RGD sequences encoded in exon 6 of the NH2-propeptide of the COL2A1 gene (22). Like other fibril-forming collagens, types I, III, V and XI, type IIB collagen is synthesized as a procollagen form (23) that is secreted from the cell into the extracellular matrix where extension propeptides (NH2-propeptides and COOH-propeptides) are removed by specific proteinases before the mature molecules are incorporated into fibrils in matrix (23). The N-propeptide of type IIB procollagen is removed from each collagen molecule by the N-proteinase, ADAMTS-3(24). Recently, we reported that PIIBNP binds to cells via αVβ3 and αVβ5 integrins and kills chondrosarcoma and other tumor cells and retards tumor growth in vivo (25). In other preliminary studies we have shown that it also inhibits angiogenesis (26) and the migration and survival of a breast cancer cell line (27). The expression of αVβ3 is limited to a small number of cell types such as endothelial cells, synovial cells, osteoclasts and some tumors (28, 29). As osteoclasts function via attachment to αVβ3, we speculated that PIIBNP might play a role in osteoclast survival and function. In this study, we analyzed the function of PIIBNP in bone cells and show that PIIBNP inhibits osteoclast, but not osteoblast or macrophage function and survival.
Materials and Methods
Cells
Mouse monocytic RAW 264.7 cells and mouse osteoblastic MC3T3-E1 cell lines were purchased from American Type Culture Collection. The cells were maintained in alpha-minimum essential medium (α-MEM) (Thermo Fisher Scientific, Pittsburgh, PA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and 100 U/ml of penicillin–streptomycin at 37° C in a humidified atmosphere of 5% CO2.
Primary bone marrow macrophages were prepared as previously described (28). Primary osteoblasts were isolated from 1-day-old newborn C57BL/6J mouse calvaria after five routine sequential digestions with 0.1% collagenase (Sigma-Aldrich, St.Louis, MO). Osteoblasts released in digestions 3-5 were pooled and cultured in α-MEM supplemented with 10% FBS at 37° C in a humidified atmosphere of 5% CO2.
Differentiation of osteoclasts
RAW 264.7 cell line
1.5 × 106 RAW 264.7 macrophage cells were cultured with 100 ng/ml of receptor activator of nuclear factor kappa-B ligand (RANKL) in 10% FBS (Pepro Tech, Rocky hill, NJ) in 10 cm tissue culture dishes for 4 days to allow differentiation into osteoclasts. After 4 days culture, differentiated osteoclasts (RAW-osteoclasts) were used for various experiments with continued culture in the presence of 100 ng/ml of RANKL and 10% FBS. Apoptosis experiments were done in the absence of serum.
Primary cells
1.5 × 106 bone marrow macrophages were prepared from bone marrow as described (30), and cultured in 10 cm culture dishes for 5 days with 100 ng/ml of RANKL and 20 ng/ml of macrophage colony stimulating factor (M-CSF) (Cell Science tech, Canton, MA) for differentiation into osteoclasts.
After differentiation of RAW or primary osteoclasts, RNA was isolated for RT-PCR or cell lysates were prepared for western blotting. The expression of β3 integrins and TRAP staining (Sigma-Aldrich) were performed to confirm differentiation and function of osteoclasts.
RT-PCR analysis
Total RNA was purified from cells cultured using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNAs were synthesized from 1 μg of total RNA using the SuperScript First-Strand Synthesis System (Invitrogen) in a volume of 20 μl. One microliter of these cDNAs was then amplified with primers specific for αV integrins (forward, 5′-ATTGGGAGTAC AAGGAAACC-3′; reverse, 5′-CCGCTTGGTGATGAGATGATT -3′), and β3 integrin (forward, 5′-ACTGCAACTGT ACTACACGCAC-3′; reverse, 5′-AGTAGCTTCCAGATGA GCAGAG-3′). After 30 cycles of 94° C (30 seconds), 60° C (30 seconds), and 72° C (30 seconds), 10 μl of PCR products were separated in a 1.5% agarose gel containing 0.5 μg/ml ethidium bromide. Real-time PCR was performed on an ABI Prism 7500 Sequence Detection system using Sybr Green PCR Master mix (Applied Biosystems) and following the manufacturer's protocols. Primer sequences used in this study were the same as described above described.
Preparation of cell lysates
Cells were washed 3 times with PBS and lysed in hypotonic lysis buffer (25 mM Tris, 1% NP-40, 150 mM NaCl, 1.5 mM EGTA) supplemented with protease inhibitors (Roche Diagnostics, Indianapolis, IN) on ice for 30 minutes (min) (31). The lysates were centrifuged at 15,000 rpm for 20 min to remove cellular debris and the supernatants were collected. Proteins were quantified by the Bradford method with protein assay reagent (BioRad, Hercules, CA), and diluted to an equal concentration with hypotonic buffer.
Western blotting
Each sample of cell lysate was mixed with 6× electrophoresis sample buffer (BioRad) and electrophoresed in a 4%-15% polyacrylamide gradient gel (BioRad) and transblotted onto PVDF membrane (Invitrogen). The membranes were blocked in 5% milk/PBS -0.1% Tween 20 for 1 hour (h) and incubated with primary antibodies at 4°C overnight followed by reaction with secondary antibodies coupled to horseradish peroxidase
The primary antibodies used were rabbit anti-αV integrin polyclonal antibody (Santa Cruz Biotechnology, Santacruz, CA), rabbit anti-β3 integrin polyclonal antibody (Santa Cruz Biotechnology), rabbit anti-active caspase 3 polyclonal Ab (Sigma Aldrich), rabbit anti-caspase 8 polyclonal antibody (Cell Signalling Technology, Danvers, MA), mouse anti-caspase 9 monoclonal antibody (Cell Signalling Technology). Horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG Ab (GE Healthcare, Picataway, NJ) or HRP conjugated rabbit anti-mouse IgG antibody (GE Healthcare) was used as a secondary antibody, and the signals were visualized by ECL plus reagent (GE Healthcare). The expression of proteins was determined by semi-quantification of digitally captured image using the Image J software. Actin was used as a control to estimate protein loading on the gel.
Site-directed mutagenesis
Recombinant fusion protein GST- exons 3-8 (PIIBNP), mutant GST-exons 3-8 (mPIIBNP), RGDRGD to RADRGD, have been described previously (25) with one exception. That is, in this study, we used RGDRGD mutated to RADRGD instead of mutating both RGD's as we found that the first RGD was the active triplet (Z. Wang, J. Bryan, L. Sandell, unpublished data). Site-directed mutagenesis was performed using Quick Change Kit (Stratagene, Santacruz, CA) to mutate the –RGDRGD- sequence to –RADRGD- (mPIIBNP) in PIIBNP. The mutated plasmid was confirmed by DNA sequence and transformed into BL21 (DE3) host strain to express mutated PIIBNP as described by (32). GST fusion proteins were prepared and isolated as described (25). Each batch of recombinant fusion protein was tested for purity and function.
Adhesion assay
96 well EIA/RIA plates were incubated with 100 μl/well of various concentrations of GST-NH2-propeptides or 10 μg/ml of bovine type I collagen (Sigma-Aldrich), human fibronectin (Promega, Madison, WI) or rat vitronectin (Sigma-Aldrich) overnight at 4° C. The plates were incubated with 0.5% BSA for 1.5 h at 4°C. Cells were lifted by scraping and resuspended in 2 × 104 cells in 100 μl of serum free α-MEM, and replated on various substrates for 1.5 h at 37° C. In some cases, GST, GST-PIIBNP, or RGD (GRGDNP), RAD (GRADNP) peptides (Calbiochem, Darmstadt, Germany) were added to cells prior to incubation. Attached cells were stained with 100 μl of 0.5% (w/v) crystal violet/25% (v/v) methanol. After gentle washing, the incorporated dye was dissolved in 0.1 M sodium citrate/50% (v/v) ethanol and measured by spectrophotometry (540 nm).
Assessment of cell death
5 × 104 cells were replated in 24-well plates coated with 10 μg/ml of type I collagen, fibronectin, or vitronectin. After plating cells for 3 h, RAW 264.7 macrophages, RAW-osteoclasts and MC3T3-E1 osteoblasts were treated with GST, GST-PIIBNP or GST-mutant PIIBNP for 16 h without serum. Primary bone marrow macrophages, osteoclasts and osteoblasts were treated for 24 h without serum. The number of dead cells was measured by counting trypan blue staining cells in the medium and on the plate separately. The number of cells detached was measured by counting cells separately in the medium and in the wells.
TUNEL staining
5× 103 RAW 264.7 osteoclasts were cultured in 8-well chamber slides (Nunc Roskilde, Denmark) coated with 10 μg/ml of type I collagen. After plating cells for 3 h, the cells were treated with GST or GST-PIIBNP for 16 h without serum. The cells were fixed with 4% neutral buffered formalin for 10 min and apoptotic cells were determined using TUNEL assay kit (Wako, Japan) according to the manufacturer's protocol.
DNA laddering
After treatment with GST or PIIBNP, 3.0 × 106 cells were resuspended in Hank's balanced salt solution and fixed in 70% ethanol at -20°C. The DNA was extracted in phosphate-citrate buffer for 3 h, treated with NP-40 and RNase for 30m, and added proteinase K for an additional 30 min. The DNA was electrophoresed on 1.5 % agarose gel containing 0.5 μg/ml ethidium bromide.
Knock down of αv and β3 integrin in osteoclasts by siRNA
αv and β3 integrin siRNA and non-specific random siRNA control (Santa Cruz Biotechnology) were transfected into RAW-osteoclasts using HiPerFect Transfection Reagent (Qiagen) according to the manufacturer's protocol with minor modifications. Briefly, 50, 100 or 150 nM siRNA was formulated with liposomes, diluted in 150 μl of serum free α-MEM and applied to 5×104 cells. After incubation for 6 h, the mixture was replaced with α-MEM supplemented with 10% FBS and 100 ng/ml of RANKL, and incubated for 24 before mRNA extraction. Prior to protein treatment, 5 × 104 cells were replated in 24 well plates coated with the extracellular matrices with serum free α-MEM after 24 h of siRNA transfection. After the replacement for 3 h, the cells were treated by 3 μM of GST and GST-PIIBNP for 16 h.
In vivo LPS injection
Eight week old C57BL/6J mice (n=12) were used in this experiment. PBS, 500 μg of lipopolysaccharide (LPS) (Sigma-Aldrich), LPS + 10 nmol of GST, or LPS + 10 nmol of PIIBNP were injected (total volume 50 μl) to the calvaria under anesthesia. Five days after injection, the mice were sacrificed in a CO2 chamber. The entire calvarial bone was dissected and fixed in 10% formalin for 24 h, decalcified by 14% EDTA for 7 days, and embedded in paraffin. TRAP staining was performed as described previously (33). The osteoclast index, which represents the number of osteoclasts per millimeter of trabecular bone surface, was measured by Image J Software. The percentage of bone surface covered by osteoclasts was also measured. These histomorphometric parameters adhere to the recommended American Society of Bone and Mineral Research (34). The entire experiment was repeated.
Collagen Type 1 Fragment Assay
Serum was collected from mice of LPS-induced bone resorption or PBS prior to sacrifice. The level of type I collagen telopeptide fragment (CTX-1) was measured by ELISA (Immuno Diagnostic Systems and Nordic Bioscience Diagnostics). ELISA was performed as previously described (35).
Statistical analysis
All data were expressed as mean ± standard deviation (SD) unless otherwise indicated. Statistical analysis was performed using one-way (Fig 1, Fig 3(b) (d), Fig 4(a), Fig 5(a), Fig 6) or two-way (Fig2, Fig3a, Fig4b and Fig5b) analysis of variance, with Tukey's post hoc test for multiple comparsions of paired samples. Mann-Whitney U test was used for comparisons between the two groups. P values less than 0.05 were considered significant.
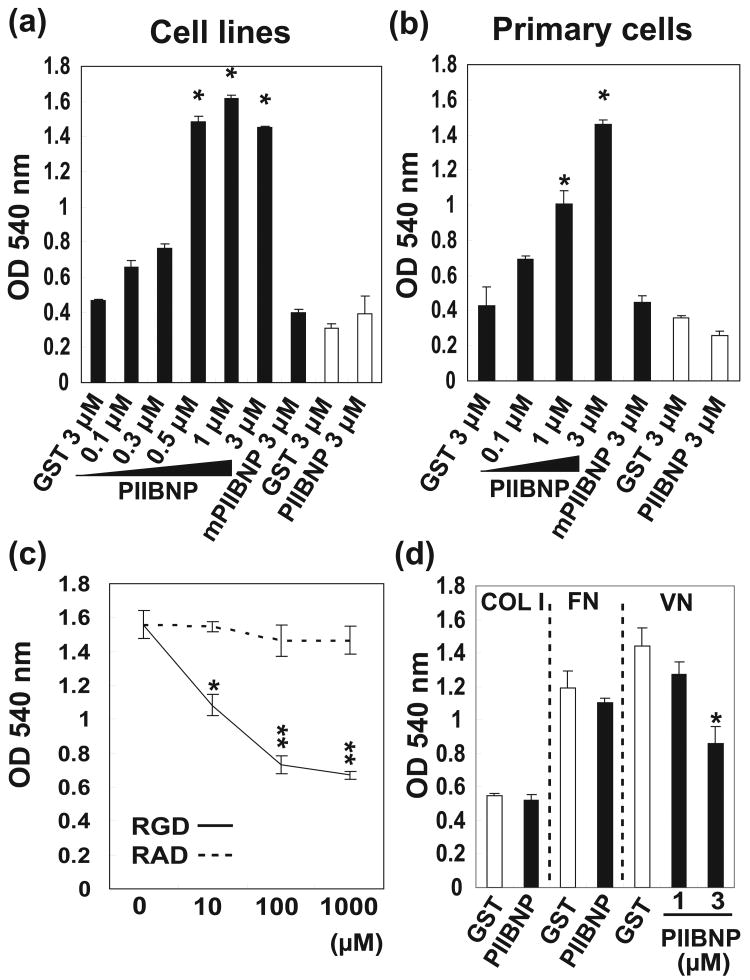
Figure 1.
(a) Adhesion of RAW-osteoclasts and MC3T3-E1 osteoblasts to GST, GST-PIIBNP and GST-mutant PIIBNP. *indicates p<0.05 versus cells treated with 3 μM GST. (b) Adhesion of primary osteoclasts and osteoblasts to GST, GST-PIIBNP and GST-mutant PIIBNP. *indicates p<0.05 versus cells treated with 3 μM GST. (c) Effect of blocking of RGD or RAD peptides on RAW-osteoclast adhesion to 0.5 μM PIIBNP. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05, and * * indicates p<0.01 versus cells treated with individual concentrations of RAD peptides.
(d) Effect of PIIBNP on osteoclast adhesion to type I collagen (COL1), fibronectin (FN) and vitronectin (VN) by PIIBNP. GST or GST-PIIBNP was added to RAW 264.7 osteoclasts before the incubation. Each assay was performed in triplicate wells and three independent experiments. Columns represent mean ± S.D. *indicates p<0.05 versus cells treated with 3 μM GST.
Figure 3.
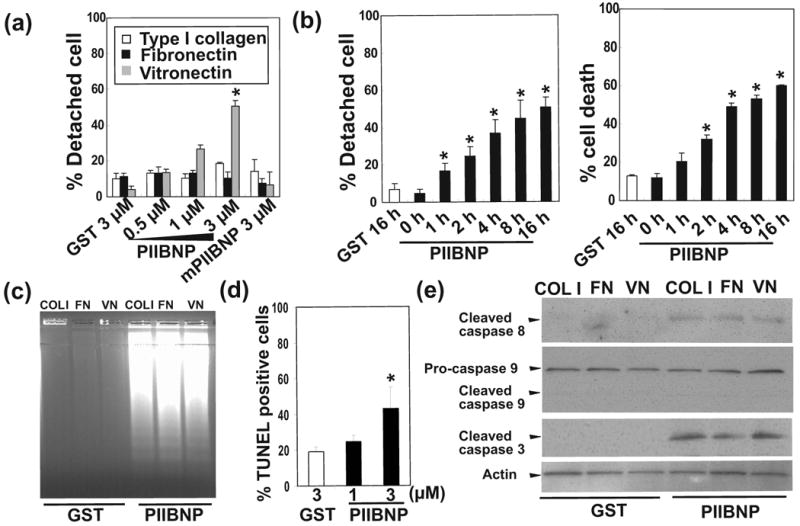
(a) Percent of detached cells of differentiated RAW osteoclasts treated for 16 h without serum on type I collagen (COL1), fibronectin (FN) and vitronectin (VN) by GST, GST-PIIBNP and GST-mutant PIIBNP (mPIIBNP). Columns represent mean ± S.D. of percent detached cells. (b) A time course experiment showing the kinetics of osteoclast detachment by PIIBNP on vitronectin (VN) coated plates. Left panel shows percent detached RAW-osteoclasts. Right panel shows percent cell death of RAW osteoclasts. Columns represent mean ± S.D. of the percent detached cells (Left panel) and percent trypan blue stained cells (Right panel). (c) DNA fragmentation in RAW-osteoclasts after 16 h of treatment with 3 μM GST and GST-PIIBNP on type I collagen (COL1), fibronectin (FN) and vitronectin (VN) coated plates. (d) Effect of GST-PIIBNP for apoptosis of osteoclasts on type I collagen. Columns represent mean ± S.D. of TUNEL positive cells, at least 300 cells were counted by a blinded observer in the same experiment. The results shown are the average of three individual samples. (e) Effect of PIIBNP on caspases 8, 9 and 3 analyzed by Western blotting. RAW-osteoclasts were treated for 16 h with 3 μM GST and GST-PIIBNP on type I collagen (COL1) fibronectin (FN) and vitronectin (VN) coated plates. The results shown represent three independent experiments. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus (a) (d) cells treated with 3 μM GST and (b) non (0h) treatment.
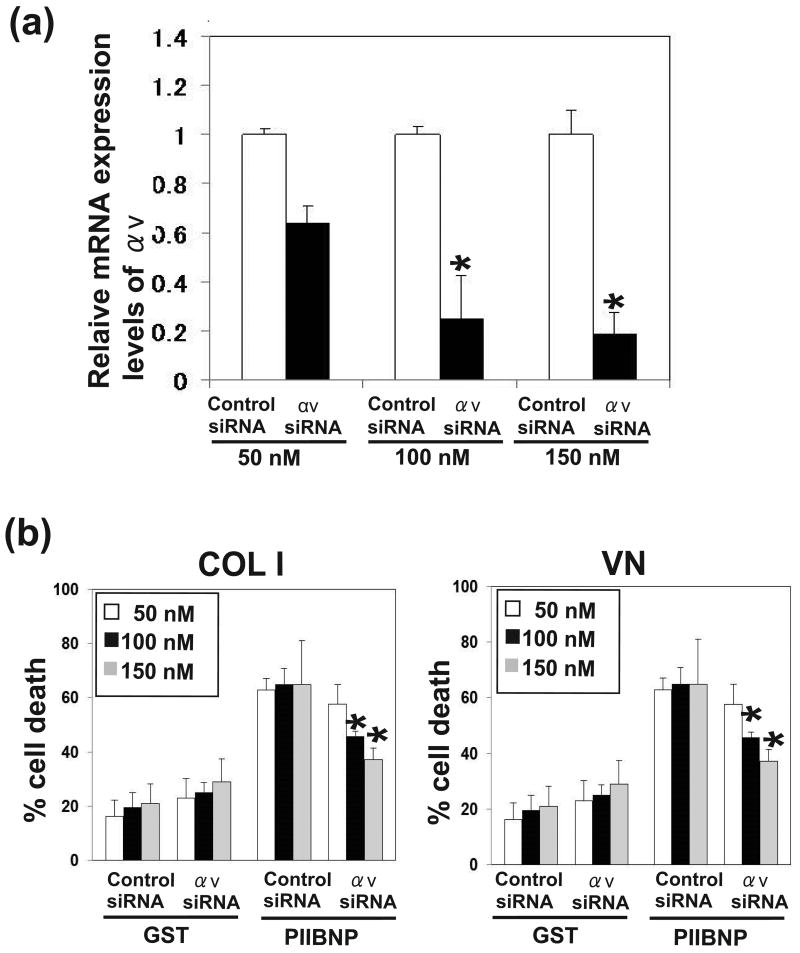
Figure 4.
The dose-dependent effect of reduction of αV integrin by siRNA on PIIBNP-induced cell death. (a) The expression levels of αV integrin mRNA were analyzed by realtime PCR. Expression ratios of αv integrin (αv siRNA/ Control siRNA) are shown. (b) The panels show percent cell death of RAW-osteoclasts without serum on type I collagen (COL 1) and vitronectin (VN) after the treatment of GST, and GST-PIIBNP for 16 h. Three concentrations of siRNA were used to reduce integrin expression. Columns represent mean ± S.D. of the percent of Trypan Blue stained cells/ total cells. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus cells treated with control siRNA.
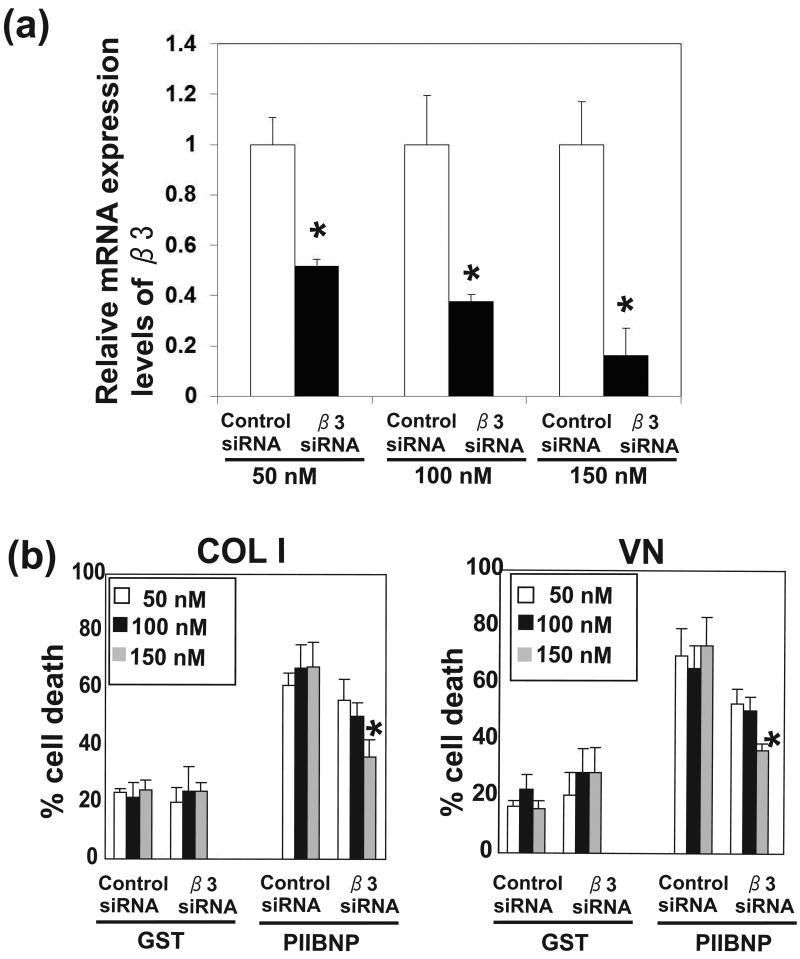
Figure 5.
The dose-dependent effect of reduction of β3 integrin by siRNA on PIIBNP-induced cell death. (a) The expression levels of β3 integrin mRNA were analyzed by realtime PCR. Expression ratios of αv integrin (β3 siRNA/ Control siRNA) are shown. (b) The panels show percent cell death of RAW-osteoclasts without serum on type I collagen (COL 1) and vitronectin (VN) after the treatment of GST, and GST-PIIBNP for 16 h. Three concentrations of siRNA were used to reduce integrin expression. Columns represent mean ± S.D. of the percent of Trypan Blue stained cells/ total cells. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus cells treated with control siRNA.
Figure 6.
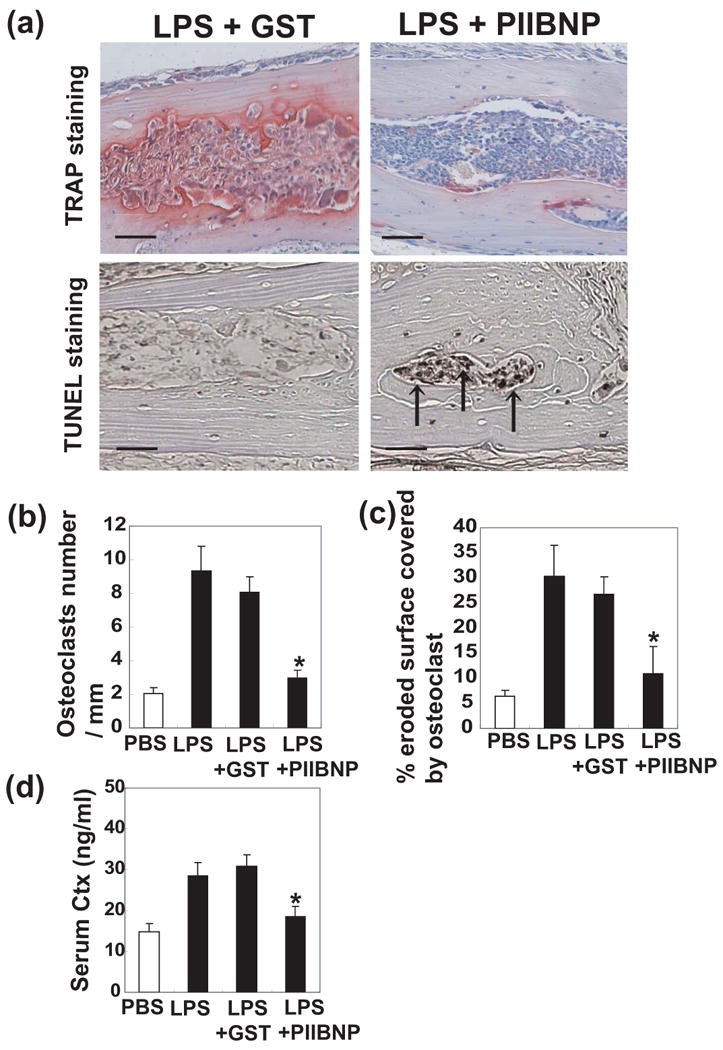
Inhibition of osteoclast bone resorptive activity by PIIBNP in vivo. The calvarial bones were harvested from mice treated with PBS, LPS, LPS + GST, and LPS + PIIBNP. (a) Upper panels showed that the histological sections stained for TRAP and counterstained with hematoxylin. Red cells are TRAP-stained osteoclasts. Lower panels showed that the histlogical staining for in situ TUNEL staining and counterstained with methyl green. Black arrow indicates TUNEL positive cells. The histological sections were fixed with 4% neutral buffered formalin for 10 min and apoptotic cells were determined using TUNEL assay kit (Wako) according to the manufacturer's protocol. Bar =100 μm. (b) Quantitation of osteoclast numbers. Columns represent mean ± S.D. of the osteoclasts number/ mm2.
(c) Percent of eroded surface occupied by osteoclasts. Columns represent mean ± S.D. of the percentage of eroded bone surface length covered by osteoclasts. For each animal, the sections with the largest diameter of bone marrow were used for calculations. (d) Quantitation of serum type I collagen fragment (C-telopeptide, Ctx) at the time of sacrifice. Columns represent mean ± S.D. of the Ctx concentration in serum. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus samples treated with LPS + GST.
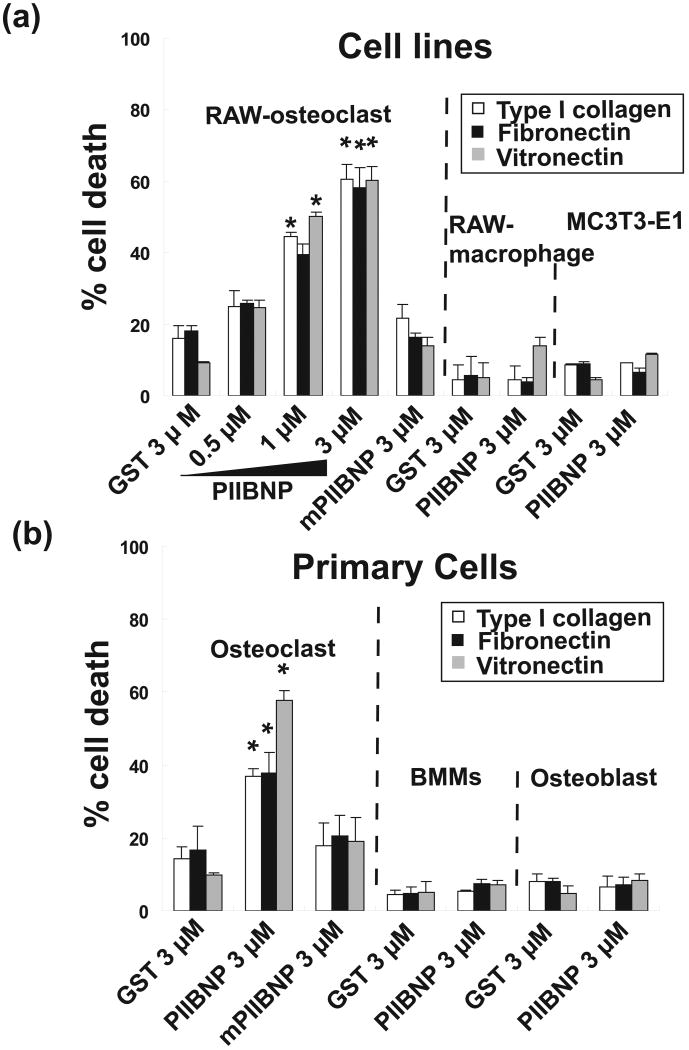
Figure 2.
(a) Percent cell death of differentiated RAW-osteoclasts and MC3T3-E1 osteoblasts on type I collagen (COL1), fibronectin (FN) and vitronectin (VN) without serum after treatment of GST, GST-PIIBNP and GST-mutant PIIBNP for 16 h. (b) Percent cell death of primary osteoclasts and osteoblasts. Columns represent mean ± S.D. of percent of trypan blue stained cells/ total cells in medium and on the plate. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus cells treated with 3 μM GST.
Results
PIIBNP adheres to osteoclasts via the RGD domain
To investigate whether the RGDRGD sequence in PIIBNP was used to adhere to osteoclasts, we performed adhesion assays to recombinant proteins PIIBNP and mutant PIIBNP. Adhesion assays performed with primary cells and cell lines of osteoclasts and osteoblasts yielded identical results (Fig. 1a,b). Adhesion assays showed that RAW 264.7-derived osteoclasts adhered to PIIBNP in a dose-dependent manner, (Fig. 1a, black columns). No adhesion was observed with the mPIIBNP where first RGD was inactivated. The MC3T3-E1 osteoblast cell line did not adhere to the PIIBNP (Fig 1a, white columns). Undifferentiated RAW macrophages did not adhere to PIIBNP (data not shown). The experiments with primary cells demonstrated that osteoclasts adhered to PIIBNP (Fig 1b, black columns), but not osteoblasts (Fig 1b, white columns). The attachment of PIIBNP and osteoclasts was inhibited by the treatment of RGD peptide in a dose dependent manner, but not by the RAD peptide (Fig 1c).
The adhesion assay was also performed on various protein substrates. Vitronectin is the classic ligand for αVβ3 integrin, while type I collagen binds to α2β1 and is not RGD-mediated, and fibronectin binds to αVβ3 and α5β1. As expected, osteoclasts did not adhere as well to type I collagen as they did to fibronectin or vitronectin (Fig. 1d). The adhesion of RAW 264.7-derived osteoclasts to vitronectin-coated plates was significantly reduced by the treatment of 3 μM PIIBNP, but not adhesion to type I collagen or fibronectin coated plates (Fig 1d). To confirm that the cells were adhering to integrins in the predicted manner, RGD peptides were used to compete for binding of the osteoclasts to the matrices (Supplemental Figure 1). Adhesion to type I collagen was not inhibited up to 500 μM RGD peptide because the α2β1 and the α1β1, the primary type I collagen-binding integrins, are not RGD-dependent (36). Binding to fibronectin was reduced by 100 μM RGD peptide while binding to vitronectin was reduced by 10 μM as demonstrated previously (19) (Supplemental Figure 1). These results suggest that PIIBNP competes for binding to vitronectin receptor, αVβ3 integrin, but does not compete with the receptors for binding to type I collagen or fibronectin, which do not bind primarily via αVβ3.
PIIBNP induced cell death of osteoclasts, but not macrophages or osteoblasts
In order to test the effect of PIIBNP on cell survival, osteoclasts and osteoblasts were cultured on various extracellular matrix molecules that have different integrin binding properties: type I collagen, fibronectin and vitronectin-coated plates. PIIBNP induced cell death of RAW 264.7-derived osteoclasts on all three matrices (Fig 2a), and mutant PIIBNP did not induce cell death of RAW 264.7-derived osteoclasts on any matrix (Fig 2a). Under these conditions, almost 60% of the cells died when treated with 3 μM of PIIBNP. 3 μM PIIBNP did not induce cell death of RAW 264.7 macrophages nor the MC3T3-E1 osteoblast cell line (Fig. 2a).
Experiments with primary osteoclasts demonstrated that almost 40% cell death was induced by treatment of 3 μM of PIIBNP on type I collagen or fibronectin coated plates and almost 55% cell death on vitronectin coated plate (Fig 2b). PIIBNP did not induce cell death of primary bone marrow macrophages nor primary osteoblasts (Fig 2b)
PIIBNP induced cell detachment when osteoclasts were treated on vitronectin-coated plates, but not on fibronectin or type I collagen
In order to begin to determine the mechanism by which cell death occurs in osteoclasts, we further investigated the role of cell detachment from the matrix. Cell death induced by cell detachment is called anoikis and occurs rapidly after addition of the agent. However, PIIBNP did not induce cell detachment from type I collagen or fibronectin coated plates (Fig 3a). On vitronectin coated plates PIIBNP induced cell detachment and cell death of RAW 264.7-derived osteoclasts in a dose-dependent manner beginning as early as 1 h (Fig 3b). Therefore, we conclude that anoikis plays a role in the cell death induced on vitronectin, but not on type I collagen or fibronectin. These results strongly suggest that detachment would not be necessary for the cell death signal in the complex matrix of bone in vivo.
PIIBNP induced apoptosis of osteoclasts on vitronectin, type I collagen and fibronectin
To analyze the mechanism of cell death, DNA fragmentation, TUNEL staining and induction of caspase cleavage were tested. The DNA ladder assay showed that PIIBNP induced DNA fragmentation of RAW 264.7-derived osteoclasts. The results did not depend on the type of matrix and showed similar DNA fragmentation for type I collagen, fibronectin and vitronectin (Fig 3c). Further evidence for apoptosis, TUNEL staining showed that PIIBNP increased TUNEL positive cells to about 40 % on COL I without cell detachment (Figure 3d). Immunoblotting for cleaved (activated) caspase 8 and caspase 3 showed that PIIBNP induced apoptosis of RAW 264.7-derived osteoclasts via the caspase 8 and 3 pathway, not via the caspase 9 pathway (Fig 3e).
Apoptosis is dependent on the αV and β3 integrin
To determine the role of αVβ3 integrin in the induction of osteoclast death by PIIBNP, the endogenous αV or β3 expression was reduced by incubation of the RAW-derived osteoclasts with siRNA specific for αV or β3 mRNA. The various concentrations of αV and β3 integrin siRNA showed that the expression of αV and β3 integrin mRNA was significantly decreased in a dose-dependent manner, at each concentration: 50 nM, 100 nM and 150 nM siRNA (Fig 4a, 5a). Fig 4b and Fig 5b demonstrate inhibition of apoptosis on type I collagen and vitronectin (Fig 4b) that was dependent on the extent of reduction of αV integrin expression. Therefore, cell death is dependent on αV integrin expression. The same experiments were conducted after inhibition of integrin β3 with similar results (Figure 5b). We also confirmed that other constructs αV or β3 siRNA transfection reduced αV or β3 integrin expression, and reduction of αV or β3 integrin inhibited PIIBNP-induced cell death (Supplemental Figure 2). These results indicated the effects of αV or β3 siRNA transfection are not caused by off-target effects.
PIIBNP inhibited bone resorption in vivo and decreased osteoclast number
Ultimately the ability of PIIBNP to inhibit bone resorption by osteoclasts must be shown in vivo. Experiments in vitro showed that PIIBNP inhibits osteoclast survival on purified matrices. To determine whether PIIBNP functioned in vivo where the bone matrix is a complex mixture of osteopontin, type I collagen, thrombospondin, fibronectin, vitronectin, and bone sialoprotein, we tested an established mouse model of lipopolysaccharide (LPS)-induced bone resorption (37). The calvarial bones of mice were treated with injections of PBS, LPS, LPS + GST or LPS + GST-PIIBNP. The calvarial bones of LPS + GST-injected mice were eroded by osteoclasts, which can be observed to reside in resorption lacunae (Fig. 6a). In contrast, the calvaria of LPS + PIIBNP-treated animals were protected from bone erosion: that is, the resorption area, indicated by the red osteoclast-specific TRAP staining, was greatly reduced in the PIIBNP-treated animals (Fig 6a upper panels). To clarify the evidence that PIIBNP inhibited LPS-induced apoptosis, we tested in situ TUNEL staining for calvarial bones. The calvarial bones of LPS + GST-injected mice were eroded by osteoclasts and TUNEL positive cells were not seen in the section. In contrast, the calvaria of LPS + PIIBNP-treated animals were protected from bone erosion and TUNEL positive cells were detected (Figure 6a lower panels).
In order to quantify the changes in bone resorption, the number of osteoclasts per millimeter of bone and the percentage of eroded bone surface covered by osteoclasts were counted and shown in Fig 6b and c. The PIIBNP reduced osteoclast number by 60%. When osteoclasts resorb bone in vivo, type I collagen fragments are released that can be measured in the serum. Therefore, to confirm that there was an alteration in bone resorption at the biochemical level, the serum level of the type I collagen telopeptide biomarker, CTX-I, was measured and found to be reduced by 40% in mice treated with LPS + PIIBNP compared to control mice (Fig 6d).
Discussion
We demonstrate here that the recombinant protein, PIIBNP, the NH2-propeptide moiety released from type II procollagen during the normal course of biosynthesis, induces apoptosis of osteoclasts via the caspase 3/8 pathway, and is a novel biological regulator of bone resorption. The specificity of PIIBNP arises from its use of the cell integrin receptor, αVβ3, an integrin that is expressed by a limited number of cells, including osteoclasts, endothelial cells, synovial cells and some tumors (28, 29). In bone, the effect on cells is very specific to osteoclasts and it does not affect circulating macrophages or osteoblasts.
In the classic model, integrin engagement by ECM ligands is necessary for survival, and the loss of cell attachment to the ECM results in a caspase-dependent apoptosis known as anoikis (38). Importantly, our studies indicate that, unlike RGD peptides, PIIBNP does not function in bone only as a mediator of cell detachment or anoikis. This conclusion is supported by the evidence that osteoclasts died by apoptosis whether cultured on vitronectin, the classic αVβ3 ligand, but also fibronectin or type I collagen. PIIBNP did not detach cells from type I collagen or fibronectin, but could still effect cell death in a β3 integrin-dependent manner over a long time period. Therefore, unlike RGD peptides that act by rapid detachment of cells from the matrix, PIIBNP is a unique protein moiety able to specifically inhibit osteoclast function and cause cell death without the requirement for rapid cell detachment.
The mechanism of action of PIIBNP is through the osteoclast integrin αVβ3. Two lines of evidence support this conclusion. First, the expression of αVβ3 integrin is correlated with the inhibitory activity of the PIIBNP and when the first RGD peptide is mutated to RAD, inhibitory activity is abrogated. Undifferentiated RAW cells, bone marrow macrophages and osteoblasts do not express αVβ3 integrin (Supplemental figure 3) and do not adhere to PIIBNP. When bone marrow macrophages are differentiated with M-CSF and RANKL, adhesion to PIIBNP is correlated with the expression of the αVβ3 integrin in the mature osteoclast. Secondly, when αV or β3 integrin expression is reduced, induction of apoptosis by PIIBNP is reduced.
Previously, we have shown that PIIBNP induced necrosis in a chondrosarcoma cell line (25). We believe that the difference in cell type response to PIIBNP is important. Tumor cells often are known to escape spontaneous and therapy-induced caspase activation due to acquired mutations in the apoptotic machinery (39). In the hCh-1 tumor cell line used in the previous publication (25), there is a mutation in the p53 gene (40).
A significant body of knowledge supports the function of αVβ3 integrin in bone resorption and several blocking studies using antibodies and competitive ligands have established that the αVβ3 integrin is critical for bone resorption in vitro and in vivo (41, 42). Several studies based on results obtained with adhesion-blocking antibodies or RGD peptides have led to antagonist-induced cell death (43, 44). Monoclonal antibody to αVβ3 integrin blocks the osteoclast-mediated bone resorption in the thyroparathyroidectomized rat (45). RGD peptide mimetic and echistatin, an RGD-containing αVβ3 specific snake venom peptide, inhibited bone loss in ovariectomized rodents (41). In comparison with these inhibitors, PIIBNP does not rely on detachment-mediated cell death, but can affect cells over a longer period of time.
An important question that arises from this work is whether this function of PIIBNP is also found in vivo? The articular cartilage is composed only of chondrocytes: osteoclasts, osteoblasts and blood vessels are excluded from cartilage tissue. αVβ3 integrin is expressed on a limited number of cell types that include angiogenic endothelial cells, some tumor cells, vascular smooth muscle cells and osteoclasts (28, 29), but not in developing chondrocytes, thereby potentially conferring resistance to cell killing by PIIBNP in cartilage in vivo (25). Therefore, we speculate that type IIB NH2-propeptides, when liberated from the collagen molecule during biosynthesis in developing cartilage, may be one of regulators that limits the cell types in cartilage by inhibition of invasion by osteoclasts and endothelial cells, and thus protects the tissue from invading and matrix-degrading osteoclasts and the vascularity that promotes bone formation; however, this is yet to be proven.
In order to demonstrate function for PIIBNP on osteoclasts in vivo, we induced calvarial osteolysis with lipopolysaccharide and treated with PIIBNP. Injection of GST PIIBNP, but not GST alone, was able to reduce osteoclast number and the extent of eroded bone surface. Thus, PIIBNP can function in vivo to reduce bone resorption. In conclusion, PIIBNP induces osteoclast apoptosis and reduces bone resorption. These findings support a strategy whereby PIIBNP could be considered as a potential therapeutic tool where the reduction of osteoclast activity is required as in osteoporosis or inflammation-induced osteolysis, without diminishing bone formation activity.
Supplementary Material
Supplemental Figure 1. This panel shows effect of adhesion blocking to COL1, FN and VN by RGD and RAD peptide. RGD or RAD peptides were added to RAW 264.7 osteoclasts before the incubation of adhesion. Each assay was performed in triplicate wells and three independent experiments. Columns represent mean ± S.D. BSA as control groups were omitted.
Supplemental Figure 2. The effect of reduction of αV or β 3 integrin by siRNA on PIIBNP-induced cell death. Another construct of 150 nM siRNA were used to reduce integrin expression. (a) The expression levels of αV or β 3 integrin mRNA were analyzed by realtime PCR. Expression ratios of αv or β3 integrin (αv siRNA/ Control siRNA, β3 siRNA/ Control siRNA) are shown. (b) The panels show percent cell death of RAW-osteoclasts without serum on type I collagen or vitronectin after the treatment of 3 μM GST, and GST-PIIBNP for 16 h. Columns represent mean ± S.D. of the percent of Trypan Blue stained cells/ total cells. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus cells treated with control siRNA.
Supplemental Figure 3. (a) Expression of integrins mRNA in macrophages, osteoclasts and osteoblasts analyzed by RT-PCR. These osteoclasts were differentiated for 4 days in RAW cells and for 5 days in primary cells. Lane 1; RAW 264.7 macrophage, 2; 4 days differentiated RAW 264.7 osteoclast, 3; MC3T3-E1, 4, 5, 6; 5 days differentiated primary osteoclast, 7, 8, 9; primary osteoblasts. (b) Expression of integrins protein in macrophages, osteoclasts and osteoblasts analyzed by Western blotting.
Highlights.
PIIBNP adheres to osteoclasts via the RGD domain.
PIIBNP induced cell death of osteoclasts, but not macrophages or osteoblasts.
PIIBNP induced apoptosis of osteoclasts via αv and β3 integrins.
PIIBNP inhibited bone resorption in vivo and decreased osteoclast number.
Acknowledgments
We thanks members of the Sandell, Roberto Civitelli, Steve Teitelbaum and Roberta Faccio laboratories for help with the osteoclast and osteoblast experiments and Dr's. Steven Teitelbaum and Debabrada Patra for critical reading of the manuscript. No authors have conflict of interest. Funding was provided from the National Institute of Arthritis, Musculoskeletal and Skin Diseases, R01 AR36994, R01 AR45550 and R01 AR050847.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;5:407–13. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 2.May H, Murphy S, Khaw KT. Aged--associated Bone Loss in Men and Women and its Relationship to weight. Age Ageing. 1994;23:235–240. doi: 10.1093/ageing/23.3.235. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Manolagas SC. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan C, Finnegan A. Osteoclasts, pro-inflammatory cytokines, RANK-L and bone remodeling in rheumatoid arthritis. Front Biosci. 2003;8:d1018–29. doi: 10.2741/1102. [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD. Biology of Osteoclast Activation in Cancer. J Clin Oncol. 2001;19:3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 7.Giancotti FG, Ruoslahti E. Integrin Signaling. Science. 1999;285:1028–1033. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–8. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 9.Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab. 2000;18:344–9. doi: 10.1007/s007740070007. [DOI] [PubMed] [Google Scholar]
- 10.Davies J, Warwick J, Totty N, Philp R, Helfrich M, Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989;109:1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue M, Namba N, Chappel J, Teitelbaum SL, Ross FP. Granulocyte Macrophage-Colony Stimulating Factor Reciprocally Regulates {alpha}v-Associated Integrins on Murine Osteoclast Precursors. Mol Endocrinol. 1998;12:1955–1962. doi: 10.1210/mend.12.12.0213. [DOI] [PubMed] [Google Scholar]
- 12.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–5. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 13.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 14.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 15.Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–36. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 16.Clezardin P, Jouishomme H, Chavassieux P, Marie PJ. Thrombospondin is synthesized and secreted by human osteoblasts and osteosarcoma cells. A model to study the different effects of thrombospondin in cell adhesion. Eur J Biochem. 1989;181:721–6. doi: 10.1111/j.1432-1033.1989.tb14783.x. [DOI] [PubMed] [Google Scholar]
- 17.Robey PG, Young MF, Fisher LW, McClain TD. Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J Cell Biol. 1989;108:719–727. doi: 10.1083/jcb.108.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin-A Possible Anchor of Osteoclast to Bone. PNAS. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helfrich MH, Nesbitt SA, Dorey EL, Horton MA. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner Res. 1992;7:335–43. doi: 10.1002/jbmr.5650070314. [DOI] [PubMed] [Google Scholar]
- 20.Rodan SB, Rodan GA. Integrin function in osteoclasts. J Endocrinol. 1997;154(Suppl):S47–56. [PubMed] [Google Scholar]
- 21.Sandell LJ, Nalin AM, Reife RA. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Developmental dynamics. 1994;199:129–40. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- 22.Fukui N, Mcalinden A, Zhu Y, Crouch E, Broekelmann TJ, Mecham RP, Sandell L. Processing of Type II Procollagen Amino Propeptide by Matrix Metalloproteinases. Journal of Cell Biology. 2002;277:2193–2201. doi: 10.1074/jbc.M105485200. [DOI] [PubMed] [Google Scholar]
- 23.van der Rest M, Garrone E. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 24.Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. Journal of Biological Chemistry. 2001;276:31502–9. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Bryan J, Franz C, Havlioglu N, Sandell LJ. Type IIB procollagen NH(2)-propeptide induces death of tumor cells via interaction with integrins alpha(V)beta(3) and alpha(V)beta(5) J Biol Chem. 2010;285:20806–17. doi: 10.1074/jbc.M110.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandell LJ, Wang Z, Franz C, Bryan J, Siegel A, Mecham R, Wagensail J, Ell B, Rapraeger A. Live-cell Imaging of Endothelial Cell Tube Formation: Inhibition by Chondrostatin. FASEB J. 2008;22:101.4. [Google Scholar]
- 27.Wang Z, Bryan J, Franz C, Siegel A, Wagenseil J, Mecham R, Sandell LJ. A Fragment of Cartilage Collagen, Chondrostatin, Inhibits Migration of Breast Cancer Cells. FASEB J. 2008;22:1029.11. [Google Scholar]
- 28.Felding-Habermann B, O'Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–8. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Ross FP, Teitelbaum SL. Unoccupied {alpha}v{beta}3 Integrin Regulates Osteoclast Apoptosis by Transmitting a Positive Death Signal. Mol Endocrinol. 2005;19:771–780. doi: 10.1210/me.2004-0161. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi S, Miura Y, Nishiyama T, Mitani M, Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S, Doita M. Decoy receptor 3 expressed in rheumatoid synovial fibroblasts protects the cells against Fas-induced apoptosis. Arthritis Rheum. 2007;56:1067–75. doi: 10.1002/art.22494. [DOI] [PubMed] [Google Scholar]
- 32.Oganesian A, Zhu Y, Sandell LJ. Type IIA procollagen amino-propeptide is localized in human embryonic tissues. J Histo & Cytochem. 1997;45:1469–1480. doi: 10.1177/002215549704501104. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest. 1999;103:517–24. doi: 10.1172/JCI5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. J Bone Miner Res. Vol. 2. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee; pp. 595–610. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VLJ, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the {alpha}v{beta}3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook A, Hook M. Multiple Binding Sites in Collagen Type I for the Integrins alpha 1beta 1 and alpha 2beta 1. J Biol Chem. 2000;275:38981–38989. doi: 10.1074/jbc.M007668200. [DOI] [PubMed] [Google Scholar]
- 37.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 38.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 39.Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23:2746–56. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- 40.Chansky H, Robbins JR, Cha S, Raskind WH, Conrad EU, Sandell LJ. Expression of cartilage extracellular matrix and potential regulatory genes in a new human chondrosarcoma cell line. Journal of Orthopaedic Research. 1998;16:521–530. doi: 10.1002/jor.1100160502. [DOI] [PubMed] [Google Scholar]
- 41.Engleman VW, Nickols GA, Ross FP, Horton MA, Griggs DW, Settle SL, Ruminski PG, Teitelbaum SL. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest. 1997;99:2284–92. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, Tucker GC, Van Obberghen-Schilling E. Blockade of {alpha}vbeta3 and {alpha}vbeta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108:3035–3044. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 44.Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M. Inhibition of alpha(v)beta3 integrin reduces angiogenesis, bone turnover, and tumor cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis. 2003;20:413–20. doi: 10.1023/a:1025461507027. [DOI] [PubMed] [Google Scholar]
- 45.Crippes BA, Engleman VW, Settle SL, Delarco J, Ornberg RL, Helfrich MH, Horton MA, Nickols GA. Antibody to beta3 integrin inhibits osteoclast-mediated bone resorption in the thyroparathyroidectomized rat. Endocrinology. 1996;137:918–924. doi: 10.1210/endo.137.3.8603604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. This panel shows effect of adhesion blocking to COL1, FN and VN by RGD and RAD peptide. RGD or RAD peptides were added to RAW 264.7 osteoclasts before the incubation of adhesion. Each assay was performed in triplicate wells and three independent experiments. Columns represent mean ± S.D. BSA as control groups were omitted.
Supplemental Figure 2. The effect of reduction of αV or β 3 integrin by siRNA on PIIBNP-induced cell death. Another construct of 150 nM siRNA were used to reduce integrin expression. (a) The expression levels of αV or β 3 integrin mRNA were analyzed by realtime PCR. Expression ratios of αv or β3 integrin (αv siRNA/ Control siRNA, β3 siRNA/ Control siRNA) are shown. (b) The panels show percent cell death of RAW-osteoclasts without serum on type I collagen or vitronectin after the treatment of 3 μM GST, and GST-PIIBNP for 16 h. Columns represent mean ± S.D. of the percent of Trypan Blue stained cells/ total cells. Each assay was performed in triplicate wells and three independent experiments. *indicates p<0.05 versus cells treated with control siRNA.
Supplemental Figure 3. (a) Expression of integrins mRNA in macrophages, osteoclasts and osteoblasts analyzed by RT-PCR. These osteoclasts were differentiated for 4 days in RAW cells and for 5 days in primary cells. Lane 1; RAW 264.7 macrophage, 2; 4 days differentiated RAW 264.7 osteoclast, 3; MC3T3-E1, 4, 5, 6; 5 days differentiated primary osteoclast, 7, 8, 9; primary osteoblasts. (b) Expression of integrins protein in macrophages, osteoclasts and osteoblasts analyzed by Western blotting.