Abstract
Background
Recent pathological studies report vascular pathology in clinically diagnosed Alzheimer's disease (AD) and AD pathology in clinically diagnosed vascular dementia (VaD). We compared magnetic resonance imaging (MRI) measures of vascular brain injury (white matter hyperintensities (WMH) and infarcts) to neurodegenerative measures (medial-temporal atrophy (MTA) and cerebral atrophy (CA)) in clinically diagnosed subjects with either AD or VaD. We then examined relationships among these measures within and between the two groups and their relationships to mental status.
Methods
Semi-quantitative MRI measures were derived from blind ratings of MRI scans obtained from participants in a research clinical trial of VaD (N=694) and a genetic epidemiological study of AD (N=655).
Results
CA was similar in the two groups, but differences in the mean of MTA and WMH were pronounced. Infarcts were significantly associated with CA in VaD, but were not in AD; MTA and WMH were associated with CA in both. WMH was associated with MTA in both groups, however MRI infarcts were associated with MTA in VaD but not with MTA among AD patients. MTA was strongly associated with MMSE scores in both groups while evidence of a modest association between WMH and MMSE was seen among VaD patients.
Conclusions
MRI data from two dementia cohorts with differing dementia etiologies find that the clinical consequences of dementia are most strongly associated with cerebral and medial-temporal atrophy suggesting that tissue loss is the major substrate of the dementia syndrome.
Keywords: Alzheimer's disease, MRI, Dementia, vascular, Hippocampus, Atrophy
1. Introduction
Dementia research has focused on understanding and differentiating dementia subtypes to identify clinical and pathophysiological characteristics unique to each disorder. However, data strongly suggest that late-life dementia, commonly attributed to Alzheimer's disease (AD) is actually a complex process often due to the combined effects of multiple pathologies.(1) Magnetic resonance imaging (MRI) is one method by which the extent, impact and possible etiology of regional brain pathology can be quantitatively assessed (2), Early attempts to compare MRI measures among clinically diagnosed AD and vascular dementia (VaD) subjects, however, were relatively small and inconclusive.(3) More recent reports of the qualitative assessment of MRI differences between AD and VaD patients with larger samples have suggested that medial-temporal atrophy (MTA), particularly hippocampal atrophy, is uniquely associated with clinical AD as opposed to VaD.(4) In fact, hippocampal atrophy is considered the imaging hallmark of clinical AD and is strongly associated with AD pathology.(5-8) Bastos-Leite et al, however, reported a high rate of MTA in a well-characterized sample of VaD patients and an association of MTA with cognitive functioning in the same group (9); similarly other MRI studies have found extensive hippocampal atrophy in patients with suspected VaD (10) and hippocampal sclerosis has been associated with severe hippocampal atrophy on MRI.(2) Global cerebral atrophy also has been associated with cognitive performance in both AD and VaD patients.(11) While VaD characteristically has been associated with white matter hyperintensities (WMH) in addition to infarction, WMH have been negatively correlated with cognitive functioning in persons with normal cognition, VaD (12), mild cognitive impairment (MCI) (2), and AD (13) suggesting that the effect of this pathology transcends clinical diagnosis.
Given the evidence for the complex nature of brain diseases underlying the dementia syndrome, we therefore sought to investigate the relative impact of cerebral atrophy, MTA, WMH, infarcts and Mini-Mental State Exam (MMSE) scores amongst cognitively normal, AD and VaD individuals. To accomplish this aim, we first characterized the different levels of atrophy and vascular pathology observed in each group. We then examined the functional relationships between atrophy, vascular pathology, and cognitive impairment within the AD and VaD groups. Given the presumption that VaD is due solely to vascular pathology, we hypothesized that MRI infarcts and WMH should be strongly associated with global cerebral atrophy (CA) in the VaD group. Conversely, given that the dementia attributable to AD is due solely to AD pathology, we hypothesized that MTA—as an excellent marker of AD pathology—would be strongly associated with CA in the AD group. To test the possibility, however, that hippocampal atrophy can result from either vascular or AD pathology (2), we also examined the relationship between MTA and vascular markers. We again hypothesized that MTA would be weakly associated with vascular markers (white matter hyperintensity and stroke) in the VaD group and unassociated with vascular markers amongst the AD subjects. Finally, we investigated the relationship between all the MRI and cognitive ability (MMSE) within the two dementia groups. We hypothesized that vascular markers would have a stronger association with MMSE in the VaD group, but that MTA would have the strongest association with MMSE in the AD group.
2. Methods
2.1. Subjects
We studied VaD patients diagnosed using NINDS-AIREN criteria, AD patients diagnosed using NINCDS-ADRDA criteria, and cognitively normal relatives (mostly siblings) of the AD patients. Eight hundred and twenty-six randomized subjects meeting NINDS-AIREN for VaD were recruited for an industry-sponsored clinical trial evaluating the safety and efficacy of donepezil in VaD of which approximately 31% met clinical criteria for probable VaD (14) (clinicaltrials.gov NCT00165737); 655 AD patients meeting NINCDS-ADRDA criteria for AD and 756 cognitively normal (CN) relatives of the AD patients were Caucasian participants of the MIRAGE Study, a multi-site, multi-ethnic, family-based genetic study of AD.(15-17) The recruitment and evaluation of MIRAGE subjects was overseen and approved by Boston University Human Subjects Protection Committee. Ethics review panels appropriate for clinical trials also oversaw VaD subject collection and study design. Written consent was obtained from all subjects (or guardians of subjects).
2.2. Data
Both the MIRAGE and VaD studies were large multi-center studies. MRI scanner strength, model, and settings used varied by collection site, necessitating the use of semi-quantitative measures of atrophy and vascular disease. All MRI scans were scored by a single rater (CD) blind to screen failure status in the VaD study and AD status in the MIRAGE study (proband vs. control). Although the rater was aware of the study for which the person was recruited, he was blind to clinical syndrome in both studies since MIRAGE included both AD and normal controls and the VaD study included both VaD and AD patients. Among the 826 VaD subjects, 132 did not receive an MRI and were excluded from comparisons other than basic descriptive statistics. Scans were scored for left and right medial-temporal atrophy (MTA) using Scheltens’ scale (0-4 integer where most severe atrophy =4), cerebral infarction (presence/absence) and number of infarcts (NI). The average MTA (left and right) was used as our measure MTA in analyses described below. Global atrophy and white matter hyperintensity (WMH) were rated on a visual analog scale (0-100), with 100 representing severe atrophy and extreme WMH respectively as previously reported.(18) The method utilized tie points that were derived from quantitative analysis of brain and WMH as shown in the Supplemental Figures 1, 2, and 3. This approach has proven to have clinical diagnostic significance for differentiating cognitively normal individuals from Alzheimer's (18) and conversion of mild cognitive impairment to dementia (19). MRI infarcts were identified based on imaging characteristics (20) and further defined as small (maximum diameter <1 cm) or large (maximum diameter ≥1 cm). Finally, the total number of infarcts (NI) was recorded and ranged from 0 to 10. Cognitive functioning in both patient samples was measured using the MMSE.(13)
2.3. Analysis Methods
Data analysis was performed with R.(21) Means were compared with an analysis of variance, and Tukey's HSD test (corrected for unequal sample sizes) was used to evaluate pair-wise comparisons. The relationship between vascular markers atrophy and cognitive functioning was evaluated in a series of regression models. The regression models and parameters examined were specified in advance, guided by the hypotheses stated in the introduction. All models examined are presented. Regression predictors were evaluated by examining the estimated β weights with all predictors in the model. Separate regression models were estimated for AD and VaD. To evaluate their relative importance, continuous predictors and responses were standardized (mean=0, variance=1) and β estimates can be interpreted as correlations. R2 estimates were compared using a normal approximation appropriate for large samples.(22) Age and sex were included as covariates in all regression models to minimize possible confounding. Sex is coded as females=0 and males=1, so that positive β estimates for the sex effect indicate higher scores for males and negative β estimates indicate higher scores for females. To compare models between AD and VaD groups, an additional pooled analysis was performed which estimated an interaction term representing the difference in be β estimates for the two groups. The significance of this term indicates the degree to which the associated predictor has a different effect in AD and VaD subjects.
3. Results
Demographic information is presented in Table 1. The CN subjects were younger than either of the dementia groups (p<0.0001). Vascular risk factors were lower in the CN and AD groups than the VaD subjects (p<0.03). Boxplots of MTA, global atrophy, WMH, and NI are shown in Figure 1. For all MRI ratings, the CN group was lowest with the VaD group showing the highest scores on global atrophy (Fdf=2,2071=303.14; p<0.0001), white matter hyperintensities (Fdf =2,2079=175.82; p<0.0001) and number of infarcts (Fdf=2,2050=282.25; p<0.0001). The AD group showed the highest MTA scores (Fdf=2,2014=466.04; p<0.0001). Differences between the VaD and AD group were significant for global atrophy (p<0.035), WMH (p<0.0001) and MTA (p<0.0001). The prevalence of infarcts for AD subjects, although higher, was not significantly different from CN (p=0.0641). The distribution of MTA scores separated by left and right hemisphere is displayed in Figure 2. While AD subjects generally had high MTA ratings and CN had low ratings, the VaD group showed a substantial proportion of subjects at each MTA rating except 0.
Table 1.
Demographic characteristics
| CN N=756 | AD N=655 | VaD N=826 | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 69.43 (SD=9.04) | 74.17 (SD=8.73) | 73.52 (SD=9.14) |
| Sex | |||
| Male | 287 (38%) | 255 (39%) | 329 (40%) |
| Female | 469 (62%) | 400 (61%) | 497 (60%) |
| Years of Education | |||
| ≤8 | 98 (13%) | 121 (19%) | 22 (12%) |
| 9-12 | 278 (37%) | 273 (42%) | 79 (41%) |
| >12 | 374 (50%) | 258 (39%) | 78 (41%) |
| Medical History | |||
| Stroke | 32 (4%) | 56 (9%) | 706 (85%) |
| Hypertension | 377 (51%) | 306 (47%) | 656 (79%) |
| Smoking | 332 (44%) | 256 (39%) | 500 (60%) |
| Diabetes | 70 (9%) | 83 (13%) | 143 (17%) |
| Hyperlipidemia | 338 (46%) | 271 (43%) | 411 (50%) |
| TIA | 17 (2%) | 27 (4%) | 114 (14%) |
| Psychometric Scores | |||
| MMSE | NA | 18.17 (0.35) | 22.65 (0.18) |
| MRI measures | CN N=756 | AD N=655 | VaD N=694 |
| Mean Atrophy (0-100) | 49.23 (SD=11.64) | 62.40 (SD=11.84) | 64.13 (SD=14.13) |
| Mean WMH (0-100) | 8.97 (SD=15.26) | 19.06 (SD=22.18) | 30.32 (SD=26.37) |
| Mean MTA (0-4) | 0.80 (SD=0.92) | 2.48 (SD=1.16) | 1.88 (SD=1.05) |
| Cerebral Infarction | 105 (14%) | 150 (23%) | 451 (66%) |
Numbers in parentheses represent standard deviation or proportion for frequency data.
Figure 1. Box plots of global cerebral atrophy ratings, medial temporal atrophy, white matter hyperintensity, and number of infarcts.
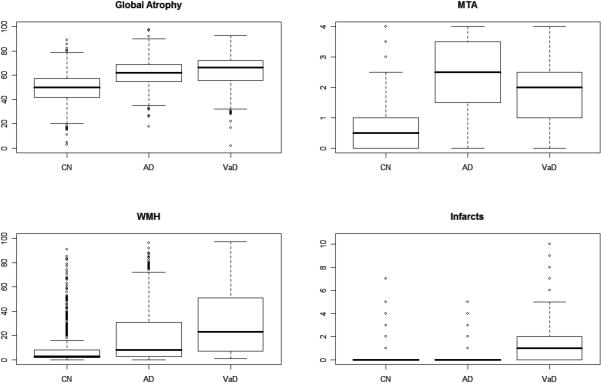
CN=cognitively normal, AD=Alzheimer's disease cases, and VaD=vascular dementia cases. Bold lines represent median, boxes show inter-quartile ranges. Whiskers extend to data within 1.5 times the inter-quartile range from the box. Outliers (points >1.5 times the inter-quartile range) are presented as circles.
Figure 2. Left and right MTA distribution for cognitively normal (CN), Alzheimer disease cases (AD), and vascular dementia cases (VaD).
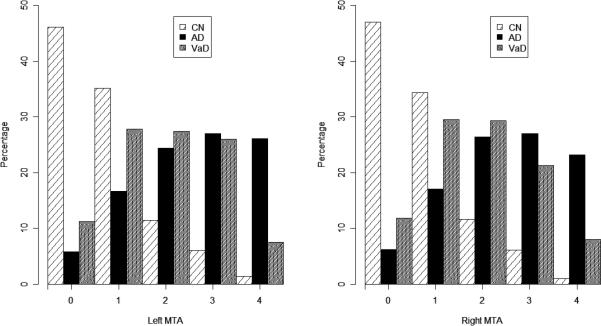
Scheltens’ scale: 0-4 integer scale with most severe atrophy =4.
As expected, VaD had a substantially higher prevalence of large infarcts (39%) compared to AD (5%) and CN (2%). Significant differences in the prevalence of both large and small infarcts between VaD and AD subjects were also noted, although 34% of VaD subjects did not have a detectable infarct on MRI.
For the next stage of the analysis, regression models were analyzed to compare the pathological processes underlying these two samples. First, we examined the relationship between WMH, NI, and MTA on global atrophy. Among VaD patients, age, sex, NI, WMH, MTA were significantly related to CA (Fdf=5,608=67.34; p<0.0001) and all predictors contributed to the model (all p<0.025). The model was also significant in the AD group (Fdf=5,612=44.45; p<0.0001) but only age, WMH, and MTA contributed to the model (p<0.02). The model accounted for CA in VaD patients (R2=0.356) significantly more precisely (p=0.025) than for AD patients (R2=0.266) (See Table 2). The model estimates for the VaD group remained nearly identical after eliminating approximately one-third of the VaD group without infarcts — those subjects most likely considered to be suffering from the combined effects of AD and vascular pathologies.
Table 2.
Regression Models
| Model 1: Predictors of CA in the VaD and AD cohorts. | ||||||
|---|---|---|---|---|---|---|
| Cohort: | VaD | AD | Comparison | |||
| Response | Predictor | β estimate | Pr(>|t|) | β estimate | Pr(>|t|) | Pr(>|t|) |
| CA | Sex | -0.16 | 0.020 | 0.14 | 0.052 | 0.0027 |
| Age | 0.23* | < 10-5 | 0.094* | 0.016 | 0.014 | |
| Infarcts (count) | 0.094* | 0.0059 | -0.065* | 0.076 | 0.0015 | |
| WMH (1-100) | 0.18* | < 10-5 | 0.15* | 1.6 × 10-4 | 0.61 | |
| MTA | 0.38* | < 10-5 | 0.41* | < 10-5 | 0.66 | |
| Model 2: Predictors of MTA in the VaD and AD samples. | ||||||
|---|---|---|---|---|---|---|
| Cohort: | VaD | AD | Comparison | |||
| Response | Predictor | β estimate | Pr(>|t|) | β estimate | Pr(>|t|) | Pr(>|t|) |
| MTA | Sex | -0.19 | 0.016 | -0.015 | 0.84 | 0.11 |
| Age | 0.28* | < 10-5 | 0.33* | < 10-5 | 0.41 | |
| WMH (1-100) | 0.21* | < 10-5 | 0.16* | < 10-5 | 0.35 | |
| Stroke (Yes/No) | -0.23 | 0.033 | 0.095 | 0.48 | 0.06 | |
| Model 3: Impact of MTA on cognitive ability (MMSE score) | ||||||
|---|---|---|---|---|---|---|
| Cohort: | VaD | AD | Comparison | |||
| Response | Predictor | β estimate | Pr(>|t|) | β estimate | Pr(>|t|) | Pr(>|t|) |
| MMSE | Sex | -0.0064 | 0.93 | 0.10 | 0.33 | 0.40 |
| Age | -0.035* | 0.37 | 0.067* | 0.25 | 0.14 | |
| MTA | -0.34* | < 10-5 | -0.38* | < 10-5 | 0.56 | |
| Model 4: Impact of vascular disease markers on cognitive ability (MMSE score) | ||||||
|---|---|---|---|---|---|---|
| Cohort: | VaD | AD | Comparison | |||
| Response | Predictor | β estimate | Pr(>|t|) | β estimate | Pr(>|t|) | Pr(>|t|) |
| MMSE | Sex | 0.018 | 0.82 | 0.083 | 0.46 | 0.63 |
| Age | -0.12* | 0.0021 | -0.094* | 0.12 | 0.71 | |
| WMH (1-100) | -0.13* | 6.1 × 10-4 | -0.049* | 0.42 | 0.25 | |
| Stroke (Yes/No) | -0.081 | 0.44 | 0.019 | 0.92 | 0.65 | |
indicates β estimate can be interpreted as a correlation coefficient.
Next, we explored the relationship between MTA and vascular markers (WMH and stroke (yes/no)) (See Table 2). Among VaD patients, both presence of stroke and WMH contributed significantly to MTA (p<0.04; R2=0.163), but in opposing directions with increasing WMH associated with worsening MTA scores, but the presence of stroke associated with improved MTA scores. Conversely, among AD patients, only age and WMH contributed significantly to the model predicting MTA with increasing WMH associated with worsening MTA scores (p<0.0001; R2=0.173). A summary of this model (Model 2) including coefficient estimates and their significance is given in Table 2.
Finally, we investigated the relationship between MRI measures and cognitive ability (MMSE) to test our hypothesis the various MRI measures would show different associations with cognition due to the different etiologies of dementia. Surprisingly, MMSE scores were associated with MTA in both VaD (Fdf=3,619=31.19; p<0.0001; R2=0.131) and AD (Fdf=3,317=14.79; p<0.0001; R2=0.123). Details of this model (Model 3) are shown in Table 2. Examining vascular factors in relation to MMSE scores, we found significant associations between MMSE and WMH in the VaD group (p<0.005), but this association explained only a limited amount of additional variance (R2=0.039) in MMSE. There was no association between WMH and MMSE for the AD group (p=0.2654; R2=0.017). The covariate estimates and significance levels are summarized in Table 2: Model 4.
4. Discussion
We examined brain MRI measures in large samples of VaD, AD and cognitively normal controls. Expected large differences in MTA, WMH and NI were found between VaD and AD subjects. While the mean global cerebral atrophy differed between the VaD and AD subjects, the magnitude of this difference was rather small — a difference of less than 2 on a (0-100) scale.
From these findings, we conclude that brain tissue loss accompanies clinical dementia, regardless of etiology. We also note that age was significantly associated with brain atrophy in both groups but more strongly among VaD than AD patients despite very similar age distributions. This finding suggests that normal aging and vascular disease interact in the VaD group whereas AD pathology appears to overwhelm age-related differences. In addition we note the expected finding of a significant association between infarcts and CA within the VaD group, but also a significant inverse relationship between infarcts and CA within the AD group. We hypothesize that the inverse association between infarcts detected on MRI and CA within the AD group indicates an additive effect of infarcts for dementia in AD such that less AD pathology is necessary to develop dementia among those with concomitant vascular pathology.(1, 23, 24) Other studies (25) have emphasized, in unselected samples, the importance of vascular pathology in cognitive functioning within AD samples. The lack of association with infarcts and MMSE in our AD sample may largely reflect the sampling methods used as our AD sample was selected for minimal vascular pathology.
Consistent with recent findings, MTA was strongly associated with CA in both dementia groups, even after excluding VaD patients with no infarcts to reduce the likely contamination of this group by mixed pathologies. As a consequence, we suspect that AD pathology was much less common within this latter group (26), and yet, we note that the relationship between MTA and CA did not change. The cause for this association is unclear, but hippocampal sclerosis (HS) does occur in vascular cognitive impairment (2) and is associated with profound hippocampal atrophy. It is, therefore, possible that some of the association between CA and MTA in the VaD group represented shared vascular injury to the cerebrum and hippocampus, respectively.
We observed a complex relationship between vascular disease and MTA. Stroke was not associated with MTA among AD subjects but was associated with less MTA among VaD subjects. Furthermore, WMH was significantly associated with worse MTA scores in both groups. These seemingly disparate results may be reconciled through consideration of the potential mechanisms leading to WMH and macroscopic infarcts. In a study of subcortical vascular cognitive impairment (27), Chui et al. observed greater WMH and HS in the subcortical vascular disease group compared to the AD group. Subsequently, these investigators reported significant univariate associations of WMH with HS (2) suggesting that vascular medial-temporal injury may result from small, rather than large, vessel cerebrovascular disease. The association between WMH and MTA in the VaD group found here, therefore, might reflect a similar relationship with large vessel disease causing more cortical infarcts and dementia, while preserving hippocampal integrity, but small vessel vascular disease causing increased WMH and hippocampal injury. It is also intriguing to suggest that small vessel disease association with WMH in AD may also contribute to hippocampal injury.(28) However, it is also possible that WMH in AD might result from an alternative neurodegenerative pathway (29) leading to a significant association between MTA and WMH for alternative reasons. These intriguing hypotheses require further research.
Despite pathological differences between AD and VaD, we found MTA and MMSE scores were correlated in both groups. MTA values were strongly and equally associated with cognition in both groups, explaining approximately 13% of the variance in MMSE scores while vascular measures, particularly WMH, were associated with cognition only in the VaD group, but explained only a small amount of variance (4%). These findings suggest that neuronal cell loss (as exemplified by atrophy) is specific to cognition independent of dementia etiology.
Use of a single rater for MRI-based scales of atrophy and cerebrovascular health presented a unique opportunity despite several limitations. First, the rater was not blind to the study of origin; however, again we stress that the studies contained AD subjects and either VaD subjects or controls, and the rater was blind to diagnosis within study. Second, the observed relationships among vascular measures, atrophy, and cognitive functioning in these uniquely ascertained cohorts where the diagnostic criteria are designed to minimize the inclusion in either group of subjects with pathophysiology more characteristic of the other group cannot be generalized to clinical populations. Thus, it would be inappropriate to develop diagnostic recommendations based on parameter estimates obtained here. Third, our use of semi-quantitative measures may reduce sensitivity to group differences. Of course, the large sample size compared to previous studies would mitigate any lack of power caused by using semi-quantitative measures. Moreover, the use of semi-quantitative measurements may also be a strength, as this would tend to reduce artificial differences between groups which may arise due to subtle differences in the MRI machines and specific scan parameters used at each study site. Finally, because these data are cross-sectional, one cannot assess the rate of atrophy in an individual or use these data to predict the transition from a non-demented to a demented state.
VaD is radiologically defined by large and small vessel infarction that can include multiple basal ganglia and white matter lacunes, bilateral thalamic infarctions or large hemispheric infarctions with or without accompanying extensive WMH.(30) Large cortical infarctions, multiple lacunae and thalamic infarcts are each sufficient to cause vascular dementia in the setting of limited AD pathology (26, 31), whereas the potential role of extensive WMH in dementia is less clear.(27, 32) Like previous reports (30), these analyses identified a significantly increased prevalence of vascular risk factors and MRI evidence of vascular brain injury in VaD subjects. Similar degrees of generalized brain atrophy were also found for the dementia groups. Unlike the study by Scheltens and colleagues (30), the distribution of MTA measures in these data was significantly different between VaD and AD groups, with less atrophy on average for the VaD group. Despite differences in MTA distribution, MTA measures remained significant predictors of cognition in VaD patients, confirming a previous report.(9) These results strongly suggest a consistent relationship between clinically recognized dementia and tissue loss independent of etiology, a unique finding of the current study.
These results may impact future clinical trials aimed at primary or secondary prevention of vascular or Alzheimer dementias. Although selected treatments may vary, clinical outcomes are likely to be tied to slowing or preventing brain tissue loss. Furthermore, the role of medial-temporal injury to the dementia syndrome in VaD is an understudied area. Understanding the process by which tissue loss occurs with vascular brain injury—beyond the obvious areas of necrosis from infarction—may provide important new areas for drug development.
Supplementary Material
Supplemental Figure Legends
Supplemental Figure 1 Title: Tie points used for evaluation of global cerebral atrophy.
Supplemental Figure 2 Title: Tie points used for evaluation of white matter hyperintensity.
Supplemental Figure 3 Title: Range of anterior hippocampus.
Acknowledgements
We gratefully acknowledge the contributions of the MIRAGE Study Group whose members are: Drs. Lindsay A. Farrer, Robert C. Green, L. Clinton C. Baldwin, L. Adrienne Cupples, Kathryn Lunetta, and Mark Logue (Boston University); Drs. Abimbola Akomolafe, Allison Ashley, Lorin Freedman, and Elizabeth Ofili (Morehouse School of Medicine); Dr. Helena Chui (University of Southern California); Dr. Charles DeCarli (University of California – Davis); Dr. Ranjan Duara (Mt. Sinai Medical Center, Miami); Drs. Tatiana Foroud and Martin Farlow (Indiana University School of Medicine); Dr. Robert Friedland (University of Louisville); Dr. Rodney Go (University of Alabama-Birmingham); Dr. Alexander Kurz (Technical University, Munich, Germany); Dr. Thomas Obisesan (Howard University); Drs. Helen Petrovitch and Lon White (Pacific Health Research Institute); Dr. Marwan Sabbagh (Sun Health Research Institute); Dr. Dessa Sadovnick (University of British Columbia); and Dr. Magda Tsolaki (University of Aristotle, Thessaloniki, Greece). We are indebted to Michael Wake for project coordination and John Farrell for database programming and electronic data capturing support.
We would also like to acknowledge the contributions of additional Eisai Inc. study members, including Harut Chaghasbanian, Timothy Hsu, MD, and Qin Wang, PhD.
Sources of funding
This work was supported by Eisai Inc. and by NIH grants R01-AG09029, R01-AG025259, R01-HG/AG02213, K24-AG027841 and P30-AG13846, P30-AG10129.
Abbreviations List
- AD
Alzheimer's disease
- VaD
vascular dementia
- WMH
white matter hyperintensities
- MTA
medial-temporal atrophy
- CA
cerebral atrophy
- MRI
Magnetic resonance imaging
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Exam
- CN
cognitively normal
- CD
single rater
- NI
total number of infarcts
- HS
hippocampal sclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
S. Hurt has provided consultation to Eisai concerning this and other studies. H. Posner was a previous employee of Eisai Medical Research Inc and is a current employee of Pfizer Inc. H. Zou and M. Moline are employees of Eisai, Inc. R. Green, M. Logue, L. Farrer, A. Cupples, and K. Lunetta, and C. DeCarli have no financial relationships or other competing interests that could be perceived as biasing the study whether or not this support was related to the subject of the manuscript.
REFERENCES
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 2.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills S, Cain J, Purandare N, Jackson A. Biomarkers of cerebrovascular disease in dementia. Br J Radiol. 2007;80:S128–45. doi: 10.1259/bjr/79217686. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 4.Du AT, Schuff N, Laakso MP, Zhu XP, Jagust WJ, Yaffe K, et al. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology. 2002;58:1635–41. doi: 10.1212/wnl.58.11.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR, Jr., Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr., Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–89. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr., Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastos-Leite AJ, van der Flier WM, van Straaten EC, Staekenborg SS, Scheltens P, Barkhof F. The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke. 2007;38:3182–5. doi: 10.1161/STROKEAHA.107.490102. [DOI] [PubMed] [Google Scholar]
- 10.Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–35. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser DJ, Kanz JE, Garrett KD. White Matter Hyperintensities and Cognition. In: Paul RH, Cohen R, Ott BR, Salloway S, editors. Vascular Dementia: Cerebrovascular Mechanisms and Clinical Management. Humana Press, Inc.; Totowa, NJ: 2005. pp. 223–9. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Roman GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–21. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demissie S, Green RC, Mucci L, Tziavas S, Martelli K, Bang K, et al. Reliability of information collected by proxy in family studies of Alzheimer's disease. Neuroepidemiology. 2001;20:105–11. doi: 10.1159/000054768. [DOI] [PubMed] [Google Scholar]
- 16.Farrer LA, Cupples LA, Blackburn S, Kiely DK, Auerbach S, Growdon JH, et al. Interrater agreement for diagnosis of Alzheimer's disease: the MIRAGE study. Neurology. 1994;44:652–6. doi: 10.1212/wnl.44.4.652. [DOI] [PubMed] [Google Scholar]
- 17.Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. Jama. 2002;287:329–36. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 18.Cuenco KT, Green RC, Zhang J, Lunetta K, Erlich PM, Cupples LA, et al. Magnetic resonance imaging traits in siblings discordant for Alzheimer disease. J Neuroimaging. 2008;18:268–75. doi: 10.1111/j.1552-6569.2007.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeCarli C, Frisoni GB, Clark CM, Harvey D, Grundman M, Petersen RC, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–15. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.R Development Core Team R: A language and environment for statistical computing. 2008.
- 22.Olkin I, Finn JD. Correlations redux. Psychological Bulletin. 1995;118:155–64. [Google Scholar]
- 23.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–8. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 25.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–7. [PubMed] [Google Scholar]
- 26.Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, et al. Vascular dementia in a population-based autopsy study. Arch Neurol. 2003;60:569–75. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 27.Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60:677–87. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–70. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 29.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheltens P, Kittner B. Preliminary results from an MRI/CT-based database for vascular dementia and Alzheimer's disease. Ann N Y Acad Sci. 2000;903:542–6. doi: 10.1111/j.1749-6632.2000.tb06411.x. [DOI] [PubMed] [Google Scholar]
- 31.Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–44. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 32.Leys D, Erkinjuntti T, Desmond DW, Schmidt R, Englund E, Pasquier F, et al. Vascular dementia: the role of cerebral infarcts. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S38–48. doi: 10.1097/00002093-199912003-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends
Supplemental Figure 1 Title: Tie points used for evaluation of global cerebral atrophy.
Supplemental Figure 2 Title: Tie points used for evaluation of white matter hyperintensity.
Supplemental Figure 3 Title: Range of anterior hippocampus.


