Abstract
Background
Despite known gender differences in recovery, few studies have examined symptom management (SM) interventions or responses by gender after coronary artery bypass surgery (CABS).
Objective
The purpose of this subanalysis was to describe and evaluate differences in response by gender to an SM intervention on the presence and burden of symptoms, physical activity, and physical functioning in elderly CABS patients during the early discharge period (3 and 6 weeks after CABS, and 3 and 6 months after CABS).
Methods
The parent study whose data were analyzed to examine gender differences involved a two-group, randomized clinical trial design. The 6-week early recovery SM telehealth intervention was delivered by the Health Buddy. Measures included the Cardiac Symptom Survey, a Modified 7-Day Activity Interview, an RT3 accelerometer, an Activity Diary, and the Medical Outcomes Study Short Form 36. This study was not powered for a gender × group analysis, and we used descriptive statistics, χ2 tests, t tests, and analysis of variance for statistical analyses.
Results
Subjects (n = 232) included 192 men and 40 women, with a mean age of 71.2 SD, 7 years. The intervention group consisted of 86 men and 23 women, and the usual care (UC) group consisted of 106 men and 17 women. Data trends suggest that the SM intervention exerted greater impact on women than on men for symptoms such as fatigue, depression, sleep problems, and pain. Again, men exhibited higher levels of physical activity than did women. However, women in the SM group generally had higher scores than did women in the UC group.
Conclusion
Although the parent study found no effect of an early recovery SM intervention, this exploratory secondary analysis indicated that women in the intervention group demonstrated more improvement in measures of physical activity than did those in the UC group. Further study, using a larger sample, is necessary to test these preliminary results.
Keywords: Gender Differences, Symptoms, Physical Functioning, Physical Acclivity
Cardiovascular disease (CVD) is the largest single cause of death among women, accounting for one third of women’s deaths.1 Women differ from men in their risk profile for CVD and cardiovascular outcomes. Women manifest a greater symptom burden, poorer functional ability, more healthcare needs, and more adverse outcomes.2 Specifically, women who undergo cardiac surgery have a lower functional capacity before and after surgery,3 experience more impaired physical functioning, engage in less physical activity, and are slower to return to normal functioning, compared with men.4 Women have a higher prevalence and persistence of cardiac-related symptoms after cardiac surgery,4 and a higher incidence of adverse long-term outcomes after coronary artery bypass surgery (CABS).5 Although intervention studies were conducted after cardiac surgery, none considered gender differences in symptoms and functioning before surgery, or involved adequate sample sizes to compare gender differences according to intervention group on recovery outcomes after surgery. Therefore, this report describes the results of a subanalysis that examined the influence of gender on the impact of a symptom management (SM) intervention, compared with usual care (UC), among elderly patients (>65 years old) during the early recovery period after CABS. We specifically studied (1) the presence and burden of symptoms at 3 and 6 weeks and at 3 months, (2) physical activity, and (3) physical functioning at 3 and 6 weeks, and at 3 and 6 months.
Related Literature
The literature relating to gender differences in symptoms after CABS and the impact of symptoms on physical function and activity will be discussed. SM interventions that were reported for cardiac patients (including those undergoing CABS) and the influence of gender on the intervention will also be discussed.
Influence of Gender Differences After CABS on Symptoms, Physical Activity, and Functioning
Fatigue most often occurs during the early recovery period, from 2 to 6 weeks after cardiac surgery,6-8 and often persists for 1 to 2 years after cardiac surgery.9 Fatigue was more prevalent among women compared with men after CABS.4 During the first 6 months after CABS, fatigue was consistently reported to exert the greatest interference in both physical activity and enjoyment of life.10 Fatigue limits cardiac patients’ ability to be physically active, and is a constant reminder of their ongoing heart problem.11 Fatigue is also associated with more impaired psychosocial functioning at 3 weeks after CABS, and with more impaired role physical functioning at 6 weeks after CABS, as measured by the Medical Outcomes Study Short Form 36 (MOS SF-36).12
Approximately 50% of patients experience sleep disturbance during the first few weeks and months after CABS.8,13 Women experience poorer sleep quality than men,4,14 which in turn may be associated with decreased daytime physical functioning, impaired emotional well-being, longer sleep onset latency, more difficulty falling asleep, later morning awakenings, more awakenings, and total waking time.13-15
Women often experience more pain16 and higher intensities of pain17 from 1 to 6 weeks after CABS.18-20 When pain persisted during the first 7 weeks after CABS, it also interfered with quality of sleep.21 Although postoperative pain may dissipate over time, the literature suggests that women may experience more pain and have different responses to its impact on their daily lives (eg, interference with household tasks).
Anxiety and depression are the most frequently reported symptoms associated with impaired physical functioning22 and with psychosocial functioning between hospital discharge and 6 weeks23 and 6 months24 after CABS. Women experience more anxiety before surgery and after CABS: specifically, within the first 8 weeks25 to 3 months4 and up to 1 year26 after CABS, compared with men. The reported higher rates of anxiety among women may be related to perceptions of feeling alone in response to their cardiac event, and perceived lack of spousal support.27 Anxiety can cause additional burdens on patients, because preoperative distress is negatively related to quality of life preoperatively (r = −.35, P < .01), at 1 month after cardiac surgery (r = −.45, P < .01), and 6 months after cardiac surgery (r = −.40, P < .01).28 Higher preoperative levels of anxiety (State-Trait Anxiety Inventory trait scores >45) were associated with higher mortality rates in a prospective study of patients after CABS (n = 180).29 Women experienced more depressive symptoms, and were more likely to develop depressive symptoms or remain depressed from 1 to 8 weeks after CABS compared with men.26,30 Furthermore, females with depression demonstrated significantly poorer physical functioning.31,32
Symptom Management Interventions
The impact of symptoms on patients’ physical activity, functioning, and health-related quality of life (HRQoL) underscore the critical need for interventions to manage postoperative symptoms. SM interventions were conducted to alleviate psychosocial symptoms (depression and anxiety)33-36 and physical symptoms such as pain.17,37 Some studies reported conflicting results on the significant effects of SM interventions in improving patients’ depressive symptoms,33,34,36 whereas others demonstrated no efficacy.17,35,38
Methods commonly used to deliver SM interventions include telephone contact,33 face-to-face interviews,35,36,38 and video combined with face-to-face interviews.34 Other innovative methods of delivering SM interventions include telehealth modalities, and these modalities may be advantageous for reaching patients in a wide range of settings (eg, rural).39-41
Influence of Gender on Cardiac Interventions
Evidence supports a gender difference in the efficacy of cardiac rehabilitation (CR) programs in the coronary artery disease population.42-44 After CR, women demonstrated greater improvement in psychosocial outcomes, such as anxiety, depression,42 and social isolation,43 whereas men exhibited greater improvement in functional capacity.44 Moreover, women were reported to demonstrate greater long-term adherence to a dietary intervention component.44 One study found that women in a gender-specific CR program had higher attendance rates, compared with nongender-tailored CR programs.45
In postcardiac surgical patients (coronary artery bypass graft and percutaneous coronary intervention), women were more responsive to an exercise component, with greater improvement in total cholesterol levels and exercise capacity, whereas men were more responsive to the stress management component and had greater reduction in weight, lipids levels, and hemoglobin A1c levels.46
For post-CABS patients, only a few studies examined gender differences in response to an intervention.4,17,33 Rollman et al reported that men with depression benefited more from a nurse-led SM intervention delivered by telephone, compared with women.33 However, none of these studies were designed or powered to examine the impact of gender on the intervention. No study has been conducted to examine the specific impact of gender on an SM intervention for the postoperative CABS population.
Conceptual Framework
The conceptual model for SM was developed with a primary focus on subjective symptom experiences, and selected conceptual definitions of the conceptual model of SM were used in this study.47 The three interrelated dimensions in the model include symptom experience, SM strategies, and symptom outcomes. SM often requires changes in strategies over time or in response to acceptance of a strategy. Multidimensional indicators of symptom outcomes include symptom status, functional status, emotional status, self-care ability, cost (financial status), quality of life, morbidity and comorbidity, and mortality. In this study, symptoms were viewed as a subjective experience reflecting changes in a person’s biopsychosocial functioning, sensation, or cognition. This analysis was conducted to determine the influence of gender on the impact of an SM intervention on 3 study outcomes: (1) the presence and burden of symptoms, (2) physical activity, and (3) physical functioning. Symptom status was defined as the presence or absence of a symptom and symptom burden (mean severity and frequency ratings of each individual symptom). Other outcomes in this study related to functional status, such as physical activity and physical functioning. SM strategies were incorporated into the intervention to influence one or more of the desired outcomes (symptom burden and functioning). According to an underlying assumption of the conceptual model of SM, all troublesome symptoms are in need of management through professional and self-care strategies because they may prevent or delay a positive outcome (such as relief of symptoms or increased physical activity and enhanced functional status in the CABS population).
Materials and Methods
Design
Data for this subanalysis were drawn from a study that examined the effects of an SM intervention with UC, compared with UC only, on postoperative physical activity and functioning among older adults (aged 65 years or older). The SM intervention used a device from Bosh Healthcare called the Health Buddy (HB) as an innovative means to communicate with the patient. Details of this intervention were reported previously.39,48 The HB is a 6 × 9 inch device that attaches to the patient’s telephone (for downloading and transferring data). It is a simple, user-friendly, visible, efficient means of communication. The purposes of the intervention were to assess and manage patient symptoms, provide positive reinforcement to increase self-efficacy, promote patient knowledge of self-care, educate patients, and monitor patient response to the intervention. Each day for 6 weeks, patients received a generic script (a set of questions and health-related information or suggestions) relevant to the management of their overall recovery. Tailored scripting was provided based on patient responses, but was standard for the response. Patients responded to questions by pushing 1 of 4 buttons on their screen. Results of the primary study did not reveal significant differences in physical activity or functional outcomes.48 Therefore, the present subanalysis focused on determining if the effects of the SM intervention compared with UC differed by gender in terms of symptom presence, symptom burden, physical activity, and physical functioning after surgery.
Sample
Subjects were recruited from 4 Midwestern tertiary hospitals. Subjects met several criteria, ie, they were well-oriented to persons, places, and time; were able to hear, see, speak, and read English; were aged 65 years or older and had undergone CABS; had a phone with nonrotary phone service; had been discharged within 7 days after surgery; had no physical impairments that would limit their physical functioning; and were not receiving home healthcare.
Measurement of Variables
The Cardiac Symptom Survey (CSS) was used to measure the symptom presence and symptom burden of cardiac-related symptoms (angina, shortness of breath, fatigue, depression, problems sleeping, incision pain, leg swelling, fluttering/rapid heartbeats (palpitations), anxiety, and poor appetite). Symptom presence (presence or absence of each symptom) was measured first. Symptom burden was then measured as a mean of the frequency and severity ratings of each symptom (range of 0 to 10, with 0 indicating absence of the symptom, and 10 indicating very frequent or very severe symptom). The content validity of the CSS was established through interviews and questionnaires with CABS patients and nurse researchers, and through reviews of the literature.8,49 The psychometric properties of the CSS were reported.49
Data on physical activity were collected using the Modified 7-Day Activity tool, the RT3 accelerometer (Stayhealthy, Inc., Monrovia, CA) and an Activity Diary. The Modified 7-Day Activity Interview measured a subject’s baseline level of physical activity before CABS (total kcal/day expended, average kcal/kg/day expended, and average minutes/day spent in moderate or greater activity).50 These variables were derived from subjects’ reported number of hours per day in sleep and in light, moderate, hard, and very hard activity (based on metabolic equivalent levels). This instrument demonstrated concurrent validity when used in older adults.50 The RT3 triaxial accelerometer measures body motion (specifically, the electrical energy of acceleration and deceleration) during activities that involve energy cost. The RT3 is approximately the size of a pager, and was worn continuously, except during sleep, for 3 consecutive days during each data collection period. The RT3 yields “activity counts” of a subject’s amount of activity. These counts were converted to kcal expended, to derive measures of average kcal expended/day and kcal/kg/day expended. Triaxial accelerometers have documented validity, with correlations between the triaxial accelerometer and indirect calorimetry ranging from .86 to .96,51,52 and a correlation of .85 between RT3 activity counts and saturated venous oxygen saturation.53 The reliability coefficients for the RT3, based on a 3-day period of data collection at 3 different data collection points over time, were high (.85 to .97).54
An activity diary was completed by subjects, who recorded their amount of time spent in various physical activities (light, moderate, hard, or very hard) and asleep. The diary was used on those same 3 days when subjects wore the RT3 accelerometer during times of follow-up. The diary provided data on the mean number of minutes spent in light, moderate, hard, and very hard levels of physical activity. Three-day activity diaries such as this were successfully used to obtain activity data of free-living adults.55,56
The Medical Outcomes Study Short Form-36 (MOS SF-36, version 2.0) was used to measure physical function.57 The MOS SF-36 provides an 8-scale health functioning profile of both psychosocial and physical function (general health, physical, role physical, role emotional, social, bodily pain, mental health, and vitality functioning). Subscale scores range from 0 to 100, with higher scores indicating better or higher functioning. Satisfactory reliability was estimated by Cronbach’s α at .78 to .93.58,59 Physical function in this study was measured using 3 subscales: physical functioning, role-physical functioning, and vitality. Cronbach’s α in this study for the subscales ranged from .89 to .90 for all data collection times.
Data Collection
Approval from the Institutional Review Committees at the facilities was obtained. Every month, the project coordinator randomly checked (eg, made site visits, observed subject enrollment, monitored telephone follow-up calls) on different components of the data collection process, to assure the integrity of the methods used in this study.
Charge nurses on cardiovascular units identified and approached patients who met the study criteria. If patients were interested, a research assistant fully explained the study, invited them to participate, and obtained written, informed consent. Each potential subject was randomly assigned to the intervention or UC group stratified by gender, using a previously generated randomization schedule. The research assistant completed the Demographic and Patient Characteristic tool, and at baseline verbally administered the MOS SF-36, the CSS, and Modified 7-Day Activity Recall by asking subjects to reflect on their responses to these measures before undergoing cardiac surgery. At discharge, subjects in the SM group and their family members were provided specific and thorough instructions on the hookup and use of the HB device, and return demonstration and validation of their ability to read the screen and respond to questions were determined. A demonstration on how to wear and care for the RT3 accelerometer, along with a teaching booklet on wearing the RT3, was given to the patients.
Subjects were contacted by telephone at 3 and 6 weeks, and 3 and 6 months, after discharge, at which times the MOS SF-36 and CSS tools were completed by interview. Subjects were mailed the RT3 and diary to wear and complete, respectively, at each time point.
Data Analysis
The original trial was powered to examine the effects of an SM intervention on the primary outcomes, ie, physical activity and functioning (SF-36). Our previous data showed that women demonstrated a higher prevalence and persistence of cardiac-related symptoms after cardiac surgery.4 This study was not powered to examine the interactions between gender and the intervention. Because of the small sample size of women and severely non-normal distributed symptom variables, we used descriptive statistics, χ2 tests, t tests, and analyses of variance to describe differences at baseline and the trends of gender differences in response to the SM intervention on recovery outcomes (symptoms, physical activity, and physical functioning).
Research Findings
Sample Demographics and Baseline Clinical Variables
Out of 280 subjects, 232 completed the study and were followed for up to 6 months after CABS, with a total of 192 men and 40 women (86 men and 23 women in the SM intervention group, and 106 men and 17 women in the UC group). Approximately 24% of subjects in the SM group did not complete the study, compared with 11% in the UC group. The reasons for dropping out included subject burden (n = 36), rehospitalization or transfer to an extended care facility (n = 4), equipment malfunction (n = 3), an inability to reach the subject for follow-up (n = 2), and an inability to complete the intervention protocol (n = 3). Compared with subjects who completed the study, dropouts were more likely to be older (Mean [M], 73; SD, 5.6 years vs. M, 71; SD, 4.9 years), female (38% vs. 17%), unmarried (34% vs. 19%), and assigned to the intervention group (32% vs. 10%). Dropouts had fewer years of schooling (12 SD, 2.3 vs. 13 SD, 3), slightly fewer comorbidities (1.44 vs. 1.11), and a lower level of physical activity (kcal/kg/day expended M = 26 vs. 28, and minutes spent in moderate or greater activity M = 106 vs. 160) at baseline.
Among 232 subjects, men were significantly (P < .05) more likely to be married (93% vs. 55%) and working (49% vs. 24%). The average age for men (M, 71.4; SD, 4.5 years) and women (M, 72.2; SD, 5.1 years) was not significantly different. Educational levels were similar for men (M = 13.7 years) and women (M = 13.3 years). Both men and women had similar frequency distributions of risk factors (hypertension, high cholesterol, and family history of coronary artery disease) and body mass indices (which ranged from 28.0 to 29.5).
Symptom Differences by Gender Before the SM Intervention
Preoperatively, according to χ2 analysis, women reported a significantly (P < .05) higher presence and burden of symptoms in terms of fatigue, trouble sleeping, anxiety, and problems with appetite. In addition, women had significantly lower baseline scores in functional capacity duke activity status index, levels of physical activity (average kcal/kg/day expended and average minutes/day spent in moderate or greater activity), and physical functioning (physical and vitality subscales of the MOS SF-36). Postoperatively and before the SM intervention, no other gender differences in symptom presence were evident, except for shortness of breath. Women in the SM group had a higher percentage of reported shortness of breath (52%) than women in the UC group (12%), men in the SM group (25%), and men in the UC group (29%) (χ2 = 9.049, P = .029).
Symptom Differences by Gender During and After the SM Intervention
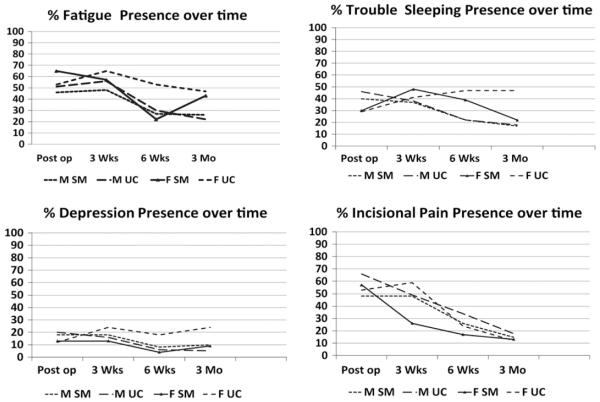
Women in general had a greater presence and burden of symptoms than men in terms of most symptoms. Data trends suggest that the SM intervention had more impact on women than men for symptoms such as fatigue, depression, sleep problems, and pain. Women in the SM group had less of a presence and burden of these symptoms compared with women in the UC group at 3 weeks, 6 weeks, and 3 months. For instance, although no significant difference at baseline was evident between the 4 groups (men in UC and SM, and women in UC and SM), the occurrence of fatigue was reduced in women in the SM group (65% at baseline, decreasing to 22% at 6 weeks and 43% at 3 months) compared with women in the UC group (53% at both baseline and 6 weeks, and 47% at 3 months). Fatigue scores decreased for men in the SM group (46% at baseline, and 26% at 3 months) and men in the UC group (from 51% at baseline to 22% at 3 months). Women in the SM group exhibited less depression (13% vs. 24% at 3 weeks, 4% vs. 18% at 6 weeks, and 9% vs. 24% at 3 months), less trouble sleeping (39% vs. 47% at 6 weeks, 22% vs. 47% at 3 months, and 26% vs. 35% at 6 months), and less pain (17% vs. 24% at 6 weeks), compared with women in the UC group who had demonstrated a similar baseline symptom profile. Women in the SM group were similar to men in both groups regarding depression. See Figure 1 for the presence of symptoms according to gender and group.
Figure 1.
Symptom presence by gender and group.
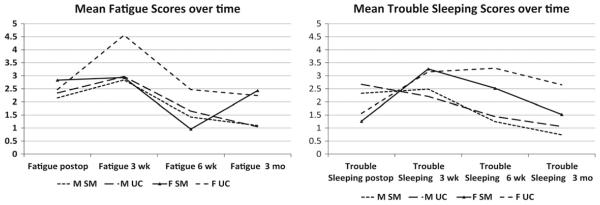
Regarding symptom burden (severity/frequency mean rating, 0 to 10), the overall mean scores were low (scores of 0 were calculated in means). Women in the UC group had higher scores for fatigue at 3 weeks (M, 4.56; SD, 3.68) and 6 weeks (M, 2.47; SD, 2.75) and lower baseline fatigue (M, 2.47; SD, 2.97), compared with women in the SM group (M, 2.93; SD, 2.81 at 3 weeks, and M, 0.96; SD, 1.94 at 6 weeks), with baseline scores of M, 2.83; SD, 2.65. Men in general had lower fatigue scores at baseline and during follow-up (Figure 1). In relation to trouble sleeping, women in the UC group had higher mean scores at 6 weeks (M, 3.29; SD, 3.82) and 3 months (M, 2.65; SD, 3.5), compared with women in the SM group (2.52 SD, 3.6 at 6 weeks, and 1.52 SD, 3.0 at 3 months) and men in the SM group (M, 1.25; SD, 2.65 at 6 weeks, and M, 0.74; SD, 1.953 months) and men in the UC group (M, 1.44; SD, 2.94 at 6 weeks, and M, 1.06; SD, 2.52 at 3 months). In general, men at baseline had higher scores than women (Figure 2). Similar trends were evident for other symptoms (eg, depression and pain). Similar means were evident between men in the SM and UC groups at 3 weeks, 6 weeks, and 3 months. These findings were similar to the pattern of symptom presence already described.
Figure 2.
Mean symptom scores for fatigue and trouble sleeping.
Physical Activity and Functioning Differences by Gender After SM Intervention
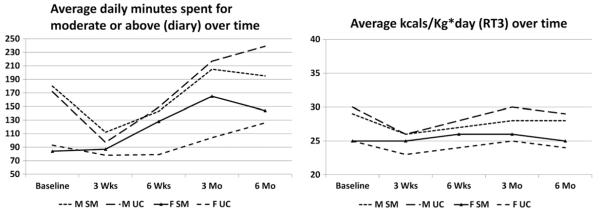
The Modified 7-Day Activity Interview measured a subject’s baseline level of physical activity before and after CABS. Levels of physical activity, measured according to energy expenditure (kcal/kg/day, using the RT3) and minutes spent in moderate or greater intensity activity (diary), showed a gender disparity after CABS. Men again had higher levels of physical activity than women, with women’s activity levels increasing at a much slower pace over time. In addition, measures of physical activity showed the impact of gender on the intervention. Women in the SM group had a higher average energy expenditure (kcal/kg/day) at 3 and 6 weeks and 3 and 6 months, and more minutes spent in moderate or greater intensity activity (diary) at 6 weeks and 3 months, than women in the UC group, given that no differences were evident at baseline between women in the UC and SM groups (Figure 3).
Figure 3.
Means physical activity scores over time by group and gender.
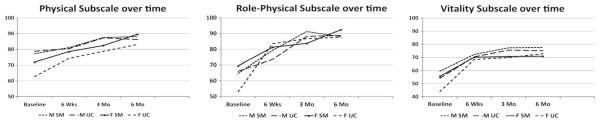
After accounting for baseline differences, no differences in measures of physical function (physical, role physical, or vitality subscales) were evident in terms of gender or group. Women did not reach levels of functioning as high as men, and their patterns of functional recovery were similar to men during the 6 months after CABS. Men and women in both groups improved in measures of physical function during the first 6 months after CABS (Figure 4).
Figure 4.
Means physical functioning scores over time by group and gender.
Discussion
Our study supports previous evidence regarding gender differences in recovery outcomes (symptoms, physical activity, and physical function) in patients after CABS. Consistent with findings by other researchers,30,32,60-63 women in this study had a higher presence and burden of symptoms, as well as a lower level of physical activity, than men. Preoperative factors that may explain the gender discrepancy among women for outcomes after CABS include a higher number of comorbidities (eg, heart failure, hypertension, peripheral vascular disease, or diabetes),64,65 more impaired functioning and cardiac condition before CABS,3 and delays in treatment66 attributable to atypical presentations of cardiac symptoms in women.67 In contrast to these findings, women had a similar comorbidity profile at baseline compared with men, which did not support the assumption that higher comorbidity in women leads to their suboptimal CABS outcomes.
Postoperative factors that may contribute to the gender differences experienced by women during CABS recovery include the differences in social roles and responsibilities in home management performed by women compared with men.30,68 Women traditionally have more responsibility in home management than men, and may feel greater disruption than men when they cannot resume their roles upon returning home after surgery. This may also explain the greater need for home care after discharge and the more frequent hospital readmissions among women in our study.
This study provides an important and unique perspective on the gender differences in response to a home-based SM intervention delivered by a telehealth device in the early recovery period after CABS. Women in the SM group had a lower incidence and burden of fatigue, trouble sleeping, depression, and pain during the early recovery period, compared with women in the UC group. Similar improvements associated with the SM intervention were not evident among male subjects in the SM group. Regarding physical activity, women in the SM group were more responsive to the intervention, and experienced higher levels of physical activity over time compared with women in the UC group. Women in the SM group were also able to achieve levels of physical activity that were comparable to those of men in both the SM and UC groups. Regarding measures of physical function using the SF-36, no gender difference was evident in response to the SM. Arguably, the SF-36 is a generic measure, commonly used to assess health status and HRQoL in the general population, and may not be very sensitive to changes of function in the post-CABS population.
The findings in this study are consistent with the literature that supports an association between symptoms and physical activity/function in cardiac populations.10,14,22,31,69-72 The underlying mechanism involves the alleviation of symptoms that are barriers or constraints to physical activity and function, resulting in higher levels of physical activity and function among postoperative CABS patients. Although several studies examined gender differences in CABS outcomes,4,30,63 little evidence is available regarding gender differences in response to post-CABS interventions and the potential explanatory factors. In this study, women were more responsive to the SM intervention, perhaps because women were more receptive and attentive than men to the educational information related to post-CABS care.17 Because women are more motivated to learn postoperative care strategies and engage in self-care activities, women may be more likely to use the strategies programmed into the SM intervention devices to manage their postoperative problems and seek the ongoing reassurance to safely resume housework and physical activity sooner. Women undergoing CABS were reported to be more likely older, widowed, and lacking family support.32,73 Men undergoing CABS are more likely to be married, to be cared for by their spouses, and to have more family support and fewer immediate household responsibilities. The social attention and support offered by the SM program may also have alleviated women’s feelings of isolation, and reduced their depression. Because our study was not originally designed to answer the question of why the intervention had a different effect on men and women in terms of recovery outcomes, further study in this area needs to be undertaken.
Limitations
The limitations of this study included its small sample size of women, and its use of convenience sampling. Because of the small sample size of women, this study was not powered for more sophisticated analytical methods that may have resulted in significant findings. The dropouts contributed to the baseline differences between the SM and UC groups, and skewed the study results. The convenience sample in this study was predominately white males, which affected the generalizability of results. However, this proportion of males to females is typical for the distribution of the CABS population. In summary, as in the study of Rollman et al,33 the results of our study generated from an unplanned post hoc analysis could only show some trends of gender differences in response to the SM intervention. To obtain significant evidence, a randomized, controlled trial is needed, with larger cohorts of women to examine the impact of gender on the SM interventions used to improve outcomes after CABS.
Conclusions and Recommendations
Our study was unique in providing descriptive evidence to suggest a gender disparity in response to an SM intervention for recovery outcomes after CABS, which was not previously reported in the literature, to the best of our knowledge. Further research, using a larger sample size of women, is needed to examine whether women and men exhibit different responses to an SM intervention after CABS. If a significant difference exists between women and men in response to SM interventions, more thorough study is needed to understand what factors contribute to these differences, what works better for men, and what works better for women. This information will provide insights into the development of gender-specific SM interventions to optimize outcomes for those who are at high risk for poor post-CABS outcomes. Our study demonstrated a plateau in physical activity/functioning measures, with increased depression and sleep problems at 3 and 6 months for both genders and groups. These findings support the need to consider a longer duration of intervention or a possible booster intervention at 3 months, to prevent the recurrence of symptoms and to help sustain and improve physical function and activity after CABS.
Acknowledgments
This study was funded by the National Institute of Nursing Research of the National Institutes of Health (grant R01 NR07759; L.Z., principal investigator).
Footnotes
This study’s contents are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
References
- 1.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Bugiardini R, Merz CNB. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guru V, Fremes SE, Austin PC, Blackstone EH, Tu JV. Gender differences in outcomes after hospital discharge fro coronary artery bypass grafting. Circulation. 2006;113:507–16. doi: 10.1161/CIRCULATIONAHA.105.576652. [DOI] [PubMed] [Google Scholar]
- 4.Schulz P, Zimmerman L, Barnason S, Nieveen J. Gender differences in recovery after coronary artery bypass graft surgery. Prog Cardiovasc Nurs. 2005;20:58–64. doi: 10.1111/j.0889-7204.2005.03868.x. [DOI] [PubMed] [Google Scholar]
- 5.Hassan M, Smith JM, Engel AM. Predictors and outcomes of sternal wound complications in patients after coronary artery bypass graft surgery. Am Surg. 2006;72:515–20. [PubMed] [Google Scholar]
- 6.Barnason S, Zimmerman L, Nieveen J, Hertzog M. Impact of a telehealth intervention to augment home health care on functional and recovery outcomes of elderly patients undergoing coronary artery bypass grafting. Heart Lung. 2006;35:225–33. doi: 10.1016/j.hrtlng.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Crane PB. Fatigue and physical activity in older women after myocardial infarction. Heart Lung. 2005;34:30–8. doi: 10.1016/j.hrtlng.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman L, Barnason S, Brey BA, Catlin S, Nieveen J. Comparison of recovery patterns for patients undergoing coronary artery bypass grafting and minimally invasive direct coronary artery bypass in the early discharge period. Prog Cardiovasc Nurs. 2002;17:132–41. doi: 10.1111/j.0889-7204.2002.00764.x. [DOI] [PubMed] [Google Scholar]
- 9.Chocron S, Tatou E, Schjoth B, Naja G, Clement F, Viel J, et al. Perceived health status in patients over 70 before and after open-heart operations. Age Ageing. 2000;29:329–34. doi: 10.1093/ageing/29.4.329. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman L, Barnason S, Nieveen J, Schmaderer M. Symptom management intervention in elderly coronary artery bypass graft patients. Outcomes Manage. 2004;8:5–12. [PubMed] [Google Scholar]
- 11.Friedman MM. Gender differences in the health related quality of life of older adults with heart failure. Heart Lung. 2003;32:327. doi: 10.1016/s0147-9563(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 12.Barnason S, Zimmerman L, Nieveen J, Schulz P, Miller C, Hertzog M, et al. Relationships between fatigue and early postoperative recovery outcomes over time in elderly patients undergoing coronary artery bypass graft surgery. Heart Lung. 2008;37:245–56. doi: 10.1016/j.hrtlng.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redeker N, Ruggiero J, Hedges C. Patterns and predictors of sleep pattern disturbance after cardiac surgery. Res Nurs Health. 2004;27:217–24. doi: 10.1002/nur.20023. [DOI] [PubMed] [Google Scholar]
- 14.Edell-Gustafson U, Svanbor E, Swahn E. A gender perspective of sleeplessness behavior, effects of sleep loss, and coping resources in patients with stable coronary artery disease. Heart Lung. 2006;35:75–89. doi: 10.1016/j.hrtlng.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Redeker N, Hedges C. Sleep during hospitalization and recovery after cardiac surgery. J Cardiovasc Nurs. 2002;17:56–68. doi: 10.1097/00005082-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Yorke J, Wallis M, McLean B. Patients’ perceptions of pain management after cardiac surgery in an Australian critical care unit. Heart Lung. 2004;33:33–41. doi: 10.1016/j.hrtlng.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Watt-Watson J, Stevens B, Katz J, Costello J, Reid GJ, David T. Impact of preoperative education on pain outcomes after coronary artery bypass graft surgery. Pain. 2004;109:73–85. doi: 10.1016/j.pain.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Miller KH, Grindel CG. Comparison of symptoms of younger and older patients undergoing coronary artery bypass surgery. Clin Nurs Res. 2004;13:179–93. doi: 10.1177/1054773804265693. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher R, McKinley S, Dracup K. Post discharge problems in women recovering from coronary artery bypass graft surgery. Aust Crit Care. 2004;17:160–5. doi: 10.1016/s1036-7314(04)80021-3. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman L, Barnason S, Schulz P, Nieveen J, Miller C, Hertzog M, et al. The effects of a symptom management intervention on symptom evaluation, physical functioning, and physical activity for women after coronary artery bypass surgery. J Cardiovasc Nurs. 2007;22:493–500. doi: 10.1097/01.JCN.0000297379.06379.b6. [DOI] [PubMed] [Google Scholar]
- 21.Hartford K. Telenursing and patients’ recovery from bypass surgery. J Adv Nurs. 2005;50:459–68. doi: 10.1111/j.1365-2648.2005.03427.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee GA. Determinants of quality of life five years after coronary artery bypass graft surgery. Heart Lung. 2009;38:91–9. doi: 10.1016/j.hrtlng.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Doering LV, Moser DK, Lemankiewicz W, Luper C, Khan S. Depression, healing, and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;14:316–24. [PubMed] [Google Scholar]
- 24.Goyal TM, Idler EL, Krause TJ, Contrada RJ. Quality of life following cardiac surgery: impact of the severity and course of depressive symptoms. Psychosom Med. 2005;67:759–65. doi: 10.1097/01.psy.0000174046.40566.80. [DOI] [PubMed] [Google Scholar]
- 25.McCrone S, Lenz E, Tarzian A, Perkins S. Anxiety and depression: incidence and patterns in patients after coronary artery bypass graft surgery. Appl Nurs Res. 2001;14:155–64. doi: 10.1053/apnr.2001.24414. [DOI] [PubMed] [Google Scholar]
- 26.Lindquist R, Dupuis G, Terrin M, Hoogwerf B, Czajkowski S, Herd J, et al. Comparison of health-related quality-of-life outcomes of men and women after coronary artery bypass surgery through 1 year: findings from the POST CABG Biobehavioral Study. Am Heart J. 2003;146:1038. doi: 10.1016/S0002-8703(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 27.Orth-Gomér K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: the Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–14. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 28.Panagopoulou E, Montgomery A, Benos A. Quality of life after coronary artery bypass grafting: evaluating the influence of preoperative physical and psychosocial functioning. J Psychosom Res. 2006;60:639–44. doi: 10.1016/j.jpsychores.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Szekely A, Balog P, Benko E, Breuer T, Szekely J, Kertai MD. Anxiety predicts mortality and morbidity after coronary artery and valve surgery: a 4-year follow-up study. Psychosom Med. 2007;69:625–31. doi: 10.1097/PSY.0b013e31814b8c0f. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V, Lin ZQ, Kasl SV, Mattera JA, Roumanis SA, Abramson JL, et al. Gender differences in recovery after coronary artery bypass surgery. J Am Coll Cardiol. 2003;41:307–14. doi: 10.1016/s0735-1097(02)02698-0. [DOI] [PubMed] [Google Scholar]
- 31.Plach SK, Hendrich SM, Jeske L. Fatigue representations in women with heart failure. Res Nurs Health. 2006;29:452–64. doi: 10.1002/nur.20156. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen EA, Wang F. Social support, depression, functional status, and gender differences in older adults undergoing first-time coronary artery bypass graft surgery. Heart Lung. 2009;38:306–17. doi: 10.1016/j.hrtlng.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF., III The bypassing the blues treatment protocol: stepped collaborative care for treating post-CABG depression. Psychosom Med. 2009;71:217–30. doi: 10.1097/PSY.0b013e3181970c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørlie T, Busund R, Sexton J, Sexton H, Sørlie D. Video information combined with individualized information sessions: effects upon emotional well-being following coronary artery bypass surgery—a randomized trial. Patient Educ Couns. 2007;65:180–8. doi: 10.1016/j.pec.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Lie I, Arnesen H, Sandvik L, Hamilton G, Bunch EH. Effects of a home-based intervention program on anxiety and depression 6 months after coronary artery bypass grafting: a randomized controlled trial. J Psychosom Res. 2007;62:411–8. doi: 10.1016/j.jpsychores.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Davila-Roman VG, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. 2009;66:387–96. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cebeci F, Celik SS. Discharge training and counselling increase self-care ability and reduce postdischarge problems in CABG patients. J Clin Nurs. 2008;17:412–20. doi: 10.1111/j.1365-2702.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 38.Deyirmenjian M, Karam N, Salameh P. Preoperative patient education for open-heart patients: a source of anxiety? Patient Educ Couns. 2006;62:111–7. doi: 10.1016/j.pec.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman L, Barnason S. Use of a telehealth device to deliver a symptom management intervention to cardiac surgical patients. J Cardiovasc Nurs. 2007;22:32–7. doi: 10.1097/00005082-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Brennan PF, Moore SM, Bjornsdottir G, Jones J, Visovsky C, Rogers M. HeartCare: an internet-based information and support system for patient home recovery after coronary artery bypass graft (CABG) surgery. J Adv Nurs. 2001;35:699–708. doi: 10.1046/j.1365-2648.2001.01902.x. [DOI] [PubMed] [Google Scholar]
- 41.Kleinpell A. Integrating telehealth as a strategy for patient management after discharge for cardiac surgery: results of a pilot study. J Cardiovasc Nurs. 2007;22:38–42. [PubMed] [Google Scholar]
- 42.Casey A. A model for integrating a mind/body approach to cardiac rehabilitation: outcomes and correlators. J Cardiopulm Rehabil Prev. 2009;29:230. doi: 10.1097/HCR.0b013e3181a33352. [DOI] [PubMed] [Google Scholar]
- 43.Barth J, Volz A, Schmid JP, Kohls S, von Kanel R, Znoj H, et al. Gender differences in cardiac rehabilitation outcomes: do women benefit equally in psychological health? J Womens Health. 2009;12:2033–9. doi: 10.1089/jwh.2008.1058. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R. Outcomes at one-year follow-up of women and men with coronary artery disease discharged from cardiac rehabilitation: what benefits are maintained? J Cardiopulm Rehabil Prev. 2007;27:11. doi: 10.1097/01.hcr.0000265015.44210.bf. [DOI] [PubMed] [Google Scholar]
- 45.Beckie TM. Predicting cardiac rehabilitation attendance in a gender-tailored randomized clinical trial. J Cardiopulm Rehabil Prev. 2010;30:147. doi: 10.1097/HCR.0b013e3181d0c2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daubenmier JJ. The contribution of changes in diet, exercise, and stress management to changes in coronary risk in women and men in the multisite cardiac lifestyle intervention program. Ann Behav Med. 2007;33:57. doi: 10.1207/s15324796abm3301_7. [DOI] [PubMed] [Google Scholar]
- 47.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–76. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 48.Barnason S, Zimmerman L, Nieveen J, Schulz P, Miller C, Hertzog M, et al. Influence of a symptom management telehealth intervention on older adults: early recovery outcomes following coronary artery bypass surgery (CABS) Heart Lung. 2009;38:364. doi: 10.1016/j.hrtlng.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieveen JL, Zimmerman LM, Barnason SA, Yates BC. Development and testing of the Cardiac Symptoms Scale in postoperative coronary artery bypass graft surgical patients. Heart Lung. 2008;37:17–27. doi: 10.1016/j.hrtlng.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Hellman EA, Williams MA, Thalken L. Modifications of the 7-Day Activity Interview for use among older adults. J Appl Gerontol. 1996;15:116–32. [Google Scholar]
- 51.Jakicic JM, Winters C, Lagally K, Ho J, Robertson RJ, Wing RR. The accuracy of the Tritac-R3D accelerometer to estimate energy expenditure. Med Sci Sports Exerc. 1999;31:754. doi: 10.1097/00005768-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Sherman WM, Morris DM, Kirby TE, Petrosa RA, Smith BA, Frid DJ. Evaluation of a commercial accelerometer (Tritrac-R3D) to measure energy expenditure during ambulation. Int J Sports Med. 1998;19:43–7. doi: 10.1055/s-2007-971878. [DOI] [PubMed] [Google Scholar]
- 53.Rowlands AV, Thomas PW, Eston RG, Topping R. Validation of the RT3 triaxial accelerometer for the assessment of physical activity. Med Sci Sports Exerc. 2004;36:518–24. doi: 10.1249/01.mss.0000117158.14542.e7. [DOI] [PubMed] [Google Scholar]
- 54.Hertzog MA, Nieveen JL, Zimmerman LM, Barnason SA, Schulz PM, Miller CL, et al. Longitudinal field comparison of the RT3 and an activity diary with cardiac patients. J Nurs Meas. 2007;15:105–20. doi: 10.1891/106137407782156363. [DOI] [PubMed] [Google Scholar]
- 55.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37:461–7. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 56.Sirard J, Melanson E, Li L, Freedson P. Field evaluation of the Computer Science and Applications, Inc. physical activity monitor. Med Sci Sports Exerc. 2000;32:695–700. doi: 10.1097/00005768-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Ware JE. The MOS 36-Item Short Form Health Survey (SF-36). 1. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 58.Jette D, Downing J. Health status of individuals entering a cardiac rehabilitation program as measured by the Medical Outcomes Study 36-Item Short-Form Study (SF-36) Phys Ther. 1994;74:521–7. doi: 10.1093/ptj/74.6.521. [DOI] [PubMed] [Google Scholar]
- 59.McHorney C, Ware J, Rachel J, Sherbourne C. The MOS 36-Item Short Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Moore SM, Dolansky MA. Randomized trial of a home recovery intervention following coronary artery bypass surgery. Res Nurs Health. 2001;24:93–104. doi: 10.1002/nur.1012. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell RHB, Robertson E, Harvey PJ, Nolan R, Rodin G, Romans S, et al. Sex differences in depression after coronary artery bypass graft surgery. Am Heart J. 2005;150:1017–25. doi: 10.1016/j.ahj.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 62.DiMattio MJ, Tulman L. A longitudinal study of functional status and correlates following coronary artery bypass graft surgery in women. Nurs Res. 2003;52:88–97. doi: 10.1097/00006199-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Sawatzky JA, Naimark BJ. The coronary artery bypass graft surgery trajectory: gender differences revisited. Eur J Cardiovasc Nurs. 2009;8:302–8. doi: 10.1016/j.ejcnurse.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Kim C, Redberg RF, Pavlic T, Eagle KA. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin Cardiol. 2007;30:491–5. doi: 10.1002/clc.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawatzky JA, Naimark BJ. Coronary artery bypass graft surgery: exploring a broader perspective of risks and outcomes. J Cardiovasc Nurs. 2009;24:198–206. doi: 10.1097/JCN.0b013e31819b534e. [DOI] [PubMed] [Google Scholar]
- 66.Nau DP, Ellis JJ, Kline-Rogers EM, Mallya U, Eagle KA, Erickson SR. Gender and perceived severity of cardiac disease: evidence that women are “tougher”. Am J Med. 2005;118:1256–61. doi: 10.1016/j.amjmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Zbierajewski-Eischeid SJ, Loeb SJ. Myocardial infarction in women: promoting symptom recognition, early diagnosis, and risk assessment. Dimens Crit Care Nurs. 2009;28:1–8. doi: 10.1097/01.DCC.0000325090.93411.ce. [DOI] [PubMed] [Google Scholar]
- 68.Vaccarino V, Lin ZQ, Kasl SV, Mattera JA, Roumanis SA, Abramson JL, et al. Sex differences in health status after coronary artery bypass surgery. Circulation. 2003;108:2642–7. doi: 10.1161/01.CIR.0000097117.28614.D8. [DOI] [PubMed] [Google Scholar]
- 69.Plach SK, Napholz L, Kelber ST. Depression during early recovery from heart surgery among early middle-age, midlife, and elderly women. Health Care Women Int. 2003;24:327–39. doi: 10.1080/07399330390191698. [DOI] [PubMed] [Google Scholar]
- 70.McSweeney JC, Coon S. Women’s inhibitors and facilitators associated with making behavioral changes after myocardial infarction. MEDSURG Nurs. 2004;13:49–56. [PubMed] [Google Scholar]
- 71.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER Registry. Am Heart J. 2009;157:1042–9. doi: 10.1016/j.ahj.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: testing dyadic dynamics using actor-partner interdependence model. J Psychosom Res. 2009;67:29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norris CM, Ghali WA, Galbraith PD, Graham MM, Jensen LA, Knudtson ML, et al. Women with coronary artery disease report worse health-related quality of life outcomes compared to men. Health Qual Life Outcomes. 2004;2:21. doi: 10.1186/1477-7525-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]