Abstract
Objective
Neovascularization is a physiological repair process that is partly dependent on nitric oxide. Extracellular superoxide dismutase (EcSOD) is the major scavenger of superoxide and thus is an important regulator of nitric oxide bioavailability and thus protects against vascular dysfunction. We hypothesized that overexpression of EcSOD in skeletal muscle would improve recovery from hind-limb ischemia.
Methods
Adeno associated virus (AAV) vectors expressing EcSOD or luciferase (control) from the Cytomegalovirus (CMV) promoter were cross-packaged into AAV9 capsids and injected IM into hind-limb muscles (1×1011 viral genomes(vg)/limb) of 12 wk-old mice. Ischemia was then induced after IM injections. Limb perfusion was serially measured by laser Doppler on days 0, 7 & 14 post-injection and values were expressed as a ratio relative to the non-ischemic limb. EcSOD expression was measured by Western blotting. Capillary density was documented by immunohistochemical staining for platelet endothelial cell adhesion molecule (PECAM). Apoptosis was assessed by Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and necrosis was visually evaluated daily.
Results
EcSOD expression was 2-fold up-regulated in EcSOD treated vs. control ischemic muscles at day 14. Capillary density was 1.9-fold higher in treated (1.65±0.02 capillaries/fiber) vs. control muscle (0.78±0.17 capillaries/fiber, p<0.05). Recovery of perfusion ratio at day 14 post-ischemia was 1.5-fold greater in EcSOD vs. control mice (p<0.05). The percentage of apoptotic nuclei was 1.3 ± 0.4% in EcSOD treated mice as compared to 4.2± 0.2% in controls (p<0.001). Limb necrosis was also significantly lower in EcSOD vs. control mice.
Conclusion
AAV9-mediated overexpression of EcSOD in skeletal muscle significantly improves recovery from hind-limb ischemia in mice, consistent with improved capillary density and perfusion ratios in treated mice.
Keywords: Extracellular superoxide dismutase, AAV-9, hind-limb ischemia, nitric oxide
Introduction
Peripheral arterial disease (PAD) is a major health care problem in the United States with an age-adjusted prevalence of 12%, that is expected to increase because of the aging population (1, 2). Intermittent claudication and critical limb ischemia are the two major clinical manifestations of PAD.
Medical treatments for patients with PAD include anti-platelet agents, angiotensin-converting enzyme inhibitors, and cholesterol lowering medications (statins). These treatments have no significant effect on limb outcomes, such as risk for major amputation (1,2). Angiogenesis is the growth and development of new capillaries from a pre-existing vasculature, and therapeutic angiogenesis seeks to harness this phenomenon to improve blood flow and treat disorders of inadequate tissue perfusion (3). The administration of cytokine growth factors to promote therapeutic angiogenesis, have been met with limited clinical success (4). Bone marrow (BM)-derived stem, and progenitor, cells are potential new therapeutic options to improve leg perfusion and/or wound healing which have shown promising results and several large randomized, placebo-controlled, double-blind studies are currently ongoing (5,6).
Neovascularization can be an adaptive mechanism which preserves tissue integrity despite vascular occlusions and the resulting ischemic injury. Extracellular superoxide dismutase (EcSOD) serves as a scavenger of superoxide which increases nitric oxide (NO) bioavailability in the blood vessel wall (7) by protecting it against superoxide-mediated conversion to peroxynitrite. In vessels with high levels of superoxide, NO is converted to peroxynitrite and may fail to reach smooth muscle cells, thereby producing endothelial dysfunction. EcSOD, which constitutes a large portion of total SOD in the vessel wall, is strategically located to protect NO during its diffusion to smooth muscle (7, 8). Kim et al. (9) showed that impaired neovascularization in EcSOD−/− mice is associated with enhanced superoxide production, TUNEL-positive apoptotic cells and decreased levels of NO2−/NO3− and cGMP in ischemic tissues as compared with wild-type mice.
Based on the fundamental biological properties of EcSOD and our recent findings from AAV9-mediated overexpression of EcSOD in the heart (10), we hypothesized that overexpression of EcSOD in skeletal muscle would similarly increase capillary density and thereby improve recovery from hind-limb ischemia in BALB/c mice, a strain has been shown to have poor perfusion recovery and is prone to develop necrosis of the affected limb (11).
Recombinant viral vectors based on the non-pathogenic human parvovirus, adeno-associated virus (AAV), have a number of attractive features including lack of cytotoxicity, ability to transduce both dividing and non-dividing cells, and long-term transgene expression (12). AAV serotype 9, which is under development for gene therapy applications, shows significantly enhanced transduction efficiency in muscle.(13). We therefore selected AAV9 as a vector for delivering EcSOD to ischemic skeletal muscles in the murine model of hind-limb ischemia.
Methods
Mice and Experimental Protocol
All protocols and procedures conformed to the Guidelines for Use of Laboratory Animals published by the United States Department of Health and Human Services and were approved by the University of Virginia Animal Care and Use Committee. All animals received care in accordance with Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985). All age-matched (12 to 16 weeks old) male mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Plasmids
The AAV vectors used in this study were derived from pACS as described previously (14). To create an AAV vector-generating plasmid in which the CMV IE promoter drives the expression of the firefly luciferase gene (pACMVLuc), a 2.2 kb HindIII-BamHI fragment carrying the firefly luciferase cDNA was excised from plasmid pGL3 (Promega, Madison, WI, USA) and inserted between the HindIII and BamHI sites of pACS. Plasmid pACMVEcSOD, an AAV-generating plasmid harboring mouse EcSOD driven by the CMV promoter, was constructed by inserting a mouse EcSOD cDNA between the Nhe I and BamHI sites of pACS.
AAV vector production and purification
AAV vectors were packaged into AAV9 capsids by the triple transfection method in HEK 293 cells. Helper plasmids pAdΔF6 (providing the three adenoviral helper genes) and plasmid p5E18-VD2/9 (providing AAV2 rep and AAV9 capsid genes) were kindly provided by Dr. James M. Wilson (15, 16). The AAV-generating plasmid and helper plasmids were transfected into HEK 293 cells by the calcium-phosphate co-precipitation method. A total of 50µg of plasmid DNA (mixed in equimolar ratio) was used per 15cm cell culture plate. Three days following transfection, AAV vectors were purified by ammonium sulfate fractionation and iodixanol gradient centrifugation (17). Virus particles were resuspended in 1 ml of DPBS-MK, dialyzed against DPBS-MK and stored at −80°C.
Determination of AAV vector titer
Titers for the AAV vectors (viral genomes/ml) were determined by quantitative real-time PCR as described previously (18). The primers used for amplifying luciferase were: 5’-AGAACTGCCTGCGTGAGATT-3’ (forward) and 5’-AAAACCGTGATGGAATGGAA-3’ (reverse). EcSOD was similarly amplified using: 5’CCTAGCAGACAGGCTTGACC-3’ (forward) and 5’-CCATCCAGATCTCCAGCACT-3’ (reverse). Known copy numbers (105–108) of the respective plasmids (pACMVLuc, or pACMVEcSOD) carrying the appropriate cDNAs were used to construct standard curves.
Bioluminescence imaging
Bioluminescence imaging was performed using C57BL/6 mice (n=6). Animals were anesthetized and maintained on 1–1.2% isoflurane in oxygen. D-luciferin (0.15mg/g body weight; (Xenogen Corp, Alameda, CA) was administered to mice by intraperitoneal injection. Eight minutes following D-luciferin administration, all mice were imaged using an IVIS 100 imaging system (Xenogen). Photons emitted from the mice were integrated for a period of 1 min. 3 serial recordings were done for each mouse. Images were processed using Living Image software (Xenogen) as described previously (18).
In vitro luciferase assay
Skeletal muscles, livers, hearts, lungs, kidneys and spleens from the mice injected with AAV/CMV/Luc were collected after bioluminescence imaging at 28 days post-injection. In vitro luciferase assays were performed as described previously (18) using a standardized luciferase assay (Promega Corp, Madison, WI).
Induction of hind-limb Ischemia
Mice were anesthetized using 2.5% isoflurane for induction and the maintenance dose during the surgical procedure was 1.5% isoflurane in oxygen. Unilateral femoral artery ligation and excision, resulting in hind-limb ischemia, was performed as described previously (19,22). Immediately after surgery, animals were given a subcutaneous injection of buprenorphine (0.05mg/kg). The mice received buprenorphine twice a day for 48 hours and were closely monitored for any signs of distress. In addition, post-operative mice were given tylenol (2 mg/ml), trimethoprim (16 mg/ml) and sulfamethoxazole (80mg/ml) in their drinking water.
Perfusion Recovery and Necrosis Scores
Blood flow in the ischemic and contralateral nonischemic limbs was measured as described previously with a laser Doppler perfusion imaging system (Perimed, Stockholm, Sweden) (19). Bilateral hind-limb perfusion measures were performed after light anesthesia with intraperitoneal injections of a ketamine/xylazine mix (7ml saline + 2ml ketamine (100mg/ml) + 1ml xylazine (20mg/ml): 0.1–0.15ml/25g mouse). Perfusion was expressed as the ratio of the left (ischemic) to right (nonischemic) hind-limbs. Necrosis was visually assessed every day for 7 days after surgery. The extent of necrosis was scored as follows: grade I, involving only toes; grade II, extending to dorsum pedis; grade III, extending to crus (lower leg, extending from the ankle to knee); and grade IV, extending to thigh or complete necrosis (20). Mice in which necrosis had spread to the plantar surface of the foot, or had Grade III necrosis, were euthanized in a CO2 chamber
Gene Transfer
Gene transfer was accomplished just prior to the induction of hind limb ischemia by two 10-second intramuscular injections (total: 0.04 ml) to the tibialis anterior (TA) and gastrocnemius (GA) muscles. A single operator performed all of the injections.
Tissue procurement, histological sections, and Western blotting
After the final perfusion measures were performed, an overdose of anesthetic was given, and the ischemic and contralateral TA and GA muscles were surgically excised from tendon to tendon. Euthanasia was confirmed using a CO2 chamber. The mid portions of the muscle were placed in OCT and snap frozen in liquid nitrogen, mounted and cryostat sections (5 um) were prepared. The remainder was snap frozen in liquid nitrogen. Protein lysates were prepared and protein concentrations were determined. The muscle homogenates were then separated on polyacrylamide gels, transferred to PVDF membranes, blocked and blotted with antibodies against EcSOD and GAPDH. EcSOD protein expression was determined in both non-ischemic C57BL/6 and BALB/c mice as well as ischemic BALB/c hind-limbs. EcSOD expression was normalized against GAPDH expression for quantitative analysis.
Capillary density, apoptosis, inflammatory infiltrate and cGMP in skeletal muscle
In skeletal muscles, vascular density was analyzed by immunofluorescent labeling using a rat anti-mouse CD31 antibody (BD Pharmigen, San Diego, CA) and visualized using goat anti-rat conjugated with Alexa Fluor 488 (Molecular Probes, Carlsbad, CA). Fibers were identified using Phalloidin toxin conjugated with Alexa Fluor-555 (Molecular Probes). In eight random 200× magnification fields, capillary density was expressed as capillaries/fiber. Apoptotic cells were stained using an in situ terminal deoxynucleotidyl transferase (TdT) biotin-dUTP nick-end labeling (TUNEL) apoptosis kit, ApopTag (Millipore, Temecula, CA), and quantified as described previously (20). β-gal histochemistry was performed as described previously. (18) Immunohistochemistry was performed on frozen sections using polyclonal rabbit anti-EcSOD antibody (Sigma-Aldrich), and rat monoclonals CD45 (BD Biosciences) and CD68 (AbD Serotec). For light microscopy, antibodies were detected using the ABC method (Vector Laboratories, Burlingame, CA) and visualized using DAB (DAKO Corp, Carpinteria, CA). Sections were then counterstained with Harris hematoxylin (Richard-Allen Scientific, Kalamazoo, MI). For immunfluoresence microscopy, visualization was performed using a goat anti-rabbit conjugated with Alexa Fluor-555 (Molecular Probes) and nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI; Molecular Probes). Sections were analyzed by light and fluorescence microscopy using an Olympus BX51 microscope and Image Pro Plus 3.0 software (Media Cybernetics, Silver Spring, MD). Infiltration of CD45 and CD68 positive cells was graded as: 1. 3 to 5 cells: normal infiltrate (score: 0); 6 to 8 cells: mild (score: 1); 9 to 10 cells: moderate (score: 2); >10 cells: severe (score: 3). Tissue cyclic GMP levels were determined using an enzyme immunoassay system (R&D Systems, Minneapolis, MN).
Statistical analysis
Data were expressed as medians ± SEM. For statistical comparisons of perfusion, capillary density, apoptosis, cGMP and EcSOD; expression between two groups of mice was compared using Student’s t test. Comparison of perfusion within each group at different time points was analyzed by repeated-measures ANOVA. The significance of the necrosis scores was assessed using the non-parametric Mann-Whitney U test. In all cases, P<0.05 was considered statistically significant.
Results
Time course of AAV9-mediated luciferase expression in murine hind-limb non ischemic skeletal muscles
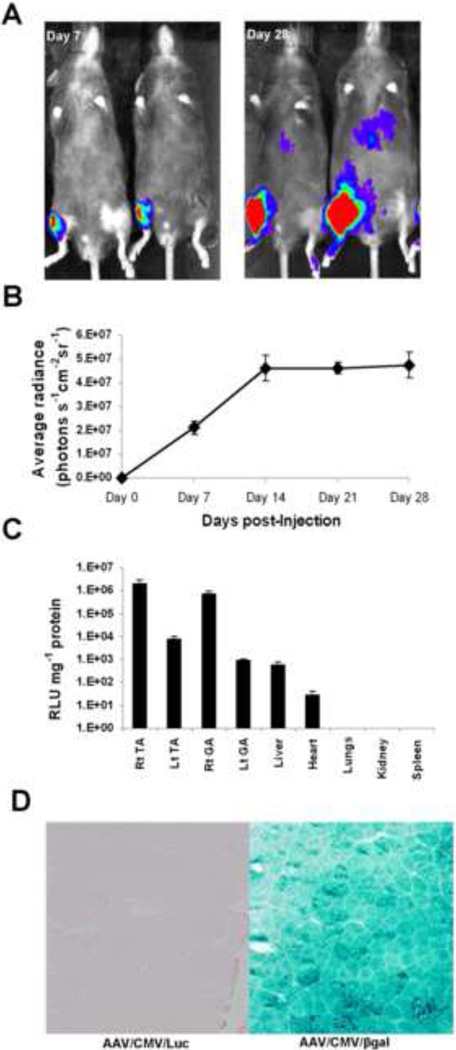
First we sought to determine the kinetics of AAV9-mediated gene expression in normal skeletal muscles. An AAV9 vector expressing the firefly luciferase gene under the control of cytomegalovirus promoter (AAV/CMV/Luc) was injected into right hind-limb (TA and GA) muscles of C57BL/6 mice (n=6/group). Seven days after vector injection, D-luciferin-dependent bioluminescence was detected in the right TA and GA but not in the left hind-limbs (Fig. 1A). The luciferase activity plateaued by 14 days-post injection (Fig. 1B). After 28 days, luciferase activity was 272-fold higher in Rt TA and 257-fold higher in Rt GA as compared to Lt TA and GA, respectively (Fig. 1C). Luciferase activities were at background levels in liver, heart, lungs, kidneys and spleen. In a separate experiment, mice (n=4/group) were injected with either AAV/CMV/Luc or AAV/CMV/βgal vectors and were euthanized 7 days after injection. Robust β-gal staining indicating virtually complete transduction of all muscle fibers was observed in mice injected with AAV/CMV/βgal, as opposed to no detectable β-gal staining in mice injected with AAV/CMV/Luc (Fig. 1D).
Figure 1.
A. Bioluminescence imaging of vector-injected mice: A. Mice were injected with AAV/CMV/Luc in the tibialis anterior and gastrocnemius muscles (5× 1010 vg/muscle). Luciferase expression in these mice was monitored for up to 6 weeks by in vivo bioluminescence imaging using an IVIS imaging system as detailed in Materials and Methods. B. Time course of in vivo AAV9-mediated luciferase expression: graph showing the time course of AAV9-mediated gene expression in mice injected with AAV9/CMV/Luc in tibialis anterior and gastrocnemius muscles. The mean values of bioluminescence as average radiance (photons/s/cm2/sr) were obtained from the regions of interest and plotted against time. C. In vitro luciferase assays were performed on protein extracts from skeletal muscles, liver, heart, lungs and spleen at 6 weeks following indicated vector injection. Luciferase activity was expressed as RLU per milligram of protein and was plotted on a log scale. D. Wild-type mice received a single intramuscular (IM) injection of AAV/CMV/Luc or AAV/CMV/βgal (1 × 1011 vg) in tibialis anterior muscles. βgal staining after 7 days shows efficient transduction of skeletal muscle fibers.
EcSOD overexpression in non-ischemic skeletal muscles after AAV9/CMV/EcSOD intramuscular injections
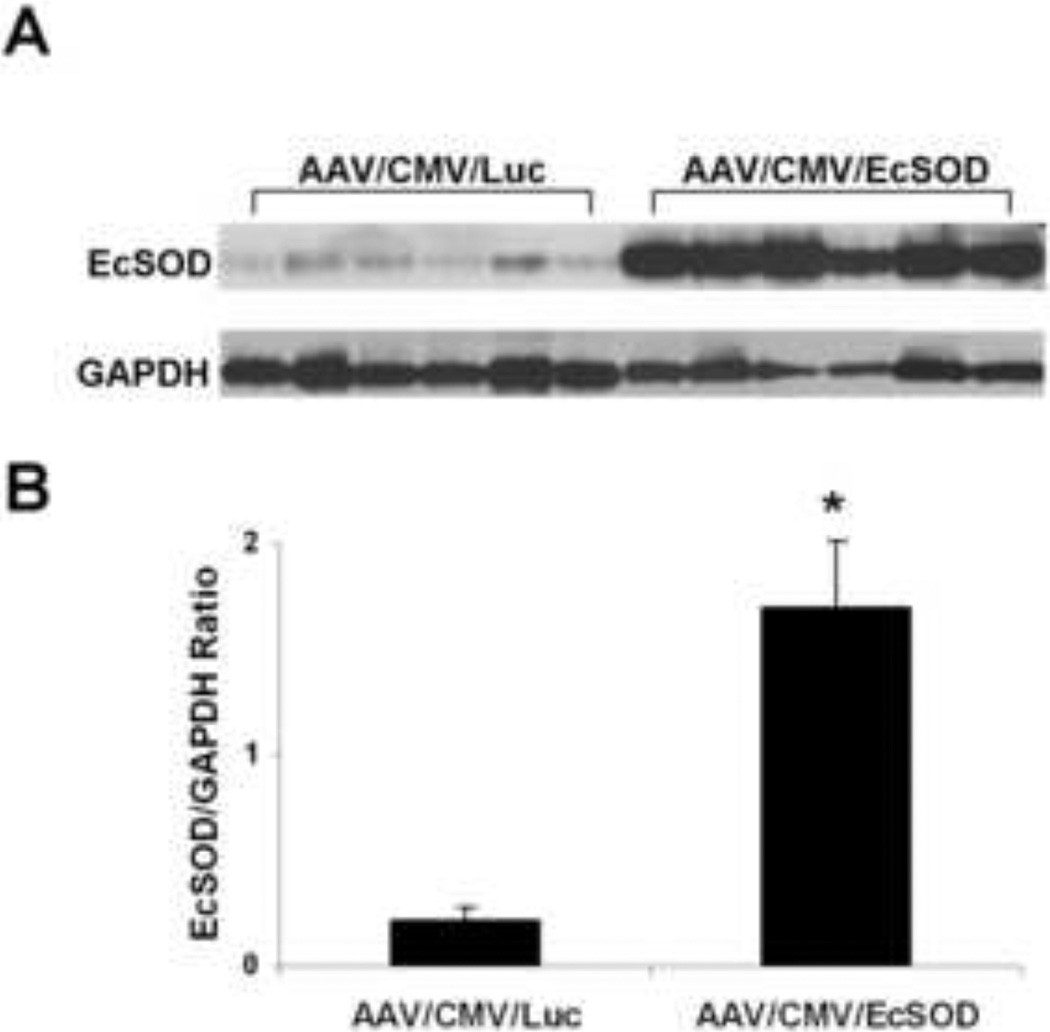
Left hind-limb skeletal muscles (TA and GA) of BALB/c mice were injected with AAV9/CMV/EcSOD or AAV9/CMV/Luc (n=10/group), 5×1010 (vg)/muscle, in 20 microliters). Mice were euthanized 14 days post injection and protein expression was assessed by Western blot analysis. EcSOD expression was normalized to GAPDH. EcSOD expression was 7.9-fold higher (P<0.002) in the EcSOD group as compared to the Luc group (Fig. 2). EcSOD expression was also determined in non-ischemic C57BL/6 hind limbs and an 8.2-fold increase in EcSOD expression was observed in AAV9/CMV/EcSOD injected limbs vs. its control.
Figure 2.
Quantitation of AAV9-mediated EcSOD expression in normal hind-limb muscles: A. Mice were injected IM with AAV9/CMV/Luc or AAV9/CMV/EcSOD (1 × 1011 vg/mouse) into the tibialis anterior and gastrocnemius muscles. 14 days following vector administration, EcSOD expression was detected by Western blot analysis. Levels of EcSOD expression were normalized to GAPDH protein. B. Bar graph showing the quantitation of EcSOD expression by Western blot analysis. EcSOD expression was 7.9-fold higher in the EcSOD-treated group as compared to controls (*P<0.002).
EcSOD protein expression in ischemic tissues is increased in response to hind-limb ischemia
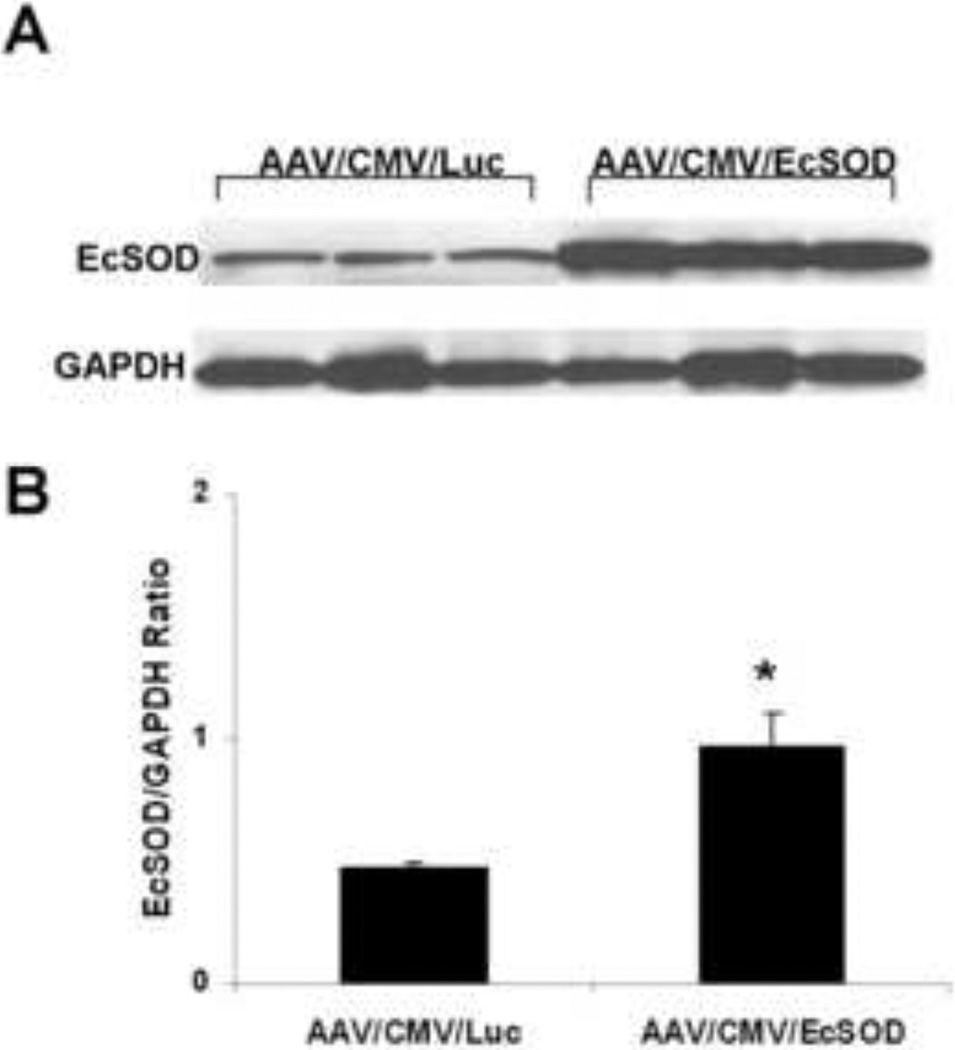
EcSOD expression in the ischemic limbs of AAV9/CMV/EcSOD treated mice (n=10/group) was 2-fold higher (P<0.05) as compared to the ischemic limbs of AAV9/CMV/Luc treated mice (Fig. 3). EcSOD expression was normalized to GAPDH.
Figure 3.
Quantitation of AAV9-mediated EcSOD expression in ischemic hind-limb muscles: A. Mice were injected IM with AAV9/CMV/Luc or AAV9/CMV/EcSOD (1 × 1011 vg/mouse) into the tibialis anterior and gastrocnemius muscles at the time of hind-limb ischemia surgery. 14 days following vector administration EcSOD expression was detected by Western blot analysis. Levels of EcSOD expression were normalized to GAPDH protein. B. Bar graph showing the quantitation of EcSOD expression by Western blot analysis. EcSOD expression was 2-fold higher in the EcSOD-treated ischemic muscles as compared to controls (n=10 per group, *P<0.05).
EcSOD gene transfer improves hind-limb perfusion recovery and reduces necrosis after hind-limb ischemia
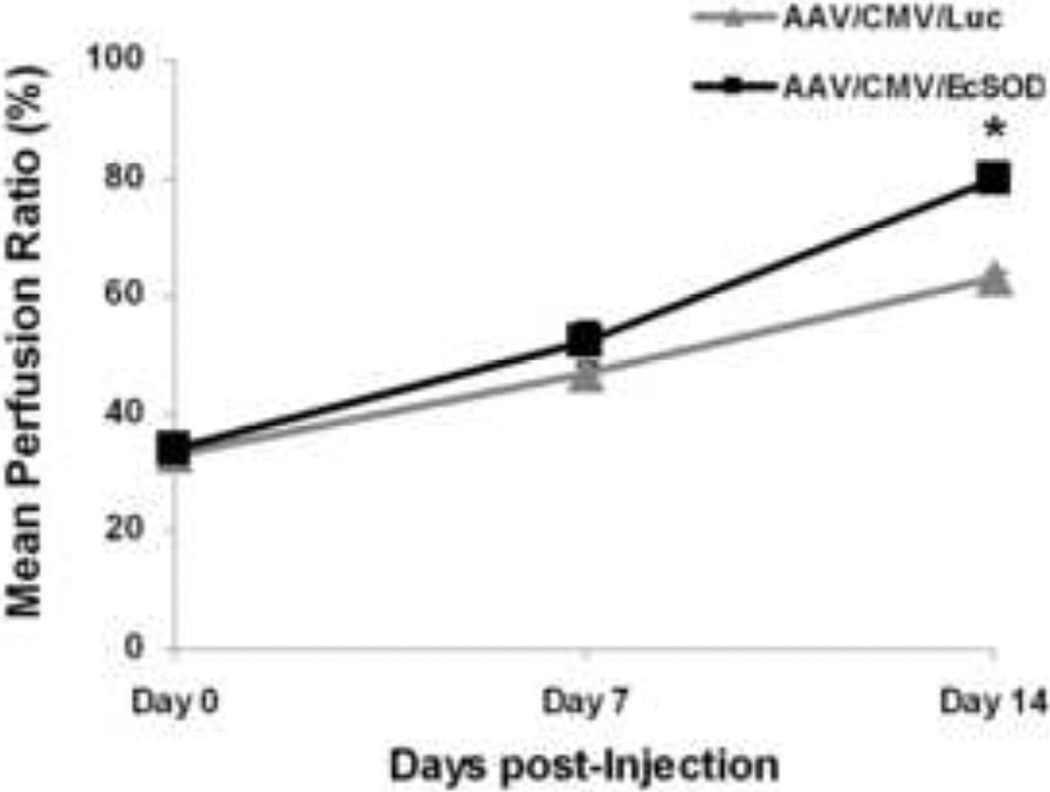
Hind-limb ischemia was surgically induced in left hind-limbs of EcSOD or Luc treated BALB/c mice (n=10/group). Intramuscular injections of AAV/CMV/EcSOD (5×1010 (vg)/muscle, in 20 microliters) were given immediately prior to surgery. The entire surgical procedure was completed in 20–25 minutes. Immediately after surgery on day 0, the perfusion ratio was 33.65 ±1.7% for EcSOD and 32.80±1.9% for Luc groups. At day 7, EcSOD injected mice had a perfusion ratio of 52.4±3.5%, whereas the perfusion ratio in the Luc group was 47.2±2.0%. Thus, no significant difference in perfusion rate was found between EcSOD and Luc groups after surgery (day 0) or on day 7 post-surgery. However, at day 14 post-surgery, EcSOD injected mice showed a perfusion ratio of 79.5±1.7%, whereas the perfusion ratio in Luc group was only 63.4±1.1% (P<0.01) (Fig. 4).
Figure 4.
Perfusion is improved in EcSOD-treated hind-limbs. Mean perfusion ratio was 33.7 ± 1.7% for EcSOD and 32.8 ± 1.9% for control groups at baseline on day 0 after hind-limb ischemia surgery and vector injection. The EcSOD group demonstrated significantly better perfusion recovery at the day 14 time point as compared to the control group (*P<0.01).
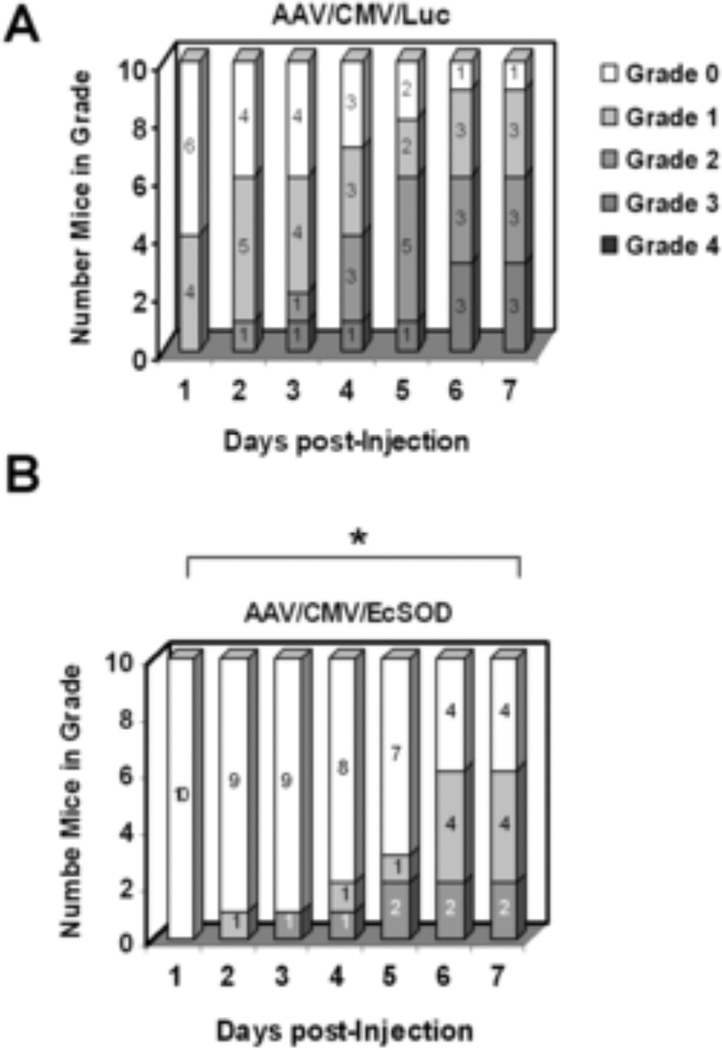
Figure 5 shows the number of mice with any necrosis and the degree of necrosis post-operatively. For example, on day 7 post injection 4 out of 10 mice in the EcSOD group showed no visible signs of necrosis, whereas only 1 mouse in the Luc group was similarly unafflicted. Furthermore, on day 7 no mice in the EcSOD group had grade 3 necrosis, whereas 3 mice in the Luc group had progressed to grade 3 at this time point. A non-parametric Mann-Whitney U test revealed significant differences in necrosis between the two groups at all time points (P<0.05)
Figure 5.
Necrosis scores were improved in EcSOD treated mice. A. Nine out of 10 mice showed some degree of necrosis in the AAV/CMV/Luc control group 7 days after vector injection, including 3 mice with grade 3 necrosis. B. In the AAV/CMV/EcSOD treated group, only 6 mice showed some degree of necrosis, and none had grade 3 necrosis (* P< 0.05).
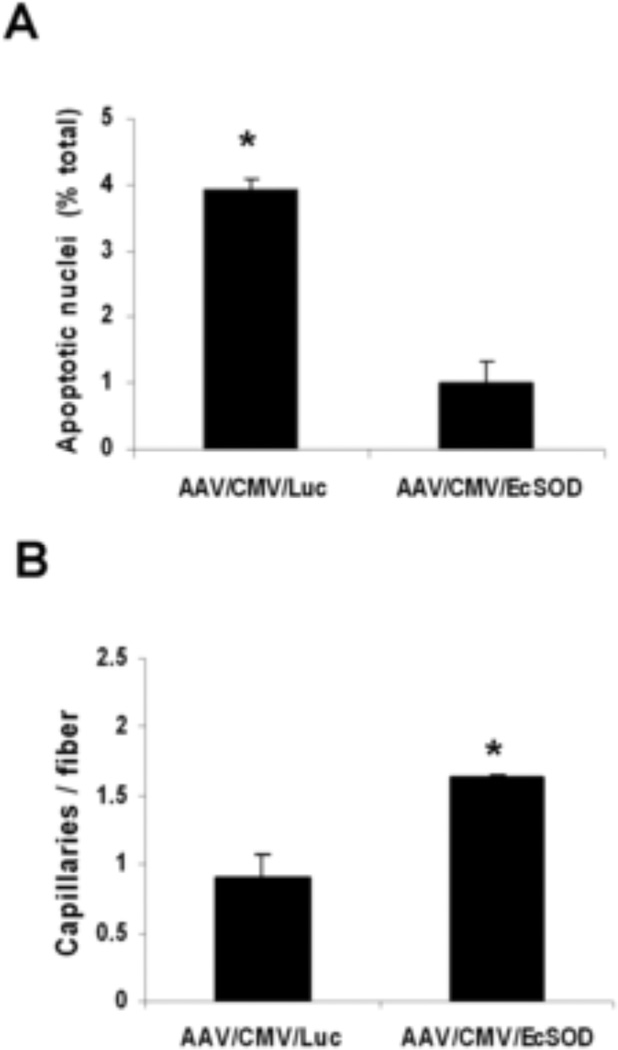
EcSOD modulates apoptosis and vascular density with no change in inflammation
To determine whether the improved perfusion rate in the EcSOD group was associated with a reduction in cellular apoptosis, apoptotic cells were stained using the TUNEL assay (n=10/group) The rate of TUNEL-positive apoptotic nuclei was 1.3±0.4% in the EcSOD group while in the Luc group it was 4.15±0.2% (P<0.001), (Fig. 6A). To determine if the improved perfusion in EcSOD group involved angiogenesis, we sought to determine capillary density at 14 days after hind-limb ischemia. Immunflouresence staining was performed for CD31 and F-actin was detected on the same slides with phalloidin. Vascular density (n=10/group) in the EcSOD group (1.65±0.02 capillaries/muscle fiber) was significantly higher as compared to the Luc group (0.78±0.17 capillaries/muscle fiber, P<0.05) (Fig. 6B). In order to determine whether differences in the inflammatory response played a significant role in recovery from hind-limb ischemia we first looked for inflammatory infiltrate in the non-ischemic EcSOD injected skeletal muscles. Mild inflammation was observed on H&E staining in both EcSOD and control groups. Immunostaining for CD45 positive monocytes and CD68 positive macrophages produced similar results. (Fig. 7, online only). In ischemic EcSOD injected and control hind limbs at day 14 post operatively both groups showed Grade 3 for CD45 or CD 68 positive cells but there was no difference between the control and EcSOD injected groups. (Fig. 8, online only).
Figure 6.
(A) Quantitative assessment of apoptosis expressed as the percentage of TUNEL positive nuclei divided by the total number of nuclei (n=10 per group, *P<0.001). (B) Quantitative analysis of capillary density expressed as the number of capillaries per muscle fiber (n=10 per group, *P<0.02)
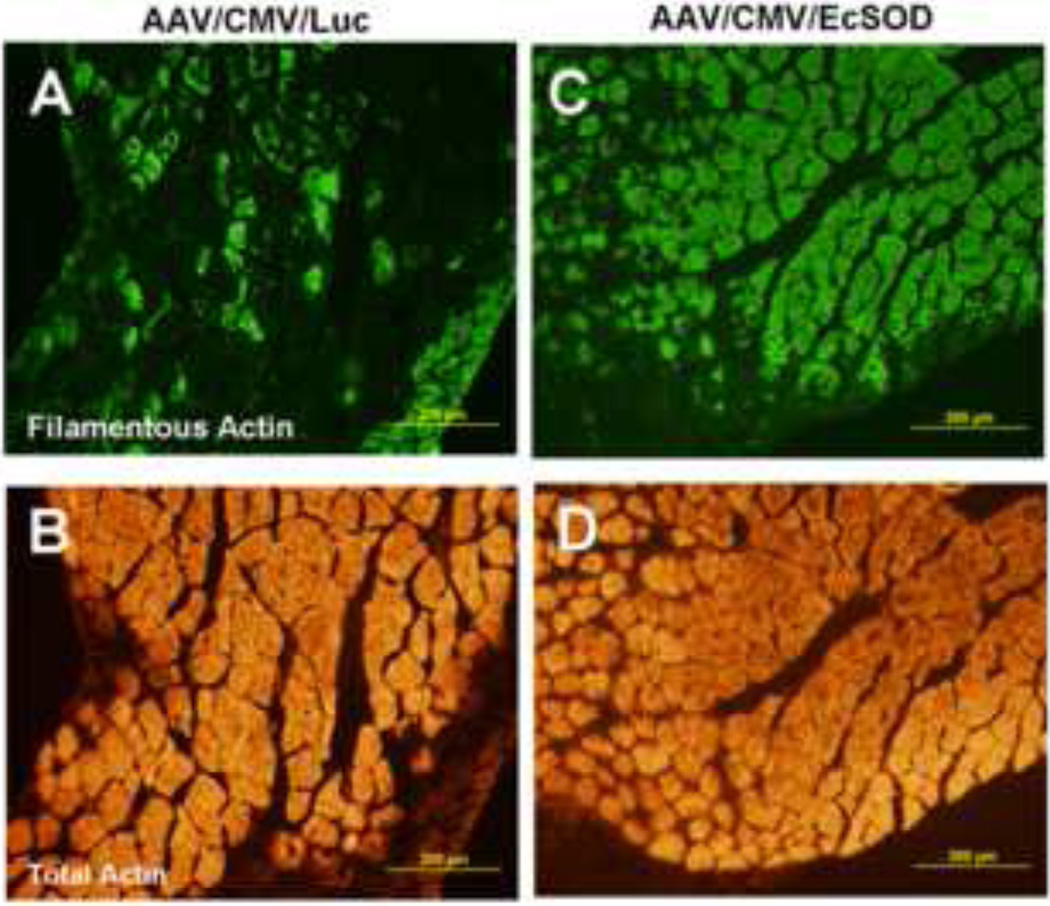
EcSOD overexpression preserves muscle integrity after ischemic surgery
We investigated the tissue integrity of the ischemic skeletal muscles in both groups by staining for total actin and filamentous actin (F-actin, i.e. polymerized form of actin, n=10/group). While fluorescence immunostaining of skeletal muscles from both groups indicated very similar patterns for total actin, filamentous actin was significantly far more abundant and its distribution more homogeneous in EcSOD-treated muscles than in the control ischemic muscles (Fig. 9).
Figure 9.
The preservation of filamentous actin (green) is markedly improved in EcSOD-treated (C) vs. control (A) in ischemic limbs, while the levels of total actin (orange) are comparable in both EcSOD-treated (D) and control (B) groups.
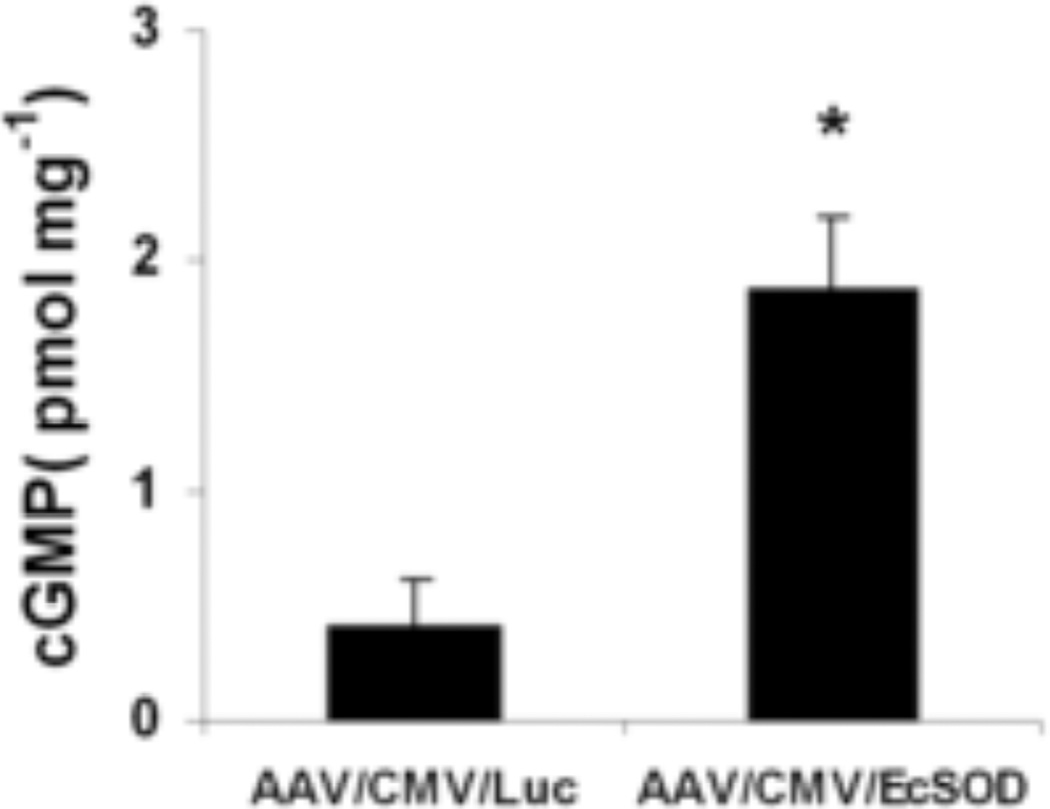
EcSOD increases cGMP levels in ischemic muscle
NO exerts its effects by the stimulation of NO sensitive guanylyl cyclase (GC) which by activation of NO-sensitive GC leads to enhanced production of the intracellular messenger cGMP (21). We assessed levels of cGMP, the product of NO-activated guanylate cyclase. cGMP levels were 4.6-fold higher (P<0.01) in ischemic hind-limbs from the EcSOD group (1.7±0.32 pmol/mg) compared to Luc group (0.41±0.20 pmol/mg, Fig. 10, n=3/group) thus providing evidence that EcSOD overexpression increases cGMP levels in ischemic muscles.
Figure 10.
Levels of cGMP (pmol/mg tissue) in ischemic tissues from control and EcSOD-treated mice at 14 days post-ischemia show a significant increase in cGMP in the EcSOD-treated group as compared to the control group (n=3 per group, *P<0.01).
Discussion
Therapeutic angiogenesis remains an investigative approach for improving limb perfusion and clinical outcomes in patients with established PAD. In this study, we used a preclinical model of acute limb ischemia to evaluate the effects of intramuscular injection(s) of an AAV9 vector directing the expression of EcSOD. There were several notable findings:
There was evidence of therapeutic angiogenesis, with an increase in perfusion to ischemic hind-limbs in the EcSOD treatment group even in a mouse strain known to have a limited capacity to recover perfusion following hind-limb ischemia.
Treatment with AAV9/CMV/EcSOD led to an increase in capillary density and a reduction in apoptosis in the ischemic muscles, all findings that are consistent with an angiogenic response.
EcSOD treatment not only increased the levels of EcSOD associated with the vasculature and interstitial space, it also resulted in detectable levels of EcSOD within the muscle fibers that were efficiently transduced by the AAV 9 vector.
Ischemic skeletal muscles from the EcSOD treatment group showed a 4.6-fold increase in cGMP levels compared to those from the Luc group
In this study, we demonstrated that tissue-restricted overexpression of EcSOD promoted therapeutic angiogenesis in a preclinical model of acute limb ischemia using BALB/c mice. The BALB/c strain was intentionally chosen for these studies as a stringent test case because they have been previously documented to have extremely poor perfusion recovery after hind-limb ischemia (11). Recovery from hind limb ischemia in BALB/c mice is worse than ApoE−/− mice as documented by a study conducted by Xie et al where no evidence of necrosis is seen in a similar model of hind limb ischemia (22). A Medline review of the past 17 years (1993–2010) revealed only 4 instances in which therapeutic angiogenesis for acute limb ischemia had been attempted in BALB/c mice (23, 24, 25, 26).
For therapeutic angiogenesis, several different strategies have been examined to deliver therapeutic agents in animal models. In some cases, the recombinant protein itself was administered but the utility of protein is limited by the relatively short circulating half-life, as well as by the large quantity needed for therapeutic effect (27). Though our study was not designed to compare adenovirus to AAV, the degree of transduction achieved with intramuscular injection of AAV9 is substantially higher than that achieved with naked DNA or adenovirus. With naked DNA, only 2% of muscle fibers are transfected after an intramuscular injection (28) while an intramuscular injection of adenovirus shows 1–2% overall transduction of muscle fibers, and 30–40% transduction is seen in muscle fibers adjacent to the injection site 7 days post injection (29, 30). In the present study, we showed robust transduction of skeletal muscles by AAV9 and gene expression was documented for at least 6 weeks. AAV2 has previously been deployed in pre-clinical models of acute limb ischemia (31–33), but this is the first study to utilize the highly efficient AAV9 serotype. Phase 1 clinical trials for heart failure using other recently discovered serotypes of AAV (AAV1/SERCA2a and AAV6/SERCA2a) are currently in progress (34, 35).
It has been previously demonstrated that endogenous EcSOD plays an essential role in post ischemic neovascularization (9). Indeed, a recent study showed that EcSOD gene transfer promotes blood flow recovery and improves capillary/muscle fiber ratio in a hind-limb ischemia model that employed C57Bl/6 mice (36). However, this study used recombinant adenovirus to deliver EcSOD and may be confounded by local inflammatory damage since Ad5 has long been known to provoke a vigorous inflammatory response in skeletal muscle (37). Our study shows that AAV9 did not provoke detectable inflammation in skeletal muscle. Mild inflammation was detected with H&E, but no difference was found between EcSOD-injected and control groups. CD45 staining for monocytes and CD68 staining for macrophages also showed similar results, making it unlikely that vector inflammation accounted for the results.
The messenger molecule NO exerts its effects by the stimulation of NO sensitive guanylyl cyclase (GC) which leads to enhanced production of the intracellular messenger cGMP (21). Our study demonstrated cGMP was significantly more abundant in ischemic muscles that received EcSOD treatment. It has been suggested that cGMP activates the HIF-1 transactivation activity at hypoxia. HIF-1 plays a crucial role in VEGF synthesis by binding to the HRE in the VEGF promoter region and by subsequently up regulating VEGF transcription. (38, 39). A more precise mechanism may become apparent by looking at these downstream mediators of cGMP. eNOS KO mice may also be used to confirm the involvement of NO/cGMP pathway in post ischemic angiogenesis in EcSOD injected mice.
The present study demonstrates that EcSOD plays an important role in neovascularization in settings of hind limb ischemia. These findings provide novel insight into use of exogenous EcSOD driven by the highly specific skeletal muscle vector AAV9 as a potential therapeutic agent for critical limb ischemia (PAD).
Conclusions
In summary, the present study demonstrated that AAV mediated gene transfer into mouse skeletal muscles results in efficient and stable gene expression. We also demonstrated that AAV-mediated EcSOD gene transfer stimulates angiogenesis and thereby improves blood flow and hence perfusion recovery in a murine hind-limb ischemia model.
Supplementary Material
Figure 7 (online only). Assessment of non-ischemic skeletal muscle after AAV/CMV/EcSOD injection: Hematoxylin & Eosin staining of the control (A) and (B) EcSOD-injected skeletal muscles revealed no detectable inflammation. Similarly, CD45 and CD68 immunostaining of control (C&E) and EcSOD-injected muscles (D&F) did not indicate leukocytic infiltrates.
Figure 8 (online only). Immunostaining for CD45 positive leukocytes in ischemic controls (A) and EcSOD-injected (B) ischemic skeletal muscles. CD68 staining in controls (C) and EcSOD-injected (D) ischemic skeletal muscles showed similar results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Vray M, Eschwege E. Epidemiology of peripheral arterial disease. J Cardiovasc Pharmacol. 1994;23:S8–S16. [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Kuhlmann MT, Klocke R, Nikol S. Therapeutic angiogenesis for peripheral artery disease: cytokine therapy. Vasa. 2007;36:253–260. doi: 10.1024/0301-1526.36.4.253. [DOI] [PubMed] [Google Scholar]
- 5.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 8.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–H2605. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kim Ha Won, Lin Angela, Guldberg RobertE, Ushio-Fukai Masuko, Fukai Tohru. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circulation Research. 2007;101:409. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 10.Prasad K-MR, Xu Y, Beyers RJ, Vandsburger MH, Harris RH, Epstein FH, French BA. Cardiac-specific over expression of EcSOD from an AAV9-pseudotyped vector increases capillary density and reduces infarct size. Circ. 2009;120:S592–S593. [Google Scholar]
- 11.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143–147. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 12.Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 13.Prasad K-MR, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: In vivo gene delivery follows a Poisson distribution. Gene Therapy. 2010 doi: 10.1038/gt.2010.105. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad KM, Yang Z, Bleich D, Nadler JL. Adeno-associated virus vector mediated gene transfer to pancreatic beta cells. Gene Therapy. 2000;7:1553–1561. doi: 10.1038/sj.gt.3301279. [DOI] [PubMed] [Google Scholar]
- 15.Gao G-P, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of adeno-associated viruses are widely disseminated in human tissues. Journal of Virology. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ried MU, Girod A, Leike K, Buning H, Hallek M. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. Journal of Virology. 2002;76:4559–4566. doi: 10.1128/JVI.76.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K-MR, Xu Y, Yang Z, Toufektsian M-C, Berr SS, French BA. Topoisomerase inhibition accelerates gene expression after adeno-associated virus-mediated gene transfer to the mammalian heart. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2007;15:764–771. doi: 10.1038/sj.mt.6300071. [DOI] [PubMed] [Google Scholar]
- 19.Dai Q, Huang J, Klitzman B, Dong C, Goldschmidt-Clermont PJ, March KL, Rokovich J, Johnstone B, Rebar EJ, Spratt SK, Case CC, Kontos CD, Annex BH. Engineered zinc finger-activating vascular endothelial growth factor transcription factor plasmid DNA induces therapeutic angiogenesis in rabbits with hindlimb ischemia. Circulation. 2004;110:2467–2475. doi: 10.1161/01.CIR.0000145139.53840.49. [DOI] [PubMed] [Google Scholar]
- 20.Dokun AyotundeO, MD, PhD*, Keum Sehoon, MS*, Hazarika Surovi, MD, PhD, Li Yongjun, MD, Lamonte GregoryM, MS, Wheeler Ferrin, PhD, Marchuk DouglasA, Ph D†, Annex BrianH., MD† A Quantitative Trait Locus (LSq-1) on Mouse Chromosome7 Is Linked to the Absence of Tissue Loss after Surgical Hindlimb Ischemia. Circulation. 2008;117:1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aicher A, Heeschen C, Feil S, Hofmann F, Mendelsohn ME, Feil R, Dimmeler S. cGMP-dependent protein kinase I is crucial for angiogenesis and postnatal vasculogenesis. PLoS One. 2009;4:e4879. doi: 10.1371/journal.pone.0004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg. 2006;44:166–175. doi: 10.1016/j.jvs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Layman H, Spiga MG, Brooks T, Pham S, Webster KA, Andreopoulos FM. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials. 2007;28:2646–2654. doi: 10.1016/j.biomaterials.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Okada H, Takemura G, Esaki M, Kobayashi H, Kanamori H, Kawamura I, Maruyama R, Fujiwara T, Fujiwara H, Tabata Y, Minatoguchi S. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol. 2009;23(53):2378–2388. doi: 10.1016/j.jacc.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Sawada T, Kubota K. Asialoerythropoietin is a strong modulator of angiogenesis by bone-marrow cells. J Invest Surg. 2007;20:357–362. doi: 10.1080/08941930701772181. [DOI] [PubMed] [Google Scholar]
- 26.Urano T, Ito Y, Akao M, Sawa T, Miyata K, Tabata M, Morisada T, Hato T, Yano M, Kadomatsu T, Yasunaga K, Shibata R, Murohara T, Akaike T, Tanihara H, Suda T, Oike Y. Angiopoietin-related growth factor enhances blood flow via activation of the ERK1/2-eNOS-NO pathway in a mouse hind-limb ischemia model. Arterioscler Thromb Vasc Biol. 2008;28:827–834. doi: 10.1161/ATVBAHA.107.149674. [DOI] [PubMed] [Google Scholar]
- 27.Rissanen TT, Vajanto I, Yla-Herttuala S. Gene therapy for therapeutic angiogenesis in critically ischemic lower limb on the way to the clinic. Eur J. Clin. Invest. 2001;31:651–666. doi: 10.1046/j.1365-2362.2001.00864.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;15(94):3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 29.Nalbantoglu J, Larochelle N, Wolf E, Karpati G, Lochmuller H, Holland PC. Muscle-specific over expression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J Virol. 2001;75:4276–4282. doi: 10.1128/JVI.75.9.4276-4282.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Ylä-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;30(92):1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 31.Shimpoa M, Ikedaa U, Maedaa Y, Takahashia M, Miyashita H, Mizukami H, Urabe M, Kume A, Takizawa T, Shibuya M, Ozawa K, Shimada K. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc Res. 2002;53:993–1001. doi: 10.1016/s0008-6363(01)00546-6. [DOI] [PubMed] [Google Scholar]
- 32.Chang DavidS, Su Hua, Tang GaleL, Brevetti LucyS, Sarkar Rajabrata, Wang Rong, Kan YuetW, Messina LouisM. Adeno-associated viral vector-mediated gene transfer of VEGF normalizes skeletal muscle oxygen tension and induces arteriogenesis in ischemic rat hindlimb. Mol Ther. 2003;7:44–51. doi: 10.1016/s1525-0016(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Tan Z, Dong CY, Chen X, Guo SF. Adeno-associated virus vectors simultaneously encoding VEGF and angiopoietin-1 enhances neovascularization in ischemic rabbit hind-limbs. Acta Pharmacol Sin. 2007;28:493–502. doi: 10.1111/j.1745-7254.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 34.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, Ly H, Kawase Y, Wagner K, Borow K, Jaski B, London B, Greenberg B, Pauly DF, Patten R, Starling R, Mancini D, Jessup M. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Therapy. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 36.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerette B, Moisset PA, Huard C, Tardif F, Gravel C, Tremblay JP. Inflammatory damage following first-generation replication-defective adenovirus controlled by anti-LFA-1. J Leukocyte Biol. 1997;61:533–538. doi: 10.1002/jlb.61.4.533. [DOI] [PubMed] [Google Scholar]
- 38.Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30:1315–1324. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia inducible factor. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 7 (online only). Assessment of non-ischemic skeletal muscle after AAV/CMV/EcSOD injection: Hematoxylin & Eosin staining of the control (A) and (B) EcSOD-injected skeletal muscles revealed no detectable inflammation. Similarly, CD45 and CD68 immunostaining of control (C&E) and EcSOD-injected muscles (D&F) did not indicate leukocytic infiltrates.
Figure 8 (online only). Immunostaining for CD45 positive leukocytes in ischemic controls (A) and EcSOD-injected (B) ischemic skeletal muscles. CD68 staining in controls (C) and EcSOD-injected (D) ischemic skeletal muscles showed similar results.