Abstract
Alterations of multiple G protein-mediated signaling pathways are detected in schizophrenia. G protein-coupled receptor kinases (GRKs) and arrestins terminate signaling by G protein-coupled receptors exerting powerful influence on receptor functions. Modifications of arrestin and/or GRKs expression may contribute to schizophrenia pathology. Cortical expression of arrestins and GRKs was measured postmortem in control and subjects with schizophrenia or schizoaffective disorder. Additionally, arrestin/GRK expression was determined in elderly patients with schizophrenia and age-matched control. Patients with schizophrenia, but not schizoaffective disorder, displayed reduced concentration of arrestin and GRK mRNAs and GRK3 protein. Arrestins and GRK significantly decreased with age. In elderly patients, GRK6 was reduced, with other GRKs and arrestins unchanged. Reduced cortical concentration of GRKs in schizophrenia (resembling that in aging) may result in altered G protein-dependent signaling, thus contributing to prefrontal deficits in schizophrenia. The data suggest distinct molecular mechanisms underlying schizophrenia and schizoaffective disorder.
Keywords: G protein-coupled receptor kinase, arrestin, schizophrenia, schizoaffective disorder, postmortem, protein expression, RNAse protection assay, mRNA expression
Introduction
Schizophrenia is a severe mental illness afflicting about 1% of the population. The dysadaptations of cortical G protein-coupled receptors (GPCRs) in schizophrenia has been the focus of many studies. The up-regulation of D1 receptors in the dorsolateral prefrontal cortex (Abi-Dargham et al., 2002) and abnormalities of D2-like receptors in the cortex in schizophrenia have been reported (Goldsmith et al., 1997; Schmauss, 1996). Postmortem studies consistently found changes in the cortical serotonin receptors, and many antipsychotic drugs target these receptors [reviewed in (Abi-Dargham, 2007)]. Complex subtype- and brain region-specific changes in muscarinic receptors have also been reported (Scarr and Dean, 2008). Thus, the functional state of multiple cortical GPCR subtypes is modified in schizophrenia, although molecular mechanisms or functional consequences of these modifications remain unknown.
Signaling by GPCRs is controlled by multiple desensitization mechanisms. The main desensitization pathway involves activation-dependent receptor phosphorylation by G protein-coupled receptor kinases (GRKs) followed by arrestin binding that blocks G protein-mediated signaling [reviewed in (Gainetdinov et al., 2004; Gurevich and Gurevich, 2006; Gurevich and Gurevich, 2003)]. Two arrestin subtypes, arrestin2 and arrestin3, and four GRK subtypes, GRK2, 3, 5, and 6, are ubiquitously expressed in the brain, each with a distinct distribution pattern (Arriza et al., 1992; Attramadal et al., 1992; Benovic and Gomez, 1993; Bezard et al., 2005; Gurevich et al., 2002; Gurevich et al., 2004; Premont et al., 1994). The functional specificity of arrestins and GRK is a matter of controversy. Although arrestin and GRK isoforms interact with many GPCRs in vitro, studies in vivo suggest that receptors are preferentially regulated by specific GRKs (Eckhart et al., 2000; Gainetdinov et al., 1999; Iaccarino et al., 1998a; Iaccarino et al., 1998b; Iwata et al., 2005; Koch et al., 1995; Luo et al., 2008; Naik et al., 2005; Rockman et al., 1996) and arrestins (Bohn et al., 2000; Bohn et al., 2003; Bohn et al., 1999; Kohout et al., 2001; Oakley et al., 2000). The signaling via GPCRs depends heavily on the cellular concentration of arrestins and GRKs, which in turn is influenced by the receptor usage (Ahmed et al., 2010; Ahmed et al., 2008a; Ahmed et al., 2008b; Gainetdinov et al., 2003; Gainetdinov et al., 1999; Gainetdinov et al., 2004).
Here we tested the hypothesis that schizophrenia is associated with changes in the expression of arrestin and GRK isoforms in cortical areas implicated in schizophrenia pathology. The dorsolateral prefrontal cortex was the focus of the study, since an impressive body of evidence demonstrates deficits in this region in schizophrenia [reviewed in (Lewis and González-Burgos, 2008)]. We have investigated the expression of arrestins and GRKs in the prefrontal cortex of patients with schizophrenia in comparison with patients with schizoaffective disorder and normal control, to assess the contribution of mood disturbance in addition to psychosis. Additionally, we have examined the expression of arrestins and GRKs in elderly patients with the life-long history of schizophrenia.
Methods and Materials
Post mortem samples
Human brain tissue from three different cohorts was used for these studies. The cohorts were obtained from NIH-supported brain banks. The tissue collection was undertaken with the written approval of each subject and approved by the institutional ethics committees. The first set was obtained from the Harvard Brain Tissue Resource Center (Director – Dr. F. Benes). The set includes 10 control cases, 9 cases with schizophrenia (SZ), and 7 cases with schizoaffective disorder (SA) matched for age, postmortem interval (PMI), and hemisphere (Table 1). The tissue from the prefrontal cortex (Brodmann’s area [BA] 9/46) and caudate nucleus was available for these patients. All patients in the Harvard cohort had lived independently or in assisted housing and none were hospitalized for psychiatric reason at the time of death. The diagnoses of SZ or SA disorder were made based on retrospective review of medical records and an extensive family questionnaire that included medical, psychiatric, and social history of the subjects. Special care was taken to ensure low incidence of substance abuse in both control and psychiatric cases (Deep-Soboslay et al., 2010). SZ was diagnosed based on the criteria of Feighner et al. (Feighner et al., 1972); the diagnosis of SA was made based on DMS-III-R criteria. The second cohort was from the Mount Sinai Medical Center Brain Bank (kindly provided by Dr. V. Haroutunian) and included 10 control and 10 schizophrenic cases (Table 2). The tissue from BA9/46, 22, 7 and 17 was available. Pharmacological treatment received by all patients within one year of their death is presented in Tables 1 and 2. Noncompliance was frequently noted in the records for the Harvard cohort but was not relevant to the Mount Sinai cases, since their treatment was supervised. Table 1 contains information on antemortem substance abuse based on extensive medical records for the Harvard cohort. There were no cases with substance abuse history in the Mount Sinai cohort. The third cohort was obtained from the Human Brain and Spinal Fluid Resource Center at the University of California, Los Angeles. This cohort contained only control subjects. Originally, 10 cases were received; however, one case was removed due to antemortem treatment with antidepressants and benzodiazepines. There were no cases with substance abuse history.
Table 1.
Characteristics of subjects in the Harvard cohort
| Diagnosis | Sex | Age, years | PMI, hours | 28S:18S RNA ratio |
Substance abuse | Psychotropic Medication/Dose (mg/day) (antipsychotic drugs are underlined) | Cause of death |
|---|---|---|---|---|---|---|---|
| SA1 | M | 52 | 14.8 | 1.2 | alcohol | Fluphenazine decanoate 25 mg byweekly; Divalproex – 500; Mirtazapine – 30; Benztropine – 2. | Cardiac arrest |
| SA | F | 49 | 23 | 1.29 | none noted | Haloperidol – 10; Clozapine – 150. | Pneumonia |
| SA | M | 48 | 7.6 | 1.47 | none noted | Quetapin – 100; Divalproex – 500; Venlafaxine – 37.5; Benztropine – 2. | Pneumonia |
| SA | M | 58 | 8.3 | 1.8 | none noted | Haloperidol – 10; Artane – 2. | Cardiac arrest |
| SA | M | 29 | 4.8 | 1.6 | none noted | Mesoridazine – 350. | Suicide (jump) |
| SA | M | 46 | 10.5 | 1.78 | none noted | Haloperidol – 19; Chlorpromazine – 600; Thioridazine – 100; Alprazolam – 0.25; Phenytoin – 300. | Sudden fatal cardiac arrhythmia followed by cardiorespiratory arrest |
| SA | M | 24 | 10 | 1.48 | none noted | Clozapine – 400. | Suicide by fire |
| N=7 | 6M+1F | 43.7±4.73 | 11.3±2.74 | 1.52±0.095, 6 | |||
| SZ2 | M | 32 | 7.8 | 1.22 | cannabis, alcohol, marijuana | Clozapine – 150; Divalproex – 500; Clonazepam – 0.5. | Acute pancreatitis |
| SZ | M | 68 | 21.4 | 1.31 | none noted | Quetapin – 100; Aripiprazole – 20; Divalproex – 1000. | Cardiac arrest |
| SZ | M | 36 | 18 | 1.25 | none noted | Clozapine – 350; Fluoxetine – 60. | Suicide by overdose |
| SZ | F | 83 | 9.3 | 1.45 | none noted | Trifluopenazine – 10; Sertraline – 75. | Cerebral vascular aneurism |
| SZ | M | 47 | 17.8 | 1.42 | none noted | Quetapin – 700; Sertraline – 100. | Multiple myeloma |
| SZ | M | 64 | 15.4 | 1.2 | none noted | Fluphenazine decanoate – 25. | Cardiac arrest |
| SZ | F | 56 | 18.7 | 1.71 | none noted | Olanzapine – 10; Divalproex – 500; Lorazepam – 0.5. | Lung cancer |
| SZ | F | 48 | 22 | 1.53 | none noted | Risperidone – 3; Olanzapine – 5; Clozapine – 400; Divalproex – 500; Fluoxetine – 60; Clonazepam – 1. | Sudden death at home, cardiorespiratory in nature, natural |
| SZ | M | 42 | 10.3 | 1.58 | none noted | Quetapin – 200; Haloperidol decanoate - 100 mg, last injections – 20 days before death. | Homicide (gunshot) |
| N=9 | 6M+3F | 52.9±5.5 | 15.6±1.7 | 1.41±0.06 | |||
| Control | M | 69 | 21.1 | 1.41 | none | none | Myocardial infarction |
| Control | F | 53 | 16.6 | 1.29 | none | none | Cardiac arrest |
| Control | F | 86 | 6.9 | 1.59 | none | none | Myocardial infarction |
| Control | F | 51 | 23.1 | 1.42 | none | none | No cause of death |
| Control | M | 52 | 13.1 | 1.83 | none | none | No cause of death |
| Control | M | 24 | 21.3 | 1.4 | none | none | Cardiac arrest |
| Control | M | 61 | 10.1 | 1.26 | none | none | Myocardial infarction |
| Control | M | 28 | 23.3 | 1.6 | none | none | Unwitnessed cardiac arrest at home |
| Control | M | 48 | 12.1 | 1.56 | none | none | Myocardial infarction |
| Control | M | 24 | 5 | 1.92 | none | none | Homicide (gunshot) |
| N=10 | 7M+3F | 49.6±6.4 | 15.3±2.1 | 1.53±0.07 |
– SA – schizo-affective disorder;
– SZ – schizophrenia;
– There are no significant differences in Age among groups (F(2,23)=0.57, p=0.572).
- There are no significant differences in PMI among groups (F(2,23)=1.195, p=0.32).
- There are no significant differences in 28S:18S ratio among groups (F(2,23)=0.924, p=0.41).
- There is a tendency to reduced 28S:18S ratio with increased PMI across groups, but no significant correlation (r=-0.352, p=0.0778) and no significant correlation between 28S:18S ratio and age (r=-0.147, p=0.48).
Table 2.
Characteristics of subjects in the Mount Sinai cohort.
| Diagnosis | Sex | Age, years | PMI hours | pH | Psychotropic Medication/Dose (mg/day) | Cause of death |
|---|---|---|---|---|---|---|
| SZ1 | F | 80 | 6.9 | 6.8 | Thiothixene – 5; Chlorpromazine – 100; | Acute pancreatitis, renal failure. |
| SZ | M | 58 | 6.7 | 6.2 | Haloperidol – 2 or 4 | Cardio pulmonary arrest. |
| SZ | M | 84 | 5.25 | 7.0 | Haloperidol - 2 | Cardio pulmonary arrest. |
| SZ | M | 58 | 22.3 | 6.9 | Haloperidol – from 0.5 to 20 | Cardiac Arrest |
| SZ | M | 63 | 6.2 | 5.9 | Haloperidol - 8 | Gastrointestinal hemorrhage |
| SZ | F | 83 | 20.4 | 7.1 | Thioridazine; Thiothixene | Cancer of pancreas |
| SZ | F | 79 | 9.9 | 6.8 | Thiothixene - 4 | Atherosclerotic Heart Disease |
| SZ | F | 70 | 29.9 | 6.5 | Risperidone – 2–6 | Cardiac arrest |
| SZ | M | 69 | 23.5 | 6.7 | Loxapine – 10–15; Perphenazine – 4–24 | Acute renal failure |
| SZ | F | 77 | 9.7 | 6.0 | Risperidone - 5 | Myocardial infarction |
| N=10 | 5F+5M | 72.1±3.12 | 14.1±2.83 | 6.5±0.094 | ||
| Control | F | 82 | 5.7 | 6.1 | none | Myocardial infarction |
| Control | F | 80 | 4.75 | 6.2 | none | Sepsis |
| Control | F | 85 | 4.3 | 6.3 | none | Myocardial infarction |
| Control | F | 102 | 7.1 | 6.5 | none | Myocardial infarction, Ovarian tumors |
| Control | M | 101 | 4.7 | 6.8 | none | Congestive Heart Failure |
| Control | F | 78 | 10 | 6.2 | none | Myocardial infarction |
| Control | M | 95 | 4.1 | 6.5 | none | Renal failure |
| Control | M | 65 | 3.8 | 6.8 | none | Renal Failure |
| Control | F | 83 | 6.2 | 6.8 | none | Atherosclerotic Heart Disease |
| Control | M | 66 | 7.6 | 6.6 | none | Congestive Heart Failure |
| N=10 | 6F+4M | 83.7±4.1 | 5.8±0.6 | 6.6±0.13 |
– SZ – schizophrenia;
– Age is significantly lower in the SZ group (t(18)=2.26, p=0.036).
– PMI is significantly longer in the SZ group (t(18)=2.84, p=0.011).
– There are no significant differences in brain pH between the groups (t(18)=0.693, p=0.496).
The tissue from the Harvard and Los Angeles cohorts was in blocks containing only the brain areas of interest. To prepare samples, grey matter from the block was rapidly chopped off on dry ice, weighed, and lysed in Lysis solution (Ambion, Austin, TX) to make 1:10 w/v homogenate. The samples from the Mount Sinai cohort received already chopped were weighed and lysed in Lysis solution at 1:10 w/v. The Lysis solution effectively lyses the tissue while inhibiting enzymatic activity. The advantage of the procedure is that the same lysates can be used to measure protein and mRNA by RNAse protection assay (RPA). The protein concentration in lysates was measured by Bradford method (Bio-Rad, Hercules, CA). The samples in Lysis solution were stored at −80°C until used.
Western Blotting
For Western blots, samples were prepared as described (Ahmed et al., 2008a; Bezard et al., 2005; Bychkov et al., 2008). Briefly, protein from tissue lysates in Lysis buffer were precipitated with 9 volumes of methanol, collected by centrifugation, the pellet washed in 90% methanol, air dried and dissolved in Laemmli buffer at 0.5 mg protein/ml. Electrophoresis, transfer, and detection were performed as described previously (Ahmed et al., 2008a; Bezard et al., 2005; Bychkov et al., 2008). Arrestins were detected with arrestin2- (Mundell et al., 1999) (1:6,000) or arrestin3-specific (Orsini and Benovic, 1998) (1:700) affinity-purified rabbit polyclonal antibodies. We used rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) to quantify GRK2 (catalog # sc-562; 1:500), GRK3 (sc-563; 1:300), GRK5 (sc-565; 1:500) and GRK6 (sc-566, 1:300). GRK5 was selectively detected with rabbit antibody from R&D Systems (Minneapolis, MN). The specificity of the antibodies to human arrestin and GRK isoforms was demonstrated previously (Bychkov et al., 2008). For quantification of arrestins, dilutions of standards containing 1:1 mix of Escherichia coli-expressed purified bovine arrestin2 and arrestin3 in sample buffer were used. For quantification of GRKs, we used bovine GRK2 and GRK3 (Kim et al., 1993), human GRK5 (Kunapuli et al., 1994) and GRK6 (Loudon and Benovic, 1994), purified as described in the given references. Appropriate dilutions of GRK proteins were loaded onto each gel alongside the samples to generate calibration curves to allow for quantification of GRKs in samples in absolute units as described previously (Ahmed et al., 2008a; Bezard et al., 2005; Bychkov et al., 2008). For other kinases, the following antibodies were used: rabbit polyclonal anti-ERK1/2, anti-MEK, anti-Akt, anti-JNK/SAPK, anti-PDK1, and anti-mTOR (1:1000; all from Cell Signaling Technology, Danvers, MA). To quantify ERK1/2, we used recombinant ERK1/2 (kindly provided by Dr. Kevin Dalby, University of Texas at Austin, Austin, TX) purified as described (Waas et al., 2003). Since no purified standards were available to quantify other proteins, we used serial dilutions of the human striatal lysate to ensure that all samples were in linear range. The values for these proteins are expressed in arbitrary units. Total protein was used as loading control. The effectiveness of sample loading, electrophoresis, and transfer was controlled by staining the membrane upon transfer with Novex reversible membrane stain (Invitrogen, Carlsbad, CA). Standard purified proteins of known concentration loaded on each gel serve as additional control for the quality of the gel and transfer procedure.
RNAse protection assay (RPA)
The brain samples in Lysis solution were used directly for hybridization with the labeled probes. The probes for the RPA were labeled with [32P]UTP by in vitro transcription. Arrestin2 and arrestin3 mRNAs were measured in one hybridization reaction and all four GRKs – in another reaction. The probes for human arrestins and GRKs were described previously (Bychkov et al., 2008). The probes were designed to yield the same protected fragments with all known splice variants of respective arrestins and GRKs (Parruti et al., 1993; Sterne-Marr et al., 1993). RPA was performed as described (Ahmed et al., 2008a; Bychkov et al., 2008) using Direct Protect Kit (Ambion, Austin, TX, USA). Calibration curves were constructed using unlabeled sense mRNA (5–100 pg) for arrestins and GRKs synthesized in vitro with full size cDNAs as templates. A series of calibration samples was included in each experiment and run alongside experimental samples on each gel. This procedure allowed for determination of absolute expression levels of each message and direct comparison of concentrations of different messages.
Direct Protect RPA method does not allow for the determination of RNA quality. In order to characterize the degree of RNA degradation, in a separate experiment, we isolated total RNA for each case from ~75 mg tissue using RNAqueous kit (Ambion, Austin, TX, USA). RNA was run on 1% denaturing agarose gel using NorthernMax kit with Millennium RNA markers (both from Ambion). The gels were stained with ethidium bromide, and scanned with GelDoc (BioRad, Hercules, CA). Table 1 shows 28S:18S rations for the Harvard cohort and Table 3 – for the Los Angeles cohort. No extra tissue from Mount Sinai cases used in the study was available for RNA isolation.
Table 3.
Characteristics of control subjects in all cohorts
| Diagnosis | Sex | Age, years | PMI, hours | 28S:18S RNA ratio |
brain pH | Cause of death |
|---|---|---|---|---|---|---|
| Harvard cohort | ||||||
| Control | M | 24 | 21.3 | 1.4 | - | Cardiac arrest |
| Control | M | 24 | 5 | 1.92 | - | Homicide (gunshot) |
| Control | M | 28 | 23.3 | 1.6 | - | Unwitnessed cardiac arrest at home |
| Control | M | 48 | 12.1 | 1.56 | - | Myocardial infarction |
| Control | F | 51 | 23.1 | 1.42 | - | No cause of death in the records |
| Control | M | 52 | 13.1 | 1.83 | - | No cause of death in the records |
| Control | F | 53 | 16.6 | 1.29 | Cardiac arrest | |
| Control | M | 61 | 10.1 | 1.26 | - | Myocardial infarction |
| Control | M | 69 | 21.1 | 1.41 | - | Myocardial infarction |
| Control | F | 86 | 6.9 | 1.59 | - | Myocardial infarction |
| N=10 | 3F+7M | 49.6±6.41 | 15.3±2.12 | 1.53±0.073 | - | |
| Mount Sinai cohort | ||||||
| Control | M | 65 | 3.8 | - | 6.8 | Renal Failure |
| Control | M | 66 | 7.6 | - | 6.6 | Congestive Heart Failure |
| Control | F | 78 | 10 | - | 6.2 | Myocardial infarction |
| Control | F | 80 | 4.75 | - | 6.2 | Sepsis |
| Control | F | 82 | 5.7 | - | 6.1 | Myocardial infarction |
| Control | F | 83 | 6.2 | - | 6.8 | Atherosclerotic Heart Disease |
| Control | F | 85 | 4.3 | - | 6.3 | Myocardial infarction |
| Control | M | 95 | 4.1 | - | 6.5 | Renal failure |
| Control | M | 101 | 4.7 | - | 6.8 | Congestive Heart Failure |
| Control | F | 102 | 7.1 | - | 6.5 | Myocardial infarction, Ovarian tumors |
| N=10 | 6F+4M | 83.7±4.1 | 5.8±0.6 | -4 | 6.6±0.13 | |
| VA Los Angeles cohort | ||||||
| Control | M | 58 | 9 | 1.82 | - | Colon cancer |
| Control | F | 67 | 11.8 | 1.25 | - | Chronic obstructive pulmonary disease |
| Control | M | 68 | 10.5 | 1.25 | - | Lung and liver metastatic cancer, respiratory arrest |
| Control | F | 73 | 12 | 1.41 | - | No cause of death in the records |
| Control | M | 74 | 13.6 | 1.25 | - | Cardiopulmonary arrest |
| Control | M | 78 | 18.3 | 1.2 | - | Esophageal cancer. Progressive weakness. Bronchitis. |
| Control | M | 80 | 14 | 1.2 | - | Bladder cancer |
| Control | M | 81 | 14 | 1.47 | - | Non-Hodgkin's lymphoma. Progressive weakness. |
| Control | M | 85 | 14 | 1.53 | - | Cardiopulmonary failure. |
| N=9 | 3F+7M | 73.8±2.8 | 13.0±0.9 | 1.38±0.07 |
– The effect of Cohort on Age is significant (F(2,26)=14.05, p<0.0001). According to Bonferroni/Dunn post hoc comparison of means, the Harvard has significantly lower age than Mount Sinai (p<0.01) and Los Angeles (p<0.05) cohorts, which are not significantly different from each other.
– The effect of Cohort on PMI is significant (F(2,26)=12.43, p=0.0002). According to Bonferroni/Dunn post hoc comparison of means, the Mount Sinai cohort has significantly shorter PMI than Harvard (p<0.001) and Los Angeles (p<0.01) cohorts, which are not significantly different from each other.
– There is no significant difference in 28S;18S ration between the Harvard and Los Angeles cohorts (t=1.56, p=0.136).
– No extra tissue from the Mount Sinai cohort was available for RNA isolation. However, considering the short PMI and good values for the brain pH, a surrogate measure of RNA quality (Lipska et al., 2006; Mexal et al., 2006), the RNA quality is likely to be acceptable.
Subcellular fractionation
Subcellular fractions were prepared essentially as described previously (Ahmed et al., 2008a). Briefly, approximately 25 mg of cortical tissue (BA9/46; Harvard cohort) was homogenized in ice-cold HEPES-buffered sucrose containing protease inhibitor cocktail. Homogenate (H) was centrifuged at 1000xg for 10 min at 4°C to remove nuclei and large debris (P1). Supernatant (S1) was centrifuged at 10,000xg for 15 min to obtain crude synaptosomal fraction (P2) and supernatant (S2). The synaptosomal pellet was lysed by hypo-osmotic shock in 9 volume of ice cold HEPES-buffer for 30 min. The lysate was centrifuged at 25,000g for 20 min at 4°C to obtain synaptosomal membrane fraction (LP1) and crude synaptic vesicle fraction (LS1). Supernatant (S2) was centrifuged at 165,000xg for 2 hours to obtain cytosolic fraction (S3) and light membrane fraction (P3). Protein concentration in the samples was measured with Bradford reagent (Bio-Rad, Hercules, CA). Samples were then precipitated with 90% (v/v) methanol. The protein was pelleted by centrifugation [10,000xg, 10 min at room temperature (RT)], washed with 1 ml of 90% methanol, dried, and dissolved in sodium dodecyl sulfate sample buffer at the final concentration of 0.5 mg/ml.
Data Analysis
For Western blots and RPA, the gray values of the bands were measured on X-ray film using Versadoc 4 (Bio-Rad, Hercules, CA). The density of 28S and 18S bands on scanned agarose RNA gels was determined using Versadoc 4 software. To account for the non-specific signal, the local background subtraction algorithm (the background determined in the area immediately surrounding the band of interest) provided by Versadoc software was used. The optical densities of the bands corresponding to arrestins, GRKs, or their messages were converted into nanograms of the respective protein or picogram of mRNA per milligram of protein using the calibration curves constructed with protein standards of known concentrations. Calibration curves were fitted to linear equations using Prism 4.0 (GraphPad Software, San Diego, CA).
For the statistical analysis, StatView software (SAS Institute, Cary, NC) was used. The data were analyzed for each brain region by ANCOVA with Diagnosis as main factor followed by Bonferroni/Dunn post-hoc test with correction for multiple comparisons where appropriate. Age and PMI were treated as co-variates, and Sex as a random factor. Since the experimental design involves multiple comparisons of values (6 brain regions in two cohorts for 6 arrestin and GRK isoforms) that could potentially generate false positive results, we verified each original significant finding in two additional independent Western blot experiments with specific null hypotheses. The difference was considered significant only if obtained in all experiments. The Pearson correlation and Fisher’s r to z transformation were used examine the relationship between the arrestin/GRK expression and age in control samples. An alternative analysis was performed by two-way ANCOVA with Age as a co-variate and Cohort as a random factor to account for potential confounding effect of the cohort. In all cases, p<0.05 was considered significant.
Results
Age-related changes in the expression of arrestin and GRK isoforms in the human cerebral cortex
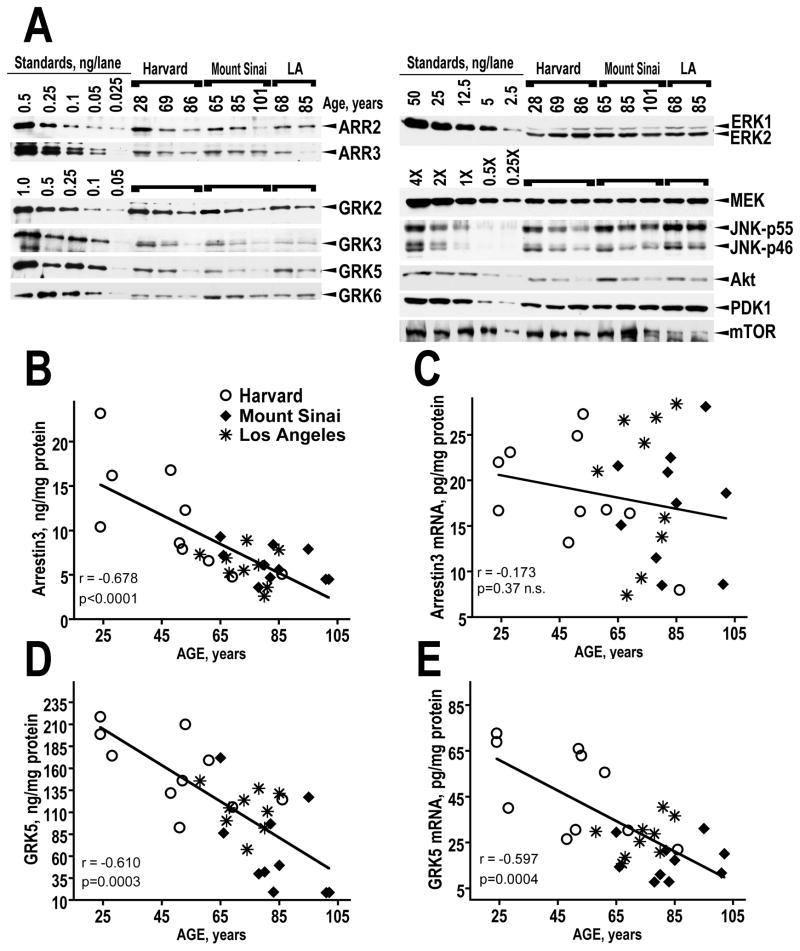
We took advantage of the wide range of ages of control subjects in the three patient cohorts (range 24–102 years) to evaluate the effect of age on the concentrations of arrestin and GRK isoforms in the dorsolateral prefrontal cortex (Brodmann area 9/46) of the control human brain. Table 3 presents the characteristics of control subjects from all cohorts for comparison. To improve reliability, we examined the samples from all cohorts side-by-side on the same blot. The levels of both arrestin isoforms significantly decreased with age (Fig. 1A,B and Table 4). The concentrations of GRKs 2, 3 and 5 also significantly declined with age (Fig. 1A, D and Table 4). Most correlations found significant by Fisher’s r to z transformation remained significant after the confounding effect of Cohort was accounted for by ANCOVA analysis. The only exception was a significant negative correlation of the GRK6 concentration with age rendered non-significant, when the contribution of Cohort was taken into account (Table 4). Importantly, samples from individuals of the same age from all three cohorts show similar levels of arrestin and GRK expression confirming that the decrease is indeed age-related.
Figure 1.
The expression of arrestins and GRKs in the human dorsolateral prefrontal cortex (Brodmann area 9/46) decreases with age. (A) A representative Western blot showing comparative expression of arrestin, GRK isoforms, and other kinases in Harvard Mount Sinai, and Los Angeles cases of corresponding ages. Ages are shown above the lanes. Left 5 lanes show recombinant protein standards for arrestins, GRKs and ERK1/2 (amounts loaded are indicated by numbers in ng/lane). For other proteins, serial dilutions of the human striatal lysate were used (4X equals 2 μg total protein/μl). The following amount of total protein per lane was loaded: ARR2 - 0.5 μg; ARR3 – 4 μg; GRK2 – 0.3 mg; GRK3 – 2.5 μg; GRK5 – 2.5 μg; GRK6 – 2.5 μg; ERK1/2 and MEK – 1.5 μg; JNK - 2 μg; Akt, PDK1, and mTOR – 2.5 μg. (B) Regression plot for arrestin3 protein demonstrating significant inverse correlation of the arrestin3 protein concentration with age. Note similar arrestin3 expression levels in individuals of similar ages from the all three cohorts. (C) Regression plot for arrestin3 mRNA demonstrating the lack of significant correlation with age. (D) Regression plot for GRK5 protein demonstrating significant inverse correlation of the protein concentration with age. (E) Regression plot for GRK5 mRNA showing significant negative correlation of the mRNA concentration with age similar to that seen with GRK5 protein.
Table 4.
Pearson correlation coefficients for the correlation with Age and significance levels obtained by Fisher’s r to z transformation and ANCOVA analysis
| Protein | mRNA | |||||
|---|---|---|---|---|---|---|
| R | p (r to z)2 | p (ANCOVA)3 | R | p (r to z) | p (ANCOVA) | |
| Arrestin2 | −0.5321 | 0.004 | 0.009 | −0.168 | 0.388 | 0.24 |
| Arrestin3 | −0.678 | <0.0001 | 0.004 | −0.173 | 0.373 | 0.5 |
| GRK2 | −0.692 | <0.0001 | 0.009 | −0.292 | 0.125 | 0.41 |
| GRK3 | −0.695 | <0.0001 | 0.037 | −0.532 | 0.0025 | 0.015 |
| GRK5 | −0.610 | 0.0003 | 0.0083 | −0.597 | 0.0004 | 0.087 |
| GRK6 | −0.404 | 0.0303 | 0.24 | −0.422 | 0.0218 | 0.32 |
| ERK1/2 | −0.123 | 0.529 | 0.39 | - | - | - |
| MEK | 0.077 | 0.7 | 0.15 | - | - | - |
| JNK-p46 | −0.580 | 0.0007 | 0.008 | - | - | - |
| JNK-p54 | −0.112 | 0.566 | 0.3 | - | - | - |
| Akt | −0.453 | 0.0146 | 0.0008 | - | - | - |
| PDK1 | −0.203 | 0.294 | 0.18 | - | - | - |
| mTOR | 0.013 | 0.948 | 0.18 | - | - | - |
– Statistically significant correlations are shown in bold.
– Statistical significance of Pearson correlation coefficients was determined by Fisher’s r to z transformation.
– p values for Age obtained by two-way ANCOVA analysis with Age as a co-variate and Cohort as a random factor
Interestingly there were no significant age-related changes in the concentrations of arrestin mRNAs (Fig. 1C and Table 4), although the amounts of both arrestin proteins significantly declined with age, suggesting that age affects posttranslational processing or half-life of these proteins. GRK2 and GRK6 mRNAs were unaffected by age, whereas GRK3 and 5 mRNAs significantly declined with age (Fig. 1E and Table 4). Similarly to GRK6 protein, GRK6 mRNA showed significant negative correlation with age that was no longer significant when the effect of Cohort was accounted for (Table 4).
To examine the possibility that age-related decrease in the concentration of arrestins and GRKs reflects loss of neural tissue and/or synaptic degeneration rather than specific downregulation of arrestin and GRK proteins in the aging cortex, we quantified several other kinases in the same samples. We found that of MAP kinase ERK1/2 and MEK1/2 were completely unaltered with age (Fig. 1A and Table 4). Of multiple JNK isoforms, longer p55 isoforms, which include brain-enriched JNK3α2 isoform as well as ubiquitous JNK1α2/β2 and JNK2α2/β2, did not decrease with age, whereas shorter p46 isoforms (JNK3α1, JNK1α1/β1, JNK2α1/β1) significantly declined with age (Fig. 1A and Table 4). The concentration of Akt showed modest negative correlation with age, whereas its upstream kinase, PDK1, that phosphorylates Akt at Thr308 and activates it (Chan et al., 1999), remained unaffected as was mTOR (Fig. 1A and Table 4).
Decreased expression of arrestins and GRK in younger schizophrenia, but not schizoaffective, cases (Harvard cohort)
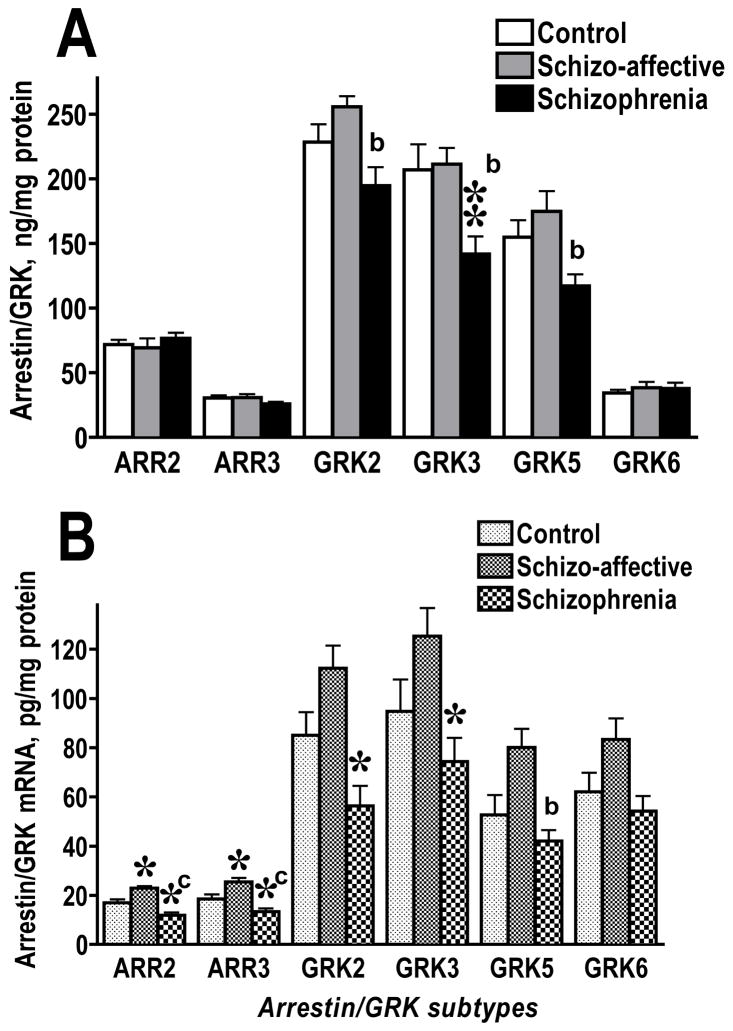
In the Harvard cohort, there were no significant differences among diagnosis groups in age or PMI (Table 1). We found no significant effect of Age, PMI, or Sex on the expression of arrestin or GRK proteins or mRNAs. The analysis of the protein levels by Diagnosis revealed no significant changes in the expression of arrestin or GRK isoforms in the caudate nucleus in either SA or SZ group (data not shown). In BA9/46 of the prefrontal cortex, the levels of arrestins were also unchanged (Fig. 2A). The ANOVA analysis detected significant effect of Diagnosis on the expression of GRK2 (F(2,23)=6.09, 5.011, and 4.58; p=0.0089, 0.0156, and 0.0211, respectively, from 3 independent experiments) and GRK3 (F=5.49, 7.93, 6.53; p=0.0112, 0.0024, 0.0057). According to post hoc comparison, GRK2 was significantly reduced in SZ group as compared to SA group, whereas neither disease group differed significantly from control (Fig. 2A). The level of GRK3 protein was decreased in SZ group as compared to both control and SA groups (Fig. 2A). The concentration of GRK5 was also significantly affected by Diagnosis (F=5.1, 5.57, 5.63; p=0.0147, 0.011, 0.0103). The GRK5 level was significantly decreased in SZ group as compared to SA group, whereas neither disease group differed significantly from control (Fig. 2A). No significant differences among all three groups were seen in the expression of GRK6. Two cases with substance abuse history have been identified, one in SZ and one in SA group (Table 1), but their values for the GRK/arrestin proteins and mRNA were within the respective group’s range.
Figure 2.
Comparison of the expression of arrestin and GRK proteins (A) and corresponding mRNAs (B) in the dorsolateral prefrontal cortex (Brodmann area 9/46) in control subjects and patients with schizophrenia or schizoaffective disorder in the Harvard cohort. The data for each isoform were analyzed by one-way ANOVA with GROUP (control, schizoaffective, schizophrenia) as main factor followed by Bonferroni/Dunn post hoc test with correction for multiple comparisons. * - p<0.05, ** - p<0.01 as compared to the corresponding control values; a – p<0.05, b – p<0.01, c – p<0.001 as compared to the values in the schizoaffective group.
We measured the concentration of arrestin and GRK mRNAs in the same samples used to measure respective proteins. There was no significant effect of 28S:18 ratio on the expression of arrestin or GRK mRNAs. In agreement with the results for proteins, we found no changes in the arrestin and GRK mRNAs in the caudate nucleus (data not shown) in either disease group. The comparison of the concentrations of respective mRNAs in BA9/46 demonstrated that changes in mRNAs mirrored, in most cases, those in the proteins. The notable exceptions were mRNAs of arrestin2 and 3. Although the expression of arrestin proteins in BA9/46 was not different among experimental groups, the levels of both arrestin2 and arrestin3 mRNAs were significantly affected by Diagnosis (F(2,23)=16.25 p<0.0001, and 12.82, p=0.002, respectively) (Fig. 2B). The concentration of arrestin2 mRNA was elevated in SA group as compared to control (p<0.05) and SZ (p<0.001) groups. At the same time, SZ group had significantly reduced concentration of arrestin2 mRNA as compared to control. The changes in arrestin3 mRNA followed a similar pattern. The levels of GRKs 2 and 3 mRNAs were significantly affected by Diagnosis (F(2,23)=10, p=0.0007, and F=5.2, p=0.014, respectively) (Fig. 2B). The amount of GRK2 mRNA was significantly reduced in SZ group as compared to control (p<0.05) and SA (p<0.001) groups, whereas there was no significant difference between control and SA groups. Similarly, the level of GRK3 mRNA significantly differed between control and SZ (p<0.05) and between SA and SZ groups (p<0.01). The pattern of the changes in the GRK5 mRNA (F=6.7 p=0.0054) was similar to that of GRK5 protein, with significant difference between SA and SZ group (p<0.05) but not between control and either group. We also detected significant, albeit weak, effect of Diagnosis on the level of GRK6 mRNA (F=4.3 p=0.0262), but there were no differences among individual groups (Fig. 2B).
Reduced expression of arrestins and GRKs in elderly schizophrenic patients (Mount Sinai cohort)
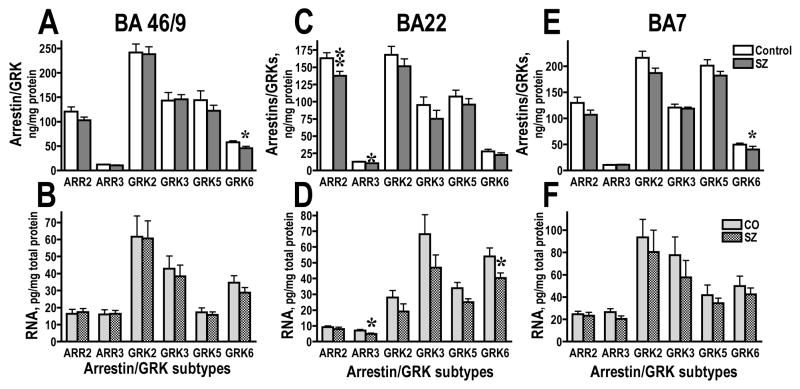
We have examined the expression of arrestin and GRK isoforms in the select cortical areas of elderly schizophrenic patients in comparison with age-matched control. In the Mount Sinai cohort, SZ patients were significantly younger than control (p<0.05), but with significantly longer PMI (Table 2). However, we detected no significant effect of age, sex, or PMI on the concentration of any protein or mRNA examined. In the BA9/46, we found reduced concentration of GRK6 (F(1,18)=5.47, 5.37, 5.019; p=0.0311, 0.0325, 0.0379 from 3 independent experiments), but no changes in any other arrestin or GRK isoform (Fig. 3A). There were no changes in mRNA or any isoform (Fig. 3B). Similar reduction in the concentration of GRK6 (F=7.9, 6.84, 5.82; p=0.0104, 0.0175, 0.0267) without changes in the corresponding mRNA was seen in the parietal area 7 (Fig. 3C,D). Interestingly, in the superior temporal cortex (BA22), the concentration of arrestin2 (F=14.9, 7.35, 10.79; p=0.0012, 0.0143, 0.004) and arrestin3 (F=11.6, 9.55, 8.17; p=0.0033, 0.0063, 0.0.1) was significantly reduced in SZ group (Fig. 3E), with corresponding reduction in the arrestin3, but not arrestin2, mRNA (F=6.1 p=0.0242) (Fig. 4F). The concentration of GRK6 (F=4.9 p=0.0401) mRNA was also significantly decreased in SZ group without the decrease of the GRK6 protein (Fig. 3E,F). We found no significant changes in the expression of arrestins and GRKs in the primary visual cortex (BA17; data not shown).
Figure 3.
Comparison of the expression of arrestin and GRK proteins (A,C,E) and corresponding mRNAs (B,D,F) in the dorsolateral prefrontal cortex (Brodmann area 9/46) (A,B), parietal cortex (Brodmann area 7) (C,D), and superior temporal cortex (Brodmann area 22) (E,F) in elderly control subjects and patients with schizophrenia in the Mount Sinai cohort. The data for each isoform were analyzed by one-way ANOVA with GROUP (control, schizophrenia) as main factor. * - p<0.05, ** - p<0.01 as compared to the corresponding control values.
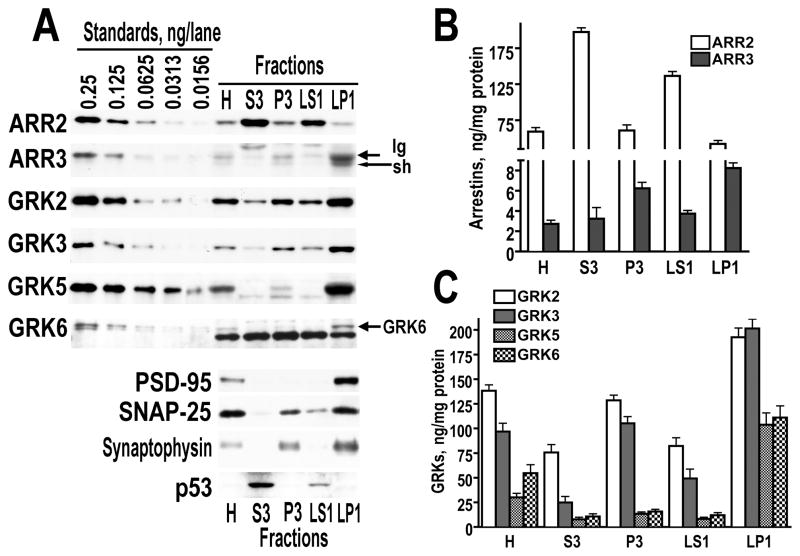
Figure 4.
The subcellular distribution of arrestin and GRK isoforms in the dorsolateral prefrontal cortex of the control human brain. Subcellular fractions were prepared as described in Methods. (A) Representative Western blots demonstration subcellular distribution of arrestins and GRKs. Left 5 lane – different amounts of purified recombinant proteins used as standards. H – Homogenate, S3 – cytosol, P3 – light membrane fraction, LS1 – crude synaptic vesicle fraction, LP1 – synaptic membrane fraction. (B) Quantitative analysis of the subcellular distribution of the two arrestin isoforms. The data (in percents of the values in corresponding homogenates) are presented as means±S.E.M. (C) Quantitative analysis of the subcellular distribution of GRK isoforms. Data are means±S.E.M in percents from homogenate.
Distinct subcellular distribution of arrestin and GRK isoforms
Based on our finding that each arrestin and GRK isoform has characteristic subcellular distribution in the rat striatum (Ahmed et al., 2008a), we performed fractionation experiments using the tissue derived from BA9/46 of control, SZ, and SA groups of the Harvard cohort (the only tissue available in sufficient quantity and suitable for fractionation). The subcellular distribution of arrestin and GRK isoforms in the human cortex was similar to that in the rat striatum. Arrestin2 was enriched in the cytosol (S3), whereas arrestin3 was largely membrane-associated, with both long and short isoforms particularly abundant in the synaptic membrane fraction (LP1) (Fig. 4A,B). All GRK isoforms we enriched in synaptic membranes. GRKs 2 and 3 were also abundant in the light membrane fraction (P3), whereas GRKs 5 and 6 were almost exclusively synaptic (Fig. 4A,C). The similarities in the distribution of individual arrestins and GRKs in the brain of the two species as far divergent as humans and rats suggest that their specific subcellular localization is functionally important.
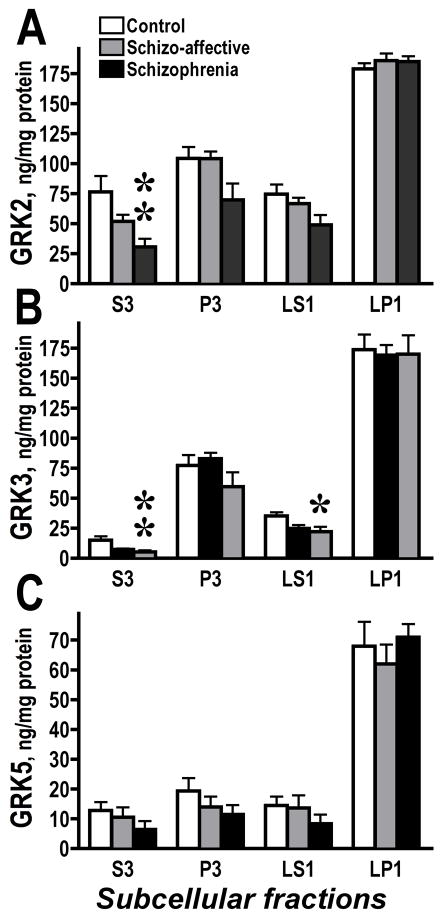
We found no alterations in the subcellular distribution of arrestins and GRK6 in SZ or SA groups as compared to control, in agreements with the lack of changes in the total level of the GRK6 protein. The concentration of GRK2 in the total cell lysate was decreased in the SZ group as compared to the SA group but not to control (Fig. 2A). However, the level of GRK2 was decreased in the SZ group as compared to control in the cytosol (F(2.23)=5.94, 3.59, 5.11; p=0.0086, 0.044, 0.015) fraction, but not in other fractions (Fig. 5A). We found decreased concentrations of GRK3 in cytosol (F=5.66, 3.74, 5.48; p=0.0104, 0.04, 0.011) and crude synaptic vesicle fraction (F=3.83, 3.46, 3.73; p=0.0373, 0.049, 0.0395) but not in other fractions (Fig. 5B). Since GRKs phosphorylate receptors at the membrane, these data may indicate a redistribution of GRKs 2 and 3 from the cytosolic pool to the membrane to compensate to reduced expression and increase functionality in the SZ group. We were unable to detect significant changes in the GRK5 concentration in any individual subcellular fraction, although there was a tendency towards a reduction in the GRK5 levels in all but synaptic membrane (LP1) fractions (Fig. 5C).
Figure 5.
The GRK concentrations in subcellular fractions in control, schizoaffective and schizophrenia groups. Subcellular fractioning of the dorsolateral prefrontal cortex (Brodmann area 9/46) was performed as described in Methods. (A) – GRK2; (B) – GRK3; (C) – GRK5. The data were analyzed separately for each subcellular fraction by one-way ANOVA with GROUP (control, schizo-affective, schizophrenia) as main factor followed by Bonferroni/Dunn post hoc test. * - p<0.05; ** - p<0.01 to control.
Discussion
Here we compared the protein and mRNA levels of ubiquitously expressed arrestin and GRK isoforms in the cortex of young and elderly patients with schizophrenia with that of age-matched control individuals. We found that specific GRK isoforms were reduced in the cortex of both young and elderly schizophrenics. In the younger group, GRK3 was the most affected isoform, whereas in the older SZ group it was GRK6. Conversely, there was no decrease of GRKs in the group diagnosed with schizoaffective disorder age-matched to the younger schizophrenia group. Moreover, the levels of GRKs 2,3, and 5 were higher in schizoaffective disorder as compared to schizophrenia. Apparently, schizophrenia, but not schizoaffective disorder, is associated with decreased expression of GRKs in the cortex, although different isoforms are affected at different stages of the disease. Since both schizophrenic and schizoaffective patients antemortem received similar antipsychotic drugs, the difference between schizoaffective and schizophrenic patients is unlikely to stem from the drug treatment. Furthermore, as we have shown previously (Ahmed et al., 2008b), chronic treatment with haloperidol has no effect on the arrestin and GRK expression in the rat prefrontal cortex, whereas clozapine upregulates GRK2 but causes no other alterations. It is conceivable that the GRK downregulation is driven by psychosis in both diseases, but in schizoaffective disorder the mood component overrides that drive resulting in normal or even exaggerated levels of GRK expression.
Although GRKs and arrestins are critical for the control of GPCR signaling, their role in psychiatric disorders remains undetermined. Here we demonstrate decreased expression of arrestins and GRKs schizophrenia. The concentration of arrestins and GRKs could be reduced to compensate for insufficient stimulation of specific GPCRs. For example, as we have previously shown, loss of stimulation of dopamine receptor following dopamine depletion results in reduced striatal expression of select GRK isoforms (Ahmed et al., 2010; Ahmed et al., 2008a). Numerous studies in cultured cells and in vivo demonstrated that downregulation of arrestins and/or GRKs result in enhanced GPCR signaling (Bohn et al., 2003; Bohn et al., 1999; Gainetdinov et al., 2003; Gainetdinov et al., 1999; Luo et al., 2008). Therefore, regardless of the cause, a reduction of key proteins involved in the negative regulation of GPCR signaling could results in deficient desensitization and increased activity of select GPCRs in the prefrontal cortex of patients with schizophrenia, which suggests that excessive activity of certain G protein-dependent signaling pathways contributes to the pathophysiology of this disorder. Conversely, the proteins involved in the positive regulation of signaling via dopamine receptors such as calcyon and neuronal calcium sensor-1 are increased (Baracskay et al., 2006; Koh et al., 2003a; Koh et al., 2003b; Levenson et al., 2003), further arguing for the possibility of enhanced activity of specific GPCR-dependent pathways in schizophrenia.
Due to lack of experimental evidence, it is impossible to determine which GPCRs or signaling pathways may be affected by downregulation of GRKs seen in schizophrenia. In younger schizophrenia patients, GRK3 was the most affected, whereas GRK6 was reduced in older schizophrenics. Although only four ubiquitous GRK isoforms are available to desensitize ~800 GPCRs, it is increasingly recognized that GRK isoforms do have specific functions [reviewed in (Gurevich and Gurevich, 2008)]. The molecular basis of isoform specificity is poorly understood. In vitro studies demonstrated that receptors could be phosphorylated by more than one GRK with similar or distinct functional consequences (Kim et al., 2005; Kim et al., 2001; Pan et al., 2003; Ren et al., 2005). GRK knockout mice have remarkably narrow phenotypes, which suggests that many, albeit not all, functions of the GRK isoforms overlap allowing remaining isoforms to compensate for the missing one. The mice lacking GRK6, but not other isoforms, are supersensitive to dopamine (Gainetdinov et al., 2003), whereas mice lacking GRK5 show muscarinic, but not dopaminergic, supersensitivity (Gainetdinov et al., 1999). Obviously, functional roles of GRK isoforms may depend on their cellular distribution. Studies of the expression of GRK isoforms in defined neuronal populations are lacking, but available data indicate that most cortical neurons are likely to possess all four GRKs, albeit in different concentrations (Ahmed et al., 2008a; Arriza et al., 1992; Gurevich et al., 2004). Among the cortical neurotransmitter receptor perturbed in schizophrenia, dopamine D1, D2 and D3 receptors (Kim et al., 2001; Macey et al., 2004; Macey et al., 2005) and muscarinic M1 receptors (Willets et al., 2004; Willets et al., 2007) are known to desensitize in the GRK/arrestin-dependent manner.
The opposite pattern of changes in schizophrenia and schizoaffective disorder is surprising. Individuals with schizoaffective disorder suffer from psychosis accompanied by mood disorder. How different is the molecular basis of schizophrenia and schizoaffective disorder? Psychosis in both schizophrenia and schizoaffective disorders is successfully treated with antipsychotic drugs, suggesting common underlying biological mechanisms (Tamminga and Davis, 2007). In rare molecular studies that include schizoaffective disorder, it is often grouped together with schizophrenia in comparison to control (Morris et al., 2008), apparently, under the assumption that these conditions have common molecular basis. This assumption may be justified when the molecular mechanisms of psychosis are of prime interest. Molecular abnormalities common for schizophrenia and schizoaffective disorder have been documented (Richardson et al., 2007). Genetic linkage studies suggest that functional psychosis is a spectrum of clinical phenotypes controlled by overlapping sets of susceptibility genes (Craddock et al., 2006; Craddock et al., 2005; Craddock and Owen, 2005; Hamshere et al., 2005). A clear difference in the mode of regulation of the GRK expression between patients with schizophrenia and schizoaffective disorder found in this work suggests that some molecular mechanisms underlying the two disorders are distinct. Apparently, the GPCR desensitization machinery in general and GRKs in particular are a part of the molecular pathways differentially affected in schizophrenia and schizoaffective disorder.
Both young and older schizophrenia groups had decreased expression of some GRK isoforms in the prefrontal cortex, but here the similarity ends. In the younger group, significant decline was noted in the expression of GRK3 and a strong tendency – in the expression of GRK2 and 5 isoforms. The only isoform that was not decreased was GRK6. In contrast, in the older group, GRK6 was the only GRK isoform significantly decreased as compared to control. One obvious difference between the two cohorts used in this study is age, which affects the neurochemistry not only of the schizophrenia group but also that of control. The other is differential psychotropic treatment of patients with schizophrenia in the two cohorts. Older patients were mostly on haloperidol, whereas younger ones received atypical antipsychotics as well as non-antipsychotic drugs such as antidepressants and benzodiazepins. Almost no information is available about the effects of different classes of drugs on the expression of arrestin and GRK isoforms. We have previously shown that typical antipsychotic haloperidol and atypical drug clozapine cause quite different patterns of change in the arrestin/GRK expression both in terms of isoforms and brain regions affected (Ahmed et al., 2008b). GRK2 and 3 and arrestin2 have been shown to respond to antidepressant treatment (Avissar et al., 2004; García-Sevilla et al., 2010). These data highlight the difficulties in proposing a uniform picture of neurochemical abnormalities associated with such a complex disorder as schizophrenia. However, both schizophrenia groups, in spite of all their differences, show changes in the GRK availability in the same direction, which suggests functional relevance of these changes.
We detected a strong age-related decline in the concentration of arrestin and GRK isoforms in the cortex of neurologically and psychiatrically normal control. Interestingly, GRK6 shows no negative correlation with age, which might be the reason why it is still altered in elderly schizophrenics as compared to age-matched controls. Conversely, no age-dependent changes in the concentration of kinases belonging to different classes such as MEK1/2, ERK1/2, JNK-p55 isoforms, PDK1, or mTOR were seen. At the same time, Akt and shorter JNK-p46 isoforms showed significant decline with age similar to that seen with GRKs and arrestins. These data suggest that each kinase is differentially affected by aging and support the specificity of the age-related decline in the arrestin/GRK expression. Similarly to arrestins and GRKs, the expression of RGS4, the protein mediating the alternative desensitization process, also decreases with age (Colantuoni et al., 2008). Aging leads to deficiencies in the functions of the prefrontal cortex such as working memory due to compromised neuroanatomical and molecular integrity of the prefrontal cortex (Hao et al., 2006). In particular, age-related impairment in working memory has been linked to hyperactivity of the protein kinase A pathway (Ramos et al., 2003). The protein kinase C pathway also becomes progressively hyperactive with aging, contributing to morphological alterations in the prefrontal cortex and cognitive deficits (Brennan et al., 2007). Massively reduced availability of arrestins and GRKs in the aging prefrontal cortex is likely to result in partial loss of control over GPCR signaling, thus causing hyperactivity of certain signaling pathways and contributing to the functional decline. In addition to impaired regulation of the G protein-dependent signaling, age-related loss of arrestins, particularly that of arrestin3, might lead to defective arrestin-mediated signaling. Arrestins act as major regulators of signaling redirecting the GPCR signaling to a multitude of G protein-independent pathways (reviewed in (Gurevich and Gurevich, 2006). In particular, arrestin3 is known to couple the D2 dopamine receptor (Beaulieu et al., 2005) and the 5-HT2A receptor to the Akt-GSK pathway (Schmid and Bohn, 2010; Schmid et al., 2008). Therefore, in addition to the desensitization deficit, age-related loss of arrestins might cause abnormalities in signaling pathways controlled by GPCRs in a G protein-independent arrestin-dependent manner. Assuming that defective desensitization is the main defect, increasing the concentration and/or activity of GRKs might be beneficial for the prefrontal cortex-dependent functions in aging individuals. The decrease in the GRK concentration in schizophrenia, although smaller, resembles the age-related decline. It is conceivable that in schizophrenia, similarly to aging, the loss of GRKs leads to hyperactivity of select signaling pathways and resulting working memory and other prefrontal deficits typical for this disorder.
In conclusion, our findings provide evidence of reduced prefrontal expression of GRKs in schizophrenia and of the loss of GRKs and arrestins with age. We also demonstrate that GRK isoforms are affected in schizophrenia and schizoaffective disorder in a dissimilar manner. It is possible that the affective component in schizoaffective disorder determines the pattern of alterations in the GRK expression. Further research is required to fully appreciate the functional significance of alterations in the expression of GRK isoforms in normal aging and psychiatric disorders.
Highlights.
The expression of arrestins and GRKs was examined at postmortem.
Expression of GRKs proteins was decreased in schizophrenia.
In some cases, arrestin and GRK mRNA was also decreased.
We found no reduction in GRK expression in schizo-affective disorder.
Expression of arrestin and GRKs decreased with age in control group.
Acknowledgments
The work was supported by NS045117 and NS065868 (EVG), GM77561 (VVG), GM47417 (JLB), by MH066392 support of the Mount Sinai Department of Psychiatry Schizophrenia Brain Bank, and by MH068855 support for the Harvard Brain Tissue Resource Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A. Alterations of serotonin transmission in schizophrenia. Int Rev Neurobiol. 2007;78:133–164. doi: 10.1016/S0074-7742(06)78005-9. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MR, et al. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2010;2:28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MR, et al. Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. J Neurochem. 2008a;104:1622–36. doi: 10.1111/j.1471-4159.2007.05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MR, et al. Haloperidol and clozapine differentially affect the expression of arrestins, receptor kinases, and extracellular signal-regulated kinase activation. J Pharmacol Exp Ther. 2008b;325:276–83. doi: 10.1124/jpet.107.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, et al. The G-protein-coupled receptor kinases bARK1 and bARK2 are widely distributed at synapses in rat brain. J Neurosci. 1992;12:4045–4055. doi: 10.1523/JNEUROSCI.12-10-04045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attramadal H, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–90. [PubMed] [Google Scholar]
- Avissar S, et al. Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry. 2004;161:2066–2072. doi: 10.1176/appi.ajp.161.11.2066. [DOI] [PubMed] [Google Scholar]
- Baracskay KL, et al. Dopamine receptor signaling molecules are altered in elderly schizophrenic cortex. Synapse. 2006;60:271–279. doi: 10.1002/syn.20292. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Gomez J. Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family. J Biol Chem. 1993;268:19521–19527. [PubMed] [Google Scholar]
- Bezard E, et al. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol Dis. 2005;18:323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bohn LM, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–3. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–73. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Brennan AR, et al. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov E, et al. Arrestins and two receptor kinases are upregulated in Parkinson's disease with dementia. Neurobiol Aging. 2008;29:379–396. doi: 10.1016/j.neurobiolaging.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, et al. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annual Review of Biochemistry. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, et al. Age-related changes in the expression of schizophrenia susceptibility genes in the human prefrontal cortex. Brain Struct Funct. 2008 doi: 10.1007/s00429-008-0181-5. In press. [DOI] [PubMed] [Google Scholar]
- Craddock N, et al. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, et al. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genetics. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, et al. Psychiatric Brain Banking: Three Perspectives on Current Trends and Future Directions. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.05.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart AD, et al. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000;86:43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- Feighner JP, et al. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, et al. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–36. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, et al. Desensitization of G protein-coupled receptors and neuronal function. Ann Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, et al. Reduced platelet G protein-coupled receptor kinase 2 in major depressive disorder: antidepressant treatment-induced upregulation of GRK2 protein discriminates between responder and non-responder patients. Eur Neuropsychopharmacol. 2010;20:721–730. doi: 10.1016/j.euroneuro.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Goldsmith SK, et al. Disrupted pattern of D2 dopamine receptors in the temporal lobe in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1997;54:649–658. doi: 10.1001/archpsyc.1997.01830190077008. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, et al. Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience. 2002;109:421–436. doi: 10.1016/s0306-4522(01)00511-5. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, et al. Arrestin2 expression selectively increases during neural differentiation. J Neurochem. 2004;91:1404–16. doi: 10.1111/j.1471-4159.2004.02830.x. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The new face of active receptor bound arrestin attracts new partners. Structure. 2003;11:1037–1042. doi: 10.1016/s0969-2126(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. Rich tapestry of GPCR signaling and regulatory mechanisms. Mol Pharm. 2008 doi: 10.1124/mol.108.049015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere ML, et al. Genomewide Linkage Scan in Schizoaffective Disorder: Significant Evidence for Linkage at 1q42 Close to DISC1, and Suggestive Evidence at 22q11 and 19p13. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Hao J, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino G, et al. Myocardial overexpression of GRK3 in transgenic mice: evidence for in vivo selectivity of GRKs. Am J Physiol. 1998a;275:1298–1306. doi: 10.1152/ajpheart.1998.275.4.H1298. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, et al. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by b-adrenergic receptor stimulation and blockade. Circulation. 1998b;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- Iwata K, et al. Bimodal regulation of the human H1 histamine receptor by G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:2197–204. doi: 10.1074/jbc.M408834200. [DOI] [PubMed] [Google Scholar]
- Kim CM, et al. Expression and characterization of two beta-adrenergic receptor kinase isoforms using the baculovirus expression system. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Nat Acad Sci USA. 2005;102:142–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, et al. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Koch WJ, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Koh PO, et al. Up-regulation of the D1 dopamine receptor-interacting protein, calcyon, in patients with schizophrenia. Arch Gen Psychiatry. 2003a;60:311–319. doi: 10.1001/archpsyc.60.3.311. [DOI] [PubMed] [Google Scholar]
- Koh PO, et al. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Nat Acad Sci USA. 2003b;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout TA, et al. Beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–6. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli P, et al. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK5. J Biol Chem. 1994;269:1099–1105. [PubMed] [Google Scholar]
- Levenson R, et al. Dopamine receptor-interacting proteins: The Ca2+ connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Benovic JL. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK6. J Biol Chem. 1994;269:22691–22697. [PubMed] [Google Scholar]
- Luo J, et al. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol mol107044750. 2008 doi: 10.1124/mol.107.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, et al. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;66:1635–42. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- Macey TA, et al. Dopamine D1 receptor interaction with arrestin3 in neostriatal neurons. J Neurochem. 2005;93:128–134. doi: 10.1111/j.1471-4159.2004.02998.x. [DOI] [PubMed] [Google Scholar]
- Morris HM, et al. Alterations in Somatostatin mRNA Expression in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia or Schizoaffective Disorder. Cereb Cortex. 2008 doi: 10.1093/cercor/bhm186. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, et al. Characterization of G protein-coupled receptor regulation in antisense mRNA-expressing cells with reduced arrestin levels. Biochemistry. 1999;38:8723–8732. doi: 10.1021/bi990361v. [DOI] [PubMed] [Google Scholar]
- Naik S, et al. Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J Biol Chem. 2005;280:8722–32. doi: 10.1074/jbc.M413014200. [DOI] [PubMed] [Google Scholar]
- Oakley RH, et al. Differential affinities of visual arrestin, barrestin1, and barrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Benovic JL. Characterization of dominant negative arrestins that inhibit beta-2-adrenergic receptor internalization by distinct mechanisms. J Biol Chem. 1998;273:34616–34622. doi: 10.1074/jbc.273.51.34616. [DOI] [PubMed] [Google Scholar]
- Pan L, et al. The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- Parruti G, et al. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J Biol Chem. 1993;268:9753–9761. [PubMed] [Google Scholar]
- Premont RT, et al. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- Ramos BP, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Nat Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MA, et al. Analysis of plasma biopterin levels in psychiatric disorders suggests a common BH4 deficit in schizophrenia and schizoaffective disorder. Neurochem Res. 2007;32:107–113. doi: 10.1007/s11064-006-9233-5. [DOI] [PubMed] [Google Scholar]
- Rockman HA, et al. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Nat Acad Sci USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–1195. doi: 10.1111/j.1471-4159.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- Schmauss C. Enhanced cleavage of an atypical intron of dopamine D3-receptor pre-mRNA in chronic schizophrenia. J Neurosci. 1996;16:7902–7909. doi: 10.1523/JNEUROSCI.16-24-07902.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, et al. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R, et al. Polypeptide variants of beta-arrestin and arrestin3. J Biol Chem. 1993;268:15640–15648. [PubMed] [Google Scholar]
- Tamminga CA, Davis JM. The Neuropharmacology of Psychosis. Schizophr Bull. 2007:11. doi: 10.1093/schbul/sbm063. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waas WF, et al. Two rate-limiting steps in the kinetic mechanism of the serine/threonine specific protein kinase ERK2: a case of fast phosphorylation followed by fast product release. Biochemistry. 2003;42:12273–12286. doi: 10.1021/bi0348617. [DOI] [PubMed] [Google Scholar]
- Willets JM, et al. Imaging of muscarinic acetylcholine receptor signaling in hippocampal neurons: evidence for phosphorylation-dependent and -independent regulation by G-protein-coupled receptor kinases. J Neurosci. 2004;24:4157–62. doi: 10.1523/JNEUROSCI.5506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willets JM, et al. The regulation of M1 muscarinic acetylcholine receptor desensitization by synaptic activity in cultured hippocampal neurons. J Neurochem. 2007;103:2268–2280. doi: 10.1111/j.1471-4159.2007.04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]