Abstract
Following vessel wall injury, platelets adhere to the exposed subendothelium, become activated and release mediators such as TXA2 and nucleotides stored at very high concentration in the so-called dense granules. Released nucleotides and other soluble agents act in a positive feedback mechanism to cause further platelet activation and amplify platelet responses induced by agents such as thrombin or collagen. Adenine nucleotides act on platelets through three distinct P2 receptors: two are G protein-coupled ADP receptors, namely the P2Y1 and P2Y12 receptor subtypes, while the P2X1 receptor ligand-gated cation channel is activated by ATP. The P2Y1 receptor initiates platelet aggregation but is not sufficient for a full platelet aggregation in response to ADP, while the P2Y12 receptor is responsible for completion of the aggregation to ADP. The latter receptor, the molecular target of the antithrombotic drugs clopidogrel, prasugrel and ticagrelor, is responsible for most of the potentiating effects of ADP when platelets are stimulated by agents such as thrombin, collagen or immune complexes. The P2X1 receptor is involved in platelet shape change and in activation by collagen under shear conditions. Each of these receptors is coupled to specific signal transduction pathways in response to ADP or ATP and is differentially involved in all the sequential events involved in platelet function and haemostasis. As such, they represent potential targets for antithrombotic drugs.
Keywords: Haemostasis, Thrombosis, ADP, P2Y1, P2Y12, P2X1, Antiplatelet drugs, Thienopyridine, Clopidogrel, Prasugrel, AZD6140, Cangrelor, Ticagrelor
Introduction
The main role of blood platelets is to ensure primary haemostasis, which means the maintenance of blood vessel integrity and the rapid cessation of bleeding in the event of loss of vascular integrity. They are also responsible for the formation of pathogenic thrombi at sites of rupture or erosion of an atherosclerotic plaque, promoting atherothrombotic diseases including acute coronary syndromes, ischaemic stroke and peripheral artery disease [1]. Platelets also play an important role in inflammation and can influence the phenotype of other blood and vascular cells, thereby contributing to many other non-haemostatic disorders, from cystic fibrosis and arthritis to diabetes, atherosclerosis and cancer [2–7].
Extracellular nucleotides and their receptors are important components of the cardiovascular system and regulate a broad range of physiological processes like the control of vascular tone, smooth muscle cell proliferation and platelet activation [8]. Adenosine 5′-disphosphate (ADP) plays crucial roles in the physiological process of haemostasis and in the development and extension of arterial thrombosis [9]. As compared to strong agonists such as thrombin or collagen, ADP is, by itself, a weak agonist of platelet aggregation inducing only reversible responses. However, ADP, stored at a very high concentration in platelet dense granules and released upon activation at sites of vascular injury, constitutes an important so-called secondary agonist, which greatly amplifies most of the platelet responses and contributes to the stabilization of the thrombus.
Addition of ADP to washed platelets results in shape change, reversible aggregation at physiological concentrations of calcium (2 mM) and finally desensitization [10, 11]. Transduction of the ADP signal involves a transient rise in free cytoplasmic calcium, due to mobilization of internal stores, secondary store-mediated influx and a concomitant inhibition of adenylyl cyclase activity [12]. ATP induces an extremely rapid influx of calcium from the extracellular medium associated to platelet shape change [13, 14].
Starting from the concept of a unique P2T receptor (T for thrombocyte) originally postulated on the basis of pharmacological data [15], a model of three platelet P2 receptors progressively emerged [16]. These are the P2X1 cation channel, which is activated by ATP and two G protein-coupled receptors, P2Y1 and P2Y12, both activated by ADP. Each of these receptors has a specific function during platelet activation and aggregation, which naturally has implications for their involvement in thrombosis.
The respective roles of the three platelet P2 receptors during platelet activation
The P2Y1 receptor
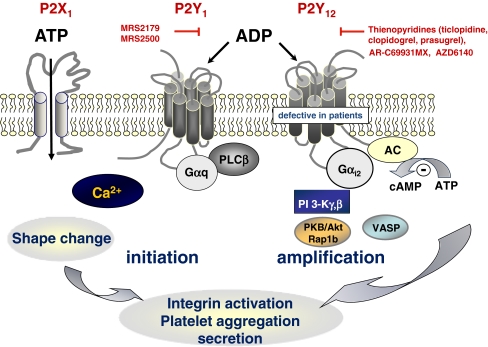
The P2Y1 receptor is widely distributed in many tissues including heart, blood vessels and blood cells, neural tissue, testis, prostate and ovary (Fig. 1) [17]. About 150 P2Y1 receptor-binding sites are expressed per platelet [18, 19], which is very low as compared for instance to the TP receptors or the thrombin receptor PAR-1 (1,000 to 2,000 sites per platelet). As it is coupled to Gαq, the P2Y1 receptor triggers the mobilization of calcium from internal stores, which results in platelet shape change and weak, transient aggregation in response to ADP [20–22]. The P2Y1 receptor is absolutely required for ADP-induced platelet aggregation. Its pharmacological inhibition or genetic deficiency results in complete absence of platelet aggregation and shape change in response to ADP. As a consequence, at the intracellular level, the calcium signal is abolished, while the ability of ADP to inhibit cAMP formation is preserved [20, 23]. The P2Y1 receptor also participates in the aggregation response to collagen and plays a key role in collagen-induced shape change when TXA2 formation is prevented [23, 24]. Overall, the P2Y1 receptor mediates weak responses to ADP but is nevertheless a crucial factor in the initiation of the platelet activation induced by ADP or collagen.
Fig. 1.
Schematic representation of the current model of platelet activation induced by adenine nucleotides (ATP and ADP)
Several selective antagonists of this receptor have been described [25], namely the adenine nucleotide analogues A2P5P, A3P5P or A3P5PS [26]; MRS2179 (N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate) [18, 27–30]; MRS2279 [31] and MRS2500 (2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate) [19, 29, 32]. The latter displays the highest affinity for P2Y1 and constitutes to date the most valuable tool to investigate the role of the P2Y1 receptor in platelet function (for review, please see Kenneth A. Jacobson, same issue).
Besides platelets, the P2Y1 receptor is also expressed on endothelial cells, where it contributes to nucleotide-induced relaxation [33, 34], and was recently shown to be involved in endothelial cell migration [35]. It is also expressed on leukocytes, where its role is less well established, although a role in the phagocytic activity of macrophages has recently been highlighted [36].
The P2Y12 receptor
The P2Y12 receptor, despite being well known and characterized on the basis of both pharmacological and genetic evidence, was the last platelet P2 receptor to be cloned [37, 38]. Its tissue distribution is very limited, although not entirely restricted to platelets as it is also present in brain [37], glial cells [39] and possibly in vascular smooth muscle cells, where it could contribute to vessel contraction [40, 41]. This receptor is defective in patients with selective defects in platelet activation by ADP [42] (for review, please see Marco Cattaneo, same issue). ADP is the natural agonist of this receptor, while ATP and a wide range of its triphosphate analogues behave as antagonists [43, 44]. It is the molecular target of the antiplatelet drugs clopidogrel and prasugrel, two thienopyridine compounds that covalently bind to the receptor, and of ticagrelor (AZD6140), cangrelor (AR-C69931MX) and elinogrel (PRT060128), which are competitive antagonists of the receptor [42, 45]. The P2Y12 receptor is responsible for completion of the platelet aggregation response to ADP initiated by P2Y1 [46] and for the ADP-dependent amplification of platelet aggregation induced by other agents such as Gq-coupled serotonin receptors [22], Gq and G12/13-coupled TXA2 and PAR-1 receptors [47, 48], immune complexes [49, 50] or when platelets are activated by collagen through GPVI/tyrosine kinase/PLCγ2 pathway [51]. The P2Y12 receptor is also responsible for the ability of ADP to restore collagen-induced aggregation in Gαq-deficient mouse platelets [52]. The P2Y12 receptor is also involved in potentiation of platelet secretion independently of TXA2 generation and macroaggregate formation [53, 54] and mediates the stabilization of platelet aggregates induced by thrombin [55–57] or TXA2 [58]. The requirement of this receptor for completion of aggregation in response to ADP but also for the ADP-dependent amplification of aggregation induced by other agents was confirmed in P2Y−/−12 mice [37, 59]. The bleeding time is markedly prolonged in these mice [37, 59], as it is in patients with severe P2Y12 deficiency [42], as well as in animals treated with high doses of clopidogrel or other P2Y12 antagonists.
The P2Y12 receptor is coupled to inhibition of adenylyl cyclase activity through activation of a Gαi2 G protein subtype [60, 61], which is a critical component of the signalling pathway for integrin αIIbβ3 activation [61, 62]. However, adenylyl cyclase inhibition and lowering cAMP levels are not sufficient to cause platelet aggregation [63–65]; thus, other signalling events are required for full activation of the αIIbβ3 integrin and subsequent aggregation [66]. One important intracellular pathway which regulates Gi-dependent integrin αIIbβ3 activation is constituted by phosphoinositide 3-kinase (PI 3-K) [56, 67–70]. PI 3-K isoform p110β regulates integrin activation through a classical lipid kinase-dependent mechanism, involving the small GTPase Rap1 and/or the serine-threonine protein kinase B/Akt (PKB/Akt) [71–76], whereas p110γ appears to regulate integrin principally through a non-catalytic signalling mechanism [77, 78]. Whether other PI3K class I isoforms such as the p110α or PI3K class II or III isoforms, which are highly expressed in blood platelets, play a role in integrin αIIbβ3 activation remains to be determined. Another way by which P2Y12 could contribute to modulate aggregation through Gαi2 may involve inhibition of the cAMP-dependent protein kinase (PKA)-mediated phosphorylation of the vasodilator-stimulated phosphoprotein (VASP), an intracellular actin regulatory protein that is a negative modulator of αIIbβ3 integrin activation [79].
Co-activation of the P2Y1 and P2Y12 receptors is necessary for normal ADP-induced platelet aggregation since separate inhibition of either of them with selective antagonists results in a dramatic decrease in aggregation [22, 46, 80]. The P2Y1 and P2Y12 receptors are differentially involved in platelet aggregation induced by other agonists, with the P2Y1 playing only a minor role, except in the case of collagen-induced activation, while P2Y12 supports amplification of these responses. This is also the case in the procoagulant activity of platelets. While both receptors are indirectly involved through their role in platelet P-selectin exposure and in the formation of platelet-leukocyte conjugates leading to leukocyte tissue factor exposure [81, 82], the P2Y12 receptor is also directly implicated in the exposure of phosphatidylserine at the surface of platelets [81, 83, 84].
The P2X1 receptor
The third component of the platelet P2 receptors is P2X1, a ligand-gated cation channel responsible for a fast calcium entry induced by ATP [14, 85]. Although unable to trigger platelet aggregation by itself, the P2X1 receptor induces transient shape change [13] and participates in collagen- and shear-induced aggregation [86–88]. A comprehensive review of its role in platelet function is provided by Martyn Mahaut-Smith (this issue).
Desensitization
An important phenomenon in controlling thrombus growth is the regulation of platelet reactivity after stimulation, and receptor desensitization is one general mechanism used by cells to adapt their responsiveness. It has long been known that after being exposed to ADP, platelets become unresponsive to a second stimulation with ADP with a resultant loss of shape change and aggregation. This so-called refractory state of platelets to ADP is transient and, depending on the experimental conditions, lasts 15 to 30 min provided an enzymatic system degrades ADP in the medium. In the absence of such a system, platelets do not recover responsiveness to ADP. The molecular mechanisms of this phenomenon have been studied in detail, but consensus has not been reached, and two different views have not yet been reconciled. On the one hand, it is thought that the phenomenon of platelet refractoriness to ADP is due to selective desensitization and internalization of the P2Y1 receptor, while the P2Y12 receptor remains functional with the ability of ADP to induce amplification of the platelet aggregation induced by other agonists [89–91]. Desensitization of the P2Y1 receptor has been shown to be dependent on receptor C-terminal phosphorylation sites, β-arrestin-2 interaction and protein kinase C (PKC) activity [92, 93]. The in vivo consequence is that under conditions of platelets refractory to stimulation by ADP, the P2Y12 receptor remains functional and able to promote their reactivity at sites of injury, thus preventing loss of haemostatic function. On the other hand, it is reported that both P2Y1 and P2Y12 receptors undergo desensitization, and that P2Y12 desensitization is mediated by G protein-coupled receptor kinases (GRK) [93, 94]. Further studies are required to solve the apparent contradiction of these reports.
Finally, the P2X1 receptor is also desensitized, and this occurs very quickly and requires lower concentrations of nucleotides than for the metabotropic receptor P2Y1 [95, 96]. The physiological implications of P2X1 desensitization are still not well understood but might be related to the need to confine thrombus growth to the site of a lesion and prevent uncontrolled extension of the platelet aggregates.
Genetic polymorphisms of the P2Y receptors
Apart from the P2Y12 receptor defects in patients with mild to severe haemorrhagic diathesis (reviewed by Marco Cattaneo, this issue), P2Y1 and P2Y12 have been shown to display gene sequence variations, which have been proposed to be associated with increased platelet responsiveness to ADP. In P2Y12, the polymorphisms are in the intronic part of the gene and have no obvious impact on the coding sequence. Two haplotypes have been identified, designated as H1 and H2, the latter being proposed to be linked to enhanced platelet reactivity to ADP [97] and to a diminished response to clopidogrel [98] and associated with increased risks for peripheral arterial disease [99] and coronary artery disease [100]. However, these results were not confirmed in latter studies [101–103]. It thus appears that polymorphisms of the non-coding region of the P2Y12 receptor gene do not have any impact on the receptor function nor on the individual responsiveness to clopidogrel. Concerning the P2Y1 receptor, a silent polymorphism was identified at position 1622 (A/G) of the coding sequence, which led to increased platelet aggregation in response to a low concentration of ADP (0.1 μM) in subjects carrying the G allele [104]. Again, these results were not confirmed in a large population of CAD patients treated with clopidogrel [105]. Overall, whether polymorphism of the P2Y1 and P2Y12 receptors exists, which have an impact on the platelet physiology or in clinical pharmacology, probably requires further studies.
The platelet P2 receptors as molecular targets for antithrombotic drugs
The P2Y12 receptor
Long before its molecular cloning, the pharmacological importance of this receptor in haemostasis and thrombosis was well recognized. This was due to the fact that the potent antithrombotic thienopyridine compounds ticlopidine and clopidogrel, of which an active liver metabolite selectively and irreversibly targets the P2Y12 receptor, were used as molecular tools to characterize platelet responses to ADP and the role of the latter in thrombosis [106]. The thienopyridine compounds are prodrugs which have to be metabolized by the liver in order to generate active metabolites. The active metabolite of clopidogrel [107] covalently binds cysteine residues of the P2Y12 receptor, thus precluding the binding of ADP [108–110]. Moreover, it has been recently reported that clopidogrel's active metabolite disrupts homopolymers of the P2Y12 receptor expressed in lipid rafts and partitions them out of lipid rafts [111], pointing to the importance of oligomerization and membrane localization on the function of this receptor. Further studies are however required to confirm these findings.
Clopidogrel treatment leads to a dose-dependent inhibition of platelet aggregation in response to ADP with conserved shape change and transient weak aggregation driven by P2Y1. At the intracellular level, P2Y12 blockade results in the inhibition of the ability of ADP to inhibit cyclic AMP production while calcium signalling is preserved [46]. Platelet aggregation in response to strong activators is also strongly inhibited through the effect on released ADP.
Large-scale clinical trials have demonstrated the beneficial effects of thienopyridines in the prevention of major cardiac events after coronary artery stent insertion and in the secondary prevention of major vascular events in patients with a history of cerebrovascular, coronary or peripheral artery disease [106, 112].
Prasugrel (CS-747, LY640315) is a third-generation thienopyridine compound which has higher efficacy and faster onset of action than clopidogrel. This is due to a slightly different metabolic pathway and better rate of active metabolite generation as compared to clopidogrel [113]. A large-scale clinical trial, TRITON-TIMI 38, including 13,609 patients planed for percutaneous coronary intervention (PCI) demonstrated the overall superiority of prasugrel (60 mg loading dose followed by 10 mg maintenance dose) in comparison to clopidogrel (300 mg loading dose, 75 mg maintenance dose) with a total of 19% reduction of ischemic events with, particularly, 52% decreased stent thrombosis [114], but with a 32% increase of major bleeding, including fatal bleeding. Although not really surprising, these results had an important impact in the practises of interventional cardiologists [115].
Competitive P2Y12 antagonists cangrelor (AR-C69931MX) and ticagrelor (AZD6140) are in various phases of development, the latter being orally active while cangrelor requires intravenous administration [45, 116, 117]. Theoretically, use of such molecules would have an advantage mainly in acute situations like myocardial infarction, where fast blockade of the ADP receptor should be beneficial as compared to the delayed action of thienopyridine compounds. The rapid cessation of activity would also be beneficial in terms of safety. A second theoretical advantage of using competitive P2Y12 antagonists could be if there is less inter-individual variability in the response to the treatment. Cangrelor underwent two phase III trials (CHAMPION-PCI and CHAMPION-PLATFORM) which were stopped early for lack of efficacy over placebo or clopidogrel, respectively, in patients undergoing PCI. Cangrelor is still being studied as a bridge for patients on clopidogrel who need to go off of drug to undergo surgery [118]. Ticagrelor was in a phase III trial (PLATO) assessing whether this agent has clinical efficacy superior to clopidogrel in the management of ACS. Ticagrelor demonstrated improved cardiovascular outcomes, including a reduction in myocardial infarctions and vascular events as compared to clopidogrel. The main adverse events with ticagrelor are bleeding and dyspnoea, the latter of which is of unclear aetiology and of unknown long-term clinical concern [119, 120]. For a complete review, please see Collet et al. (this issue).
The P2Y1 receptor as a target for new antiplatelet compounds
A consideration of the role of P2Y1 in platelet aggregation and experimental thrombosis provides the rational for suggesting this receptor to be a relevant target for new antiplatelet compounds. P2Y1 knockout mice display resistance in various models of thrombosis such as the systemic thromboembolism induced by infusion of a mixture of collagen and adrenaline [23, 121] or of tissue factor [84] or in localized thrombosis after ferric chloride- or laser-induced injury of mouse mesenteric arteries [122]. Similar protection is observed in animals treated with selective P2Y1 antagonists such as the adenine nucleotide analogues MRS2179 [18, 27, 84] or MRS2500 [123]. However, due to their limited bioavailability for long-term treatment, new P2Y1 receptor antagonists with improved pharmacokinetic profile will need to be developed. Several non-nucleotide antagonists of this receptor have been described such as tetrahydro-quinolinamine inhibitors [124], aryl-urea inhibitors [125] and benzofuran-substituted urea derivatives [126] which display however lower affinity for the receptor. Whether these compounds fulfil these latter criteria and are effective in vivo remain to be investigated.
Moreover, a combination of P2Y1 deficiency or inhibition and clopidogrel treatment has been found to confer better thromboresistance than either condition alone, suggesting that a combination of P2 receptor antagonists could improve antithrombotic strategies [122, 123]. It is worthy to note that inhibition of the P2Y1 receptor results in only moderate prolongation of the bleeding time, which could be advantageous in terms of safety. Additional advantages of targeting the P2Y1 receptor rely on its role in vascular inflammation [127] and in atherosclerosis [128] (see below). As a consequence, this receptor could represent an attractive and original target for drugs with multiple sites of action in atherothrombosis and beyond in other inflammatory diseases.
The P2X1 receptor as a target for new antiplatelet compounds
P2X1-deficient mice have in fact no prolongation of their bleeding time as compared to the wild type, indicating that they conserve normal haemostasis. In contrast, they display resistance to the systemic thromboembolism induced by injection of a mixture of collagen and adrenaline and to localized laser-induced injury of the vessel wall of mesenteric arteries [88]. Since in vitro the P2X1 receptor plays an important role in thrombus formation only under high shear conditions, it might represent the ideal target for an antithrombotic drug. Conversely, increased systemic thrombosis has been reported in mice overexpressing the human P2X1 receptor [129]. Moreover, the P2X1 antagonist NF449 [130] has an inhibitory effect on platelet activation ex vivo and thrombosis in vivo [131]. These results clearly indicate that the P2X1 receptor should also be considered as a potential target for antiplatelet strategies, with the interesting feature that P2X1 antagonists should be effective only at sites of severe stenosis where shear forces are very high, without having a deleterious effect on normal haemostasis. However, further work is required to conclusively establish this point.
The platelet P2 receptors beyond haemostasis (Fig. 2)
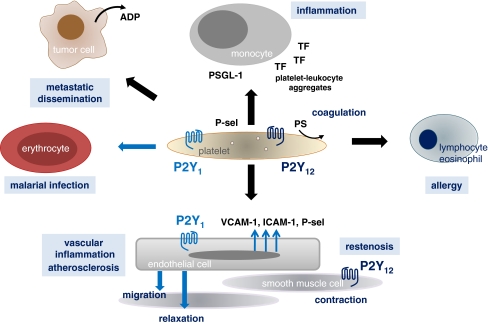
Fig. 2.
Role of P2Y1 and P2Y12 receptors beyond haemostasis
Vascular inflammation plays a central role in both the progressive and acute components of atherothrombotic disease. It is now appreciated that activated platelets contribute to inflammation since platelets are an important source of inflammatory mediators, compounds with trophic activity such as PDGF, expose P-selectin, CD40 and CD40 ligand (CD40L) which allow interaction with leukocytes and subsequent leukocyte activation and release of a range of inflammatory cytokines and exposure of tissue factor [4]. Thus, the clinical efficacy of antiplatelet drugs might also be related to blockade of the contribution of platelets to inflammation [132]. The role of the P2Y12 receptor not only in platelet aggregation but also in the activation of multiple inflammatory and trophic processes may be expected to result in its direct involvement in the progression of atherosclerosis and restenosis, which has been reported recently in rabbit and in mice [133–137].
Concerning P2Y1, its role in platelet function and its presence in all cell types and tissues involved in inflammation and atherosclerosis questioned its involvement in these diseases [138, 139]. Using P2Y−/−1 mice crossed with ApoE−/− mice, a role of the P2Y1 receptor in the development of atherosclerosis was demonstrated [128]. Interestingly, bone marrow transplantation experiments showed that the platelet receptor is not involved in this process, suggesting a possible role of the P2Y1 receptor expressed in endothelial cells [128]. We recently reported that the P2Y1 receptor contributes to the upregulation of adhesion molecule (P-selectin, VCAM-1 and ICAM-1) exposure in TNFα-stimulated ECs by a mechanism involving p38 MAP kinase signalling pathway, which in turn facilitates recruitment of monocytes and their transmigration. In addition, we found that the endothelial P2Y1 receptor contributes to TNFα-induced leukocyte recruitment in experimentally inflamed arteries in a mouse model in vivo [127]. Thus, the P2Y1 receptor might be an original target for new anti-inflammatory strategies.
In addition to vascular inflammation, through their capability of interacting with many other cells, platelets are involved in many physiological and pathological processes which we will not all cover here but will just point some of these. Platelets play a role in allergic asthma [140]. They are necessary for lung leukocyte recruitment in a murine model of allergic inflammation, and platelet-leukocyte aggregates are formed in circulating blood of patients with asthma after allergen exposure [141]. It has been reported that the P2Y12 receptor is required for proinflammatory actions of the stable abundant mediator LTE4 in allergic asthma and has been suggested to be a novel potential therapeutic target for asthma [142]. The specific contribution of the platelet P2 receptors in this disease warrants further studies as the P2Y1 receptor has also been proposed to have a role in airways inflammation [143].
Platelets are strongly involved in cancer and especially in metastatic dissemination [144, 145] through complex mechanisms including the ability to mask tumour cells to the immune system and to contribute to tumoural angiogenesis. In particular, tumour cells release nucleotides which, among other stimuli, activate platelets. Here too, animal models and clinical trials should be undertaken to evaluate existing P2 receptor targeting drugs as adjuvant anticancer therapies.
Finally, platelets are also involved in innate immunity [146, 147]. In particular, platelets play a critical role in the pathogenesis of malarial infections. However, recent work suggests that they are also involved in protection against this infection and that killing of parasite-infected erythrocytes was dependent on platelet activation via the P2Y1 receptor, but not P2Y12 [148]. Whether the platelet P2 receptors display specific roles in diseases where platelets are involved requires further studies.
Conclusions
All the effects of nucleotides on platelets appear to lay on three distinct receptors, namely two ADP receptors P2Y1 and P2Y12, and one ATP receptor P2X1. They mediate selective transduction pathways responsible for the different function of platelets. Each of these receptors plays also a role in thrombosis. The P2Y12 receptor is an established target for antithrombotic drugs. The challenge for the future will be to determine whether the two other nucleotide receptors P2Y1 and P2X1 constitute solely or in combination with P2Y12, attractive target for new antithrombotic drugs. Roles of blood platelets beyond haemostasis also involve the platelet P2 receptors, which should now be studied in more details.
References
- 1.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102(2):248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–2367. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Sullivan BP, Linden MD, Frelinger AL, 3rd, Barnard MR, Spencer-Manzon M, Morris JE, Salem RO, Laposata M, Michelson AD. Platelet activation in cystic fibrosis. Blood. 2005;105(12):4635–4641. doi: 10.1182/blood-2004-06-2098. [DOI] [PubMed] [Google Scholar]
- 6.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180(93):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60(1):12–20. [PubMed] [Google Scholar]
- 9.Born GV. Adenosine diphosphate as a mediator of platelet aggregation in vivo: an editorial view. Circulation. 1985;72(4):741–746. doi: 10.1161/01.cir.72.4.741. [DOI] [PubMed] [Google Scholar]
- 10.Mustard JF, Perry DW, Kinlough-Rathbone RL, Packham MA. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- 11.Jones S, Evans RJ, Mahaut-Smith MP. Extracellular Ca(2+) modulates ADP-evoked aggregation through altered agonist degradation: implications for conditions used to study P2Y receptor activation. Br J Haematol. 2011;153(1):83–91. doi: 10.1111/j.1365-2141.2010.08499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86(1):222–232. [PubMed] [Google Scholar]
- 13.Rolf MG, Brearley CA, Mahaut-Smith MP. Platelet shape change evoked by selective activation of P2X1 purinoceptors with alpha, beta-methylene ATP. Thromb Haemost. 2001;85(2):303–308. [PubMed] [Google Scholar]
- 14.Mahaut-Smith MP, Tolhurst G, Evans RJ. Emerging roles for P2X1 receptors in platelet activation. Platelets. 2004;15(3):131–144. doi: 10.1080/09537100410001682788. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 17.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baurand A, Raboisson P, Freund M, Léon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412(3):213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 19.Ohlmann P, Castro S, Brown GG, Jr, Gachet C, Jacobson KA, Harden TK. Quantification of recombinant and platelet P2Y(1) receptors utilizing a [(125)I]-labeled high-affinity antagonist 2-iodo-N(6)-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([(125)I]MRS2500) Pharmacol Res. 2010;62(4):344–351. doi: 10.1016/j.phrs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechler B, Léon C, Vial C, Vigne P, Frelin C, Cazenave JP, Gachet C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92(1):152–159. [PubMed] [Google Scholar]
- 21.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273(4):2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 22.Savi P, Beauverger P, Labouret C, Delfaud M, Salel V, Kaghad M, Herbert JM. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;422(3):291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 23.Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest. 1999;104(12):1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangin P, Ohlmann P, Eckly A, Cazenave JP, Lanza F, Gachet C. The P2Y receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost. 2004;2(6):969–977. doi: 10.1111/j.1538-7836.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15(13–14):570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50(5):1323–1329. [PubMed] [Google Scholar]
- 27.Baurand A, Gachet C. The P2Y(1) receptor as a target for new antithrombotic drugs: a review of the P2Y(1) antagonist MRS-2179. Cardiovasc Drug Rev. 2003;21(1):67–76. doi: 10.1111/j.1527-3466.2003.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 28.Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124(1):1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46(23):4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y(1) receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62(5):1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N(6)-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y(1) receptor antagonist. Br J Pharmacol. 2002;135(8):2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68(10):1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser RA, Buxton IL. Nucleotide-mediated relaxation in guinea-pig aorta: selective inhibition by MRS2179. Br J Pharmacol. 2002;135(2):537–545. doi: 10.1038/sj.bjp.0704476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guns PJ, Korda A, Crauwels HM, Assche T, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146(2):288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen J, DiCorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res. 2008;102(4):448–456. doi: 10.1161/CIRCRESAHA.107.165795. [DOI] [PubMed] [Google Scholar]
- 36.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216(1–2):1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 38.Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, Palmer K, Bayne M, Monsma FJ., Jr ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem. 2001;276(11):8608–8615. doi: 10.1074/jbc.M009718200. [DOI] [PubMed] [Google Scholar]
- 39.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 40.Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004;24(10):1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- 41.Hogberg C, Svensson H, Gustafsson R, Eyjolfsson A, Erlinge D. The reversible oral P2Y12 antagonist AZD6140 inhibits ADP-induced contractions in murine and human vasculature. Int J Cardiol. 2010;142(2):187–192. doi: 10.1016/j.ijcard.2008.12.091. [DOI] [PubMed] [Google Scholar]
- 42.Cattaneo M. The platelet P2Y receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011;117(7):2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- 43.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108(2):180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Kauffenstein G, Hechler B, Cazenave JP, Gachet C. Adenine triphosphate nucleotides are antagonists at the P2Y12 receptor. J Thromb Haemost. 2004;2(11):1980–1988. doi: 10.1111/j.1538-7836.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 45.Michelson AD. New P2Y12 antagonists. Curr Opin Hematol. 2009;16(5):371–377. doi: 10.1097/MOH.0b013e32832ea2f2. [DOI] [PubMed] [Google Scholar]
- 46.Hechler B, Eckly A, Ohlmann P, Cazenave JP, Gachet C. The P2Y1 receptor, necessary but not sufficient to support full ADP-induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol. 1998;103(3):858–866. doi: 10.1046/j.1365-2141.1998.01056.x. [DOI] [PubMed] [Google Scholar]
- 47.Nieswandt B, Schulte V, Zywietz A, Gratacap MP, Offermanns S. Costimulation of Gi- and G12/G13-mediated signaling pathways induces integrin alpha IIbbeta 3 activation in platelets. J Biol Chem. 2002;277(42):39493–39498. doi: 10.1074/jbc.M207256200. [DOI] [PubMed] [Google Scholar]
- 48.Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277(49):47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 49.Polgar J, Eichler P, Greinacher A, Clemetson KJ. Adenosine diphosphate (ADP) and ADP receptor play a major role in platelet activation/aggregation induced by sera from heparin-induced thrombocytopenia patients. Blood. 1998;91(2):549–554. [PubMed] [Google Scholar]
- 50.Gratacap MP, Herault JP, Viala C, Ragab A, Savi P, Herbert JM, Chap H, Plantavid M, Payrastre B. FcgammaRIIA requires a Gi-dependent pathway for an efficient stimulation of phosphoinositide 3-kinase, calcium mobilization, and platelet aggregation. Blood. 2000;96(10):3439–3446. [PubMed] [Google Scholar]
- 51.Nieswandt B, Bergmeier W, Eckly A, Schulte V, Ohlmann P, Cazenave JP, Zirngibl H, Offermanns S, Gachet C. Evidence for cross-talk between glycoprotein VI and Gi-coupled receptors during collagen-induced platelet aggregation. Blood. 2001;97(12):3829–3835. doi: 10.1182/blood.v97.12.3829. [DOI] [PubMed] [Google Scholar]
- 52.Ohlmann P, Eckly A, Freund M, Cazenave JP, Offermanns S, Gachet C. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Galphaq. Blood. 2000;96(6):2134–2139. [PubMed] [Google Scholar]
- 53.Cattaneo M, Lombardi R, Zighetti ML, Gachet C, Ohlmann P, Cazenave JP, Mannucci PM. Deficiency of (33P)2MeS-ADP binding sites on platelets with secretion defect, normal granule stores and normal thromboxane A2 production. Evidence that ADP potentiates platelet secretion independently of the formation of large platelet aggregates and thromboxane A2 production. Thromb Haemost. 1997;77(5):986–990. [PubMed] [Google Scholar]
- 54.Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol. 2000;20(11):E101–E106. doi: 10.1161/01.atv.20.11.e101. [DOI] [PubMed] [Google Scholar]
- 55.Cattaneo M, Canciani MT, Lecchi A, Kinlough-Rathbone RL, Packham MA, Mannucci PM, Mustard JF. Released adenosine diphosphate stabilizes thrombin-induced human platelet aggregates. Blood. 1990;75(5):1081–1086. [PubMed] [Google Scholar]
- 56.Trumel C, Payrastre B, Plantavid M, Hechler B, Viala C, Presek P, Martinson EA, Cazenave JP, Chap H, Gachet C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood. 1999;94(12):4156–4165. [PubMed] [Google Scholar]
- 57.Humbert M, Nurden P, Bihour C, Pasquet JM, Winckler J, Heilmann E, Savi P, Herbert JM, Kunicki TJ, Nurden AT. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol. 1996;16(12):1532–1543. doi: 10.1161/01.atv.16.12.1532. [DOI] [PubMed] [Google Scholar]
- 58.Eckly A, Gendrault JL, Hechler B, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2YT receptors in the morphological changes of platelet aggregation. Thromb Haemost. 2001;85(4):694–701. [PubMed] [Google Scholar]
- 59.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107(12):1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohlmann P, Laugwitz KL, Nurnberg B, Spicher K, Schultz G, Cazenave JP, Gachet C. The human platelet ADP receptor activates Gi2 proteins. Biochem J. 1995;312(Pt 3):775–779. doi: 10.1042/bj3120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking G alpha(i2) J Clin Invest. 2001;108(3):477–483. doi: 10.1172/JCI12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy AR, Jones ML, Mundell SJ, Poole AW. Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104(6):1745–1752. doi: 10.1182/blood-2004-02-0534. [DOI] [PubMed] [Google Scholar]
- 63.Haslam RJ. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- 64.Savi P, Pflieger AM, Herbert JM. cAMP is not an important messenger for ADP-induced platelet aggregation. Blood Coagul Fibrinolysis. 1996;7(2):249–252. doi: 10.1097/00001721-199603000-00035. [DOI] [PubMed] [Google Scholar]
- 65.Daniel JL, Dangelmaier C, Jin J, Kim YB, Kunapuli SP. Role of intracellular signaling events in ADP-induced platelet aggregation. Thromb Haemost. 1999;82(4):1322–1326. [PubMed] [Google Scholar]
- 66.Yang J, Wu J, Jiang H, Mortensen R, Austin S, Manning DR, Woulfe D, Brass LF. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem. 2002;277(48):46035–46042. doi: 10.1074/jbc.M208519200. [DOI] [PubMed] [Google Scholar]
- 67.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108(9):3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 68.Gratacap MP, Guillermet-Guibert J, Martin V, Chicanne G, Tronchere H, Gaits-Iacovoni F, Payrastre B. Regulation and roles of PI3Kbeta, a major actor in platelet signaling and functions. Adv Enzyme Regul. 2011;51(1):106–116. doi: 10.1016/j.advenzreg.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Jackson SP, Yap CL, Anderson KE. Phosphoinositide 3-kinases and the regulation of platelet function. Biochem Soc Trans. 2004;32(Pt 2):387–392. doi: 10.1042/bst0320387. [DOI] [PubMed] [Google Scholar]
- 70.Schoenwaelder SM, Ono A, Sturgeon S, Chan SM, Mangin P, Maxwell MJ, Turnbull S, Mulchandani M, Anderson K, Kauffenstein G, Rewcastle GW, Kendall J, Gachet C, Salem HH, Jackson SP. Identification of a unique co-operative phosphoinositide 3-kinase signaling mechanism regulating integrin alpha IIb beta 3 adhesive function in platelets. J Biol Chem. 2007;282(39):28648–28658. doi: 10.1074/jbc.M704358200. [DOI] [PubMed] [Google Scholar]
- 71.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11(6):507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 72.Lova P, Paganini S, Sinigaglia F, Balduini C, Torti M. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J Biol Chem. 2002;277(14):12009–12015. doi: 10.1074/jbc.M111803200. [DOI] [PubMed] [Google Scholar]
- 73.Lova P, Paganini S, Hirsch E, Barberis L, Wymann M, Sinigaglia F, Balduini C, Torti M. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J Biol Chem. 2003;278(1):131–138. doi: 10.1074/jbc.M204821200. [DOI] [PubMed] [Google Scholar]
- 74.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277(26):23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 75.Larson MK, Chen H, Kahn ML, Taylor AM, Fabre JE, Mortensen RM, Conley PB, Parise LV. Identification of P2Y12-dependent and -independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood. 2003;101(4):1409–1415. doi: 10.1182/blood-2002-05-1533. [DOI] [PubMed] [Google Scholar]
- 76.Martin V, Guillermet-Guibert J, Chicanne G, Cabou C, Jandrot-Perrus M, Plantavid M, Vanhaesebroeck B, Payrastre B, Gratacap MP. Deletion of the p110beta isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood. 2010;115(10):2008–2013. doi: 10.1182/blood-2009-04-217224. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Zhang G, Breton GC, Gao X, Malik AB, Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem. 2003;278(33):30725–30731. doi: 10.1074/jbc.M301838200. [DOI] [PubMed] [Google Scholar]
- 78.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J. 2001;15(11):2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 79.Geiger J, Brich J, Honig-Liedl P, Eigenthaler M, Schanzenbacher P, Herbert JM, Walter U. Specific impairment of human platelet P2Y(AC) ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arterioscler Thromb Vasc Biol. 1999;19(8):2007–2011. doi: 10.1161/01.atv.19.8.2007. [DOI] [PubMed] [Google Scholar]
- 80.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95(14):8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Léon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23(10):1941–1947. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- 82.Léon C, Alex M, Klocke A, Morgenstern E, Moosbauer C, Eckly A, Spannagl M, Gachet C, Engelmann B. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood. 2004;103(2):594–600. doi: 10.1182/blood-2003-05-1385. [DOI] [PubMed] [Google Scholar]
- 83.Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110(4):925–934. doi: 10.1046/j.1365-2141.2000.02208.x. [DOI] [PubMed] [Google Scholar]
- 84.Léon C, Freund M, Ravanat C, Baurand A, Cazenave JP, Gachet C. Key role of the P2Y(1) receptor in tissue factor-induced thrombin-dependent acute thromboembolism: studies in P2Y(1)-knockout mice and mice treated with a P2Y(1) antagonist. Circulation. 2001;103(5):718–723. doi: 10.1161/01.cir.103.5.718. [DOI] [PubMed] [Google Scholar]
- 85.Vial C, Hechler B, Léon C, Cazenave JP, Gachet C. Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thromb Haemost. 1997;78(6):1500–1504. [PubMed] [Google Scholar]
- 86.Cattaneo M, Marchese P, Jacobson KA, Ruggeri Z.New insights into the role of P2X1 in platelet function Haematologica 2002871013–14.12412384 [Google Scholar]
- 87.Oury C, Sticker E, Cornelissen H, Vos R, Vermylen J, Hoylaerts MF. ATP augments von Willebrand factor-dependent shear-induced platelet aggregation through Ca2+-calmodulin and myosin light chain kinase activation. J Biol Chem. 2004;279(25):26266–26273. doi: 10.1074/jbc.M402032200. [DOI] [PubMed] [Google Scholar]
- 88.Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, Cazenave JP, Cattaneo M, Ruggeri ZM, Evans R, Gachet C. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198(4):661–667. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, Gachet C. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84(3):484–491. [PubMed] [Google Scholar]
- 90.Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with beta-arrestin-1 and −2. J Biol Chem. 2008;283(45):30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Leon C, Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67(3):721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 92.Reiner S, Ziegler N, Leon C, Lorenz K, Hayn K, Gachet C, Lohse MJ, Hoffmann C. beta-Arrestin-2 interaction and internalization of the human P2Y1 receptor are dependent on C-terminal phosphorylation sites. Mol Pharmacol. 2009;76(6):1162–1171. doi: 10.1124/mol.109.060467. [DOI] [PubMed] [Google Scholar]
- 93.Mundell SJ, Jones ML, Hardy AR, Barton JF, Beaucourt SM, Conley PB, Poole AW. Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol. 2006;70(3):1132–1142. doi: 10.1124/mol.106.023549. [DOI] [PubMed] [Google Scholar]
- 94.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105(9):3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 95.MacKenzie AB, Mahaut-Smith MP, Sage SO. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J Biol Chem. 1996;271(6):2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- 96.Roberts JA, Vial C, Digby HR, Agboh KC, Wen H, Atterbury-Thomas A, Evans RJ. Molecular properties of P2X receptors. Pflugers Archiv: Eur J Physiol. 2006;452(5):486–500. doi: 10.1007/s00424-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 97.Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108(8):989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 98.Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009;133(3):341–345. doi: 10.1016/j.ijcard.2007.12.118. [DOI] [PubMed] [Google Scholar]
- 99.Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108(24):2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 100.Cavallari U, Trabetti E, Malerba G, Biscuola M, Girelli D, Olivieri O, Martinelli N, Angiolillo DJ, Corrocher R, Pignatti PF. Gene sequence variations of the platelet P2Y12 receptor are associated with coronary artery disease. BMC Med Genet. 2007;8:59. doi: 10.1186/1471-2350-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bura A, Bachelot-Loza C, Ali FD, Aiach M, Gaussem P. Role of the P2Y12 gene polymorphism in platelet responsiveness to clopidogrel in healthy subjects. J Thromb Haemost. 2006;4(9):2096–2097. doi: 10.1111/j.1538-7836.2006.02113.x. [DOI] [PubMed] [Google Scholar]
- 102.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119(3):355–360. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Zee RY, Michaud SE, Diehl KA, Chasman DI, Emmerich J, Gaussem P, Aiach M, Ridker PM. Purinergic receptor P2Y, G-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197(2):694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Hetherington SL, Singh RK, Lodwick D, Thompson JR, Goodall AH, Samani NJ. Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler Thromb Vasc Biol. 2005;25(1):252–257. doi: 10.1161/01.ATV.0000148708.44691.27. [DOI] [PubMed] [Google Scholar]
- 105.Sibbing D, Beckerath O, Schomig A, Kastrati A, Beckerath N. P2Y1 gene A1622G dimorphism is not associated with adenosine diphosphate-induced platelet activation and aggregation after administration of a single high dose of clopidogrel. J Thromb Haemost. 2006;4(4):912–914. doi: 10.1111/j.1538-7836.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- 106.Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost. 2005;31(2):174–183. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- 107.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84(5):891–896. [PubMed] [Google Scholar]
- 108.Savi P, Laplace MC, Herbert JM. Evidence for the existence of two different ADP-binding sites on rat platelets. Thromb Res. 1994;76(2):157–169. doi: 10.1016/0049-3848(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 109.Gachet C, Cattaneo M, Ohlmann P, Hechler B, Lecchi A, Chevalier J, Cassel D, Mannucci PM, Cazenave JP. Purinoceptors on blood platelets: further pharmacological and clinical evidence to suggest the presence of two ADP receptors. Br J Haematol. 1995;91(2):434–444. doi: 10.1111/j.1365-2141.1995.tb05319.x. [DOI] [PubMed] [Google Scholar]
- 110.Mills DC, Puri R, Hu CJ, Minniti C, Grana G, Freedman MD, Colman RF, Colman RW. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler Thromb. 1992;12(4):430–436. doi: 10.1161/01.atv.12.4.430. [DOI] [PubMed] [Google Scholar]
- 111.Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Herve C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, Herbert JM. The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA. 2006;103(29):11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res. 2007;100(9):1261–1275. doi: 10.1161/01.RES.0000264509.36234.51. [DOI] [PubMed] [Google Scholar]
- 113.Tomasello SD, Tello-Montoliu A, Angiolillo DJ. Prasugrel for the treatment of coronary thrombosis: a review of pharmacological properties, indications for use and future development. Expert Opin Investig Drugs. 2011;20(1):119–133. doi: 10.1517/13543784.2010.538381. [DOI] [PubMed] [Google Scholar]
- 114.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 115.Bhatt DL. Prasugrel in clinical practice. N Engl J Med. 2009;361(10):940–942. doi: 10.1056/NEJMp0806848. [DOI] [PubMed] [Google Scholar]
- 116.Cattaneo M. New P2Y(12) inhibitors. Circulation. 2010;121(1):171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 117.Storey RF. Pharmacology and clinical trials of reversibly-binding P2Y12 inhibitors. Thromb Haemost. 2011;105(Suppl 1):S75–S81. doi: 10.1160/THS10-12-0769. [DOI] [PubMed] [Google Scholar]
- 118.Ferreiro JL, Ueno M, Angiolillo DJ. Cangrelor: a review on its mechanism of action and clinical development. Expert Rev Cardiovasc Ther. 2009;7(10):1195–1201. doi: 10.1586/erc.09.101. [DOI] [PubMed] [Google Scholar]
- 119.Nawarskas JJ, Clark SM. Ticagrelor: a novel reversible oral antiplatelet agent. Cardiol Rev. 2011;19(2):95–100. doi: 10.1097/CRD.0b013e3182099d86. [DOI] [PubMed] [Google Scholar]
- 120.Gurbel PA, Kereiakes DJ, Tantry US. Ticagrelor for the treatment of arterial thrombosis. Expert Opin Pharmacother. 2010;11(13):2251–2259. doi: 10.1517/14656566.2010.511175. [DOI] [PubMed] [Google Scholar]
- 121.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5(10):1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 122.Lenain N, Freund M, Léon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1(6):1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 123.Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316(2):556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morales-Ramos AI, Mecom JS, Kiesow TJ, Graybill TL, Brown GD, Aiyar NV, Davenport EA, Kallal LA, Knapp-Reed BA, Li P, Londregan AT, Morrow DM, Senadhi S, Thalji RK, Zhao S, Burns-Kurtis CL, Marino JP., Jr Tetrahydro-4-quinolinamines identified as novel P2Y(1) receptor antagonists. Bioorg Med Chem Lett. 2008;18(23):6222–6226. doi: 10.1016/j.bmcl.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 125.Pfefferkorn JA, Choi C, Winters T, Kennedy R, Chi L, Perrin LA, Lu G, Ping YW, McClanahan T, Schroeder R, Leininger MT, Geyer A, Schefzick S, Atherton J. P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg Med Chem Lett. 2008;18(11):3338–3343. doi: 10.1016/j.bmcl.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 126.Thalji RK, Aiyar N, Davenport EA, Erhardt JA, Kallal LA, Morrow DM, Senadhi S, Burns-Kurtis CL, Marino JP., Jr Benzofuran-substituted urea derivatives as novel P2Y(1) receptor antagonists. Bioorg Med Chem Lett. 2010;20(14):4104–4107. doi: 10.1016/j.bmcl.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 127.Zerr M, Hechler B, Freund M, Magnenat S, Lanois I, Cazenave JP, Léon C, Gachet C. Major contribution of the P2Y1 receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation. 2011;123:2404–2413. doi: 10.1161/CIRCULATIONAHA.110.002139. [DOI] [PubMed] [Google Scholar]
- 128.Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Gachet C. Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2Y1 receptors. Circulation. 2008;118(7):754–763. doi: 10.1161/CIRCULATIONAHA.108.788927. [DOI] [PubMed] [Google Scholar]
- 129.Oury C, Kuijpers MJ, Toth-Zsamboki E, Bonnefoy A, Danloy S, Vreys I, Feijge MA, Vos R, Vermylen J, Heemskerk JW, Hoylaerts MF. Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood. 2003;101(10):3969–3976. doi: 10.1182/blood-2002-10-3215. [DOI] [PubMed] [Google Scholar]
- 130.Kassack MU, Braun K, Ganso M, Ullmann H, Nickel P, Boing B, Muller G, Lambrecht G. Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist. Eur J Med Chem. 2004;39(4):345–357. doi: 10.1016/j.ejmech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 131.Hechler B, Magnenat S, Zighetti ML, Kassack MU, Ullmann H, Cazenave JP, Evans R, Cattaneo M, Gachet C. Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4,4′,4″,4‴-(carbonylbis(imino-5,1,3-benzenetriylbis-(carbonylimino)))t etrakis-benzene-1,3-disulfonic acid octasodium salt] J Pharmacol Exp Ther. 2005;314(1):232–243. doi: 10.1124/jpet.105.084673. [DOI] [PubMed] [Google Scholar]
- 132.Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Luscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med. 2007;12(2):113–122. doi: 10.1177/1358863X07077462. [DOI] [PubMed] [Google Scholar]
- 133.Li M, Zhang Y, Ren H, Zhu X. Effect of clopidogrel on the inflammatory progression of early atherosclerosis in rabbits model. Atherosclerosis. 2007;194(2):348–356. doi: 10.1016/j.atherosclerosis.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Afek A, Kogan E, Maysel-Auslender S, Mor A, Regev E, Rubinstein A, Keren G, George J. Clopidogrel attenuates atheroma formation and induces a stable plaque phenotype in apolipoprotein E knockout mice. Microvasc Res. 2009;77(3):364–369. doi: 10.1016/j.mvr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Schulz C, Konrad I, Sauer S, Orschiedt L, Koellnberger M, Lorenz R, Walter U, Massberg S. Effect of chronic treatment with acetylsalicylic acid and clopidogrel on atheroprogression and atherothrombosis in ApoE-deficient mice in vivo. Thromb Haemost. 2008;99(1):190–195. doi: 10.1160/TH07-03-0235. [DOI] [PubMed] [Google Scholar]
- 136.Evans DJ, Jackman LE, Chamberlain J, Crosdale DJ, Judge HM, Jetha K, Norman KE, Francis SE, Storey RF. Platelet P2Y(12) receptor influences the vessel wall response to arterial injury and thrombosis. Circulation. 2009;119(1):116–122. doi: 10.1161/CIRCULATIONAHA.107.762690. [DOI] [PubMed] [Google Scholar]
- 137.Patil SB, Jackman LE, Francis SE, Judge HM, Nylander S, Storey RF. Ticagrelor effectively and reversibly blocks murine platelet P2Y12-mediated thrombosis and demonstrates a requirement for sustained P2Y12 inhibition to prevent subsequent neointima. Arterioscler Thromb Vasc Biol. 2010;30(12):2385–2391. doi: 10.1161/ATVBAHA.110.210732. [DOI] [PubMed] [Google Scholar]
- 138.Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 139.Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135(4):831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gresele P, Grasselli S, Todisco T, Nenci GG. Platelets and asthma. Lancet. 1985;1(8424):347. doi: 10.1016/s0140-6736(85)91126-2. [DOI] [PubMed] [Google Scholar]
- 141.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105(5):2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- 142.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206(11):2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maitre B, Freund M, Hechler B, Léon C, Heim V, Cazenave JP, Hanau D, Gachet C (2008) Involvement of the P2Y1 receptor in asthmatic airway inflammation (abstract). In: Purinergic signalling. Purines 2008 Meeting, 29 June–2 July 2008, Coppenhagen, Denmark, pp. S1–S210
- 144.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 145.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67(4):525–544. doi: 10.1007/s00018-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nature reviews. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 148.McMorran BJ, Marshall VM, Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323(5915):797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]