Abstract
Platelets contain at least five purinergic G protein-coupled receptors, e.g., the pro-aggregatory P2Y1 and P2Y12 receptors, a P2Y14 receptor (GPR105) of unknown function, and anti-aggregatory A2A and A2B adenosine receptor (ARs), in addition to the ligand-gated P2X1 ion channel. Probing the structure–activity relationships (SARs) of the P2X and P2Y receptors for extracellular nucleotides has resulted in numerous new agonist and antagonist ligands. Selective agents derived from known ligands and novel chemotypes can be used to help define the subtypes pharmacologically. Some of these agents have entered into clinical trials in spite of the challenges of drug development for these classes of receptors. The functional architecture of P2 receptors was extensively explored using mutagenesis and molecular modeling, which are useful tools in drug discovery. In general, novel drug delivery methods, prodrug approaches, allosteric modulation, and biased agonism would be desirable to overcome side effects that tend to occur even with receptor subtype-selective ligands. Detailed SAR analyses have been constructed for nucleotide and non-nucleotide ligands at the P2Y1, P2Y12, and P2Y14 receptors. The thienopyridine antithrombotic drugs Clopidogrel and Prasugrel require enzymatic pre-activation in vivo and react irreversibly with the P2Y12 receptor. There is much pharmaceutical development activity aimed at identifying reversible P2Y12 receptor antagonists. The screening of chemically diverse compound libraries has identified novel chemotypes that act as competitive, non-nucleotide antagonists of the P2Y1 receptor or the P2Y12 receptor, and antithrombotic properties of the structurally optimized analogues were demonstrated. In silico screening at the A2A AR has identified antagonist molecules having novel chemotypes. Fluorescent and other reporter groups incorporated into ligands can enable new technology for receptor assays and imaging. The A2A agonist CGS21680 and the P2Y1 receptor antagonist MRS2500 were derivatized for covalent attachment to polyamidoamine dendrimeric carriers of MW 20,000, and the resulting multivalent conjugates inhibited ADP-promoted platelet aggregation. In conclusion, a wide range of new pharmacological tools is available to control platelet function by interacting with cell surface purine receptors.
Keywords: Purines, GPCR, Ion channel, Structure–activity relationship, Molecular modeling, Mutagenesis
Introduction: subtypes of P2X and P2Y receptors
Extracellular nucleotides activate cell surface P2 receptors which are widely distributed and participate in the regulation of nearly every physiological process, including in the immune, inflammatory, cardiovascular, muscular, and central and peripheral nervous systems [1, 2]. Closely related to these processes are the adenosine receptors (ARs), all four subtypes of which are G protein-coupled receptors (GPCRs). The P2 receptors are divided into two distinct families: fast P2X receptors (ligand-gated ion channels) and P2Y receptors (GPCRs). The P2Y receptors respond to various naturally occurring adenine and uracil mono- and dinucleotides. The P2X receptors are more structurally restrictive in native ligand selectivity than P2Y receptors and are activated principally by ATP. These extracellular nucleotides are produced in response to tissue stress and cell damage during neurotransmitter release and as a result of hemichannel formation. The concentration of extracellular nucleotides that act as P2 receptor agonists can vary dramatically depending also on the aforementioned circumstances. Thus, the state of activation of these receptors can be highly dependent on the stress conditions or disease states affecting a given organ or tissue.
The P2X receptors consist of seven subtypes that are numbered P2X1 through P2X7. Activation of P2X receptors leads to an influx of cations such as sodium and calcium, which depolarize excitable cells and activate cytosolic enzymes, respectively. The P2X7 receptor, in addition to forming the usual cation channel, and upon prolonged agonist exposure, also opens a large pore which can pass organic cations and dye molecules. The active ligand-gated ion channels of the P2X receptors are composed of trimeric aggregates of subunits. Both heterotrimers and homotrimers have been characterized [3], and the homotrimers and heterotrimers of a given subtype may have entirely different structural requirements for agonists or antagonists. For example, the P2X1 receptor can form heteromers with the P2X2, P2X4, and P2X5 receptors [4–7].
The eight human P2Y receptor subtypes are numbered P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14, which leaves some gaps due to premature assignment of numbers to certain putative P2Y receptors that were later shown to be either species homologs or entirely different types of GPCRs. The structures of representative native adenine and uracil (1–4 and 23–25 in Fig. 1) nucleotides that activate P2Y receptors are shown, and each of the native nucleotides may activate one or more P2Y receptor subtypes. In some cases, the same nucleotide might activate one subtype and antagonize another. The adenine nucleotide ATP 2 is a full agonist at two human P2Y subtypes (P2Y2 and P2Y11 receptors), and the corresponding diphosphate ADP 1 activates three different subtypes (P2Y1, P2Y12, and P2Y13 receptors). The uracil nucleotide UTP 24 activates two subtypes (P2Y2 and P2Y4 receptors), while UDP 23, previously thought to activate only a single subtype (P2Y6 receptor), is also now known to activate P2Y14 receptors along with the originally designated native agonist UDP-glucose 25 [8]. The naturally occurring dinucleotide Ap3A 3 and its homologues, including uracil derivatives, also activate various P2 receptors [9, 10]. Efforts have been made to identify other naturally occurring nucleotides that interact with known P2Y receptors and to deorphanize related GPCR sequences that have not yet been assigned a native agonist [2, 11].
Fig. 1.
Nucleotide derivatives that activate P2X and P2Y receptors, with emphasis on agonist ligands for studying these receptors in platelets. a Adenine nucleotides. b Uracil nucleotides. Phosphate derivatives would exist predominantly in an ionized form under physiological conditions
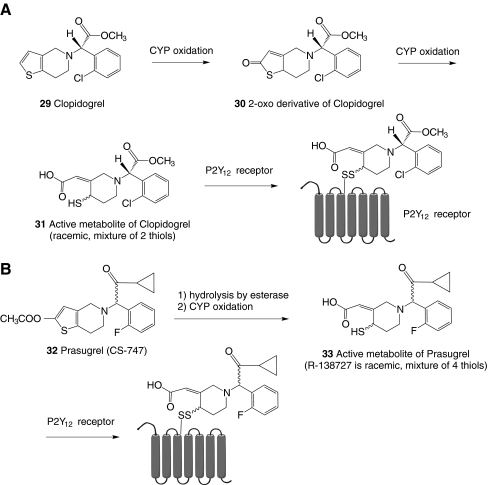
Platelets contain at least five purinergic GPCRs, e.g., the ADP-activated P2Y1 and P2Y12 receptors, the P2Y14 receptor (pyrimidine-selective), and A2A and A2B ARs, in addition to the ligand-gated P2X1 ion channel (Table 1). Co-activation of P2Y1 and P2Y12 receptors is required for the aggregatory effect of ADP [12]. The P2Y12 receptor is the site of action of the important thienopyridine antiplatelet drugs Clopidogrel 29 and Prasugrel 32 (Fig. 2). Activation of the P2X1 receptor by ATP is also pro-aggregatory, but only transiently and under high shear stress conditions [13]. Antagonists of the P2X1 receptor inhibit the platelet shape change induced by α,β-meATP 5 [80]. A P2Y14 receptor was also detected in platelets, but its role is undetermined [14].
Table 1.
Subtypes of platelet purine receptors and their representative ligands (potency at the human homologs, unless noted r = rat)
| Receptor | Main distribution | Agonists (native in bold, pEC50) | Antagonists (pIC50) |
|---|---|---|---|
| P2Y1 | Brain, epithelial and endothelial cells, platelets, immune cells, osteoclasts | MRS2365 9.4 > 2-MeSADP 8.2 >> ADPβS 7.0> ADP5.1 >ATP | MRS2500 9.0> MRS2279 7.3> MRS2179 6.5 |
| P2Y12 | Platelets, brain (glial cells), microglial cells | 2-MeSADP 7.9 ≥ ADP7.2 | AR-C69931MX 9.4 > AZD 6140 7.9, INS 50589 7.8 > RB2 7.6 (r) > 2-MeSAMP 4.0 |
| P2Y14 | Placenta, mast cells, adipose tissue, stomach, intestine, discrete brain regions | MRS2905 8.7 > MRS2690 7.3 > UDP6.8, UDP-glucose6.5 > UDP-galactose 6.2 | Compound 808.7 |
| P2X1 | Smooth muscle, platelets, cerebellum, dorsal horn spinal neurons | BzATP 8.7 > ATP7.3, 2-MeSATP 7.3, α,β-MeATP 6.7 (rapid desensitization) >> CTP 4.4 | NF 449 9.5 > Ip5I 8.8 > TNP-ATP 8.2 > Ro 0437626 > NF 279 7.7 |
| A2A | Striatum, platelets, lymphocytes | CGS21680 7.6 > adenosine6.5 | ZM241385 8.8 > SCH442416 8.4 > CSC 7.3 > theophylline 5.6 > caffeine 4.6 |
| A2B | Colon, fibroblasts, endothelial cells, astrocytes, bronchial smooth muscle, intestinal epithelial cells, mast cells, platelets | BAY 60-6583 8.0 > adenosine4.8 | PSB603 9.3 > MRS1754 8.7 > MRE2029-F20 8.3 > theophylline 5.0 > caffeine 4.5 |
Fig. 2.
Thienopyridines as non-nucleotide antagonists of the P2Y12 receptor that require activation in vivo. Enzymatic formation of the active metabolites precedes the formation of the disulfide bond with the target P2Y12 receptor on platelets. CYP=cytochrome P450
The first purine receptor detected in platelets was the Gs-coupled A2A AR, at which adenosine has an anti-aggregatory function [15]. The SAR of AR ligands will be covered only briefly in this review. There are a number of excellent reviews of A2A AR ligands [16, 17]. Recently, a novel role in platelet aggregation was proposed for the Gs-coupled A2B AR [18], which is expressed at low levels in mouse platelets [19]. This subtype is upregulated in platelets under injury or stress conditions in vivo, and it downregulates the expression and function of the P2Y1 receptor by raising cyclic AMP.
Structure and regulation of purine receptors that are expressed in platelets
The knowledge of P2X receptor structures was recently advanced with the X-ray crystallographic determination of the P2X4 subunit [20]. However, the crystal form did not contain a bound ligand, so predicting the orientation of ligands in the extracellular loop (EL) region of the P2X receptors is still subject to modeling. The X-ray structure of the P2X1 receptor in platelets has not yet been determined, but the structure–function relationships of various P2X subtypes have been probed using site-directed mutagenesis [21].
The structure, signaling, and regulation of P2Y receptors have been explored pharmacologically. Two subfamilies of P2Y1-like (five members: P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors) and P2Y12-like receptors (three members: P2Y12, P2Y13, and P2Y14 receptors) have been defined. The P2Y1 receptor was first cloned from chick brain in 1994 [22], and the platelet P2Y12 receptor was first cloned in 2001 [23]. These subfamilies constitute two distinct groups based on signaling pathways and similarities in the function of key amino acid residues, but not on ligand structure [24, 25]. Thus, both adenine and uracil nucleotides are found as agonists in each P2Y subfamily.
The signaling pathways of P2Y receptors have been extensively characterized. The preferential coupling of the first subfamily of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors is to Gq, leading to the activation of phospholipase Cβ and to a rise in intracellular calcium. The P2Y11 receptor alternately couples to Gs. The second subfamily of P2Y12, P2Y13, and P2Y14 receptors couples to Gi, resulting in the inhibition of adenylyl cyclase to decrease production of cyclic AMP. Therefore, activation of the P2Y1-like receptors leads to a rise in intracellular calcium. The amino acid residues R333 and R334 in the carboxy terminal region of the human P2Y1 receptor were found using site-directed mutagenesis to be crucial for Gq coupling [26]. Activation of the P2Y1 receptor can inhibit ion channels, such as the M-type K+ current in hippocampal neurons [27].
Desensitization of P2Y receptors in response to prolonged agonist exposure has been studied pharmacologically [28, 29]. In platelets, which express two ADP-responsive P2Y subtypes, the P2Y1 receptor desensitizes more rapidly than the P2Y12 receptor. The P2Y1 receptor is desensitized mainly through protein kinase C-dependent processes, and the P2Y12 receptor is a good substrate for the GPCR kinases leading to arrestin binding.
Factors that affect the localization of P2Y receptors within the cell have been studied. In kidney cells, the role of residues on the cytosolic C-terminal domain of the P2Y1 receptor in basolateral sorting has been probed [30]. Deletion of this sorting signal that contains nine charged residues or alteration of the total number of charges caused a redirection of the receptor to the apical membrane. A P2Y1-like receptor response in calcium transport has been detected on mitochondrial membranes [31].
Dimerization of GPCRs is a general phenomenon that can lead to major effects on the pharmacology of a given receptor subtype. Homodimerization of the P2Y1 receptor expressed in human embryonic kidney (HEK)-293 cells was detected using fluorescence resonance energy transfer donor photobleaching analysis [32]. In membranes prior to agonist exposure, 44% of the P2Y1 receptors existed in the dimeric state; upon exposure to agonist, a reversible rise to 85–100% dimerization was observed. Both monomeric P2Y1 receptors and constitutive dimers were fully active. The terminal 19 amino acids of the cytosolic terminal region were essential for both dimerization and internalization, but activation by agonists was not reduced until >39 amino acids were removed from this region. Various heterodimers of P2Y receptors with other P2Y and non-P2Y GPCRs have been proposed. For example, a dimer of the A1 AR and the P2Y1 receptor was characterized [33]. The internalization of the P2Y11 receptor is dependent on the co-expression of the rapidly desensitizing P2Y1 receptor, suggesting the occurrence of P2Y1/P2Y11 receptor heterodimers [34].
Molecular recognition in the P2Y1 and P2Y12 receptors, i.e., ligand binding and activation functions, has been extensively explored using mutagenesis [35–37]. Homology modeling of the P2Y receptors based on bovine rhodopsin or one of the more recent template structures followed by small molecule docking has provided insight into the possible ligand-binding modes. Comparisons of the structural characteristics and functionally important amino acid residues within the family have been described. Molecular models of these two subtypes are compared in Fig. 3. In each of the subfamilies, specific cationic, anionic, aromatic, and other residues in the helical transmembrane (TM) region and on the ELs have conserved functions in coordinating the bound nucleotide [25].
Fig. 3.
a, b Molecular model of the human P2Y1 receptor based on the structure of the β2-adrenergic receptor. The P2Y1 receptor is shown in complex with a selective antagonist (MRS2500) (b) and a selective agonist (MRS2365) (a). The residues shown are those that, when mutated, lead to a decrease of potency of 20 times or higher. The helices are color coded as TM1 (red), TM2 (orange), TM3 (yellow), TM4 (light green), TM5 (dark green), TM6 (cyan), and TM7 (purple). c, d Molecular model of the human P2Y12 receptor based on the structure of the A2A adenosine receptor. The P2Y12 receptor is shown in complex with a nonselective agonist (2-MeSADP) (c) and a selective non-nucleotide antagonist (PSB-0739) (d). The helices are color-coded in a progression from TM1 (red) to TM7 (yellow). Two disulfide bridges and a salt bridge give rigidity to the extracellular domains of the P2Y1 and P2Y12 receptors. In the antagonist-bound state of the P2Y1 receptor, a salt-bridge between R128 and D204 provides an additional link between TM3 and EL2. Our models suggest that agonist binding causes a disruption of this additional bridge as well as a counter-clockwise rotation (when observed from the extracellular side) of Lys280(6.55). Molecular modeling of these receptors was reported previously [24, 25, 35, 36, 38, 40, 41, 67, 89, 117]
The homology model of the P2Y1 receptor has been constructed using a two-template strategy, as described in de Castro et al. [38]. In particular, our experimentally supported rhodopsin-based P2Y1 homology model [24] was used for the construction of almost the entire receptor, while the newly reported A2A adenosine receptor structure (PDB ID: 3EML) [39] was used as the template for the conformation of the portion of second extracellular loop (EL2) downstream of C202, the conserved Cys that links EL2 to the third TM domain (TM3). The remaining portion of the loop, for which the published crystal structures of GPCRs suggest a greater variability among the receptors, was instead modeled without the use of a template. By using this approach, we obtained a model that closely resembles our previous one [24], but with a more solvent-exposed EL2. Receptor–ligand interactions were refined through Monte Carlo conformational searches, starting from our published experimentally supported binding modes [24].
Mutagenesis of the P2Y1 receptor has concentrated on the identification of residues involved in reversible binding of agonist and antagonist ligands and its regulation. Figure 3a, b shows the human P2Y1 receptor in complex with a selective antagonist (MRS2500) and a selective agonist ((N)-methanocarba-2-MeSADP, MRS2365), respectively. The residues shown are those that, when mutated, lead to a decrease of potency of 20 times or higher of nucleotide agonists. The main differences between models of the antagonist-bound and agonist-bound complexes are shown. When the agonist is bound, K280 rotates counterclockwise and the salt bridge between R128 and D204 breaks. The P2Y1 homology model indicated that the ribose moiety of nucleotide ligands was situated in a hydrophilic pocket between TM3 and TM6. The studies also indicated that the adenine ring of the ligands interacts with residues from TM7 and points in the direction of TM1. Q307(7.36) (using Ballesteros numbering for each TM) [40] was found to form a critical H-bond with the N6-amino group for both agonists and antagonists. The three critical cationic residues, i.e., R128(3.29), K280(6.55), and R310(7.39), appeared to form ionic interactions with both the bisphosphate chains of the antagonist and the diphosphate chain of the agonists. In particular, in the antagonist-bound complex, R128 interacted with the 5′-phosphate, K280 with both the 3′ and the 5′-phosphates, and R310 with the 3′-phosphate. Two of these interactions underwent significant rearrangement upon agonist docking. In particular, R128 interacted with the α- and β-phosphates, causing the disruption of the salt bridge with D204 (in EL2) that was found in the antagonist-bound complex, while K280 interacted with the β-phosphate, undergoing a significant rotation in the counterclockwise direction, when observed from the extracellular side. Instead, R310 underwent only a minor movement to interact with the α-phosphate group.
Mutagenesis of the P2Y12 receptor has concentrated on the identification of residues involved in binding of agonist and antagonist ligands, covalent binding of thiol-reactive ligands, and desensitization. The residues R256(6.55), Y259(6.58), and, possibly, H253(6.52), as well as K280(7.35), are required for the activation of the human P2Y12 receptor [37]. R256(6.55) is involved in the recognition of nucleotide agonists and the non-nucleotide antagonist Reactive Blue-2, but not the nucleotide antagonist AR-C69931MX (Cangrelor). Hoffmann et al. [41] studied the recognition of the competitive non-nucleotide antagonist PSB-0739 at the human P2Y12 receptor. The residue R256 is involved in the interaction of this and other antagonists derived from Reactive Blue-2 with the human P2Y12 receptor.
The mutations F104S and S288P significantly increased agonist-induced receptor function without affecting the inhibition by AR-C69931MX [42]. R256 in TM6 and R265 in EL3 are more important for antagonist recognition than for agonist-induced activation. Compared to the wild-type P2Y12 receptor, R256T/Q and/or R265W mutations, which have been detected in a patient with congenital bleeding, significantly increased the sensitivity to AR-C69931MX. Both the cytosolic side of TM3 and the exofacial side of TM5 are critical for P2Y12 receptor function, which differs from the P2Y1 receptor. R256 in TM6 and R265 in EL3 appear to play a role in antagonist recognition rather than receptor activation.
Figure 3c, d shows the human P2Y12 receptor complexes with the agonist 2-MeSADP and the selective and competitive antagonist PSB-0739. Amino acid residues that were mutated corresponding to those in Fig. 3a, b are shown [43]. The three-dimensional structure of P2Y12 was built by means of comparative modeling using the crystal structure of the A2A adenosine receptor [39] as the template. The disulfide bridge between the N terminal and EL3, which is conserved in the P2Y family but is absent in the A2A receptor structure, was created on the P2Y12 model between C17 and C270. The extracellular loops were further refined. Molecular docking and Monte Carlo conformational studies were conducted with agonists and antagonists of the P2Y12 receptor to understand and possibly to explain the involvement of the residues in the binding site of P2Y12 receptor in the ligand recognition process.
The binding pocket for agonists and antagonists within the P2Y12 receptor model is formed by residues located in TM1, TM2, TM3, TM6, and TM7. The negatively charged groups of 2-MeSADP and PSB-0739 are located in the extracellular part of the binding cavity of the P2Y12 receptor, and they are surrounded by positively charged residues, including R256 in TM6 and K280 in TM7, and polar residues, including Y259 in TM6. In the complex with 2-MeSADP, the guanidinium group of R256 interacts with both phosphate groups of the agonist. The hydroxyl group of Y259 contributes to coordinate the α-phosphate group, while K280 binds to the β-phosphate group of 2-MeSADP. The residue S288(7.43) in TM7 engages in a favorable interaction with the thiomethyl group of 2-MeSADP at the bottom of the binding pocket. In the complex with the selective antagonist PSB-0739, R256 interacts directly with both the sulfonic acid groups. The sulfonic group attached to the anthaquinone moiety interacts also with the side chain of K280. The side chain of Y259 is in proximity to the ligand, but does not interact directly with the negatively charged groups. The bottom of the binding pocket of the P2Y12 receptor is characterized by a network of aromatic residues, among which are the residues H253(6.52) and F104(3.32), that embed the agonist and the antagonist molecules with favorable interactions. The S101(3.29) side chain is located in the binding pocket close to the ligands, but it is not engaged in direct interactions with either 2-MeSADP or PSB-0739.
In a direct analogy to an ionic bridge between EL2 and EL3 detected in the P2Y1 receptor [35, 36], the P2Y12 modeling suggested an ionic interaction between E181 in EL2 and R265 in EL3 that is present in both the agonist-bound and the antagonist-bound complexes. However, the question of a bridge between EL2 and TM3 is inconclusive because position 3.29 in the P2Y12 receptor is S101, not an Arg as in the P2Y1 receptor, and in EL3, the residue F177 occupies the position of D204 of the P2Y1 receptor.
Chimeric constructs of P2Y receptors have helped establish the basis for ligand recognition, activation, and regulation. For example, a chimeric hemagglutinin-tagged human P2Y12 receptor in which the cytosolic carboxy terminal region was replaced by the corresponding sequence of the P2Y1 receptor exhibited a high level of constitutive activity [44].
Ding et al. [45] compared the differential reactivity of P2Y1 and P2Y12 receptors toward thiol reagents. Although both subtypes are encoded on the same chromosome and have similar agonist activation profiles, the thiol-reactive p-chloromercuribenzene sulfonic acid and the irreversibly binding metabolites (e.g., 31 and 33) of the antiplatelet drugs Clopidogrel (Plavix©) 29 and Prasugrel (Effient©) 32 (Fig. 2) inactivated the P2Y12, but not the P2Y1 receptor. The two enantiomers of Prasugrel are similar in activity and also rapidly racemize; therefore, it is used in its racemic form. The interaction of these thiol-reactive metabolites with specific Cys residues on the human P2Y12 receptor was probed by site-directed mutagenesis. There are four Cys residues in the extracellular region of the P2Y12 receptor—at positions 17, 97, 175, and 270—which presumably form two disulfide pairs. It was speculated that the active metabolites of the thienopyridines 31 and 33, which themselves are reactive thiols, formed disulfide bridges in this extracellular region, thus inactivating the receptor. The sites of covalent modification of the P2Y12 receptor were suggested to be at C17 and C270 in the N-terminal domain and in EL3, respectively [45]. Algaier et al. [46] reached different conclusions about which Cys residues of the ELs of the P2Y12 receptor are involved in the thiol reactivity of the active metabolite of Prasugrel, R-138727 (2-[1-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4-mercapto-3-piperidinylidene]acetic acid, mixture of 4 stereoisomers) 33 [47, 48]. The mixture of (R,S) and (S,R) isomers, which is more potent than R-138727, is designated R-99224. Irreversible antagonism by R-138727 of the inhibition of forskolin-stimulated cyclic AMP production mediated by 2-MeSADP 8 was observed for both the wild-type P2Y12 receptor and C17A/C270A mutant receptors. This eliminated C17 and C270 as sites for covalent modification. However, C97A and C175A constructs lost the ability to be inhibited by R-138727. These proposed sites of action of R-138727 at C97 (top of TM3) and C175 (EL2) would be closer to the ligand-binding site in the TM region of the P2Y12 receptor than those thiol sites proposed by Ding et al. [45].
Mutagenesis and molecular modeling of A2A AR has concentrated on the identification of residues involved in the binding of agonist and antagonist ligands [49, 50]. The X-ray structure of A2A AR was reported in 2008 [39]. This advance has enabled the structure-based discovery of novel ligands of this GPCR independently by Abagyan and coworkers and by Shoichet and coworkers [51, 52]. In silico screening based on virtual docking at the A2A AR of molecules that distantly resemble other AR ligands has identified antagonist molecules having novel chemotypes. Fluorescent and other reporter groups incorporated into high-affinity ligands can enable new technology for receptor assay and imaging. A fluorescence polarization assay of binding at the A2A AR in HEK-293 cell membranes was reported [53]. This could serve as a model for the development of fluorescence polarization assays for other purine receptors that avoid the use of radioisotopes in ligand screening. The assay can be performed in real time in a well plate format.
Macromolecular conjugates of ligands for platelet purine receptors were recently introduced. The A2A agonist CGS21680 (2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine) and the P2Y1 receptor antagonist MRS2500 ((1′R,2′S,4′S,5′S)-4-(2-iodo-6-methylamino-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane) 38 were derivatized for covalent attachment to (polyamidoamine) dendrimeric carriers of MW 20,000, and the resulting multivalent conjugates inhibited ADP-promoted human platelet aggregation [38, 54]. High affinity at the receptor was maintained in the A2A AR agonist conjugates, but the multivalent conjugate of MRS2500 38 was less potent than the parent antagonist monomer. Structural modification of these dendrimer conjugates is in progress to improve their pharmacological properties.
The mouse P2X1, human P2Y1, and human P2Y12 receptors have all been purified to homogeneity using combinations of affinity chromatography and other column purification [55–57].
Selective ligands for the P2Y and P2X receptors in platelets
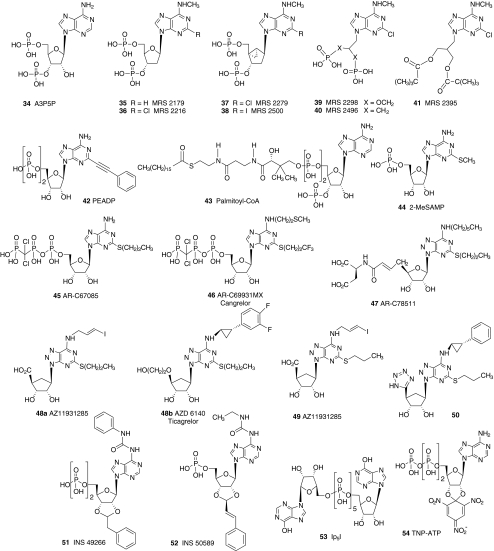
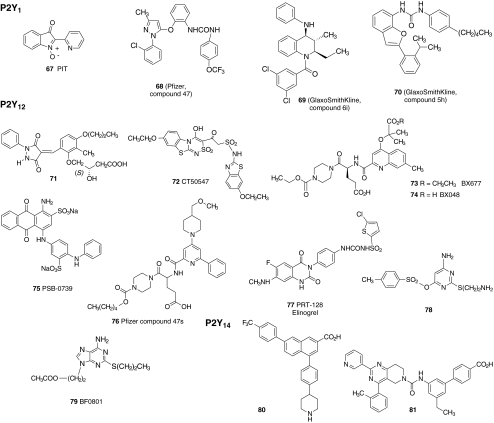
New selective agonists and antagonists have recently been identified for the P2 receptors that occur in platelets. The structures of representative nucleotide (34–54 in Fig. 4) and non-nucleotide (55–81 in Figs. 5 and 6) antagonists of the platelet P2 receptors are shown. Selective antagonist ligands for these receptors have been reported as a result of the systematic conversion of agonists into antagonists, the careful structural modification of known non-selective ligands, and, more recently, the screening of structurally diverse chemical libraries.
Fig. 4.
Nucleotide derivatives that have been useful as antagonists in the study of the P2X and P2Y receptors in platelets. Phosphate derivatives would exist predominantly in an ionized form under physiological conditions
Fig. 5.
Non-nucleotides antagonists: Nonselective antagonists and their derivatives and P2X1 selective antagonists. Phosphate, sulfonate, and carboxylate derivatives would exist predominantly in ionized forms under physiological conditions
Fig. 6.
Selective non-nucleotides that have been useful antagonists in the study of P2Y receptors in platelets. See Fig. 2 for thienopyridine antagonists of the P2Y12 receptor. Sulfonate and carboxylate derivatives would exist predominantly in ionized forms under physiological conditions
A recurrent issue in the use of typical P2 receptor ligands is the lack of specificity for a single subtype among multiple P2 receptors. Often, the complete P2 receptor selectivity profile of a given ligand is unknown. Also, many of the ligands display low bioavailability due to high molecular weight or multiple charged groups, such as phosphates and sulfonates, present in the molecules. Another drawback of many of the currently used ligands is their lability in biological systems. The use of P2 receptor ligands is also complicated by the presence of ectonucleotidases that degrade both native agonists and, often, the synthetic nucleotides that are used as agonists or antagonists [58]. Adenine nucleotides are progressively converted enzymatically to AMP and finally to adenosine, which activates its own family of four ARs. Selective inhibitors of ectonucleotidases that can serve as modulators of receptor function are being explored [59, 60]. Moreover, many P2 receptor agonists and antagonists are known to inhibit ectonucleotidases at comparable concentrations. The transformation of nucleotides can also proceed in the other direction, for example in the conversion of 5′-diphosphates to 5′-triphosphates by extracellular nucleoside diphosphokinase [61]. The purity of commercial preparations of nucleotides isolated from natural sources is variable. Thus, ATP might contain ADP and other nucleotides as contaminants. The use of synthetic and rigorously purified nucleotides minimizes this ambiguity. Finally, many of the early P2 antagonists have been found to interact intracellularly with other signaling mediators, such as G proteins at concentrations similar to those needed at P2 receptors. For example, suramin and its analogues inhibit G proteins [62]. Recently, a known P2X1-selective antagonist (NF449, 64) was found to inhibit signaling from the fibroblast growth factor receptor 3 [63]. In general, novel drug delivery methods, prodrug approaches, allosteric modulation, and biased agonism would be desirable to overcome side effects that tend to occur even with receptor subtype-selective ligands.
Generally, radioligand binding serves as a primary research tool for the screening of new ligands at a given GPCR, but most of the P2 receptors do not have viable radioligands yet. Fortunately, the platelet purine receptors, i.e., P2Y1, P2Y12, P2X1, A2A, and A2B receptors, have such high-affinity radioligand tools [17, 64–67]. Nevertheless, much of the drug discovery at these subtypes has relied on functional assays, for example, the activation of phospholipase C by nucleotides acting at the P2Y1 receptor expressed in 1321N1 astrocytoma cells [68].
P2X1 receptors
Novel P2X receptor ligands have been introduced for use as pharmacological probes and as potential therapeutic agents. Selective P2X receptor antagonists are of interest in pain control, depression, urinary incontinence, rheumatoid arthritis, chronic inflammation, and other conditions [69].
Non-selective P2X ligands
ATP 2, but not UTP 24, activates P2X receptors, and its EC50 at the various subtypes varies from the low nanomolar to the high micromolar [70]. Purified ADP 1, AMP, and adenosine are inactive at P2X receptors. 2-Methylthio-adenosine 5′-triphosphate (2-MeSATP, 9) is a potent agonist at multiple P2X receptors, for example, P2X1 (EC50 = 54 nM) and P2X3 (EC50 = 350 nM) receptors. α,β-Methyleneadenosine 5′-triphosphate (α,β-meATP, 5) activates the P2X1 receptor, but not the P2X2 receptor, and its ability to rapidly desensitize the P2X1 receptor allows it to be used in functional pharmacological experiments in place of an antagonist. A wide variety of ATP derivatives were compared in the inhibition of specific binding of [3H]α,β-meATP at the rat urinary bladder P2X1 receptor [66]. Among analogues with a substituted adenine ring, 2-(6-cyanohexylthio)-ATP 21 displayed a potent pIC50 value of 7.24. Various nucleotide derivatives were assayed functionally at P2X receptors expressed in astrocytoma cells and oocytes [71, 72]. For example, 2′, 3′-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP, 22), which is the most potent known agonist of the P2X7 receptor, was also found to potently activate the P2X1 receptor. The same nucleotide also acts as an antagonist of the P2Y12 receptor with an IC50 of 116 μM [45].
Until recently, the only antagonists of the P2X1 receptor were highly charged compounds. Various negatively charged organic dyes, such as Reactive Blue-2 59 (Fig. 5), act as non-selective and weak P2X antagonists. Reactive Blue-2 was also shown to be a potent antagonist of the P2Y12 receptor with a pKB of 7.6 [73]. The polysulfonated biphenyl derivative Evans Blue (structure not shown) acts as a P2X receptor antagonist, but it also affects other channels and amino acid binding sites [74]. Another polysulfonated derivative that has been widely used as a nonselective P2 receptor antagonist is the antiparasitic drug suramin 61. The aryldiazo-bridged pyridoxal phosphate derivative PPADS 55 was found to inhibit P2 receptors, and its potency and selectivity have been increased by chemical modification [75]. Typically, such compounds are relatively non-subtype-selective P2X antagonists that also block some P2Y subtypes [76]. Later, a positional isomer, iso-PPADS 56, was introduced and found to be more potent than PPADS at P2X receptors. The p-carboxylate analogue MRS2159 57 is somewhat selective for the P2X1 receptor, but also antagonizes the P2X3 receptor. The pyridoxal phosphate derivative lacking an aryldiazo moiety MRS2219 58 is a weak potentiator of P2X1-mediated responses [77].
A few nucleotide derivatives have been found to block P2X receptors. For example, the dinucleotide Ip5I 53 potently antagonizes the P2X1 receptor [78]. TNP-ATP 54 is a potent P2X antagonist that is selective for several subtypes [79]. It antagonizes P2X1, P2X3, and heteromeric P2X2/3 receptors with IC50 values of 6, 0.9, and 7 nM, respectively, and displays 1,000-fold selectivity for homomeric P2X3 over P2X2, P2X4, and P2X7 receptors.
P2X1 antagonists of greater selectivity have been reported in compound classes both related to the known, nonselective antagonists, and to novel chemotypes. For example, the suramin analogue NF 023 62 is a moderately selective, competitive P2X antagonist with IC50 values of 0.21 and 28.9 μM at human P2X1 and P2X3 receptors, respectively, and is inactive at the P2X2 and P2X4 receptors [80]. Another analogue NF 157 (not shown) was found to be a P2X1 antagonist, but it is not absolutely specific because it also blocks the P2Y11 receptor [81]. The P2X1 potency selectivity in the suramin series of antagonists improved in later structural iterations. Other suramin derivatives that act as selective P2X1 antagonists include PPNDS, NF 279 63, and the more highly selective P2X1 antagonist NF 449 64 [82, 83]. An additional high-affinity antagonist in this series is NF864 65 (8,8′,8″,8′″-(carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino)))tetrakis-naphthalene-1,3,5-trisulfonic acid-dodecasodium salt. The ability to inhibit the platelet P2X1 receptor displayed the following order (pA2 in shape change): NF864 (8.49) > NF449 (7.61) > or = NF110 (7.22) > NF023 (6.11) = MK-HU1 (5.98, structure not shown) = suramin (5.76) [84]. In the series of benzimidazole derivatives introduced by Roche, a 2-carboxamide derivative Ro 0437626 66 was found to be a selective P2X1 antagonist (IC50 = 3 μM) that displays >30-fold selectivity over P2X2, P2X3, and P2X2/3 receptors (IC50 > 100 μM) [85].
P2Y1, P2Y12, and P2Y14 receptors
Selective agonist and antagonist ligands for P2Y receptors are in preclinical development for pulmonary diseases, thrombosis, and other conditions [86]. The rapidly accelerating progress in this field has already resulted in new drug candidates for pulmonary diseases, dry eye disease, and thrombosis. There is much activity in the pharmaceutical industry to identify novel antagonists of the P2Y12 receptor, with the success of the antithrombotic Clopidogrel 29, which acts as a prodrug of an irreversibly binding P2Y12 receptor antagonist [87]. The P2Y1 receptor might also prove to be a useful target for antithromobotic drugs [88]. Detailed analyses of SAR have been performed on the pro-aggregatory P2Y1 and P2Y12 receptors, for which selective nucleotide ligands have been reported. Because nucleotide analogues generally have limited bioavailability and stability in vivo, selective non-nucleotide antagonists of the P2Y1 and P2Y12 receptors are also being developed as potential antithrombotic agents. Structurally diverse chemical libraries have been screened to identify novel chemotypes to act as competitive, non-nucleotide antagonists of these subtypes, which may be optimized for selectivity and potency.
P2Y1 receptor
ADP activates the P2Y1, P2Y12, and P2Y13 receptors and therefore is of limited use in characterizing subtype-specific effects. 2-MeSADP 8 (EC50 = 6 nM) is a more potent agonist at the P2Y1 receptor than ADP (EC50 = 8 μM). 2-MeSATP 9 and its 2-thioether congeners have also been shown to activate the P2Y1 receptor, although depending on conditions where 5′-triphosphates might appear to be less than full agonists. ATP, itself, has been characterized as an agonist, a partial agonist, or an antagonist at the P2Y1 receptor, depending on the model used and the level of spare receptors expressed. N6-methyl adenine nucleotides also potently activate the P2Y1 receptor, but larger substituents on the exocyclic amine reduce potency, consistent with a small hydrophobic pocket surrounding the N6-position within the binding site of the P2Y1 receptor. For example, N6-(2-phenylethyl)-ATP is inactive at the P2Y1 receptor [89]. N6-Disubstituted ATP derivatives are inactive at the P2Y1 receptor and have found application as inhibitors of ectonucleotidases, such as ARL 67156 [59].
The conformation of the ribose moiety has been the focus of recent studies of P2 receptor agonists. By comparing two isomeric conformationally restricted (i.e., rigid) equivalents of the ribose moiety in nucleotide derivatives (i.e., methanocarba ring system containing fused cyclopropane and cyclopentane rings), the favored ribose ring conformation at the P2Y1 receptor was established [90, 91]. The North (N)-methanocarba analog of 2-MeSADP (MRS2365, 14) displayed an EC50 of 0.4 nM as an agonist of the P2Y1 receptor [92] and did not activate appreciably the P2Y12 and P2Y13 receptors. Addition of the (N)-methanocarba ring system to N6-methyl-ATP in 15 enhanced its potency at the turkey P2Y1 receptor by >200-fold [91]. The preference for the (N)-methanocarba over the isomeric (S)-methanocarba nucleotide analogues was also demonstrated for the activation of the P2X1 receptor [93]. However, this conformational preference does not apply to all of the P2Y receptors; the P2Y12 receptor is not activated by the (N)-methanocarba analogue of 2-MeSADP 14 [92].
Extension of the 2-methylthio ether on adenine nucleotides to longer alkyl and arylalkyl chains was one of the first classes of favorable modifications identified to preserve or enhance P2Y1 receptor potency. Extended groups such as the p-aminophenylethylthio ether were tolerated at platelet P2Y receptors [94, 95]. Thio ethers (RS-) were found to be superior P2Y1 receptor agonists in comparison to the corresponding oxygen ethers (RO-) or amines (RNH-) at the 2 position [96].
The presence of a (N)-methanocarba ring system greatly improved the stability of the phosphate ester of the AMP derivative toward hydrolysis by an ectonucleotidase [91], and the phenomenon occurred to a lesser degree for ADP and ATP derivatives. Another means of improving hydrolytic stability is the introduction of a borano group within the phosphate moiety of P2Y receptor agonists [97]. Phosphonate groups have been included in the phosphate chain to increase the stability of the nucleotide analogues. β,γ-methylene-ATP 5 itself is inactive at the human P2Y1 receptor, but when combined with the conformationally constrained (N)-methanocarba modification, the resulting analogue 16 is a potent agonist at this subtype [91]. Analogues of 2-MeSATP 9 that contain either a β,γ-methylene modification 10 or an α-borano group 12 are more stable toward hydrolysis than 9 [98]. They activate the human P2Y1 receptor with EC50 values of 80 nM and 17.2 μM, respectively, and are inactive or only weakly active at the P2Y2, P2Y4, and P2Y6 receptors. 2-MeS-β,γ-CCl2-ATP 11 activates the P2Y1 receptor with an EC50 value of 80 nM and is resistant to a 30-min treatment with alkaline phosphatase [99].
Adenine dinucleotides, specifically diadenosine polyphosphates, are naturally occurring in secretory granules of nerve terminals. In a series of dinucleotides of varying lengths (two to six phosphates), Ap3A 3 was found to have the highest potency at the human P2Y1 receptor expressed in 1321N1 astrocytoma cells (pEC50 = 7.5), and the potency was similar to ADP [100]. The compound also displayed >1,000-fold selectivity over the human P2Y2 receptor. However, Ap3A was also reported to activate rat P2X1 and P2X3 receptors [9]. The homologue Ap4A 4, which is a constituent of platelet dense granules, was the most potent agonist in the series at the P2Y2 receptor, but was inactive as an agonist at the P2Y1 receptor. Chemically synthesized Ap4A of high purity was recently found to inhibit human platelet aggregation by antagonizing the P2Y1 receptor (fully) and the P2Y12 receptor (partially) [101]. Ap4A was also found to be a full agonist of the P2X1 receptor and a partial agonist of the P2Y12 receptor. However, a modified analogue, di-(2-MeS)-adenosine-5′,5″-P1,P4,α,β-methylene-tetraphosphate 13 was found to activate the human P2Y1 receptor with an EC50 value of 0.42 μM and was 2.5-fold more stable in human blood serum than ATP, with a t1/2 of 12.1 h [102].
Other naturally occurring nucleotides have been found to interact with the P2Y1 receptor. For example, ADP-ribose at micromolar concentrations can act as an endogenous agonist the P2Y1 receptor [103]. Also, extracellular nicotinamide adenine dinucleotide (NAD+) 18 was shown to activate the P2Y1 receptor to induce a rise in intracellular calcium ions in transfected astrocytoma cells (EC50 = 743 nM, efficacy of 77%, compared to ADP) [104]. NAD+ contains a β-blocked diphosphate group. Beta-NAD has been shown to be an inhibitory neurotransmitter that activates P2Y1 receptors in gastrointestinal smooth muscle [105]. Curiously, another blocked diphosphate derivative 19, in which the β-phosphate of 2-MeSADP was masked as photoreversible 2-nitroveratryl ester, did not activate the P2Y1 receptor until irradiated to free the 5′-diphosphate. This compound acted as a caged agonist of the P2Y1 and P2Y12 receptors for use as a tool for the light-directed facilitation of platelet aggregation [106].
Many nucleotide antagonists of the P2Y1 receptor have been introduced (Fig. 4). The initial observation was that adenosine 3′,5′-bisphosphate (A3P5P, 34) and its naturally occurring congeners acted as partial agonists or antagonists, respectively, at the turkey and human P2Y1 receptors [107]. Various chemical modifications of adenine nucleotides containing bisphosphate groups, for example N6-methyl 2′-deoxyadenosine 3′, 5′-bisphosphate MRS2179 35 (pKB = 6.99), and its 2-chloro analog MRS2216 36 (pKi = 6.69), provided potent and selective P2Y1 antagonists [108]. [33P]MRS2179 was studied as a radioligand of the P2Y1 receptor [109]. C-nucleoside pyrazolo[1,5-a]-1,3,5-triazines were prepared, and their 3′,5′-bisphosphate C-nucleotide analogues are stable in vivo as P2Y1 receptor antagonists [110].
The same conformationally constrained (N)-methanocarba modification of the ribose moiety that enhanced agonist action in MRS2365 14 also favored antagonist action in nucleotide bisphosphate derivatives. For example, the 2-chloro analogue MRS2279 37 (pKB = 8.10) and the 2-iodo analogue MRS2500 38 (pKB = 9) were selective, high-affinity antagonists of the P2Y1 receptor [111]. MRS2500 effectively inhibited platelet aggregation in vivo in the mouse and other species [112, 113]. The (N)-methanocarba antagonist [3H]MRS2279 37 was introduced as a radioligand for the P2Y1 receptor [114]. The higher affinity antagonist MRS2500 38 has been prepared as a radioligand for the P2Y1 receptor both as a 32P form and as a 125I form [64, 115].
Although the steric constraint of the (N)-methanocarba ring greatly enhanced affinity at the P2Y1 receptor, a cyclic form was not essential for P2Y1 receptor antagonism [116]. The acyclic bisphosphate derivative MRS2298 39 (IC50 = 62.8 nM) and bisphosphonate derivative MRS2496 40 (IC50 = 1.5 μM) were effective inhibitors of ADP-promoted platelet aggregation with intermediate potency [112].
Costanzi et al. [117] studied QSAR of antagonists of the P2Y1 receptor based on ligand docking models and focusing on halo and alkynyl groups at the 2 position. Other alkynyl nucleotides were evaluated at the platelet P2Y receptors. The 5′-diphosphate derivative of 2-phenylethynyladenosine (PEADP, 42) was found to interact mainly with the platelet P2Y1 receptor as an antagonist, while the corresponding 2-hexynyladenosine derivative (HEADP, 17) activated the platelet P2Y12 receptor, but not the P2Y1 receptor [118].
Thiol Coenzyme A (CoA-SH) and various drug-derived CoA derivatives antagonized the human P2Y1, but not the P2Y2, receptor expressed in Xenopus laevis oocytes [119]. Palmitoyl-CoA (16:0) 43 and CoA thioester derivatives of nafenopin and ciprofibrate, two clinically relevant hypolipidemic drugs, were more potent than CoA-SH as antagonists. This phenomenon was further studied using CoA derivatives with saturated acyl groups containing 16–18 carbons to influence the platelet aggregation and Ca2+ mobilization induced by various P2Y agonists [120]. Palmitoyl-CoA 43 was shown to act mainly as an antagonist of the P2Y1 receptor but also as a partial antagonist at the P2Y12 receptor. Not all inhibitors of the P2Y1 receptor are competitive with the binding of nucleotides at the receptor. For example, pyridyl isatogen (PIT) 67 was found to be an allosteric modulator of the P2Y1 receptor [121].
The screening of structurally diverse chemical libraries has helped identify lead compounds for the development of non-nucleotide antagonists of the P2Y1 receptor (Fig. 6). For example, the urea derivative 68 is a selective and orally bioavailable antagonist of the human P2Y1 receptor of novel chemotype with a Ki value of 90 nM [122]. Aminobenzazole derivatives from Bristol–Myers Squibb were reported as P2Y1 receptor antagonists [123]. Other structurally diverse antagonists of the P2Y1 receptor have been reported. Tetrahydro-4-quinolinamines such as 69 (Ki = 70 nM) were found to be novel P2Y1 receptor antagonists [124]. Recently, benzofuran-substituted urea derivatives such as 70 (Ki = 140 nM) were reported as novel P2Y1 receptor antagonists [125].
P2Y12 receptors
ADP (EC50 = 69 nM) and 2-MeSADP (EC50 = 0.3 nM) are potent non-selective agonists at the platelet P2Y12 receptor. [33P]2-MeSADP was utilized as a radioligand of the P2Y1 receptor [126]. Adenine nucleotides, including 5′-monophosphates, with extended 2-alkylthio groups were found to preserve or enhance the potency as agonists at the rat C6 glioma cell P2Y12 receptor [43, 127]. For example, 2-(hexenylthio)-ADP 20 displayed a pEC50 value of 83 nM and selectivity over the P2Y1 receptor of 80-fold.
The SAR of antagonists of the P2Y12 receptor has been extensively explored, resulting in clinical agents. Thienopyridines, notably the blockbuster antiplatelet drug Clopidgrel 29 (Fig. 2), act as liver-activated prodrugs that are irreversible inhibitors of the P2Y12 receptor [128]. In order to form the P2Y12 receptor antagonist species, a two-step pre-activation in vivo is required, which delays onset of action of the drug and the time required for reversal of the platelet effect after drug administration is ceased. This pre-activation process also is subject to pharmacogenomic factors, which explain the variability of the population to be effectively treated by Clopidogrel. A patient’s poor clinical response to Clopidogrel can be predicted using an in vitro platelet function assay [129]. A patient’s response to Clopidogrel depends partly on factors that affect its metabolism through cytochrome P450 in the liver. The presence of a reduced-function CYP2C19*2 allele or the co-administration of the proton pump inhibitor omeprazole decreases the effectiveness of Clopidogrel, and cigarette smoking increases its pre-activation. Another thienopyridine antagonist that has been in clinical trials, Prasugrel 32 (CS-747, LY640315), is a more potent P2Y12 antagonist than Clopidogrel and leads to a more complete inhibition of platelet function, but it also displays a longer bleeding time. Prasugrel, with less genetic variability than Clopidogrel, requires only one step of pre-activation in vivo [130] to the active metabolite R-138727 33.
As discussed above, the action of the thienopyridines depends on the covalent reaction of an active metabolite with a thiol on the P2Y12 receptor. However, directly acting and reversible P2Y12 receptor antagonists, both nucleotides and non-nucleotides, have also been reported. ATP, itself, has been characterized as an antagonist at the P2Y12 receptor, which has enabled the introduction of various 5′-triphosphate analogs as selective receptor probes and clinical candidates. Thus, the antithrombotic nucleotide derivatives from AstraZeneca, AR-C67085 45 and 5′-adenylic acid, N-[2-(methylthio)ethyl]-2-[(3,3,3-trifluoropropyl)thio]-, monoanhydride with (dichloromethylene)bis[phosphonic acid] (AR-C69931MX, Cangrelor, 46), have been tested clinically as antithrombotic agents [131, 132]. The EC50 values of these P2Y12 receptor antagonists are 30 μM and 0.4 nM, respectively. Note that these two P2Y12 receptor antagonists, AR-C67085MX 45 and AR-C69931MX 46, also activate the P2Y11 receptor. Ding et al. [44] reported that (E)-N-[1-[7-(hexylamino)-5-(propylthio)-3H-1,2,3-triazolo-[4,5-d]-pyrimidin-3-yl]-1,5,6-trideoxy-beta-D-ribo-hept-5-enofuranuronoyl]-L-aspartic acid (AR-C78511, 47) is an inverse agonist of the P2Y12 receptor, but AR-C69931MX is a neutral antagonist. [3H]2-Propylthioadenosine-5′-adenylic acid (1,1-dichloro-1-phosphonomethyl-1-phosphonyl) anhydride ([3H]PSB-0413, which is the tritiated equivalent of AR-C67085MX), is a high-affinity antagonist radioligand of the P2Y12 receptor [65].
A 5′-triphosphate group in adenine nucleotides is not strictly required for P2Y12 receptor antagonists. The P2Y12 receptor antagonists from Inspire Pharmaceuticals, INS 49266 51 (an ADP derivative with EC50 of 52 nM) and INS 50589 52 (an AMP derivative with EC50 of 11 nM) [133, 134], could be considered truncated derivatives of the proven 5′-triphosphate antagonists. The potent P2Y12 receptor antagonist and clinical candidate AZD 6140 48b (Brilinta, Ticagrelor, pIC50 = 7.9) is an uncharged cyclopentyltriazolopyrimidine analogue that was developed in an extensive SAR exploration by AstraZeneca [132, 135]. Like 47, this nucleoside derivative contains a modified base, i.e., 8-azaadenine. The antithrombotic effects of 48b compare favorably with the thienopyridines in rat and dog models and with less risk of bleeding, possibly due to its reversible receptor binding [136]. The receptor-binding properties and functional antagonism of 48b are complex (competitive toward 2-MeSADP, but not ADP) [67]. A related carbocyclic nucleoside derivative [125I](1S,2R,3S,4R)-2,3-dihydroxy-4-[7-[[(2E)-3-iodoprop-2-en-1-yl]amino]-5-(propylthio)3H-[1–3]triazolo[4,5-d]pyrimidin-3-yl]cyclopentanecarboxylic acid ([125I]AZ11931285) 48a has been proven useful as a high-affinity antagonist radioligand of the P2Y12 receptor [67]. Other uncharged analogues of nucleotides that act as potent antagonists of the P2Y12 receptor are carbocyclic nucleoside tetrazole derivatives, such as 50 [137]. An uncharged acyclic adenine diester derivative MRS2395 41 acted as a weak but selective antagonist of the P2Y12 receptor (IC50 = 3.6 μM, rat) [116]. This is in contrast to 39 and 40 which contain the same acyclic 9-alkyladenine scaffold and interact only with the P2Y1 receptor as antagonists.
Library screening has identified novel chemotypes as antagonists of the P2Y12 receptor (Fig. 6). For example, Elinogrel (PRT-128, 77) [138], a competitive and reversible antagonist with an IC50 value of 20 nM at the P2Y12 receptor, is being developed as an antithrombotic agent by Portola Pharmaceuticals. Earlier, P2Y12 receptor antagonists consisting of pyrazolidine-3,5-dione derivatives including 71 were reported in an abstract [139]. Tricyclic benzothiazolo[2,3-c]thiadiazine antagonists of the P2Y12 such as CT50547 72 (pEC50 = 6.74, also called C1330-7) were reported [140]. An ester derivative BX 667 73 and the corresponding free carboxylic acid BX 048 74 (binding IC50 values of 29 and 5.3 nM, respectively), which are derivatives of l-glutamic acid, reversibly inhibit the binding and functional effects of 2-MeSADP in platelets of several species [141, 142]. The functional selectivity of BX 667 and BX 048 for the P2Y12 receptor in comparison to P2Y1 and P2Y6 receptors was demonstrated. Parlow et al. [143–145] reported piperazinyl–glutamate–pyridine derivatives such as 76 (pEC50 = 7.82) as potent orally bioavailable P2Y12 antagonists. 6-Amino-2-mercapto-3H-pyrimidin-4-one derivatives such as 78 (IC50 = 8.1 μM) appear to antagonize the P2Y12 receptor [146]. One very potent and selective competitive antagonist of the P2Y12 receptor, the disulfonate derivative PSB 0739 75, which was derived from RB2, was recently introduced as a research tool [41, 147]. PSB-0739 was reported as the most potent competitive non-nucleotide antagonist at the human P2Y12 receptor described so far (Ki = 24.9 nM). BF0801 79 is an uncharged adenine derivative that antagonizes the P2Y12 receptor in platelets to inhibit aggregation with an IC50 of 63.3 μM and also inhibits a phosphodiesterase [148].
P2Y14 receptors
The P2Y14 receptor is structurally restrictive with respect to the modification of the nucleobase, ribose, and phosphate moieties of agonist ligands. UDP-glucose 25 (EC50 = 0.35 μM) and UDP 23 are nearly equipotent as agonists of the human P2Y14 receptor. Other naturally occurring UDP-sugars activate this receptor less potently. The SAR of analogues of both endogenous agonists was recently explored in a systematic fashion [149]. When the glucose moiety is present, there is a requirement for specific hydroxyl groups in order to potently activate the P2Y14 receptor. When the distal hexose moiety is entirely absent, very high potencies can be obtained, suggesting partly different modes of binding of the two ligand series. The 2-thiouracil modification enhances potency in both series. Thus, the 2-thio analog of UDP-glucose MRS2690 26 is a sixfold more potent agonist for the P2Y14 receptor and, unlike UDP-glucose, is inactive at the P2Y2 receptor. Because UDP activates both the P2Y6 and P2Y14 receptors, there is a need for agonist ligands that can distinguish between these two subtypes. Stabilizing phosphonate groups have facilitated this selectivity. For example, α,β-difluoromethylene-UDP, MRS2802 27, is inactive at the P2Y6 receptor and fully activates the human P2Y14 receptor with an EC50 of 63 nM. MRS2905 28 displays an EC50 of 2 nM at the human P2Y14 receptor with a selectivity of >2,000 in comparison to the P2Y6 receptor.
The heterocyclic antagonists of the P2Y14 receptor 80 (Ki = 2.2 nM) and 81 (Ki = 4.0 nM) were disclosed in patents from Merck, but the full pharmacological characterization has not yet appeared in the literature [150, 151]. A prodrug approach in the structural series of compound 80 was recently reported [152].
Conclusions
Novel ligands for the purine receptor families in platelets, including both selective agents derived from known ligands and new chemotypes, are now available for use as tools in pharmacological studies. Some of these agents have entered into clinical trials. Functional properties of the P2Y1 and P2Y12 receptors were extensively explored using mutagenesis and molecular modeling, which are useful tools in drug discovery. Detailed SAR analyses have been constructed for nucleotide and non-nucleotide ligands at the P2X1, P2Y1, P2Y12, and P2Y14 receptors. There is much pharmaceutical development activity aimed at identifying newer agents to act at the P2Y12 receptor that do not require enzymatic pre-activation in vivo. The screening of chemically diverse compound libraries has identified novel chemotypes that act as competitive non-nucleotide antagonists of the P2Y1 receptor or the P2Y12 receptor, and antithrombotic properties of the structurally optimized analogues were demonstrated. New tools have been developed for the discovery of ligands at platelet purine receptors, including in silico screening to identify novel antagonist chemotypes, fluorescent probes, and covalent attachment to dendrimeric carriers to produce multivalent conjugates that inhibit platelet aggregation. In conclusion, a wide range of new pharmacological tools is available to control platelet function by interacting with cell surface purine receptors.
Acknowledgment
Support from the NIDDK Intramural Research Program of NIH is acknowledged.
References
- 1.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev (in press) [DOI] [PMC free article] [PubMed]
- 2.Abbracchio M, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquez-Klaka B, Rettinger J, Nicke A. Inter-subunit disulfide cross-linking in homomeric and heteromeric P2X receptors. Eur Biophys J. 2009;3:329–338. doi: 10.1007/s00249-008-0325-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Townsend-Nicholson A, Jacobson KA, Burnstock G, King BF. Heteromultimeric P2X1/2 receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther. 2002;300:673–680. doi: 10.1124/jpet.300.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armaz A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–343. doi: 10.1016/j.jmb.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 6.Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 7.Torres G, Haines W, Egan T, Voigt M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- 8.Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol. 2009;76:1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildman SS, Brown SG, King BF, Burnstock G. Selectivity of diadenosine polyphosphates for the rat P2X receptor subunits. Eur J Pharmacol. 1999;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]
- 10.Shaver SR, Rideout J, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR. Structure–activity relationships of dinucleotides: potent and selective agonists of P2Y receptors. Purinergic Signal. 2005;1:183–191. doi: 10.1007/s11302-005-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciani P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 13.Mahaut-Smith MP, Tolhurst G, Evans RJ. Emerging roles for P2X1 receptors in platelet activation. Platelets. 2004;15:131–144. doi: 10.1080/09537100410001682788. [DOI] [PubMed] [Google Scholar]
- 14.Dovlatova N, Wijeyeratne YD, Fox SC, Manolopoulos P, Johnson AJ, White AE, Latif ML, Ralevic V, Heptinstall S. Detection of P2Y14 protein in platelets and investigation of the role of P2Y14 in platelet function in comparison with the EP3 receptor. Thromb Haemost. 2008;2:261–270. [PubMed] [Google Scholar]
- 15.Johnston-Cox HA, Yang D, Ravid K. Physiological implications of adenosine receptor-mediated platelet aggregation. J Cell Physiol. 2011;226:46–51. doi: 10.1002/jcp.22379. [DOI] [PubMed] [Google Scholar]
- 16.Baraldi PG, Tabrizi TA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev. 2008;108:238–263. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- 17.Cristalli G, Müller CE, Volpini R. Recent developments in adenosine A2A receptor ligands. Handb Exp Pharmacol. 2010;193:59–98. doi: 10.1007/978-3-540-89615-9_3. [DOI] [PubMed] [Google Scholar]
- 18.Ham J, Rees DA. The adenosine A2b receptor: its role in inflammation. Endocr Metabol Imm Disorders–Drug Targets. 2008;8:244–254. doi: 10.2174/187153008786848303. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Chen H, Koupenova M, Carroll SH, Eliades A, Freedman JE, Toselli P, Ravid K. A new role for the A2b adenosine receptor in regulating platelet function. J Thromb Haemost. 2010;4:817–827. doi: 10.1111/j.1538-7836.2010.03769.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts JA, Valente M, Allsopp RC, Watt D, Evans RJ. Contribution of the region Glu181 to Val200 of the extracellular loop of the human P2X1 receptor to agonist binding and gating revealed using cysteine scanning mutagenesis. J Neurochem. 2009;4:1042–1052. doi: 10.1111/j.1471-4159.2009.06035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb TE, Simon J, Bateson AN, Barnard EA. Transient expression of the recombinant chick brain P2y1 purinoceptor and localization of the corresponding . Cell Mol Biol (Noisy-le-grand) 1994;3:437–442. [PubMed] [Google Scholar]
- 23.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by anti-thrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 24.Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov AA, Costanzi S, Jacobson KA. Defining the nucleotide binding sites of P2Y receptors using rhodopsin-based homology modeling. J Comput Aided Mol Des. 2006;20:417–426. doi: 10.1007/s10822-006-9054-2. [DOI] [PubMed] [Google Scholar]
- 26.Ding Z, Tuluc F, Bandivadekar KR, Zhang L, Jin J, Kunapuli SP. Arg333 and Arg334 in the COOH terminus of the human P2Y1 receptor are crucial for Gq coupling. Am J Physiol Cell Physiol. 2005;3:C559–C567. doi: 10.1152/ajpcell.00401.2004. [DOI] [PubMed] [Google Scholar]
- 27.Filippov AK, Choi RC, Simon J, Barnard EA, Brown DA. Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons. J Neurosci. 2006;26:9340–9348. doi: 10.1523/JNEUROSCI.2635-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundell SJ, Barton JF, Mayo-Martin MB, Hardy AR, Poole AW. Rapid resensitization of purinergic receptor function in human platelets. J Thromb Haemost. 2008;8:1393–1404. doi: 10.1111/j.1538-7836.2008.03039.x. [DOI] [PubMed] [Google Scholar]
- 29.Bourdon DM, Boyer JL, Mahanty S, Jacobson KA, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–868. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff SC, Qi AD, Harden TK, Nicholas RA. Charged residues in the C-terminus of the P2Y1 receptor constitute a basolateral-sorting signal. J Cell Sci. 2010;123:2512–2520. doi: 10.1242/jcs.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belous E, Jones CM, Wakata A, Knox CD, Nicoud IB, Pierce J, Chari RS. Mitochondrial calcium transport is regulated by P2Y1- and P2Y2-like mitochondrial receptors. J Cell Biochem. 2006;99:1165–1174. doi: 10.1002/jcb.20985. [DOI] [PubMed] [Google Scholar]
- 32.Choi RC, Simon J, Tsim KW, Barnard EA. Constitutive and agonist-induced dimerizations of the P2Y1 receptor: relationship to internalization and scaffolding. J Biol Chem. 2008;283:11050–11063. doi: 10.1074/jbc.M709266200. [DOI] [PubMed] [Google Scholar]
- 33.Nakata H, Yoshioka K, Saitoh O. Hetero-oligomerization between adenosine A1 and P2Y1 receptors in living cells: formation of ATP-sensitive adenosine receptors. Drug Dev Res. 2003;58:340–349. [Google Scholar]
- 34.Ecke D, Hanck T, Tulapurkar ME, Schäfer R, Kassack M, Stricker R, Reiser G. Hetero-oligomerization of the P2Y11 receptor with the P2Y1 receptor controls the internalization and ligand selectivity of the P2Y11 receptor. Biochem J. 2008;1:107–116. doi: 10.1042/BJ20070671. [DOI] [PubMed] [Google Scholar]
- 35.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274:14639–14647. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann K, Sixel U, Pasquale F, Kügelgen I. Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12-receptor. Biochem Pharmacol. 2008;10:1201–1213. doi: 10.1016/j.bcp.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Castro S, Maruoka H, Hong K, Kilbey SM, Costanzi S, Hechler B, Brown GG, Gachet C, Harden TK, Jacobson KA. Functionalized congeners of P2Y1 receptor antagonists: 2-alkynyl (N)-methanocarba 2′-deoxyadenosine 3′, 5′-bisphosphate analogues and conjugation to a polyamidoamine (PAMAM) dendrimer carrier. Bioconjug Chem. 2010;21:1190–1205. doi: 10.1021/bc900569u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure–function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 41.Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, Kügelgen I. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther. 2009;331:648–655. doi: 10.1124/jpet.109.156687. [DOI] [PubMed] [Google Scholar]
- 42.Mao Y, Zhang L, Jin J, Ashby B, Kunapuli SP. Mutational analysis of residues important for ligand interaction with the human P2Y12 receptor. Eur J Pharmacol. 2010;644:10–16. doi: 10.1016/j.ejphar.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer JL, O’Tuel J, Fischer B, Jacobson KA, Harden TK. Potent agonist action of 2-thioether derivatives of adenine nucleotides at adenylyl cyclase-linked P2Y purinoceptors. Br J Pharmacol. 1995;116:2611–2616. doi: 10.1111/j.1476-5381.1995.tb17215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Z, Kim S, Kunapuli SP. Identification of a potent inverse agonist at a constitutively active mutant of human P2Y12 receptor. Mol Pharmacol. 2006;1:338–345. doi: 10.1124/mol.105.014654. [DOI] [PubMed] [Google Scholar]
- 45.Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood. 2003;10:3908–3914. doi: 10.1182/blood-2002-10-3027. [DOI] [PubMed] [Google Scholar]
- 46.Algaier I, Jakubowski JA, Asai F, Kügelgen I. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost. 2008;6:1908–1914. doi: 10.1111/j.1538-7836.2008.03136.x. [DOI] [PubMed] [Google Scholar]
- 47.Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H. Prasugrel metabolite: antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type Gi-linked P2T antagonist, CS-747. Br J Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa M, Sugidachi A, Ogawa T, Isobe T, Jakubowski JA, Asai F. Stereoselective inhibition of human platelet aggregation by R-138727, the active metabolite of CS-747 (prasugrel, LY640315), a novel P2Y12 receptor inhibitor. Thromb Haemost. 2005;3:593–598. doi: 10.1160/TH05-03-0208. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Wess J, Rhee AM, Shöneberg T, Jacobson KA. Site-directed mutagenesis identifies residues involved in ligand recognition in the human A2a adenosine receptor. J Biol Chem. 1995;270:13987–13997. doi: 10.1074/jbc.270.23.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Jiang Q, Glashofer M, Yehle S, Wess J, Jacobson KA. Glutamate residues in the second extracellular loop of the human A2a adenosine receptors are required for ligand recognition. Mol Pharmacol. 1996;49:683–691. [PMC free article] [PubMed] [Google Scholar]
- 51.Katritch V, Jaakola VP, Lane JR, Lin J, IJzerman AP, Yeager M, Kufareva I, Stevens RC, Abagyan R. Structure-based discovery of novel chemotypes for adenosine A2A receptor antagonists. J Med Chem. 2010;53:1799–1809. doi: 10.1021/jm901647p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlsson J, Yoo L, Gao ZG, Irwin J, Shoichet B, Jacobson KA. Structure-based discovery of adenosine A2A receptor ligands. J Med Chem. 2010;53:3748–3755. doi: 10.1021/jm100240h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kecskés M, Kumar TS, Yoo L, Gao ZG, Jacobson KA. Novel Alexa Fluor-488 labeled antagonist of the A2A adenosine receptor: application to a fluorescence polarization-based receptor binding assay. Biochem Pharmacol. 2010;80:506–511. doi: 10.1016/j.bcp.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y, Hechler B, Gao ZG, Gachet C, Jacobson KA. PEGylated dendritic unimolecular micelles as versatile carriers for ligands of G protein-coupled receptors. Bioconjug Chem. 2009;20:1888–1898. doi: 10.1021/bc9001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Hardwick JP, McPhie P, Sitkovsky M, Jacobson KA. Purification and recognition of recombinant mouse P2X1 receptors expressed in a baculovirus system. Drug Dev Res. 2000;51:7–19. doi: 10.1002/1098-2299(20000901)51:1<7::AID-DDR2>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;2:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- 57.Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol. 2003;5:1210–1216. doi: 10.1124/mol.64.5.1210. [DOI] [PubMed] [Google Scholar]
- 58.Deaglio S, Robson SC (2011) Ectonucleotidases as regulators of purinergic signaling in inflammation and thrombosis. Adv Pharmacol (in press) [DOI] [PMC free article] [PubMed]
- 59.Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol. 2007;152:141–150. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baqi Y, Lee SY, Iqbal J, Ripphausen P, Lehr A, Scheiff AB, Zimmermann H, Bajorath J, Müller CE. Development of potent and selective inhibitors of ecto-5′-nucleotidase based on an anthraquinone scaffold. J Med Chem. 2010;53:2076–2086. doi: 10.1021/jm901851t. [DOI] [PubMed] [Google Scholar]
- 61.Semianrio-Vidal L, van Hesuden C, Mugesh G, Lazarowski ER (2011) Ebselen is a potent non-competitive inhibitor of extracellular nucleoside diphosphokinase. Purinergic Signal. doi:10.1007/s11302-010-9203-x [DOI] [PMC free article] [PubMed]
- 62.Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M. Inhibition of receptor/G protein coupling by suramin analogues. Mol Pharmacol. 1996;2:415–423. [PubMed] [Google Scholar]
- 63.Krejci P, Murakami S, Prochazkova J, Trantirek L, Chlebova K, Ouyang Z, Aklian A, Smutny J, Bryja V, Kozubik A, Wilcox WR. NF449 is a novel inhibitor of fibroblast growth factor receptor 3 (FGFR3) signaling active in chondrocytes and multiple myeloma cells. J Biol Chem. 2010;27:20644–20653. doi: 10.1074/jbc.M109.083626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′, 5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Tayeb A, Griessmeier KJ, Müller CE. Synthesis and preliminary evaluation of [3H]PSB-0413, a selective antagonist radioligand for platelet P2Y12 receptors. Bioorg Med Chem Lett. 2005;15:5450–5452. doi: 10.1016/j.bmcl.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 66.Bo X, Fischer B, Maillard M, Jacobson KA, Burnstock G. Comparative studies on the affinities of ATP derivatives to P2X-purinoceptors in rat urinary bladder. Br J Pharmacol. 1994;112:1151–1159. doi: 10.1111/j.1476-5381.1994.tb13204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giezen JJJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, Greasley PJ. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7:1556–1565. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 68.Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br J Pharmacol. 1996;1:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Expert Opin Ther Pat. 2010;20:625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- 70.Jacobson KA, Jarvis MF, Williams M. Perspective: purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 72.Brown SG, King BF, Kim YC, Burnstock G, Jacobson KA. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev Res. 2000;49:253–259. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyer JL, Zohn IE, Jacobson KA, Harden TK. Differential effects of P2-purinergic receptor antagonists on phospholipase C- and adenylyl cyclase-coupled P2Y purinergic receptors. Br J Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whittenburg H, Bültmann R, Pause B, Ganter C, Kurz G, Starke K. P2-purinoceptor antagonists:II blockade of P2-purinoceptor subtypes and ecto-nuleotidase by compounds related to Evans blue and trypan blue. Naunyn-Schmiedeberg’s Arch Pharmacol. 1996;354:491–497. doi: 10.1007/BF00168441. [DOI] [PubMed] [Google Scholar]
- 75.Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure–activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8:2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- 76.Kim YC, Brown SG, Harden TK, Boyer JL, Dubyak G, King BF, Burnstock G, Jacobson KA. Structure activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J Med Chem. 2001;44:340–349. doi: 10.1021/jm9904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobson KA, Kim YC, Wildman SS, Mohanram A, Harden TK, Boyer JL, King BF, Burnstock G. A pyridoxine cyclic phosphate and its 6-axoaryl derivative selectively potentiate and antagonise activation of P2X1 receptors. J Med Chem. 1998;41:2201–2206. doi: 10.1021/jm980183o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol. 1999;128:981–988. doi: 10.1038/sj.bjp.0702876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Virginio C, Robertson G, Surprenant NRA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- 80.Soto F, Lambrecht G, Nickel P, Stühmer W, Busch AE. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- 81.Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems JM, Wolf C, Hausmann R, Schmalzing G, Kassack MU. Synthesis and structure–activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem. 2005;48:7040–7048. doi: 10.1021/jm050301p. [DOI] [PubMed] [Google Scholar]
- 82.Rettinger J, Schmalzing G, Damer S, Müller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X1 receptor. Neuropharmacology. 2000;39:2044–2053. doi: 10.1016/s0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 83.Rettinger J, Braun K, Hochmann H, Kassack MU, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. Profiling at recombinant homomeric and heteromeric rat P2X receptors identifies the suramin analogue NF449 as a highly potent P2X1 receptor antagonist. Neuropharmacology. 2005;48:461–468. doi: 10.1016/j.neuropharm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Horner S, Menke K, Hildebrandt C, Kassack MU, Nickel P, Ullmann H, Mahaut-Smith MP, Lambrecht G. The novel suramin analogue NF864 selectively blocks P2X1 receptors in human platelets with potency in the low nanomolar range. Naunyn-Schmiedeberg’s Arch Pharmacol. 2005;372:1–13. doi: 10.1007/s00210-005-1085-z. [DOI] [PubMed] [Google Scholar]
- 85.Jaime-Figueroa S, Greenhouse R, Padilla F, Dillon MP, Gever JR, Ford APDW. Discovery and synthesis of a novel and selective drug-like P2X1 antagonist. Bioorg Med Chem Lett. 2005;15:3292–3295. doi: 10.1016/j.bmcl.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 86.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cattaneo M. New P2Y12 inhibitors. Circulation. 2010;121:171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 88.Leon C, Hechler B, Freund M. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y receptor-null mice. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhee AM, Fischer B, Galen PJ, Jacobson KA. Modelling the P2Y purinoceptor using rhodopsin as template. Drug Des Discov. 1995;13:133–154. [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-K, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: high potency of northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunn PM, Kim HS, Jacobson KA, Burnstock G. Northern ring conformation of methanocarba-adenosine 5′-triphosphate required for activation of P2X receptors. Drug Dev Res. 2004;61:227–232. doi: 10.1002/ddr.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5′-triphosphate. J Med Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cristalli G, Mills DC. Identification of a receptor for ADP on blood platelets by photoaffinity labelling. Biochem J. 1993;291:875–881. doi: 10.1042/bj2910875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halbfinger E, Major DT, Ritzmann M, Ubl J, Reiser G, Boyer JL, Harden KT, Fischer B. Molecular recognition of modified adenine nucleotides by the P2Y1-receptor. 1. A synthetic, biochemical, and NMR approach. J Med Chem. 1999;42:5325–5337. doi: 10.1021/jm990156d. [DOI] [PubMed] [Google Scholar]
- 97.Nahum V, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y1 receptor agonists. J Med Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- 98.Eliahu SE, Camden J, Lecka J, Weisman GA, Sevigny J, Gelinas S, Fischer B. Identification of hydrolytically stable and selective P2Y1 receptor agonists. Eur J Med Chem. 2009;44:1525–1536. doi: 10.1016/j.ejmech.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eliahu S, Martín-Gil A, Perez de Lara MJ, Pintor J, Camden J, Weisman GA, Lecka J, Sévigny J, Fischer B. 2-MeS-β, γ-CCl2-ATP is a potent agent for reducing intraocular pressure. J Med Chem. 2010;53:3305–3319. doi: 10.1021/jm100030u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patel K, Barnes A, Camacho J, Paterson C, Boughtflower R, Cousens D, Marshall F. Activity of diadenosine polyphophates at P2Y receptors stably expressed in 1321N1 cells. Eur J Pharmacol. 2001;430:203–210. doi: 10.1016/s0014-2999(01)01401-7. [DOI] [PubMed] [Google Scholar]
- 101.Chang H, Yanachkov IB, Michelson AD, Li Y, Barnard MR, Wright GE, Frelinger AL., 3rd Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res. 2010;125:159–165. doi: 10.1016/j.thromres.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eliahu S, Barr HM, Camden J, Weisman GA, Fischer B. A novel insulin secretagogue based on a dinucleoside polyphosphate scaffold. J Med Chem. 2010;53:2472–2481. doi: 10.1021/jm901621h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gustafsson AJ, Muraro L, Dahlberg C, Migaud M, Chevallier O, Khanh HN, Li N, Islam MS (2010) ADP ribose is an endogenous ligand for the purinergic P2Y1 receptor. Mol Cell Endocrinol 333:8–19 [DOI] [PubMed]
- 104.Klein C, Grahnert A, Abdelrahman A, Müller CE, Hauschildt S. Extracellular NAD+ induces a rise in [Ca2+]i in activated human monocytes via engagement of P2Y1 and P2Y11 receptors. Cell Calcium. 2009;46:263–272. doi: 10.1016/j.ceca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao ZG, Hechler B, Besada P, Gachet C, Jacobson KA. Caged agonist of P2Y1 and P2Y12 receptors for light-directed facilitation of platelet aggregation. Biochem Pharmacol. 2008;75:1341–1347. doi: 10.1016/j.bcp.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1-receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 108.Nandanan E, Camaioni E, Jang SY, Kim Y-C, Cristalli G, Herdewijn P, Secrist JA, Tiwari KN, Mohanram A, Harden TK, Boyer JL, Jacobson KA. Structure activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baurand A, Raboisson P, Freund M, Léon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 110.Raboisson P, Baurand A, Cazenave JP, Gachet C, Schultz D, Spiess B, Bourguignon JJ. A general approach toward the synthesis of C-nucleoside pyrazolo[1,5-a]-1, 3, 5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y1-receptor antagonists. J Org Chem. 2002;67:8063–8071. doi: 10.1021/jo026268l. [DOI] [PubMed] [Google Scholar]
- 111.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a Northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cattaneo M, Lecchi A, Joshi BV, Ohno M, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hechler B, Nonne C, Roh EJ, Cattaneo M, CazenaveFranc JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective and stable antagonist of the P2Y1 receptor, with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waldo G, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji X-D, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohlmann P, Castro S, Brown GG, Gachet C, Jacobson KA, Harden TK. Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]-labeled high affinity antagonist 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′, 5′-bisphosphate ([125I]MRS2500) Pharmacol Res. 2010;62:344–351. doi: 10.1016/j.phrs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, Kim HS, Bischofberger N, Cook G, Jacobson KA. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Costanzi S, Tikhonova IG, Ohno M, Roh EJ, Joshi BV, Colson A-O, Houston D, Maddileti S, Harden TK, Jacobson KA. P2Y1 antagonists: combining receptor-based modeling and QSAR for a quantitative prediction of the biological activity based on consensus scoring. J Med Chem. 2007;50:3229–3241. doi: 10.1021/jm0700971. [DOI] [PubMed] [Google Scholar]
- 118.Cristalli G, Podda GM, Costanzi S, Lambertucci C, Lecchi A, Vittori S, Volpini R, Zighetti ML, Cattaneo M. Effects of 5′-phosphate derivatives of 2-hexynyl adenosine and 2-phenylethynyl adenosine on responses of human platelets mediated by P2Y receptors. J Med Chem. 2005;48:2763–2766. doi: 10.1021/jm0493562. [DOI] [PubMed] [Google Scholar]
- 119.Coddou C, Loyola G, Boyer JL, Bronfman M, Huidobro-Toro JP. The hypolipidemic drug metabolites nafenopin-CoA and ciprofibroyl-CoA are competitive P2Y1 receptor antagonists. FEBS Lett. 2003;536:145–150. doi: 10.1016/s0014-5793(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 120.Manolopoulos P, Glenn JR, Fox SC, May JA, Dovlatova NL, Tang SW, Thomas NR, Ralevic V, Heptinstall S. Acyl derivatives of coenzyme A inhibit platelet function via antagonism at P2Y1 and P2Y12 receptors: a new finding that may influence the design of anti-thrombotic agents. Platelets. 2008;2:134–145. doi: 10.1080/09537100701708498. [DOI] [PubMed] [Google Scholar]
- 121.Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004;68:231–237. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfefferkorn JA, Choi C, Winters T, Kennedy R, Chi L, Perrin LA, Lu G, Ping YW, McClanahan T, Schroeder R, Leininger MT, Geyer A, Schefzick S, Atherton J. P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg Med Chem Lett. 2008;18:3338–3343. doi: 10.1016/j.bmcl.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 123.Herpin TF, Morton G, Rehfuss RP, Lawrence RM, Poss MA, Roberge JY, Gungor T (2005) Aminobenzazoles as P2Y1 receptors inhibitors. WO2005070920. August 4, 2005
- 124.Morales-Ramos AI, Mecom JS, Kiesow TJ, Graybill TL, Brown GD, Aiyar NV, Davenport EA, Kallal LA, Knapp-Reed BA, Li P, Londregan AT, Morrow DM, Senadhi S, Thalji RK, Zhao S, Burns-Kurtis CL, Marino JP., Jr Tetrahydro-4-quinolinamines identified as novel P2Y1 receptor antagonists. Bioorg Med Chem Lett. 2008;18:6222–6226. doi: 10.1016/j.bmcl.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 125.Thalji RK, Aiyar NV, Davenport EA, Erhardt JA, Kallal LA, Morrow DM, Senadhi S, Burns-Kurtis CL, Marino JP., Jr Benzofuran-substituted urea derivatives as novel P2Y1 receptor antagonists. Bioorg Med Chem Lett. 2010;20:4104–4107. doi: 10.1016/j.bmcl.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 126.Mathieu R, Baurand A, Schmitt M, Gachet C, Bourguignon JJ. Synthesis and biological activity of 2-alkylated deoxyadenosine bisphosphate derivatives as P2Y1 receptor antagonists. Bioorg Med Chem. 2004;12:1769–1779. doi: 10.1016/j.bmc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 127.Boyer JL, Siddiqi S, Fischer B, Romera-Avila T, Jacobson KA, Harden TK. Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Br J Pharmacol. 1996;118:1959–1964. doi: 10.1111/j.1476-5381.1996.tb15630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of Clopidogrel. Thromb Haemost. 2002;84:891–896. [PubMed] [Google Scholar]
- 129.Michelson AD. New P2Y12 antagonists. Curr Opin Hematol. 2009;5:371–377. doi: 10.1097/MOH.0b013e32832ea2f2. [DOI] [PubMed] [Google Scholar]
- 130.Jakubowski JA, Matsushima N, Asai F, Naganuma H, Brandt JT, Hirota T, Freestone S, Winters KJ. A multiple dose study of prasugrel (CS-747), a novel thienopyridine P2Y12 inhibitor, compared with clopidogrel in healthy humans. Br J Clin Pharmacol. 2007;63:421–430. doi: 10.1111/j.1365-2125.2006.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ingall AH, Dixon J, Bailey A, Coombs ME, McInally JI, Hunt SF, Kindon ND, Theobald BJ, Willis PA, Humphries R, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 132.Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC, Chapman D, Dixon J, Guile SD, Humphries RG, Hunt SF, Ince F, Ingall AH, Kirk IP, Leeson PD, Leff P, Lewis RJ, Martin BP, McGinnity DF, Mortimore MP, Paine SW, Pairaudeau G, Patel A, Rigby AJ, Riley RJ, Teobald BJ, Tomlinson W, Webborn PJ, Willis PA. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett. 2007;17:6013–6018. doi: 10.1016/j.bmcl.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 133.Johnson FL, Boyer JL, Leese PT, Crean C, Krishnamoorthy R, Durham T, Fox AW, Kellerman DJ. Rapid and reversible modulation of platelet function in man by a novel P2Y12 ADP-receptor antagonist, INS50589. Platelets. 2007;18:346–356. doi: 10.1080/09537100701268741. [DOI] [PubMed] [Google Scholar]
- 134.Douglass J, Patel RI, Yerxa B, Shaver SR, Watson PS, Bednarski K, Plourde R, Redick C, Brubaker K, Jones AC, Boyer JL. Lipophilic modifications to dinucleoside polyphosphates and nucleotides that confer antagonist properties at the P2Y12 platelet receptor. J Med Chem. 2008;51:1007–1025. doi: 10.1021/jm701348d. [DOI] [PubMed] [Google Scholar]
- 135.Husted S, Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giezen JJ, Berntsson P, Zachrisson H, Björkman JA. Comparison of ticagrelor and thienopyridine P2Y12 binding characteristics and antithrombotic and bleeding effects in rat and dog models of thrombosis/hemostasis. Thromb Res. 2009;124:565–571. doi: 10.1016/j.thromres.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 137.Ye H, Chen C, Zhang HC, Haertlein BJ, Parry TJ, Damiano BP, Maryanoff BE. Carba-nucleosides as potent antagonists of the adenosine 5′-diphosphate (ADP) purinergic receptor (P2Y12) on human platelets. ChemMed Chem. 2003;3:732–736. doi: 10.1002/cmdc.200700310. [DOI] [PubMed] [Google Scholar]
- 138.Oestereich JH. Elinogrel, a reversible P2Y12 antagonist for the treatment of acutre coronary syndrome and prevention of secondary thrombotic events. Curr Opin Investig Drugs. 2010;11:340–348. [PubMed] [Google Scholar]
- 139.Fretz H, Houille O, Hilpert K, Peter O, Breu V, Giller T, Valdenaire O, Riederer M (2005) Novel pyrazolidine-3,5-dione derivatives are P2Y12 receptor antagonists and inhibit ADP-triggered blood platelet aggregation. 229th National Meeting of the American Chemical Soc, San Diego, CA, Abstract MEDI 80
- 140.Scarborough RM, Laibelman AM, Clizbe LA, Fretto LJ, Conley PB, Reynolds E, Sedlock M, Jantzen H-M. Novel tricyclic benzothiazolo[2,3-c]thiadiazine antagonists of the platelet ADP receptor (P2Y12) Bioorg Med Chem Lett. 2001;11:1805–1808. doi: 10.1016/s0960-894x(01)00313-4. [DOI] [PubMed] [Google Scholar]
- 141.Wang YX, Vincelette J, Cunha V, Martin-McNulty B, Mallari C, Fitch RM, Alexander S, Islam I, Buckman BO, Yuan S, Post JM, Subramanyam B, Vergona R, Sullivan ME, Dole WP, Morser J, Bryant J. A novel P2Y12 adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb Haemost. 2007;97:847–855. [PubMed] [Google Scholar]
- 142.Bryant J, Post JM, Alexander S, Wang YX, Kent L, Schirm S, Tseng JL, Subramanyam B, Buckman B, Islam I, Yuan S, Sullivan ME, Snider M, Morser J. Novel P2Y12 adenosine diphosphate receptor antagonists for inhibition of platelet aggregation (I): in vitro effects on platelets. Thromb Res. 2008;4:523–532. doi: 10.1016/j.thromres.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 143.Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD. Piperazinyl-glutamate-pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. Bioorg Med Chem Lett. 2009;16:4657–4663. doi: 10.1016/j.bmcl.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 144.Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Harris PK, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD. Part II: piperazinyl-glutamate-pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. Bioorg Med Chem Lett. 2010;20:1388–1394. doi: 10.1016/j.bmcl.2009.12.110. [DOI] [PubMed] [Google Scholar]
- 145.Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Harris PK, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD. Piperazinyl glutamate pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. J Med Chem. 2010;53:2010–2037. doi: 10.1021/jm901518t. [DOI] [PubMed] [Google Scholar]
- 146.Crepaldi P, Cacciari B, Bonache MC, Spalluto G, Varani K, Borea PA, Kügelgen I, Hoffmann K, Pugliano M, Razzari C, Cattaneo M. 6-Amino-2-mercapto-3H-pyrimidin-4-one derivatives as new candidates for the antagonism at the P2Y12 receptors. Bioorg Med Chem. 2009;17:4612–4621. doi: 10.1016/j.bmc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 147.Baqi Y, Atzler K, Köse M, Glänzel M, Müller CE. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem. 2009;52:3784–3793. doi: 10.1021/jm9003297. [DOI] [PubMed] [Google Scholar]
- 148.Zhang S, Hu L, Du H, Guo Y, Zhang Y, Niu H, Jin J, Zhang J, Liu J, Zhang X, Kunapuli SP, Ding Z. BF0801, a novel adenine derivative, inhibits platelet activation via phosphodiesterase inhibition and P2Y12 antagonism. Thromb Haemost. 2010;104:845–857. doi: 10.1160/TH10-05-0285. [DOI] [PubMed] [Google Scholar]
- 149.Das A, Ko H, Burianek LE, Barrett MO, Harden TK, Jacobson KA. Human P2Y14 receptor agonists: truncation of the hexose moiety of uridine-5′-diphosphoglucose and its replacement with alkyl and aryl groups. J Med Chem. 2010;53:471–480. doi: 10.1021/jm901432g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.International Patent Application No. PCT/CA2008/002105, WO 2009/070873 A1 (published June 11, 2009), Merck Frosst Canada Ltd., applicant
- 151.International Patent Application No. PCT/CA2008/001214, WO2009/000087 A1 (published Dec. 31, 2008), Merck Frosst Canada Ltd., applicant
- 152.Robichaud J, Fournier J-F, Gagné S, Gauthier JY, Hamel M, Han Y, Hénault M, Kargman S, Levesque J-F, Mamane Y, Mancini J, Morin N, Mulrooney E, Wu J, Black WC (2011) Applying the pro-drug approach to afford highly bioavailable antagonists of P2Y14. Bioorg Med Chem Lett. doi:10.1016/j.bmcl.2010.12.113 [DOI] [PubMed]