Introduction
Mitosis in Aspergillus nidulans is very rapid, requiring less than five minutes at 37°C in germlings (Bergen and Morris, 1983). In this time the cytoplasmic microtubules (MTs) must disassemble, the mitotic spindle assemble, function and disassemble, and cytoplasmic MTs reassemble. It follows that MTs must be extremely dynamic in this period and we were interested, in particular, in examining the processes of cytoplasmic MT disassembly in prophase and reassembly in anaphase and telophase. We observed a diploid strain that expressed GFP-α-tubulin. We used a spinning disk confocal microscope that allowed rapid image capture, which proved necessary because microtubule dynamics were extremely rapid. We found, for the first time, that microtubule severing occurs in prophase in a filamentous fungus and that catastrophe rather than nucleation limits astral microtubule growth.
Microtubule severing and astral microtubule catastrophe in mitosis in A. nidulans
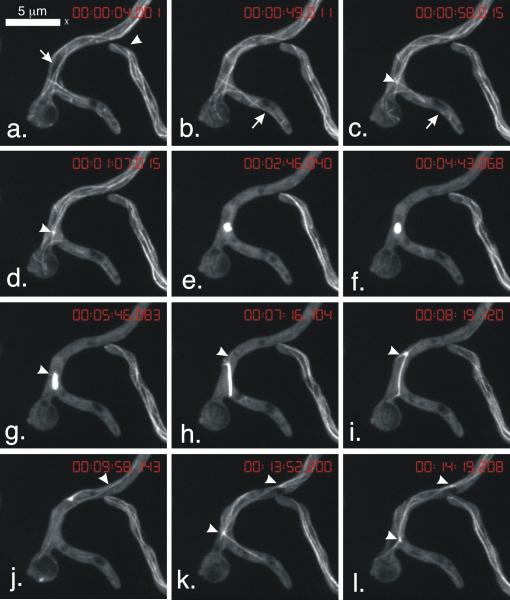
We created strain LO1052, a diploid homozygous for the GFP-TUBA (α-tubulin) fusion previously constructed by Han et al. (2001). Diploid strains are larger than haploid strains and have larger mitotic apparatuses, which facilitated our observations. It grew at rates comparable to tubA+ control diploids, indicating that the GFP-TUBA fusion supported normal growth. We were able to capture four-dimensional (Z-series stacks collected at time intervals) images of hyphae passing through the entire mitotic process. The data set shown in Video 1 and Fig. 1 illustrates our major findings. In prophase, MTs disassembled from their ends and also severed into fragments that subsequently disassembled (Fig. 1b,c). Disassembly of cytoplasmic MTs was rapid (Fig. 1a-e) and the mitotic spindle began to assemble well before the completion of the disassembly of the cytoplasmic MTs (Fig. 1c,d). Microtubule fragmentation and severing has been studied in animal cells and extracts. The key enzymes are a group of related AAA ATPase proteins that also play a role in the proliferation of MTs in plant cells and in flagellar excision (Vale, 1991; McNally and Vale, 1993; McNally, 1998, additional data reviewed in Roll-Mecak and McNally, 2010). Genes encoding AAA ATPase proteins are present in A. nidulans and it would be interesting to determine if any of them is involved in microtubule severing.
Fig. 1.
Microtubule dynamics in mitosis. Images are from Video 1 and are of strain LO1052. Each image is a maximum intensity projection of a Z-series stack. The time of completion of acquisition of each Z-series stack is shown at the top of each panel (hr:min:sec:msec). Note that internuclear distances are greater in diploid hyphae than haploid hyphae. In a haploid hypha there would be more than one mitotic nucleus in the field of view. a. Arrow shows a space of reduced tubulin fluorescence that is the nucleus that will go into mitosis. Arrowhead shows an interphase hypha. Note microtubule dynamics at tip. b,c. Arrow shows a microtubule that severs and disassembles leaving a gap in the formerly continuous microtubule. c,d. Arrowhead shows the mitotic spindle as it initially begins to form. e-i. The mitotic spindle is initially lobed but becomes a short rod that later extends into a long, thinner and somewhat flexible rod. g,h. Arrowheads show short astral MTs. i. In late anaphase the astral MTs become longer. j. An astral microtubule is adjacent to a microtubule from the adjacent spindle (out of field of view). Arrowhead shows point of intersection. k,l. Astral MTs extend from former spindle poles and apparently interact with each other.
The mitotic spindle initially appeared to be lobed (Fig.1e), probably reflecting the fact that two half spindles were in the process of fusing into a bipolar spindle. It became a short rod (Fig. 1f) and then began to elongate (Fig. 1g). As it elongated, very short astral MTs assembled (Fig. 1g,h), but they disassembled immediately (Video 1), obviously undergoing catastrophe. As the spindle lengthened, the catastrophe frequency appeared to decrease resulting in longer astral MTs (Fig. 1i). These data indicate that astral microtubule growth is limited by catastrophe frequencies rather than microtubule nucleation. As mitosis proceeded, the mitotic spindle disassembled and astral MTs grew and appeared to interact with MTs growing in from adjacent nuclei (Fig. 1j). In early G1 nuclei, the former poles (i.e. spindle pole bodies) nucleated a robust array of cytoplasmic MTs (Fig. 1k,l) with MTs from the two spindle pole bodies apparently interacting.
We also observed robust microtubule dynamics at the tip of a hypha next to the mitotic hypha. MTs did not come to a particular focus but probed the tip area, continually growing and shrinking.
Finally, it is worth noting that there is considerable variation in mitosis and microtubule dynamics in filamentous fungi. Unlike A. nidulans, which has a “semi-open” mitosis (De Souza et al., 2004) Neurospora crassa, for example, has a “closed mitosis and cytoplasmic MTs do not disassemble when nuclei undergo mitosis (Roca et al., 2010).
Methods
Image acquisition with a spinning disk confocal microscope was as previously described (Horio and Oakley, 2005) except that a 60X 1.42 N.A. Planapochromatic objective was used. LO1052 is a vegetative diploid. The genotype of one parent was GFP-tubA, sC12, pabaA1, cnxE16, wA2 and the other was GFP-tubA, pyrG89, pabaA1, choA1, fwA1.
Supplementary Material
Video Legend
Video 1. See captions for Fig. 1.
Highlights.
Microtubule severing and fragmentation occur in early mitosis in A. nidulans.
Microtubule catastrophe limits astral microtubule growth in mitosis.
Reduced catastrophe in telophase and G1 allows astral microtubule growth.
Acknowledgments
We thank Dr. Stephen Osmani for the use of his confocal system. Supported by grant GM031837 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Further Reading
- Bergen LG, Morris NR. Kinetics of the nuclear division cycle of Aspergillus nidulans. J. Bacteriol. 1983;156:155–160. doi: 10.1128/jb.156.1.155-160.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr. Biol. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Han G, Liu B, Zhang J, Zuo W, Morris NR, Xiang X. The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 2001;11:719–724. doi: 10.1016/s0960-9822(01)00200-7. [DOI] [PubMed] [Google Scholar]
- Horio T, Oakley BR. The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans. Mol. Biol. Cell. 2005;16:918–926. doi: 10.1091/mbc.E04-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- McNally F. Purification and assay of the microtubule-severing protein katanin. Meth. Enzym. 1998;298:206–218. doi: 10.1016/s0076-6879(98)98020-x. [DOI] [PubMed] [Google Scholar]
- Roca MG, Kuo HC, Lichius A, Freitag M, Read ND. Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa. Eukaryot. Cell. 2010;9:1171–1183. doi: 10.1128/EC.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr. Opin. Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991;64:827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Legend
Video 1. See captions for Fig. 1.