Abstract
Dietary restriction (DR) and reduced reproduction each extend life span in many species. Females undergoing DR typically experience a reduction in their fecundity, which raises the question of whether the two treatments are actually extending life span in overlapping ways. Life span in lubber grasshoppers has been shown to be increased by DR, and separately by ovariectomy (OVX). Here, we test the combination of these on life span. If life extension by the two treatments are additive, it would suggest that they likely act through separate pathways. The experimental groups were: fully reproductive and fully fed (ShamFD); ovariectomized and fully fed (OVX FD); fully reproductive and restricted diet (ShamDR); and ovariectomized and restricted diet (OVX DR). The median life spans of these groups were: ShamFD = 245 d, OVX FD = 285 d, ShamDR = 286 d, and OVX DR = 322 d. Feeding rate for the OVX FD group was 64% of ad libitum, not significantly different from the 70% of ad libitum that was used for ShamDR. We also measured hemolymph parameters of physiology in these same individuals. Hemolymph levels of vitellogenin (the egg yolk-precursor protein) were increased 5-fold by OVX, but were not affected by DR. In addition, hemolymph total anti-oxidant activity (per μg protein) was significantly reduced by OVX, but was not affected by DR. We show that OVX and DR produce different physiological responses in grasshoppers, despite life extensions and feeding levels that were not significantly different. These data suggest that OVX and DR might extend life span via distinct pathways.
1. Introduction
Dietary restriction (DR) and reduced reproduction each extend life span in many animals, but the relationship between the two treatments is unclear. Many animals undergoing DR also experience a reduction in their fecundity (as reviewed in Partridge et al. 2005; detailed in Carey et al. 2008). This has led to the question of whether the two treatments are actually extending life span in similar ways (e.g., Crawford et al. 2007). This can be addressed in part via a fully factorial experiment that tests whether animals subjected to both DR and directly reduced reproduction live longer than animals on either treatment alone. If the life extensions due to DR and reduced reproduction are additive, it would suggest that they act through separate mechanisms. This conclusion could be supported further by different physiological responses to DR and reduced reproduction, addressing several of the concerns laid out by Gems et al. (2002).
The fact that DR typically reduces fecundity implies that life extension via either treatment may be due simply to reduced reproduction. For instance, in C. elegans, removal of the germline stem cells nearly doubled life span, and applying DR to worms with the germline ablated did not further extend life span. The authors conclude the life extension pathways of DR and reduced reproduction are at least partly overlapping in worms (Crawford et al. 2007).
On the other hand, some studies have concluded that life span extension by DR does not act through reduced reproduction (e.g., Mair et al. 2004; reviews in Barnes and Partridge 2003; Koubova and Guarente 2003; Kenyon 2005; Partridge et al. 2005). In Drosophila melanogaster, DR was combined with experimentally suppressed reproduction. The suppression of reproduction was accomplished in three ways: via X-irradiation, which sterilized the germline; segregating females from males to remove the stress of mating; or by the ovoDI mutation, which blocks ovarian development and vitellogenesis. Dietary restriction extended the life span of all groups of female flies regardless of experimental manipulation of reproduction. This suggests that life span extension via DR in female D. melanogaster is not directly linked to reproductive activity (Mair et al. 2004).
While many studies have manipulated diet and measured fecundity, few studies have manipulated reproduction and measured individual feeding rates (similar to Shimokawa et al. 2003 for dietary restriction and growth hormone knockdown in mice). Measuring ingestion in fruit flies is contentious (but see Lee et al. 2008), yet it is important because of the possibility of compensatory feeding on a low quality diet (Carvalho et al. 2005; Mair et al. 2005; Min et al. 2006a,b). Measurements of ingestion rate in C. elegans have never been published, to our knowledge. In contrast to fruit flies or worms, the relatively large size of our experimental model, the lubber grasshopper, makes measuring individual feeding levels possible. This allows observations on the associations among food consumption, reproduction, and life span, while simultaneously tracking physiological responses in the hemolymph. Here, we address whether dietary restriction and reduced reproduction extend life span via overlapping pathways in grasshoppers.
In previous work, DR greatly extended life span in lubber grasshoppers, without changing life-time fecundity (Hatle et al. 2006). Further, DR (70% of ad libitum feeding) had no significant effect on storage protein levels in the hemolymph, a major depot for reproduction in this phytophagous insect (Hatle et al. 2001). However, the study design in Hatle et al. (2006) did not allow oviposition of eggs into suitable substrate, and this may have affected the physiology of the females.
In a separate study, ovariectomy extended life span in grasshoppers by ~22% without any difference in the amount of food ingested (Hatle et al. 2008). As with the grasshopper DR study, this design did not allow females access to oviposition substrate, which may have affected the physiology of controls. In contrast to other aging models, ovariectomy in grasshoppers only prevents the formation of eggs. Specifically, the egg yolk-precursor protein vitellogenin is produced, but because the ovary is missing, the vitellogenin accumulates to high levels in the hemolymph (Hatle et al. 2003). Hence, ovariectomy in grasshoppers permits the allocation of a limiting nutrient (viz., protein) to an early stage of reproduction. In concert with the increased life span upon ovariectomy, levels of hemolymph storage proteins were significantly higher within old ovariectomized females (Hatle et al. 2008). This is different from the lack of response of storage proteins to DR, and these contrasting results suggest that DR and ovariectomy may have different effects on the physiology of females.
In the present study, we address whether life extensions via DR and reduced reproduction act through similar mechanisms. In particular, DR and ovariectomy are combined to test whether the increased longevity due to the two treatments is additive. Feeding rates are simultaneously measured to determine whether they play a role in life extension upon reduced reproduction. Further, total anti-oxidant activity in the hemolymph and vitellogenin are measured to address whether these physiological responses to DR and ovariectomy are the same.
2. Methods
2.1. Surgery, Diet, and Data Collection
Juvenile Romalea microptera were obtained from Miami, FL, USA as in Hatle et al. (2008) and were kept en masse and fed ad libitum Romaine lettuce. Adult females were separated and serially assigned to either a fully fed, ad libitum diet (FD) or a restricted diet (DR). In most animals that have been tested, life span increases until the point of starvation (~35% ad libitum), and an effective, life-extending quantity of DR is ~70% of ad libitum (Weindruch and Walford 1988; Hatle et al. 2006). Hence, our DR treatment was 70% that of the amount eaten daily by the FD group. Approximately every 10 d, the Romaine lettuce left uneaten from the previous day by the FD individuals was collected and dried overnight at 55°C. A conversion for dry mass from wet mass was calculated from known amounts of fresh lettuce and used to determine average amount eaten daily (Hatle et al. 2006). Within these dietary regimens, females were serially assigned to ovariectomy (OVX), or a control operation (Sham), leading to a fully factorial 2×2 design of Sham FD, OVX FD, ShamDR, and OVX DR (n=40 per group). All four groups were fed their dietary regimen daily and kept in individual ventilated containers of approximately 500 cm3 on a 14L: 10D photoperiod at 32°C during the day and 24°C at night. The study was ter minated at 365 d.
Ovariectomies and sham (control) operations were performed on the first day after adult molt as previously (Hatle et al. 2003). Starting at approximately age 30 d, all sham and ovariectomized individuals were placed on sand two times a week to allow for oviposition of eggs (Hatle et al. 2001). Any females that attempted to probe into the sand and lay eggs were left undisturbed overnight at room temperature. Clutches of eggs were collected and total number of eggs, clutch number, and age at oviposition were recorded. Previous work has shown that reduced diet has little or no effect on egg size (Moehrlin and Juliano 1998). At time of death, any melanization of the ovaries was recorded. Melanization is an immune response to infection; in the ovary, it causes a black, hardened growth which encloses the ovarioles and unlaid eggs and prevents further oviposition (Beckage 2008).
2.2. Hemolymph total anti-oxidant activities and vitellogenin levels
For about one-quarter of the animals in the study, hemolymph samples were collected monthly from about four months until the end of the experiment, using established methods (e.g., Hatle et al. 2006). For anti-oxidant activity, a 1 μl sample was flash frozen in liquid nitrogen and stored at −20°C for later assay (Judd et al. 2010). Activity was measured as the reduction of trolox in an aqueous buffer (after Re et al. 1999, see also Williams et al. 2008), so this assay detects only water-soluble anti-oxidants. For vitellogenin measurement, a 5 μl hemolymph sample was placed in 250 μl of phosphate buffered saline and stored at −20°C an d later assay by ELISA as described (Borst et al. 2000; Hatle et al. 2001; 2004; 2008).
2.3. Statistics
The amount of food eaten at each sampling date for FD groups (Sham and OVX) was compared by a MANOVA with time as a dependent variable (a type of repeated measures analysis). Similarly, for all groups, body masses of each individual over time were compared with a MANOVA. Vitellogenin levels were also tested using two-way MANOVA with time as a dependent variable, while total anti-oxidant activities were tested with a two-way ANOVA. The assumptions underlying all MANOVA and ANOVA analyses were not tested, but the relatively large sample sizes for most analyses in this study means they are nonetheless likely to be robust. Further, we used the test statistic for MANOVA (i.e., Pillai’s Trace) that is the most robust for deviations from the assumptions (Scheiner 1993).
For Sham reproductive data, MANOVA was used to assess eggs per clutch and time between clutches. Student’s t-tests were performed for total fecundity (cumulative number of eggs), average eggs per clutch, average time between clutches, and average number of clutches per group. Because multiple t-tests were used, a Bonferroni correction of alpha was used, which was 0.05/4 = 0.0125. The rates of melanization were compared using an r x c chi-squared test for association with one degree of freedom (Holmes et al. 2006). All MANOVAs were performed in SAS (SAS Institute 2009) and all t-tests were done in Microsoft Excel®. An estimate of Lifetime Reproductive Effort (as in Charnov et al. 2007) was calculated using the following formula: (clutches) x (clutch size) x (average adult life span) x (offspring mass at independence)/(adult mass at first reproduction).
Survivorship curves were analyzed using Cox proportional hazards models implemented with Proc PHREG in SAS (SAS Institute 2009). The proportional hazards model is the preferred method in survival analysis, and it can tolerate censored individuals (Cox 1972; Allison 2010). Important for testing our questions, proportional hazards models allow testing of a possible interaction of diet and surgery. A significant interaction would indicate that the relationship differs significantly from an additive response (e.g., it is either synergistic or antagonistic). In contrast, a non-significant result would suggest a mathematically additive response to the two treatments. Maximum likelihood estimates approximate the contribution of the treatment to the increase in life span, with more positive numbers representing an increasing risk of mortality compared to controls (Wells and Eissenstat 2001; Allison 2010).
3. Results
3.1. Feeding and body masses
The amounts of Romaine lettuce eaten by both FD groups were not significantly different (all P>0.07) until after the first clutch of eggs was laid, at approximately 41 d (Fig. 1). From 50 d to 130 d, individuals in the ShamFD group each consumed ~4–6 g of lettuce each day, and the individuals in the OVX FD group each consumed approximately 70% of the average eaten by the ShamFD group (all P<0.02). From 131 d to 225 d, the members of the OVX FD group consumed roughly 50% of that eaten by the members of the ShamFD group. After 225 d, the amounts eaten by individuals within each group did not change noticeably and were held constant until the end of the study at 365 d, but were not measured any further due to diminishing sample sizes. Up to 330 d, 89% of the DR meals offered were consumed completely.
Figure 1.
Amount of Romaine lettuce (wet g) eaten daily by each group of adult female grasshoppers. Throughout the study (relative to the amount eaten by the ShamFD group), the ShamDR consumed 70%, the OVX FD group consumed 64% of ad libitum, and the OVX DR group consumed 44% of ad libitum.
All of the individuals in the study started adulthood at approximately the same mass (Fig. 2) and increased in mass until the first clutch. After 50 d, body masses wavered slightly, more so in Sham than OVX groups due to production and oviposition of eggs, but remained relatively constant throughout the study. There was a difference in mass due to diet (F3,104=3.31; P<0.001). In a separate study, the wet gut masses (containing unabsorbed food) of females on ad libitum diets were about 0.5 g heavier than gut masses for females on restricted diets (Macdonald AL, Hatle JD, unpublished data). Because the body mass differences after two weeks are all greater than 0.5 g, it seems likely they were true differences in somatic mass, and not due merely to undigested food in the gut. In addition, there was a significant effect of OVX (F3,104=6.98; P<0.001) but there was no interaction of DR and OVX on body mass over time (F3,104=1.22; P=0.25). ShamFD and OVX FD had body masses that did not differ significantly throughout the study (all P>0.79); however, ShamDR had lesser body masses than OVX DR for a lengthy amount of time (all P<0.04 for 45 d to 90 d and 95 d to 135 d).
Figure 2.
Body masses of each group of adult female grasshoppers show both FD groups with masses that were not significantly different throughout the study (P>0.79). ShamDR had less mass than OVX DR for a considerable amount of time (all P<0.04 for 45 d to 90 d and 95 d to 135 d).
3.2. Reproduction
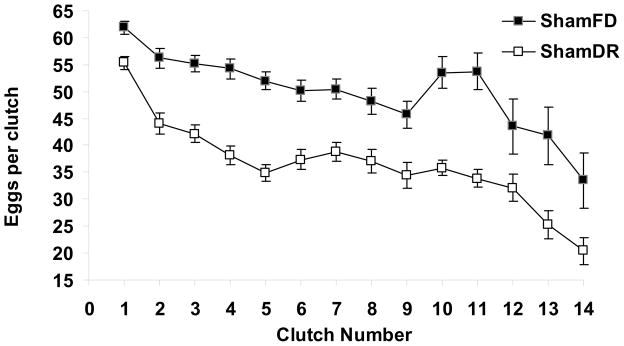
Both ShamFD and ShamDR groups were allowed to produce and oviposit eggs; therefore reproduction was measured by several variables (Table 1). The only reproductive tactic that consistently differed significantly due to diet was the number of eggs per each clutch (MANOVA run through clutch 11; n=42; all P<0.05), with ShamFD ovipositing ~30% more eggs per clutch than ShamDR (Fig. 3). The number of eggs per clutch declined significantly with time (Pillai’s Trace F10,31=11.79; P<0.0001). For the time between each clutch, MANOVA shows that only 4 out of 12 interclutch intervals were significantly longer for ShamDR (4: P=0.003; 5: P=0.004; 8: P=0.032; 9: P=0.003) while the remaining eight out of 12 interclutch intervals were not significantly affected by diet (all P>0.08; Fig. 4). Cumulative fecundity, number of clutches, rates of ovarian melanization, and lifetime reproductive effort were not significantly different (Table 1).
Table 1.
Cumulative reproductive data (mean ± SE) upon dietary restriction in grasshoppers. ShamFD is fully reproductive, fully fed; ShamDR is fully reproductive, dietary restricted. The only consistent effect of DR on reproductive tactics was the reduction in the size of each clutch. Eggs/Clutch is the grand mean of the average number of eggs per clutch for each individual. Time/Clutch is the grand mean of the average time between clutches of eggs for each individual. Melanization is an immune response in which a hardened growth begins to encase the ovaries (Beckage 2008); counts are for presence/absence. LRE is Lifetime Reproductive Effort (Charnov et al. 2007); clutch size, average adult life span, and adult mass were the factors that impacted these scores.
| Number of Eggs | Number of Clutches | Eggs/Clutch | Time/Clutch | Melanization | LRE | |
|---|---|---|---|---|---|---|
| ShamFD | 440 ± 36.5 | 9.73 ± 0.58 | 48.3 ± 1.5 | 19.0 ± 0.61 | 19 out of 40 | 1.9 |
| ShamDR | 381 ± 30.8 | 10.90 ± 0.79 | 37.4 ± 1.2 | 20.3 ± 0.65 | 12 out of 40 | 2.1 |
| P = 0.22 | P = 0.32 | P < 0.001 | P = 0.14 | P = 0.17 |
Figure 3.
Number of eggs per clutch oviposited by grasshoppers on dietary restriction and on a full diet. MANOVA suggests that ShamFD laid more eggs per clutch on all clutches (all P<0.05).
Figure 4.
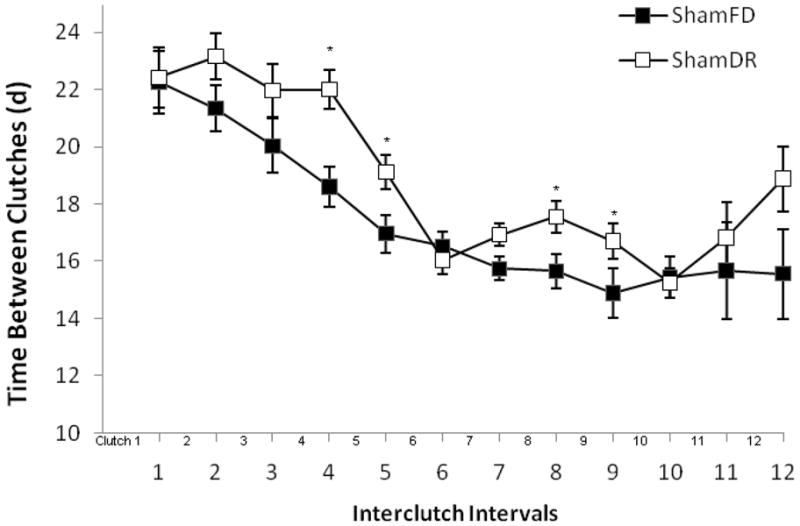
Cumulative time between clutches in grasshoppers on dietary restriction was increased at only 4 of 12 interclutch intervals. The grand mean of interclutch intervals was not significantly different from grasshoppers on full diets (P=0.14).
3.3. Life span and survivorship
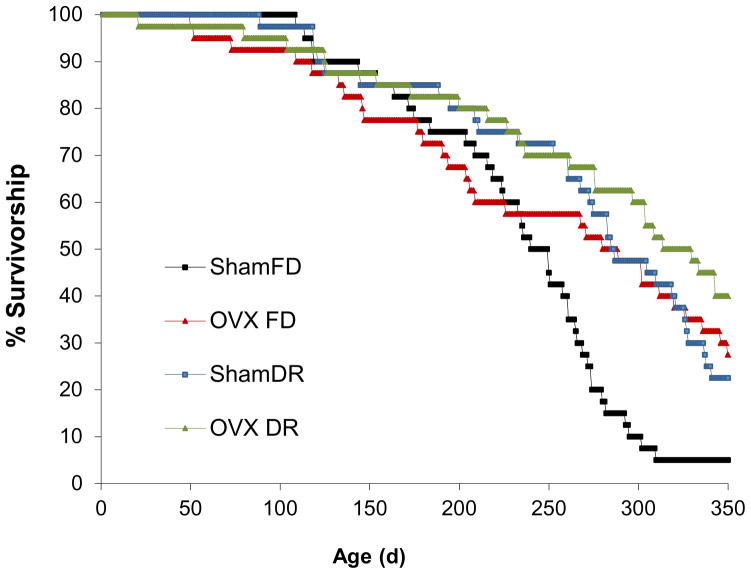
The study was carried out until day 365, when the control group, ShamFD, had 5% of the initial individuals remaining (Fig. 5). The three other treatment groups had more individuals alive at 365 d (OVX FD = 27%; ShamDR = 15%; OVX DR = 40%). Median life spans were: ShamFD = 245 d, OVX FD = 285 d, ShamDR = 286 d, and OVX DR = 322 d. Proportional hazards models revealed an effect of DR upon survivorship (χ2=11.73; P=0.0006) and an effect of OVX upon survivorship (χ2=8.60; P=0.0034). There was no interaction of DR and OVX (χ2=0.599; P=0.4388), implying an additive relationship between the two treatments. The maximum likelihood parameter estimates suggest that the strength of the effects of DR (−0.682) and OVX (−0.779) were similar.
Figure 5.
Survivorship curves for grasshoppers. ShamFD is the fully reproductive, fully fed control; OVX FD is ovariectomized and fully fed; ShamDR is fully reproductive and dietary restricted; OVX DR is ovariectomized and dietary restricted.
3.4. Total anti-oxidant activity and vitellogenin levels in the hemolymph
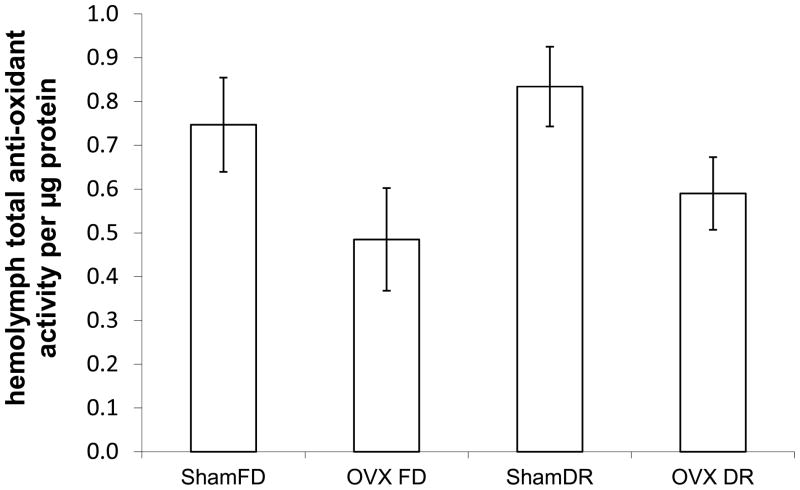
Hemolymph total anti-oxidant activities (divided by the amount of protein in the sample) were significantly decreased by OVX (two-way ANOVA; F3,34=6.33; P=0.017; Fig. 6). Anti-oxidant activities were affected by neither DR (F3,34=0.91; P=0.35) nor the interaction of OVX and DR (F3,34=0.01; P=0.93). This analysis of anti-oxidant activites was run only with samples from median age 192 d. Repeated measures analysis requires a complete set of samples for any individual included in the test. Due to lost samples, we had a limited number of individuals with a complete set of samples. Hence, we focused on the age at the beginning of increased mortality in the ShamFD group, namely 192 d. Examination of the data indicated that there were no obvious trends in anti-oxidant activity over time, supporting our conclusion that total anti-oxidant activities are lower upon ovariectomy.
Figure 6.
Hemolymph total anti-oxidant activities (as mM trolox equivalents) at 192 days of adult female grasshoppers. ShamFD is the fully reproductive, fully fed control; OVX FD is ovariectomized and fully fed; ShamDR is fully reproductive and dietary restricted; OVX DR is ovariectomized and dietary restricted. Ovariectomy significantly reduced the anti-oxidant activity per microgram of protein, whereas DR (i.e., 70% of that consumed by the respective full diet group) did not affect anti-oxidant activity.
As seen in previous experiments, vitellogenin levels were increased 5- to 10-fold by OVX (MANOVA; all four days had P<0.01; Fig. 7). In contrast, the level of DR used in this study did not significantly reduce vitellogenin levels (all four days had P>0.35). There was a mild effect of time (Pillai’s Trace F3,19=3.20; P=0.0468), likely due to higher levels at median day 112 than at median day 77. No other interactions were significant (all P>0.07).
Figure 7.
Levels of vitellogenin (from the hemolymph) of adult female grasshoppers. ShamFD is the fully reproductive, fully fed control; OVX FD is ovariectomized and fully fed; ShamDR is fully reproductive and dietary restricted; OVX DR is ovariectomized and dietary restricted. Ovariectomy increased vitellogenin levels at least 5-fold, whereas DR (i.e., 70% of that consumed by the respective full diet group) did not affect vitellogenin levels.
4. Discussion
4.1. Lifetime fecundity was not significantly altered by dietary restriction
The rate of reproduction was reduced through DR by decreasing the amount of eggs per clutch. However, on average the ShamDR group lived longer than the ShamFD group, and they laid one more clutch throughout their lifetime, which resulted in similar lifetime fecundity compared to the ShamFD group. This is consistent with evolutionary theory, which suggests that delaying reproductive output results in a longer life span, which in turn may allow greater reproductive output later in life (Kirkwood 2002).
4.2. Reduced diet and reduced reproduction may act through separate pathways
Life extension via DR and OVX are additive in lubber grasshoppers. Each treatment increases median life span (by 16 or 17%), but females subjected to both treatments increased life span by approximately 31%. This is particularly intriguing given the fact that the OVX females given free access to food consumed a similar quantity to that offered to (and consumed by) the Sham-DR females (see section 4.4). These life span data suggest that DR and OVX may act through different pathways.
Our physiological data further supports the conclusion that DR and OVX produce different effects, and could be extending life span by separate pathways. Vitellogenin levels (this paper and Hatle et al. 2008) and fat body masses (Judd et al. in review) both show responses to OVX that are not matched upon DR. Vitellogenin levels are elevated 5-fold upon ovariectomy, indicating a clear physiological difference.
Fat body in insects serves as fat storage, but also as the organ of intermediary metabolism (analogous to the vertebrate liver) and the site of synthesis of vitellogenin and hexameric storage proteins. Fat body masses can change 2-fold during the cycle of egg production in grasshoppers (Hatle et al. 2006), indicating that fat body growth and reduction can be normal processes during insect development (see also Liu et al. 2009). Upon OVX, fat body masses are doubled (Judd et al. in review). Fat body mass seems unlikely to increase upon DR. Indeed, our pilot data indicate that fat body mass is at least 2-fold higher in OVX females than in diet-matched sham females (Macdonald AL, Kellenberger JW, Viray E, Hatle JD, unpublished data). Hence, both vitellogenin levels and fat body size indicate large magnitude changes in physiology upon OVX that are not matched upon DR.
In C. elegans, reduced reproduction can extend life span and affect fat metabolism, similar to grasshoppers. In worms, reduced reproduction via ablation of the germline stem cells makes the animal leaner and long-lived (Wang et al. 2008). In contrast, ovariectomy in grasshoppers makes the animal fatter and long-lived. Wang et al. (2008) implicated induction of a lipase in decreasing fat stores in worms, and further showed that greater expression of this lipase (without germline ablation) also produces lean, long-lived worms. In mice, a DR regime in which food is provided only once daily resulted in elevated fat metabolism ~15 hr per day (Bruss et al. 2010). The fat hypertrophy observed in ovarectomized grasshoppers and ovarian knockout fruit flies (Flatt et al. 2005) could provide a good comparative model for understanding the roles of lipase in longevity.
We tested the activity of soluble anti-oxidants from the hemolymph. Most reactive oxygen species are produced in the cell, particularly during the electron transport chain (e.g., Dowling and Simmons 2009). Water-soluble anti-oxidants outside of the cells would not be able to attack these intracellular species. Our rationale for measuring hemolymph anti-oxidant activity was three-fold. First, activities (per microgram protein) were higher in the hemolymph than in either the femur muscle or the fat body in a previous study in this grasshopper (Judd et al. 2010). Second, grasshoppers have discontinuous ventilation of the spiracles to allow air into the trachea for gas exchange (e.g., Harrison 1997), and this system may serve to minimize oxygen toxicity (Hetz and Bradley 2005). This suggests that protection from reactive oxygen species in the hemolymph may be important in insects. Last, the hemolymph can be sampled nondestructively, allowing us to measure both longevity and the level of maintenance in the same individuals.
Our anti-oxidant activity data suggests that OVX decreases the potency of the combination of all hemolymph proteins in protecting from reactive oxygen species. Because anti-oxidation systems are still thought to play some role in life extension (e.g., Williams et al. 2008; although perhaps not the central role previously held: Jang et al. 2009; Zhang et al. 2009), this difference between the DR and OVX groups may be particularly important. While neither vitellogenin levels nor fat body masses are known to be related to life span (other than the special case of vitellogenin in some social insects [e.g., Amdam et al. 2004; Seehuus et al. 2006]), anti-oxidant activity has been shown to be associated with longevity in many model organisms (e.g., Muller et al. 2008). However, we see a different pattern in OVX grasshoppers, with life span increasing but anti-oxidant activity decreasing.
The direction of this difference in grasshoppers is likely due to the high levels of vitellogenin in the hemolymph of OVX females. Ovariectomized females had a higher percentage of total hemolymph protein as vitellogenin, yet these individuals had lower total anti-oxidant activity. This suggests that vitellogenin from this grasshopper is not a particularly effective anti-oxidant. While this differs from the situation in honey bees (Seehuus et al 2006), the evolutionary explanation for vitellogenin acting as an anti-oxidant in bees rests on the social structure of bees. Lubber grasshoppers are gregarious, but they are not social. Whether the anti-oxidant capability of vitellogenin co-varies with social structure across many insect species remains to be seen.
Taken together, our data on life span, vitellogenin levels, fat body masses, and total anti-oxidant activities in the hemolymph indicate that the physiological responses to DR and OVX are quite different. This in turn suggests the two treatments are extending life span via distinct mechanisms, or at least mechanisms that do not overlap completely. Our results are similar to those in flies, in which life extension via several means of reduced reproduction was further increased when combined with DR (Mair et al. 2004).
4.3. Possible pathways
We have not directly addressed the mechanistic pathways of life extension via DR and OVX. Insulin/insulin-like growth factor (IGF) signaling plays a role in extending life span in both worms (Arantes-Oliveira et al. 2002) and flies (Flatt et al. 2008) with reduced reproduction. In contrast, IGF signaling does not seem to contribute to life extension with DR in either worms (Houthoofd et al. 2003) or flies (Min et al. 2008). Hence, this pathway could in part underlie the different mechanisms of DR and OVX in grasshoppers.
4.4. Feeding rates are reduced upon ovariectomy
Feeding rates in animals manipulated to directly reduce reproductive output are rarely quantified in studies on aging. For Drosophila, when feeding rates are measured, the results often are contentious (e.g., Min et al. 2006a; Piper et al. 2007). Measuring feeding rates allows a more explicit examination of the relative roles of DR and reproduction in life extension (e.g., Tatar and Carey 1995).
On their own, the feeding and life span data could be taken to suggest that reducing reproduction does not extend life span in its own right, but instead only through reducing feeding rate. Females in the OVX FD group ate ~64% of that eaten by females in the ShamFD group. This feeding level is strikingly similar to the reduction in diet that is typically used for life extending DR and to the level of reduction in feeding rate in growth hormone knockdown mice (Shimokawa et al., 2003). The levels of reproductive investment in the ShamDR and OVX FD groups were quite different; vitellogenin levels in the OVX females are very high, but at most it is similar to five clutches, whereas the ShamDR group laid over 10 clutches. At the same time, the median life spans of the ShamDR and OVX FD groups differed by only one day (0.35%).
The life span and relative feeding rate of the OVX DR group also seem to imply that feeding rate, and not reproduction, is most important life extension. The OVX DR group ate only ~70% of that consumed by the OVX FD group, or ~45% of that consumed by the control (viz., ShamFD). Evidence from other organisms shows an increase in survivorship with reductions in feeding levels down to 35% of ad libitum (e.g., Weindruch and Wallford 1988; Koubova and Guarente 2003). Hence, the feeding and life span data alone make it appear that DR and OVX were additive not because surgery and feeding level act through different mechanisms, but that OVX freely reduced the feeding rates. This interpretation is supported by the significant correlation between age at death and average amount of food consumed daily, without regard for surgery (r=−0.46; P<0.001). However, the massive physiological differences between these treatments lead to a different conclusion. Together, this serves as a caution for making conclusions based only on reproductive and feeding rates (e.g., Tatar and Carey 1995). Our ongoing studies are aimed at comparing the physiology of sham and OVX groups with matched feeding levels.
Highlights.
Dietary restriction and ovariectomy each extend life span and reduce feeding.
Life extension via dietary restriction and ovariectomy are additive in grasshoppers.
Ovariectomy increases vitellogenin, but dietary restriction does not.
Ovariectomy decreases anti-oxidant activity, but dietary restriction does not.
Dietary restriction and ovariectomy may act via distinct pathways.
Acknowledgments
We thank Matthew Gilg, Daniel Moon, and members of the Hatle lab for critical comments, Kim Nguyen and Evan Judd for help with feeding grasshoppers, Jake Williams for insightful conversation, and Steven Juliano for help with statistics. Funding was provided by NIA grants 1 R15 AG028512-01 and 2R15AG028512-02A1 to JDH, and Orthopterists Society and UNF Graduate School awards to MDD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison PD. Survival Analysis Using SAS®: A Practical Guide. 2. SAS Institute Inc; Cary, NC: 2010. [Google Scholar]
- Amdam GV, Simões ZLP, Hagen A, Norberg K, Schrøder K, Mikkelsen Ø, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp Gerontol. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of lifespan by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Barnes AI, Partridge L. Costing reproduction. Anim Behav. 2003;66:199–204. [Google Scholar]
- Beckage NE. Insect Immunology. Elsevier; San Diego, CA: 2008. [Google Scholar]
- Borst DW, Eskew MR, Wagner SJ, Shores KM, Luker LA, Hatle JD, Hecht LB. Quantification of juvenile hormone III, vitellogenin, and vitellogenin-mRNA during the oviposition cycle of the lubber grasshopper. Insect Biochem Mol Biol. 2000;30:813–819. doi: 10.1016/s0965-1748(00)00053-9. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation. Am J Physiol Endocrinol Metab. 2010;298:E108–E116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Mőller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nature Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL, Warne R, Moses M. Lifetime reproductive effort. Am Nat. 2007;170:E129–E142. doi: 10.1086/522840. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in life span determination. Aging Cell. 2007;6:715–721. doi: 10.1111/j.1474-9726.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Dowling D, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc Royal Society B. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ line modulation of insulin signaling and lifespan. Proc Natl Acad Sci. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Gems D, Pletcher S, Partridge L. Interpreting interactions between treatments that slow aging. Aging Cell. 2002;1:1–9. doi: 10.1046/j.1474-9728.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- Harrison J. Ventilatory mechanism and control in grasshoppers. Amer Zool. 1997;37:73–81. [Google Scholar]
- Hatle JD, Andrews AL, Crowley MC, Juliano SA. Interpopulation variation in developmental titers of vitellogenin, but not storage protein, in lubber grasshoppers. Physiol Biochem Zool. 2004;77:631–640. doi: 10.1086/420946. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Eskew ME, Juliano SA. Maximum titers of vitellogenin and total hemolymph protein occur during the canalized phase of grasshopper egg production. Physiol Biochem Zool. 2001;74:885–893. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Juliano SA, Borst D. Hemolymph ecdysteroids do not affect vitellogenesis in the Lubber Grasshopper. Arch Insect Biochem Physiol. 2003;52:45–57. doi: 10.1002/arch.10067. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Paterson CS, Jawaid I, Lentz C, Wells SM, Fronstin RB. Protein accumulation underlying life span extension via ovariectomy in grasshoppers is consistent with the disposable soma hypothesis but is not due to dietary restriction. Exp Gerontol. 2008;43:900–908. doi: 10.1016/j.exger.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Wells SM, Fuller E, Allen IC, Gordy LJ, Melnyk S, Quattrochi J. Calorie restriction and late-onset calorie restriction extend life span but do not alter protein storage in female grasshoppers. Mech Ageing Dev. 2006;127:883–891. doi: 10.1016/j.mad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz S, Bradley TJ. Insects breathe discontinuously to avoid oxygen toxicity. Nature. 2005;433:516–519. doi: 10.1038/nature03106. [DOI] [PubMed] [Google Scholar]
- Holmes D, Moody P, Dine D. Research Methods for the Biosciences. Oxford University Press; Oxford, UK: 2006. [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signaling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Jang YC, Pérez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A: Biol Sci. 2009;64:1114–25. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd ET, Hatle JD, Drewry MD, Wessels FJ, Hahn DA. Allocation of nutrients to somatic tissues in young ovariectomized grasshoppers. Integ Comp Biol. 2010;50:818–828. doi: 10.1093/icb/icq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Mech Ageing Dev. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Nat Acad Sci. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Liu S, Wang S, Jiang RJ, Li S. Hormonal and nutritional regulation of insect fat body development and function. Arch Insect Biochem Physiol. 2009;71:16–30. doi: 10.1002/arch.20290. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:1305–1311. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Life span extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2006;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Moehrlin GS, Juliano SA. Plasticity of insect reproduction: testing models of flexible and fixed development in response to different growth rates. Oecologia. 1998;115:492–500. doi: 10.1007/s004420050546. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lastgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radical Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Piper M, Mair W, Partridge L. Comment by Matthew Piper, William Mair, Linda Partridge on Min, K.J., Flatt, T., Kulaots, I., Tatar, M., 2006 “Counting calories in Drosophila dietary restriction”. Exp Gerontol. 2007;42:253–255. doi: 10.1016/j.exger.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS version9.2. SAS Institute; Cary, NC: 2009. [Google Scholar]
- Scheiner SM. MANOVA: Multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. Chapman & Hall; New York: 1993. pp. 94–112. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Nat Acad Sci. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Tsuchiya T, Otani H, Komatsu T, Chiba T, Yamaza H. Lifespan extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. FASEB J. 2003;17:1108–1109. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- Tatar M, Carey JR. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosubruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- Wang M, O’Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Charles C. Thomas, Publisher; Springfield, IL: 1988. [Google Scholar]
- Wells CE, Eissenstat DM. Marked differences in survivorship among apple roots of different diameters. Ecology. 2001;82:882–892. [Google Scholar]
- Williams JB, Roberts SP, Elekonich MM. Age and natural metabolically-intensive behavior affect oxidative stress and anti-oxidant mechanisms. Exp Gerontol. 2008;43:538–549. doi: 10.1016/j.exger.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A: Biol Sci. 2009;64:1212–20. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]