Abstract
In Parkinson's disease (PD), the consequence of dopaminergic denervation is an imbalance in the activity of the direct and indirect striatofugal pathways, which include potentially important changes in opioid peptide expression and/or activity. The systemic administration of a novel glycosylated opioid peptide MMP-2200 (a.k.a. lactomorphin) was shown to have potent effects in two standard models of PD: 1) amphetamine-induced rotations in the hemi-Parkinsonian 6-hydroxydopamine (6-OHDA)-treated rat and 2) locomotion in the reserpine-treated rat. MMP-2200, an opioid mu and delta receptor agonist, reduced amphetamine-induced rotations in severely-lesioned hemi-Parkinsonian rats; this effect was fully blocked by naloxone, an opioid receptor antagonist. The selective δ-opioid receptor antagonist naltrindole only partially blocked the effect of MMP-2200. MMP-2200 alone did not induce rotations. This effect was also observed in a mild progressive rat 6-OHDA-lesion model. In animals treated with reserpine, profound akinesia was induced that was reversed with apomorphine. There was a prominent overshoot in animals that received apomorphine compared to non-reserpine treated animals, reflecting the well described phenomenon of dopamine supersensitivity indicating that apomorphine not only reversed akinesia but induced hyper-kinesia. The opioid peptide MMP-2200 blocked the apomorphine-induced hyper-kinesia. This effect of MMP-2200 was prevented by pre-administration of naloxone. MMP-2200 had no effect in preventing the reserpine-induced akinesia, nor did it affect locomotion in control animals. Taken together, the results from these two models are consistent with the glycopeptide opioid agonist MMP-2200 having a potent effect on movements related to dopaminergic hyper-stimulation following striatal dopamine depletion that are best explained by a reduction in the downstream effects of dopamine agonists in these models.
Keywords: 6-OHDA, reserpine, †-opioid receptor, μ-opioid receptor, akinesia, dopaminergic hyper-stimulation
1. Introduction
Parkinson's disease (PD) is a prevalent neurodegenerative disease characterized by a hypokinetic movement disorder with symptoms of tremor, rigidity, and bradykinesia (Olanow et al., 2009). These motor symptoms correspond to the loss of dopaminergic neurons with cell bodies located in the substantia nigra and axonal projections to the striatum. The most common treatment for PD consists of dopamine replacement therapy with levodopa and/or dopamine receptor agonists. These therapies become unsatisfactory as the disease progresses due to short- and long-term side effects that occur with dose escalation, including levodopa-induced dyskinesias (LID). Non-dopaminergic approaches may represent successful means to address motor complications of dopaminergic therapies (Olanow et al., 2009; Savitt et al., 2006).
To date, opioids have not found utility in the treatment of PD, but these compounds have profound effects on locomotion and reward behavior mediated by the basal ganglia. They can act as modulators of dopamine, GABA, and glutamate neurotransmission (Fox et al., 2006), and the striatum is rich in both μ- and δ-opioid receptors. Striatal neurons release the opioid peptides dynorphin and met-enkephalin as co-transmitters, and the levels of these peptides are greatly altered in PD (Samadi et al., 2006). The projection neurons of the indirect pathway express enkephalins derived from the precursor preproenkephalin A (PPE-A), while the neurons of the direct pathway express opioids derived from preproenkephalin B (PPE-B) (Cuello and Paxinos, 1978; Gerfen, 1992). Enkephalins derived from PPE-A are known to activate δ-opioid receptors and opioid peptides derived from PPE-B activate μ and κ subtypes of opioid receptors (Breslin et al., 1993; Seizinger et al., 1984), but the precise interactions with the opioid systems is unclear. After chronic levodopa-induced dyskinesia, both opioid peptide levels and the mRNA encoding their precursors are elevated in animal models of PD (Cenci et al., 1998; Duty and Brotchie, 1997; Engber et al., 1991; Henry et al., 1999). Postmortem studies of PD patients with motor fluctuations due to long-term levodopa use show increases in striatal PPE-A and PPE-B expression (Calon et al., 2002; Henry et al., 2003; Nisbet et al., 1995). These observations suggest that increases in enkephalin expression and release may be one of the mechanisms that could compensate for dopamine depletion in PD. Studies of opioid antagonists in MPTP-lesioned primates with dyskinesia have produced contradictory results. The non-subtype-selective opioid receptor antagonists, naloxone and naltrexone, have been variously reported to have had no effect (Gomez-Mancilla and Bedard, 1993), significantly reduced (Henry et al., 2001; Klintenberg et al., 2002) or exacerbated LID (Samadi et al., 2003). In humans low-dose oral naltrexone failed to show any effects (Rascol et al., 1994), whereas high-dose naltrexone showed minimal effects (Manson et al., 2001), neither exacerbating dyskinesia nor affecting Parkinsonian disability. A trial using i.v. infusion of 0.3 mg/kg/min naloxone, a dose known to block central opioid receptors, also failed to demonstrate any reduction in dyskinesia, but did show an extension in the duration of action of levodopa (Fox et al., 2004). This disparity has produced two conflicting concepts that endogenous opioids can either: a) cause dyskinesia, or b) be part of a homeostatic response to dopamine depletion that may be beneficial.
It is possible that the μ and δ-opioid neuronal systems have differing, complex, or opposing effects in Parkinson's disease. The δ-selective analogue SNC80 reversed drug-induced akinesia in rats treated with either reserpine or dopamine receptor antagonists (Hille et al., 2001).
Both SNC80 and the less selective, partial δ-opioid agonist tonazocine induced motor asymmetries in the unilateral 6-hydroxydopamine (6-OHDA) model (Hudzik et al., 2000). The δ-opioid agonist BW 373U68, which is structurally related to SNC80, induced motor asymmetries in rats that were pretreated with dopamine agonists (Childers et al., 1993). SNC80 was also found to have anti-Parkinsonian properties in a primate model (Hille et al., 2001). Given this background, novel opioid compounds with δ activity could provide an important nondopaminergic treatment for PD and other movement disorders. We therefore decided to test a novel glycopeptide compound with strong δ-opioid receptor activity and with mixed μ-receptor activity, expecting that the δ-opioid activity would reproduce the beneficial effects described in the studies described above. The mixed agonist properties of the test compound MMP-2200 had proven to be advantageous in studies of analgesia. Since opioid compounds with mixed δ and μ activity, had not yet been studied in models of PD we reasoned that there could be benefits of the test compound for this application as well, particularly in light of the complexity of opioid systems in the basal ganglia.
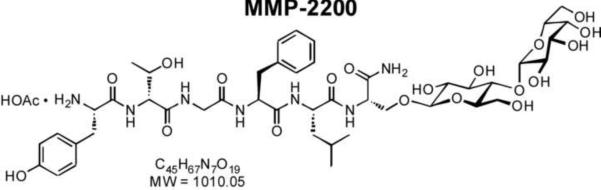
Glycopeptides based on endogenous neuropeptides that are δ-selective opioid agonists or mixed μ/δ-agonists may offer efficacy and tolerability advantages over classical μ-selective drugs (Bilsky et al., 2000; Gengo and Chang, 2004; Lipkowski et al., 2004). Recent structure-activity studies show that glycosylation increases the distribution of peptides across the blood-brain barrier (BBB), and demonstrate the enhanced potency of systemically administered (i.v., i.p., s.c.) glycopeptides to produce centrally mediated behavioral effects (Bilsky et al., 2000; Lowery et al., 2007; Lowery et al., 2011; Manson et al., 2001). For example, MMP-2200 is a glycosylated derivative of the Leu-enkephalin analog Tyr-D-Thr-Gly-Phe-Leu-Ser-CONH2 (Figure 1). Like its unglycosylated parent peptide, MMP-2200 displays similar nanomolar affinities for both δ and μ-opioid receptors, and functions as an agonist in both guinea pig ileum (GPI) and mouse vas deferens (MVD) smooth muscle assays, which are sensitive to δ- and μ-receptor agonists, respectively (Elmagbari et al., 2004). While glycosylation does not greatly alter receptor binding affinities, MMP-2200 was 10-fold more potent than the parent compound in producing antinociception in mice after systemic (i.v) administration (compare compounds 1 and 9 in Elmagbari et al., 2004). These results (Egleton et al., 2005) and more definitive studies with the closely related glucoside SAM-1095 (Egleton et al., 2001) indicate that glycosylation increases serum stability, CNS stability, as well as penetration of the blood-brain barrier, while maintaining high binding affinity receptor selectivity. These findings led us to test MMP-2200 in two standard rodent models of PD.
Figure 1.
Structure of MMP-2200.
2. Results
2.1. The glycosylated opioid peptide, MMP-2200, reduced amphetamine-mediated turning in severely-lesioned hemi-Parkinsonian rats
We first tested MMP-2200 in a standard toxin-induced model of Parkinson's disease, the rat 6-hydroxy-dopamine (6-OHDA)-induced unilateral lesion model. Greater than 90% loss of dopaminergic neurons in the substantia nigra (Figure 2A) and dopaminergic terminals in the striatum (Figure 2B) following 6-OHDA treatment are achieved in our hands. In this model, indirect dopamine agonists such as amphetamine produce rotation ipsiversive to the lesion due to release of dopamine from the un-lesioned hemisphere. The lesioned hemisphere does not respond to amphetamine due to destruction of the dopamine containing terminals of the nigrastriatal projection. This asymmetry produces easily quantifiable rotational movements. Rotations were observed for 1 min epochs over a 1 hr time course (Figure 3A). The maximum rotations induced by 5 mg/kg amphetamine were 11.7 ± 2.0 rotations/min (mean ± SEM, A+S group, n=8). The time course of the amphetamine effect in the control group (A+S) displays a rising phase which reaches a plateau 30–40 min after injection (Figure 3A). Co-injection of amphetamine and MMP-2200 (A+M group, n=8) resulted in a markedly reduced rotation rate (Figure 3A). This inhibitory effect of MMP-2200 was observed immediately after injection and became statistically significant from 20–40 min after injection (see Figure 3A, *P < 0.05 at 25 min, 35 min and 40 min and **P < 0.01 at 30 min after MMP-2200 administration compared with A+S group, n=8/group, paired t-test). The maximum rotation rate in the co-injection (A+M) group was 6.1 ± 1.3 which was a 47.6% reduction. The A+M group also showed a rising phase, but reached a maximum value at 20 min somewhat earlier than the A+S group. The apparent onset and duration of this inhibitory effect of MMP-2200 is consistent with the known pharmacokinetic properties of MMP-2200 based on earlier studies of antinociception (Elmagbari et al., 2004).
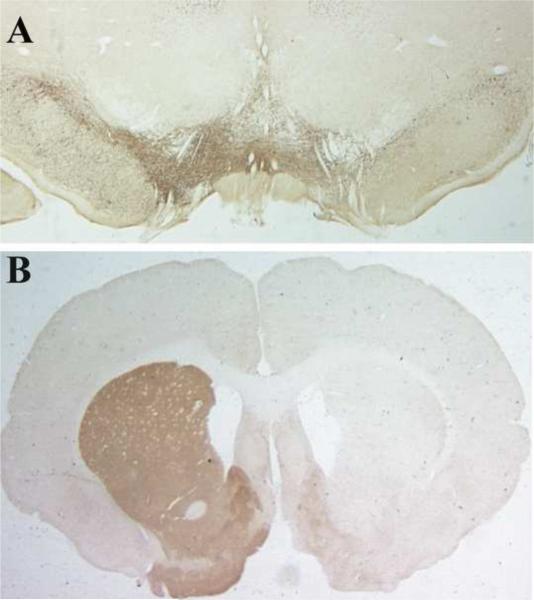
Figure 2. Unilateral injections of 6-OHDA into the striatum creates a severe lesion in the substantia nigra.
The presence of the unilateral lesion is verified using tyrosine hydroxylase (TH) antibody staining as a marker of dopaminergic neurons. The loss of cell bodies in the substantia nigra (A) and the loss of dopaminergic terminals in the striatum (B) is visible. For visualization DAB staining was performed after binding of the anti-TH antibody.
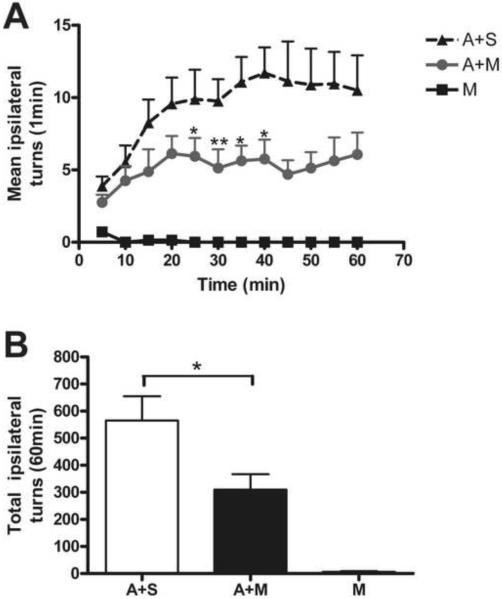
Figure 3. MMP-2200 decreased the rotations induced by amphetamine in severely 6-OHDA lesioned rats.
(A) Amphetamine injection induced turning ipsiversive to the lesion in a standard hemi-Parkinsonian model that utilized a unilateral injection of 6-OHDA (more than 90% loss of dopaminergic cell bodies in the substantia nigra). Amphetamine-induced rotation (▲) reached a plateau at 30–40 min, and then gradually decreased from 40 min onwards. Co-injection of MMP-2200 markedly reduced the amphetamine-induced rotation over the entire time-course (●). This effect was most pronounced 20–45 minutes after injection (* P < 0.05 & ** P < 0.01 compared A+M with A respectively, n=8, t-test). MMP-2200 alone (■) did not induce rotation. (B) The total number of amphetamine-induced rotations over a 1 hr period was significantly reduced by co-injection with MMP-2200 (*P < 0.05, n=8, t-test).
Unlike selective δ opioid agonists, MMP-2200 alone did not induce rotations even at 20–40 min time period where the maximum effect on amphetamine-induced rotations occurred. We also calculated the total number of rotations of the 1 hr observation period, using the data from each 1 min epoch to estimate 5 min time periods that were then summed over 1 hr (see Figure 3B). The total number of turns in the control group (A group, amphetamine plus saline to control for the co-injection) was 565 ± 96 (mean ± SEM). Co-injection of MMP-2200 (10 mg/kg, i.p.) reduced amphetamine-induced total rotations to 310 ± 61 (mean ± SEM, A+M group) representing a 45% reduction from the control (*P<0.05, n=8/group, unpaired t-test). MMP-2200 alone (10 mg/kg, i.p., M group) did not induce total rotations in 6-OHDA mediated hemi-Parkinsonian rats (n=8).
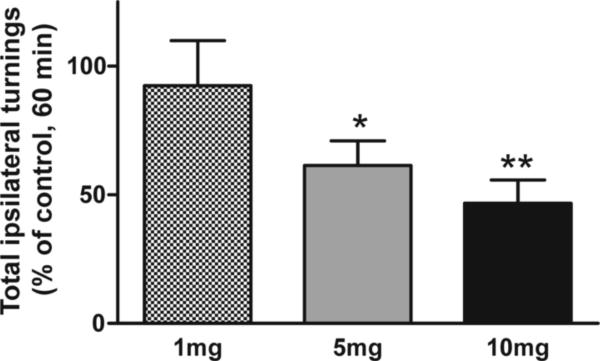
In a separate experiment a dose response study (1 mg, 5 mg and 10 mg) was included to better understand the full range of effects of MMP-2200, and showed that MMP-2200 was already effective in significantly reducing amphetamine-induced rotations at the 5 mg dose. This data is presented as % of amphetamine-only control in the plot in Figure 4. The 5 mg (*P < 0.05 vs control) and 10 mg (*P < 0.005 vs control) MMP-2200 doses showed an equivalent efficacy (n=11, repeated measures ANOVA on raw rotation data before normalization to % control).
Figure 4. Dose-response of the effect of MMP-2200 in severely 6-OHDA lesioned rats.
The total number of amphetamine-induced rotations over a 1 hr period was counted. The graph depicts the effects of 3 different MMP-2200 doses plotted as % of amphetamine-only control. For statistical analysis the raw rotation data before normalization (n=10) was subjected to a repeated measures ANOVA with a Fisher LSD post-hoc test revealing that the 5 mg (*P < 0.05) and the 10 mg (**P < 0.005) MMP-2200 doses reduced amphetamine-induced rotations significantly. The 1 mg MMP-2200 dose showed no significant effect.
2.2. The effect of MMP-2200 on amphetamine-induced turning behavior is opioid receptor mediated
2.2.1. The non-selective opioid receptor antagonist naloxone blocked the effect of MMP-2200 on amphetamine-induced turning in hemi-Parkinsonian rats
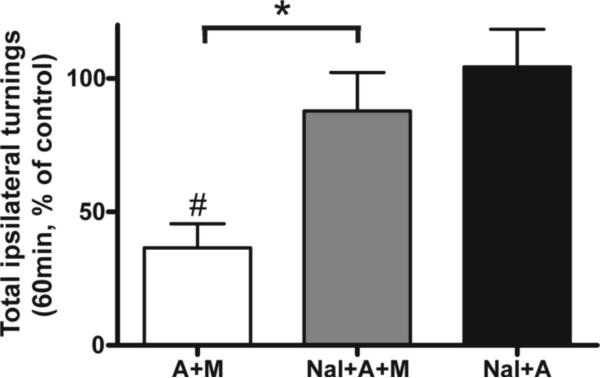
We next tested whether the prominent effect of MMP-2200 on amphetamine-induced rotation was mediated by opioid receptors. To summarize these studies we have calculated the total number of turns over the 1 hr observation period similar to Figure 3B. Lesioned rats were first injected with amphetamine alone (A) and the baseline number of turns was established. The same animals were then tested again within 1 week using a combination of MMP-2200 and opioid antagonists as shown in Figure 5. A one-way ANOVA with a Bonferroni post-hoc test was performed on the log-transformed rotation data, and revealed the following statistical significant differences. MMP-2200 was, again, demonstrated to be a potent blocker of amphetamine-induced rotation. The 51 ± 6% reduction (*P < 0.05 between the A and A+M group n=16) seen in this experiment is similar to the 45% reduction seen in the comparable experiment shown in Figure 3. This effect of MMP-2200 was completely reversed by pre-treatment with the non-subtype selective opioid receptor antagonist naloxone (3 mg/kg, i.p.; Nal+A+M group), with total rotations of 97 ± 12% relative to amphetamine control (*P < 0.05 compared with A+M group, n=16). Naloxone alone did not induce rotations (n=6) nor did it affect the amphetamine-induced rotations (n=16), and there was a significant difference between the A+M and Nal+A groups evident (P < 0.01; n=16).
Figure 5. The effect of MMP-2200 on amphetamine-induced rotation is blocked by naloxone.
Baseline rotations were established in severely-lesioned hemi-Parkinsonian rats challenged with amphetamine to establish the control level of 100%. The data are plotted as % of amphetamine-only control. MMP-2200 co-injections led to a marked decrease in amphetamine-induced rotation (A+M, #P < 0.05, one way ANOVA with a Bonferroni post-hoc test of log-transformed rotation data before normalization to % control, n=16) compared to the amphetamine-only control baseline. This effect was completely reversed by 3 mg/kg naloxone (Nal+A+M, *P < 0.05 compared with A+M group, n=16). Naloxone alone did not affect amphetamine-mediated rotations (Nal+A, P > 0.05 compared with control; n=16).
2.2.2. The selective δ-opioid receptor antagonist naltrindole partially blocked the effect of MMP-2200 on amphetamine-induced turning in hemi-Parkinsonian rats
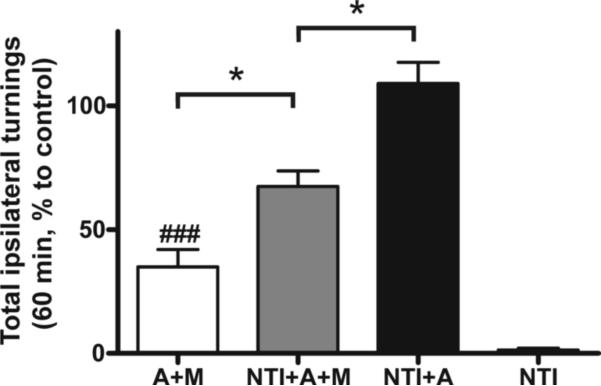
In order to further define the mechanism of action of MMP-2200, we tested the effect of the δ-opioid selective antagonist naltrindole (Figure 6). The data are plotted as % of amphetamine-only control (A). Again it was found that MMP-2200 produced a large effect on amphetamine-induced rotations. Naltrindole (3 mg/kg, i.p.) alone did not induce rotations nor did it alter the amphetamine-induced rotations. It was, however, effective in attenuating the effect of MMP-2200, although it did not completely reverse the effect as did naloxone. While MMP-2200 alone reduced amphetamine-induced rotations by 70%, pretreatment with naltrindole led to only a 30% reduction of amphetamine-induced rotations by MMP-2200. The one-way ANOVA of the raw rotation data with a Fisher LSD post-hoc test revealed the following statistical significant differences: between the A+M and the NTI+A+M groups (*P < 0.05), between the A and the NTI+A+M groups (P < 0.05), between the A and the A+M groups (P < 0.005), between the A+M and NTI+A groups (P < 0.005), between the NTI+A+M and NTI+A groups (P < 0.05), as well as between the NTI group and all 4 other groups (for groups A, NTI+A, NTI+A+M, P < 0.001; and for group A+M, P < 0.05).
Figure 6. The effect of MMP-2200 on amphetamine-induced rotations is partially reversed by the selective δ-opioid antagonist naltrindole.
Baseline rotations were measured in severely-lesioned hemi-Parkinsonian rats challenged with amphetamine to establish the 100% level. The data are plotted as % of amphetamine-only control. MMP-2200 significantly reduced the amphetamine-induced rotations (A+M, ###P < 0.005, n=15, one way ANOVA with a Fisher LSD post-hoc test of the raw rotation data before normalization to % control) compared to this baseline. Naltrindole partially reversed this effect (A+M vs NTL+A+M, n=7, *P < 0.05; NTL+A+AM vs NTL+A, *P < 0.05), confirming that MMP-2200 exerts some of its effect through the δ opioid receptor. Naltrindole alone had no effect on amphetamine-induced rotations (NTL+A; n=7) nor did it directly induce rotations (NTI; n=5).
2.3. MMP-2200, reduced amphetamine-mediated turning in mild hemi-Parkinsonian rat model
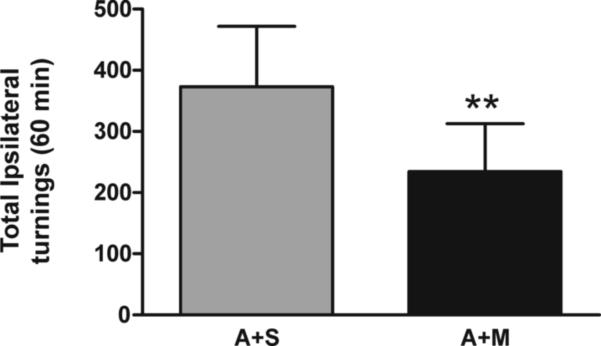
The effect of MMP-2200 was tested in a mild progressive 6-OHDA-lesion model to investigate if the benefit is maintained and rule out that worsening of deficits occurs in this model. In our hands in this model less than 50% of the dopaminergic neurons in the substantia nigra and dopaminergic terminals in the striatum following 6-OHDA (20 μg) injection were lost (Falk et al., 2011). Figure 7 depicts the results and shows that MMP-2200 also had a significant effect in reducing amphetamine-induced rotations while not worsening the deficits in this model (**P < 0.01 compared with amphetamine control injection, n=11, paired t-test).
Figure 7. MMP-2200 decreased the rotations induced by amphetamine in a mild 6-OHDA lesioned rat model.
Amphetamine injection induced turning ipsiversive to the lesion in this mild progressive unilateral 6-OHDA lesion model (less than 50% loss of dopaminergic cell bodies in the substantia nigra). The total number of amphetamine-induced rotations over a 1 hr period was significantly reduced by co-injection with MMP-2200 when compared to amphetamine alone (**P < 0.01, n=11, paired t-test).
2.4. MMP-2200 reversed apomorphine-induced hyper-kinetic movement in reserpine-mediated akinesia rats in an opioid receptor sensitive manner
We also tested the effect of MMP-2200 in the reserpinized rat model of Parkinson's disease. In this model, the nigrostriatal pathway remains anatomically intact but dopamine is depleted leading to a profound akinetic state. This model is sensitive to direct dopamine agonists that can bind to the postsynaptic dopamine-1 and dopamine-2 receptors located on the striatal neurons. These drugs will cause a reversal of the akinesia and moreover can lead to overshoot due to hyper-sensitivity of the denervated striatal neurons. This paradigm has been used extensively in screening for drugs with utility in Parkinson's disease. Indirect agonists such as amphetamine are not as effective since the normally released pool of dopamine is depleted. The behavioral measure for this model is total distance traveled when the animal is placed in a novel environment.
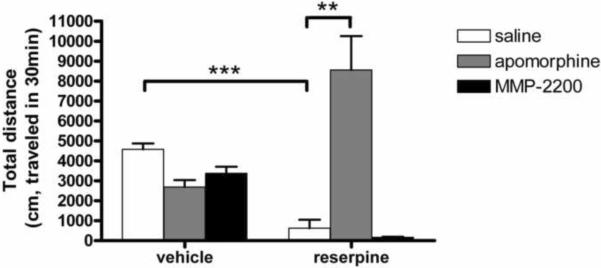
As shown in Figure 8, we first tested compounds in untreated control rats that had not received reserpine. The total movement distance in vehicle treated control rats was 4,577.1 ± 337.8 cm/30 min. MMP-2200 did not significantly alter locomotion in control rats (3,378.2 ± 383.5 cm/30 min, P > 0.05 compared with vehicle+saline group, n=4/group, one way ANOVA). There were no signs of sedation or abnormal movements that were observed in the MMP-2200-treated animals. Apomorphine (0.5 mg/kg, i.p.) alone showed a trend of decreased movement distance to 2,687.3 ± 406.4 cm/30 min in control rats (P > 0.05 compared with vehicle+saline group, n=4, one way ANOVA). This effect has been documented in the literature and may reflect a CNS stimulant effect that increases attention and focus but decreases exploration behavior (Schreur and Nichols, 1986). In the animals treated with reserpine, profound akinesia was induced: movement distance was reduced to 625±495 cm/30 min (P < 0.008 compared with vehicle control, n=4/group, one way ANOVA). As expected this akinesia was readily reversed with apomorphine, which increased movement distance to 8,557±1,953 cm/30 min (P < 0.001 compared to reserpinized rats receiving a vehicle control, n=4/group, one way ANOVA). There was a prominent overshoot in this group compared to animals not receiving reserpine, but receiving apomorphine, thus reflecting the well-described phenomenon (Friedman et al., 1975) of dopamine supersensitivity (2,687±406 cm/30 min in vehicle+apo group vs 8,557 ± 1,953 cm/30 min in res+apo group, P < 0.05, one way ANOVA, n=4/group). In this experiment the distance traveled was 218% higher than saline controls indicating that apomorphine had not only reversed akinesia but induced hyper-kinesia. On the other hand, the test drug MMP-2200 had no effect in reversing the akinesia resulting from reserpine. This result is consistent with the lack of its ability to induce rotation when used alone in the hemi-Parkinsonian model. These results are different than expected from the literature on selective δ-opioid agonists, i.e. SCNA80 (Hille et al., 2001), and indicates that MMP-2200 is not likely to have utility as a pro-kinetic drug similar to the dopamine agonists.
Figure 8. MMP-2200 alone did not induce changes in locomotion in control or reserpinized rats.
MMP-2200 induced a small but insignificant decrease in locomotion in control rats compared with vehicle control. Treatment with reserpine induced severe akinesia (***) that could be reversed with apomorphine with the expected overshoot due to dopamine super-sensitivity (**). Unlike apomorphine, MMP-2200 was not effective to reverse akinesia in reserpinized rats.
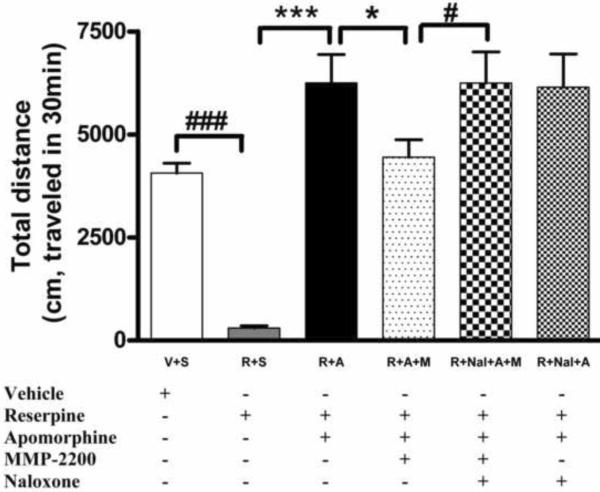
We next tested the effect of MMP-2200 on the apomorphine-induced overshoot phenomenon described above (Figure 9). In this separate experiment, we again documented the severe akinesia induced by reserpine (284.5 ± 48.3 cm/30 min verses 4,069.1 ± 243.8 cm/30 min in reserpine and control groups respectively, ###P < 0.001, n=12/group, one way ANOVA). Apomorphine (0.5mg/kg, i.p.) was then administered 18 hrs later to induce hyper-kinesia: the mean distance traveled was 6,974.84 ± 787.4 cm/30 min, which represents a 54% increase over baseline as shown in Figure 9 and is similar to the overshoot seen in Figure 8. This hyper-kinetic overshoot was abolished by 10 mg/kg i.p. MMP-2200 with the movement distance decreased to 4,445 ± 449.6 cm/30 min (res+apo+M group, *P < 0.05 compared with res+apo group, n=12/group, one way ANOVA, Figure 9). This effect of MMP-2200 was completely reversed by pre-administration of 3 mg/kg naloxone, with the movement distance increased to 6,146.8 ± 800.1 cm/30 min (#P < 0.05 compared with res+apo+M group, n=9, one way ANOVA, Figure 9), indicating that the effect is mediated by opioid receptors. Naloxone alone did not affect the overshoot (n=10).
Figure 9. The reduction of apomorphine-induced hyper-active locomotion by MMP-2200 was blocked by the non-selective opioid receptor antagonist naloxone in the reserpinized rat model.
Dopamine depletion was accomplished by systemic administration of reserpine to induce akinesia (###). Apomorphine was administered 18 hr later to induce hyper-kinesia in the reserpinized group, as shown by the overshoot of the R+A group compared to control (***). This overshoot was abolished by MMP-2200 (R+A+M, *P < 0.05, t-test). The MMP-2200-effect was totally blocked by naloxone (# P < 0.05, t-test). Naloxone alone had no effect on the apomorphine-induced hyper-kinesia (R+Nal+A).
The results using the reserpine rodent model of Parkinson's disease are consistent with MMP-2200 having a potent effect on movement related to dopaminergic stimulation and are consistent with the results from the hemi-Parkinsonian model. In both cases, MMP-2200 has no significant effect on the baseline condition but has a potent effect in reducing dopaminergic hyper-kinesia whether induced by a direct or an indirect dopamine agonist.
3. Discussion
MMP-2200 is a novel opioid glycopeptide with mixed δ- and μ-receptor agonist activity. It has excellent CNS penetration as demonstrated by the results reported in the present study as well as earlier studies focusing on its antinociceptive properties (vide infra). Since selective δ-opioid agonists have shown efficacy in rodent and primate models of PD, we tested the effects of MMP-2200 in rodent models of PD. We reasoned that the δ-opioid properties of MMP-2200 could confer similar anti-Parkinsonian benefit without the toxicity that has been associated with cyclic non-peptide δ-opioid agonists. The mixed μ/δ properties and peptide structure of this novel compound could also have undiscovered benefits. In this report we have demonstrated that MMP-2200 does in fact have significant potency in modulating dopamine-related movements in two standard PD models. We also found that MMP-2200 had a spectrum of activity that was quite different from the δ-opioid agonists previously studied and speculate that this is due to the mixed μ/δ agonist properties of the novel compound.
In the present study we found that MMP-2200 reduced amphetamine-induced rotations in hemi-Parkinsonian 6-OHDA-treated rats. MMP-2200 also reduced hyperkinetic movements produced by the dopamine agonist apomorphine in the dopamine-depleted rat. In the latter case, the motor behavior modified by MMP-2200 reflects the phenomenon of post-synaptic hypersensitivity to dopamine that develops in the denervated striatum. These effects of MMP-2200 are opioid-receptor mediated, since the non-selective opioid receptor antagonist naloxone blocked the effect completely. The selective δ-opioid receptor antagonist naltrindole partially blocked the effect of MMP-2200 indicating that both the μ and δ properties are important for this effect. Unlike the δ-opioid agonists (Hille et al., 2001), MMP-2200 did not induce movements or reverse akinesia in the rodent models we studied. Thus, the major effect of MMP-2200 is to reduce the hyper-kinetic effects of drug-induced dopaminergic stimulation. The fact that MMP-2200 was able to reverse a direct agonist as shown by the experiment with apomorphine indicates that the effect is likely downstream from the striatal dopamine receptors (Figure 10). Although this downstream mechanism is consistent with the distribution of opioid receptors in the striatum, we cannot rule out opioid-mediated effects occurring presynaptically on dopaminergic neurons without direct measurements of synaptic dopamine release which are beyond the scope of this study.
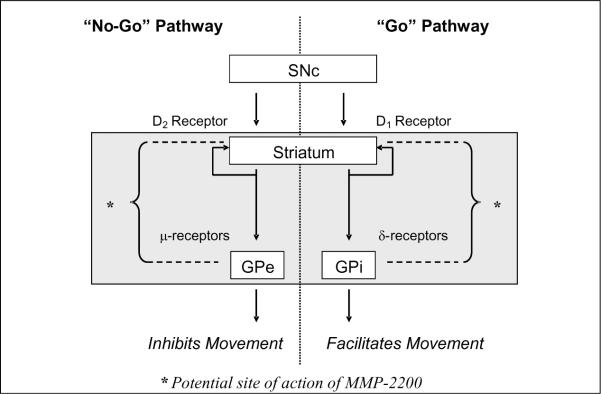
Figure 10. Schematic of MMP-2200's action on the basal ganglia circuitry.
This schematic depicts part of the classical model of basal ganglia pathways and includes the potential side of action for MMP-2200 in the direct and indirect pathways. SNc = substantia nigra pars compacta; GPe = external segment of the globus pallidus; GPi = internal segment of the globus pallidus.
One possible explanation for the lack of effect of MMP-2200 on baseline movement (as opposed to drug-induced locomotion) is that the mixed μ- and δ-opioid properties of MMP-2200 work in opposition on the direct and indirect striatofugal pathways (Figure 10), which, respectively, either facilitate or inhibit movement (Olanow et al., 2009). The direct pathway which facilitates movements, is primarily associated with δ-receptors, and stimulated by dopamine-1 receptor activation. On the other hand, the indirect pathway which inhibits movement is predominantly associated with dopamine-2 receptors and the expression μ and κ-opioid receptors (Samadi et al., 2006). The combined μ- and δ-receptor agonism of MMP-2200 could lead to a balanced effect at baseline and result in few motoric effects of MMP-2200 itself. Differential effects could arise in the condition of dopaminergic stimulation of the denervated or hyper-stimulated striatum, where the balance of direct and indirect pathway activity is likely altered (Obeso et al., 2008; Olanow et al., 2009). If the site of action of MMP-2200 is indeed downstream from the striatal dopamine receptors, this could explain the results seen in the two different models used in this study. In the case of the amphetamine-rotation model, this effect would be to limit the downstream effect of excessive dopaminergic stimulation of the normal hemisphere perhaps by differentially affecting the direct and indirect striatal output pathways. In the case of the apomorphine overshoot phenomenon, dopamine super-sensitivity causes both hemispheres to respond abnormally. Yet, the same downstream effect of MMP-2200 in differentially affecting the direct and indirect pathways could counterbalance the effect of the very high dopamine response in this model as well. The key point is that MMP-2200 may be able to selectively modify abnormally high (but not normal) dopamine signal throughput at the striatal level regardless of the cause.
Since MMP-2200 was not able to induce movements or reverse akinesia in the standard rodent models of Parkinson's disease we employed, it is not likely be useful as a prokinetic alternative to dopamine replacement therapies. Instead, the results from our study are consistent with MMP-2200 having a potent effect on movements related to dopaminergic hyper-stimulation following striatal dopamine depletion. The phenomenon of dopamine hyper-sensitivity may share physiological overlap with the occurrence of levodopa-induced dyskinesia that occurs in human Parkinson's disease. In this regard, drugs that can modify excessive dopamine-induced movements are also of therapeutic interest. This should be further investigated by testing MMP-2200 in the standard rat levodopa-induced dyskinesia model. The novel spectrum of effects found with MMP-2200 suggest that there may be great utility in studying a range of synthetic opioid glycopeptides with differing spectra of receptor subtype specificity.
Chronic opioid therapy may be considered controversial from the standpoint of tolerance, addiction and potential abuse. However several factors suggest that manipulation of the opioid peptidergic pathways of the basal ganglia is feasible: 1) We expect that the most useful compounds for PD treatment will feature mixed μ/δ or high δ-receptor selectivity. Studies with selective δ-receptor agonists have indicated a much improved safety and side-effect profile compared to compounds with greater μ-receptor agonism as typified by morphine and many other μ-agonists in current clinical use (Rischitelli and Karbowicz, 2002). 2) Clinical effects on movement disorders may occur at sub-analgesic doses. 3) As detailed below, analgesic studies of opiate peptide and glycopeptide agonists may intrinsically possess improved therapeutic indices with respect to the side effects normally associated with opioid use. For example, compared to morphine, MMP-2200 shows a better side effect profile in several pain and dependence models: The antinociceptive dose- and time-response curves for morphine and MMP-2200 in the mouse 55°C tail-flick test following s.c. administration showed comparable responses. Morphine dose-dependently induced increases in locomotor activity, stereotypical circling, and Straub tail. MMP-2200 did not induce these behaviors and, produced less antinociceptive tolerance compared to equivalent dosing with morphine (repeated s.c. administration in the 55°C tail-flick assay). MMP-2200 produced significantly less dependence liability than equivalent doses of morphine in a model for acute physical dependence. MMP-2200 produced significantly fewer signs of chronic physical dependence than equivalent treatments with morphine, as indicated by naloxone precipitated withdrawal (Lowery et al., 2011).
Taken together the results from this study are consistent with the mixed δ/μ-opioid glycopeptide MMP-2200 having a potent effect on movements related to dopaminergic hyper-stimulation following striatal dopamine depletion.
4. Experimental Procedure
4.1. Glycopeptide MMP-2200, lactomorphin
The mixed μ/δ-opioid agonist MMP-2200, or lactomorphin, is a glycopeptide analog of leu-enkephalin. This lead compound emerged from earlier studies that produced glycosylated enkephalin analogs with low nM affinity and high efficacy at both DOR and MOR (μ + δ analgesic), showing activity similar to morphine in both acute thermal nociceptive assays and chronic pain studies (Do Carmo et al., 2008; Egleton et al., 2001). Brain uptake rates on a series of I[135]-labeled glycopeptides including the iodinated derivative of MMP-2200 were measured in mice using in situ perfusion techniques (Egleton et al., 2005). Pharmacokinetics (PK) can be inferred from the observation of centrally-mediated analgesic effects (Lowery et al., 2011), and stability has been determined in the presence of mouse and rat serum and CSF, but these experiments were performed in vitro. Studies indicated that SAM-1095 (the glucoside analogue of MMP-2200) was quite stable (Egleton et al, 2001). SAM-995 is the naked peptide and SAM-1095 is the corresponding glucoside. MMP-2200 is the corresponding lactoside, and seems to be even more stable. The material used in this study was GMP-compliant material synthesized by PolyPeptide Labs in Torrence, CA.
4.2. Surgical protocol for producing the unilateral 6-OHDA lesioned rat hemi-Parkinsonian model
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Arizona and conformed to the guidelines of the National Institutes of Health. The number of animals used and their suffering was minimized. Male Sprague-Dawley rats (250 g; Charles River, Wilmington, MA) were anesthetized with ketamine:xylazine (80 mg/ml:12 mg/ml) at 1.0 ml/kg.
(1) For the severe unilateral 6-OHDA lesion a total volume of 4.0 μl freshly made 6-hydroxydopamine hydrobromide (Sigma, St. Louis, MO; 6.7 μg per 1.0 μl 0.9% sterilized saline with 0.01% ascorbic acid) was injected into the ventrolateral striatum at the following coordinates: 0.8 mm anterior to bregma, 2.5 mm lateral to midline and 5.2 mm ventral to the cerebral surface according to the atlas of Paxinos and Watson (Paxinos and Watson, 1986). The injection was carried out at a rate of 0.5 μl per min by using a Stoelting microinjector (Stoelting Co., Wood Dale, IL) and the needle was left in place for a further 5 min to prevent the backflow of the solution. Rats were pretreated (30 min prior to the infusion of 6-OHDA) with 12.5 mg/kg (i.p.) of desipramine to prevent damage to noradrenergic neurons. A 2-week recovery period was allowed, following which rats were screened using the following behavioral paradigm.
(2) For the mild unilateral 6-OHDA lesion the same protocol as describe above was employed while reducing the concentration of 6-OHDA to 5 μg per 1.0 μl 0.9% sterilized saline with 0.01% ascorbic acid. This is a standard mild progressive lesion model protocol (Sauer and Oertel, 1994; Bove et al., 2005) and details of the methods in our hands have been published (Falk et al., 2011).
4.3. Immunohistochemistry for tyrosine hydroxylase (TH)
To verify the extent of the severe 6-OHDA-induced lesions we conducted a histochemical analysis. Rats were perfusion-fixed using 4% paraformaldehyde, brains were dissected, and further fixed over night in 4% paraformaldehyde. 40 μm sections yielding the substantia nigra and the striatum were cut on a vibratome (Pelco 101 Series-1000, Pelco, Clovis, CA) and mounted on slides for staining with a rabbit anti-TH antibody (1:10,000 for 24 hrs at 4°C) and biotinylated donkey anti-rabbit immunoglobulin G antibody (1:1,000 for 1hr at RT). Antibodies from Chemicon and Santa Cruz Biotechnology, Santa Cruz, CA were used. To eliminate nonspecific binding prior to staining endogenous peroxidase was blocked with 0.3 % H2O2/Methanol for 30 min at RT, the slides were then washed 3 times for 5 min with Tris-HCl (pH 7.6) and submerged in the blocking solution Tris-D (1 % normal donkey solution, 0.1 % Triton-X-100, Tris-HCl buffer, pH 7.6) for 1 hr at 4°C. The secondary antibodies were amplified with the ABC reagent (VECTASTAIN Elite ABC Kit, PK-6100) according to the manufacturer's protocol. We used DAB substrate (Vector Laboratories, Burlingame, CA) for visualization. Example pictures showing the severity of the lesion for both substantia nigra and striatum are shown in Figure 2. There was a > 90% lesion of dopaminergic neurons and terminals evident.
4.4. Amphetamine-induced rotations
For testing the unilateral 6-OHDA lesioned rats were injected with amphetamine (5.0 mg/kg, i.p.) to induce asymmetrical dopamine release. The numbers of ipsiversive rotations were counted for a total of 60 min after the injection. Two weeks after surgery, rats showing an average of ≥5 full turns/min ipsilateral to the lesion were selected for further experiments. This has previously been shown to also correspond to levels of striatal dopamine depletion > 90% (Dekundy et al., 2007).
The opioid receptor specificity of the effects of the glycopeptides was investigated with antagonists: the non-selective opioid antagonist naloxone (Sigma, 3 mg/kg, i.p.) and the δ-selective opioid antagonist naltrindole (Sigma, 3 mg/kg, i.p.) were administrated 10 min prior to the test. We based the selection of the opioid receptor antagonists on the literature, specifically on Lowery, et al. (2011) and Bilsky, et al. (2000)
4.5. Locomotion in the reserpine-treated dopamine-depleted rat
Another common model of PD utilizes reserpine to deplete dopamine from the nigrostriatal terminals bilaterally, producing profound akinesia that can be quantified by measuring locomotion (distance traveled/time). In principle, this model is similar to testing for reversal of the akinesia induced by neuroleptic dopamine receptor antagonists. In this model, compounds of interest will restore the degree of overall locomotion. In animals treated with reserpine, reversal of akinesia was used to probe the efficacy of the glycosylated opioids as a prokinetic treatment. Secondarily, hyper-kinesia was induced with apomorphine to probe for potential anti-dyskinetic effects.
Induction of Akinesia by Administration of Reserpine
Male, Sprague-Dawley rats were housed in reversed light cycle for one week before baseline tests of locomotion were begun. Two experiments were designed. Experiment 1 evaluated the effect of apomorphine and tested opioid glycopeptides in rats with reserpine-induced akinesia. The following 6 groups were tested: (i) vehicle + saline, (ii) vehicle + apomorphine, (iii) vehicle + MMP-2200, (iv) reserpine + saline, (v) reserpine + apomorphine and (vi) reserpine + MMP-2200. Experiment 2 determined the effects of MMP-2200 and the opioid receptor antagonist naloxone to apomorphine mediated hyper-kinesia in rats. Six groups/compound were tested: vehicle + saline, reserpine + saline, reserpine + apomorphine, reserpine + apomorphine + MMP-2200, reserpine + naloxone + apomorphine and reserpine + naloxone + apomorphine + MMP-2200 Baseline tests were conducted four times in the two weeks before inducing akinesia. Rats were lightly anesthetized by inhalation of volatile anesthetic (Isofluothane) and injected with freshly-prepared reserpine (Sigma, 3 mg/kg) s.c. 18 h prior to experimentation. On the day of testing, rats treated with MMP-2200 (10 mg/kg, i.p.) and/or apomorphine (Tocris, 0.5 mg/kg, i.p.) were immediately placed in TruScan activity monitors (Coulborn Instruments, Whitehall, PA). Locomotion activity were measured over 30 min. Each animal was used only once. Each treatment was tested at least three times.
Assessment of Motor Activity of Rats Using TruScan Locomotion Monitorst
Animals were taken to the test room 30 min before drug treatments and behavioral measurements. The locomotion of animals was assessed using the TruScan photobeam Lincsystem (Coulbourn Instruments, Whitehall, PA). The Floor Plane Sensor Measures (FP) was logged every minute, over a period of 30 min, and analyzed using TruScan 2.0 software. Six major indicators were measured simultaneously, including: total movements, total movement time, total movement distance, average distance per move, mean velocity, and latency to first movement. Total movement distance was chosen as the primary endpoint to evaluate the drug effects in this experiment.
4.6 Statistical analysis
One-way ANOVA, two-way ANOVA or repeated measures ANOVA tests, followed with appropriate post-hoc tests as indicated in the text, were performed to evaluate statistical differences between treatment groups. Unpaired two tailed t-tests were used to evaluate the statistical difference between control and treated groups in the amphetamine-induced ipsilateral rotation experiments as well as the locomotion data measured by the TruScan Locomotion Monitor in rats treated with reserpine. Paired two-tailed t-tests were used to evaluate the statistical difference between control and treated groups at different time points in the amphetamine-induced ipsilateral rotation experiments (Figure 3), as well as in the mild progressive 6-OHDA lesion experiment. For the experiment evaluating the effect of naloxone to block the effect of MMP-2200 in the amphetamine-induced rotation test visual inspection of the histogram of the raw rotation data revealed a positive skew that was verified with a Skewness analysis (Skewness > 2). A Kolmogorov-Smirnov test of normality proved that the data were not normally distributed (P < 0.001). In order to address this, the data were submitted to a standard natural logarithmical transformation. A one-way ANOVA with a Bonferroni post-hoc test was then performed on the log-transformed data. The null hypothesis was rejected when P < 0.05. Statistical evaluations were performed using GraphPad Prism 4.0 software (GraphPad Software, Inc., La Jolla, CA) and SPSS software, 16.0 (SPSS, Chicago, IL).
Research Highlights
Novel glycosylated opioid peptide MMP-2200 is opioid mu and delta receptor agonist
Systemically given it reduced amphetamine-induced rotation in hemi-Parkinsonian rat
Systemic administration blocked apomorphine-induced hyper-kinesia after reserpine
MMP-2200 affects movements resulting from dopaminergic hyper-stimulation
Acknowledgements
We would like to thank K.M. Harlé, MPA, MA, for help with statistical analysis. This work was supported by the American Parkinson's Disease Association (T.F. and S.J.S.), the Office of Naval Research (Grants 14-02-01-0471, 14-05-1-0807), the National Science Foundation (CHE-607917) and the National Institutes of Health (NINDS-NS-052727) (R.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43:2586–90. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- Bové J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Lindberg I, Benjannet S, Mathis JP, Lazure C, Seidah NG. Differential processing of proenkephalin by prohormone convertases 1(3) and 2 and furin. J Biol Chem. 1993;268:27084–93. [PubMed] [Google Scholar]
- Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bedard PJ, Di Paolo T. Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. J Neuropathol Exp Neurol. 2002;61:186–96. doi: 10.1093/jnen/61.2.186. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–706. [PubMed] [Google Scholar]
- Childers SR, Fleming LM, Selley DE, McNutt RW, Chang KJ. BW373U86: a nonpeptidic delta-opioid agonist with novel receptor-G protein-mediated actions in rat brain membranes and neuroblastoma cells. Mol Pharmacol. 1993;44:827–34. [PubMed] [Google Scholar]
- Cuello AC, Paxinos G. Evidence for a long Leu-enkephalin striopallidal pathway in rat brain. Nature. 1978;271:178–80. doi: 10.1038/271178a0. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Polt R, Bilsky EJ, Rice KC, Negus SS. Behavioral pharmacology of the mu/delta opioid glycopeptide MMP2200 in rhesus monkeys. J Pharmacol Exp Ther. 2008;326:939–48. doi: 10.1124/jpet.108.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty S, Brotchie JM. Enhancement of the behavioral response to apomorphine administration following repeated treatment in the 6-hydroxydopamine-lesioned rat is temporally correlated with a rise in striatal preproenkephalin-B, but not preproenkephalin-A, gene expression. Exp Neurol. 1997;144:423–32. doi: 10.1006/exnr.1997.6431. [DOI] [PubMed] [Google Scholar]
- Egleton RD, Mitchell SA, Huber JD, Palian MM, Polt R, Davis TP. Improved blood-brain barrier penetration and enhanced analgesia of an opioid peptide by glycosylation. J Pharmacol Exp Ther. 2001;299:967–72. [PubMed] [Google Scholar]
- Egleton RD, Bilsky EJ, Tollin G, Muthu D, Lowery JJ, Alves I, Davis P, Porreca F, Yamamura HI, Yeomans L, Keyari CM, Polt R. Biousian glycopeptides penetrate the blood-brain barrier. Tetrahedron: Asymmetry. 2005;16:65–75. [Google Scholar]
- Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, Dhanasekaran M, Keyari CM, Yamamura HI, Porreca F, Hruby VJ, Polt R, Bilsky EJ. Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharmacol Exp Ther. 2004;311:290–7. doi: 10.1124/jpet.104.069393. [DOI] [PubMed] [Google Scholar]
- Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 1991;552:113–8. doi: 10.1016/0006-8993(91)90667-k. [DOI] [PubMed] [Google Scholar]
- Falk T, Yue X, Zhang SL, McCourt AD, Yee BJ, Gonzalez RT, Sherman SJ. Vascular endothelial growth factor B is neuroprotective in an in vivo model of Parkinson's disease. Neurosci Lett. 2011;496:43–47. doi: 10.1016/j.neulet.2011.03.088. [DOI] [PubMed] [Google Scholar]
- Fox S, Silverdale M, Kellett M, Davies R, Steiger M, Fletcher N, Crossman A, Brotchie J. Non-subtype-selective opioid receptor antagonism in treatment of levodopa-induced motor complications in Parkinson's disease. Mov Disord. 2004;19:554–60. doi: 10.1002/mds.10693. [DOI] [PubMed] [Google Scholar]
- Fox SH, Lang AE, Brotchie JM. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord. 2006;21:1578–94. doi: 10.1002/mds.20936. [DOI] [PubMed] [Google Scholar]
- Friedman E, Rotrosen J, Gurland M, Lambert GA, Gershon S. Enhancement of reserpine-elicited dopaminergic supersensitivity by repeated treatment with apomorphine and alpha-methyl-p-tyrosine. Life Sci. 1975;17:867–73. doi: 10.1016/0024-3205(75)90437-3. [DOI] [PubMed] [Google Scholar]
- Gengo PJ, Chang K-J. Mixed opioid receptor agonists as a new class of agents for the treatment of moderate to severe pain. In: Chang K-J, Porreca F, Woods JH, editors. In The Delta Receptor. Marcel Dekker, Inc.; New York: 2004. pp. 231–244. Vol. [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–9. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Bedard PJ. Effect of nondopaminergic drugs on L-dopa-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol. 1993;16:418–27. doi: 10.1097/00002826-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–20. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Henry B, Fox SH, Crossman AR, Brotchie JM. Mu- and delta-opioid receptor antagonists reduce levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Exp Neurol. 2001;171:139–46. doi: 10.1006/exnr.2001.7727. [DOI] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM. Increased striatal preproenkephalin B expression is associated with dyskinesia in Parkinson's disease. Exp Neurol. 2003;183:458–68. doi: 10.1016/s0014-4886(03)00064-5. [DOI] [PubMed] [Google Scholar]
- Hille CJ, Fox SH, Maneuf YP, Crossman AR, Brotchie JM. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson's disease. Exp Neurol. 2001;172:189–98. doi: 10.1006/exnr.2001.7763. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Howell A, Payza K, Cross AJ. Antiparkinson potential of delta-opioid receptor agonists. Eur J Pharmacol. 2000;396:101–7. doi: 10.1016/s0014-2999(00)00209-0. [DOI] [PubMed] [Google Scholar]
- Klintenberg R, Svenningsson P, Gunne L, Andren PE. Naloxone reduces levodopa-induced dyskinesias and apomorphine-induced rotations in primate models of parkinsonism. J Neural Transm. 2002;109:1295–307. doi: 10.1007/s00702-002-0715-6. [DOI] [PubMed] [Google Scholar]
- Lipkowski AW, Carr DB, Bonney I, Misicka A. Biphalin: A multireceptor opioid ligand. In: Chang K-J, Porreca F, Woods JH, editors. In The Delta Receptor. Marcel Dekker, Inc.; New York: 2004. pp. 245–261. Vol. [Google Scholar]
- Lowery JJ, Yeomans L, Keyari CM, Davis P, Porreca F, Knapp BI, Bidlack JM, Bilsky EJ, Polt R. Glycosylation improves the central effects of DAMGO. Chem Biol Drug Des. 2007;69:41–7. doi: 10.1111/j.1747-0285.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J. Pharm. Exp. Therap. 2011;336:767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson AJ, Katzenschlager R, Hobart J, Lees AJ. High dose naltrexone for dyskinesias induced by levodopa. J Neurol Neurosurg Psychiatry. 2001;70:554–6. doi: 10.1136/jnnp.70.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet AP, Foster OJ, Kingsbury A, Eve DJ, Daniel SE, Marsden CD, Lees AJ. Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and in Parkinson's disease. Neuroscience. 1995;66:361–76. doi: 10.1016/0306-4522(94)00606-6. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72:S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. Academic Press; New York: 1986. Vol. [Google Scholar]
- Rascol O, Fabre N, Blin O, Poulik J, Sabatini U, Senard JM, Ane M, Montastruc JL, Rascol A. Naltrexone, an opiate antagonist, fails to modify motor symptoms in patients with Parkinson's disease. Mov Disord. 1994;9:437–40. doi: 10.1002/mds.870090410. [DOI] [PubMed] [Google Scholar]
- Rischitelli DG, Karbowicz SH. Safety and efficacy of controlled-release oxycodone: a systematic literature review. Pharmacotherap. 2002;22:898–904. doi: 10.1592/phco.22.11.898.33628. [DOI] [PubMed] [Google Scholar]
- Samadi P, Gregoire L, Bedard PJ. Opioid antagonists increase the dyskinetic response to dopaminergic agents in parkinsonian monkeys: interaction between dopamine and opioid systems. Neuropharmacology. 2003;45:954–63. doi: 10.1016/s0028-3908(03)00249-1. [DOI] [PubMed] [Google Scholar]
- Samadi P, Bedard PJ, Rouillard C. Opioids and motor complications in Parkinson's disease. Trends Pharmacol Sci. 2006;27:512–7. doi: 10.1016/j.tips.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neurosci. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–54. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreur PJ, Nichols NF. Two automated locomotor activity tests for dopamine autoreceptor agonists. Pharmacol Biochem Behav. 1986;25:255–61. doi: 10.1016/0091-3057(86)90263-7. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Grimm C, Hollt V, Herz A. Evidence for a selective processing of proenkephalin B into different opioid peptide forms in particular regions of rat brain and pituitary. J Neurochem. 1984;42:447–57. doi: 10.1111/j.1471-4159.1984.tb02698.x. [DOI] [PubMed] [Google Scholar]