Abstract
The regulation of vertebrate striated muscle contraction involves a number of different molecules, including the thin filament accessory proteins tropomyosin and troponin that provide Ca2+-dependent regulation by controlling access to myosin-binding sites on actin. Cardiac myosin-binding protein C (cMyBP-C) appears to modulate this Ca2+-dependent regulation and has attracted increasing interest due to links with inherited cardiac diseases. A number of single amino acid mutations linked to clinical disease occur in the N-terminal region of cMyBP-C, including domains C0 and C1 that previously have been shown to bind to F-actin. This N-terminal region also has been shown to both inhibit and activate actomyosin interactions in vitro. Using electron microscopy and three-dimensional reconstruction, we show that C0 and C1 can each bind to the same two different positions on F-actin. One position aligns well with the previously reported binding site that clashes with the binding of myosin to actin, but would force tropomyosin into an “on” position that exposes myosin binding sites along the filament. The second position identified here would not interfere with either the binding of myosin or tropomyosin positioning. It thus appears that the ability to bind to at least two distinctly different positions on F-actin, as observed for tropomyosin, may be more common than previously considered for other actin-binding proteins. These observations help to explain many of the seemingly contradictory results obtained with cMyBP-C and show how cMyBP-C can provide an additional layer of regulation to acto-myosin interactions. They also suggest a redundancy of C0 and C1 that may explain the absence of C0 in skeletal muscle.
Introduction
Biological macromolecules have evolved to perform an enormous range of functions, from catalysis of chemical reactions to the generation of mechanical force. Over the course of evolution the regulation of such activity has become more complex, and the study of biology can be seen in some manner largely as the study of layered regulatory systems. The primary regulatory system in vertebrate striated muscle involves the Ca2+ activated troponin – tropomyosin system that either allows or prevents myosin heads from binding to F-actin. The troponin-tropomyosin system involves the troponin complex binding Ca2+ which causes it to shift the position of tropomyosin on F-actin from an ‘off’ or blocked state to an ‘on’ state which allows myosin S1 to bind. This original steric-blocking model1–4 is supported by abundant structural data and yet the molecular details of the interactions are still not known. More recently, the role of MyBP-C in modulating this primary regulatory signal has been the subject of intense investigation5, 6 as within cardiac muscle mutations in the MYBPC3 gene can cause serious heart disorders. Approximately ~ 42%7 of cases of familial hypertrophic cardiomyopathy8 (HCM) involved mutations in cMyBP-C, with a number of the single amino acid substitutions (n=24) occurring in the N-terminal ‘regulatory’ region that comprise the first four domains of the protein which modulate myosin/actin interactions to fine-tune heart muscle contraction9. A very recent tomographic reconstruction from intact muscle fibers showed that MyBP-C does actually span the space between the thick and thin filaments in striated muscle10, establishing that the N-terminal region of MyBP-C does contact the thin filament within intact muscle.
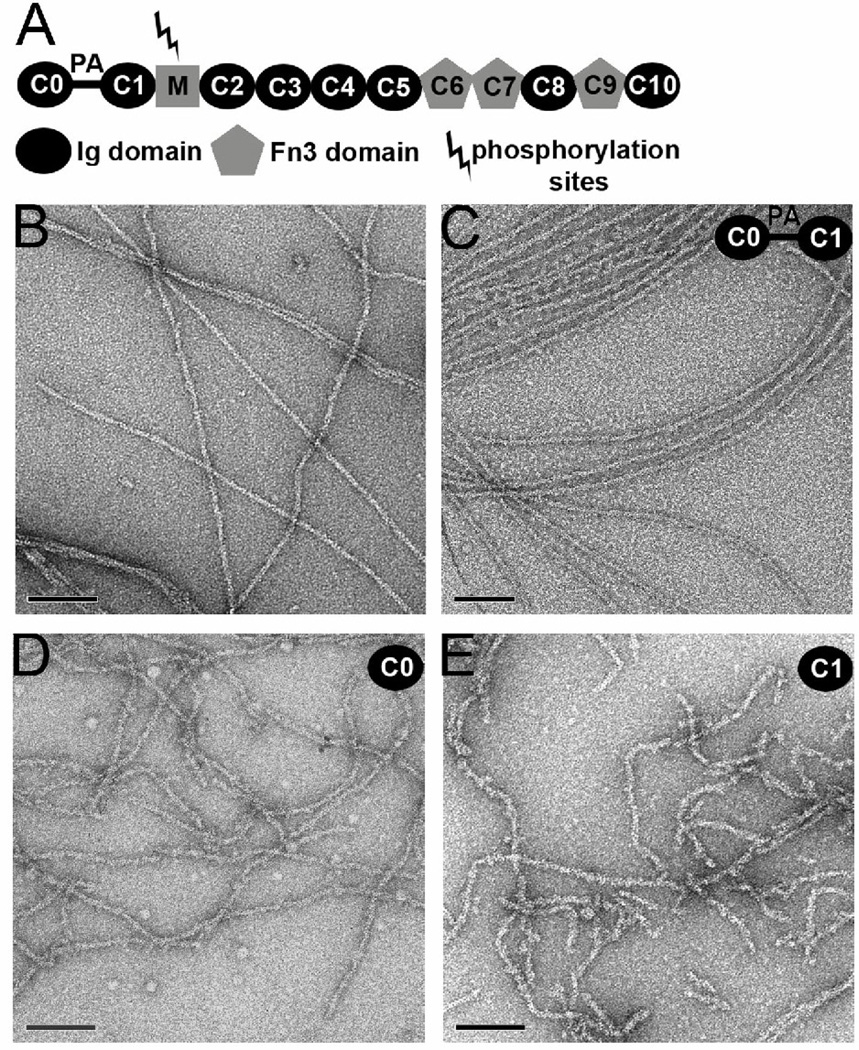
MyBP-C is a large modular protein (128–137kDa) that belongs to the immunoglobulin superfamily containing repeating immunoglobulin Ig-like and fibronectin-like type 3 modules (designated C1 through C10, Fig. 1A), plus a ~ 100 residue motif of unknown structure between the C1 and C2 domains. The cardiac isoform has an additional Ig domain, C0, at the N-terminus that is not present in the skeletal striated muscle isoform. A proline/alanine-rich linker (PA) connects C0 and C1 and it has been found that the percentage of proline + alanine content correlates with the heart-rate requirement of different species11 as well as conferring different effects on thin filament activation. In combination with C1, this linker can also modulate thin filament activated S1 ATPase activity12. Furthermore, a knock-in mouse model that carried N-terminal-shortened cardiac MyBP-C (without PA-C1, but retaining the C0 domain) exhibited an increased Ca2+-sensitivity in force development, whereas maximal active force levels remained unchanged13.
Figure 1.
The modular structure of c-MyBP-C includes multiple Ig and fibronectin domains (A). A proline/alanine rich (PA) region links C0 with C1. Electron micrographs of negatively stained F-actin (B), and F-actin decorated with C0-C1 (C), C0 alone (D) and C1 alone (E). The space bars are 1,000 Å. When C0-C1 is added to F-actin extensive cross-linking of filaments is observed (C, inset), but regions can still be found where images of single filaments can be used for three-dimensional reconstruction.
It has been shown using biochemical assays, electron microscopy and neutron scattering that the N-terminal portion of cMyBP-C (containing either C0-C2 or C1-C2) can bind F-actin11, 12, 14–16. When exogenous N-terminal fragments of cMyBP-C were added to skinned cardiac muscle fibers, these were also shown to bind actin in the intact myofibrillar lattice17 A model for the mouse C0-C2/actin interaction has been proposed based on neutron scattering data with C0 and C1 binding subdomain 1 of one actin protomer and subdomain 2 of an adjacent actin protomer, such that these cMyBP-C domains would clash with the S1 binding site on actin and with the ‘off’ position of tropomyosin. The model suggested that actin bound C0-C2 could interfere with myosin S1 attachment as well as activate thin filaments independent of Ca2+/troponin by shifting tropomyosin toward an ‘on’ position. As the C0-C2 construct has also been shown to bundle F-actin18, it has been proposed that multiple F-actin binding sites exist within the N-terminal regulatory region. The possibility that human C0-C2 bundles actin due to a dimerization is excluded by small angle x-ray scattering data (C.J. and J.T., unpublished) showing that human C0-C2 is monomeric in solution, as was previously shown for mouse C0-C216.
We have used electron microscopy (EM) and three-dimensional reconstruction to analyze complexes of human C0-C1, and both C0 and C1 alone, with F-actin. Our results are quite surprising, and show that each of these two Ig domains can bind to the same two distinctly different positions on each F-actin protomer. Our results show how these two binding modes would differently impact the binding of myosin and tropomyosin to F-actin, and help to resolve a number of seemingly contradictory observations in the literature. This dual mode of binding also indicates that the ability of an actin-binding protein to interact in multiple modes with actin is not limited to tropomyosin.
Results
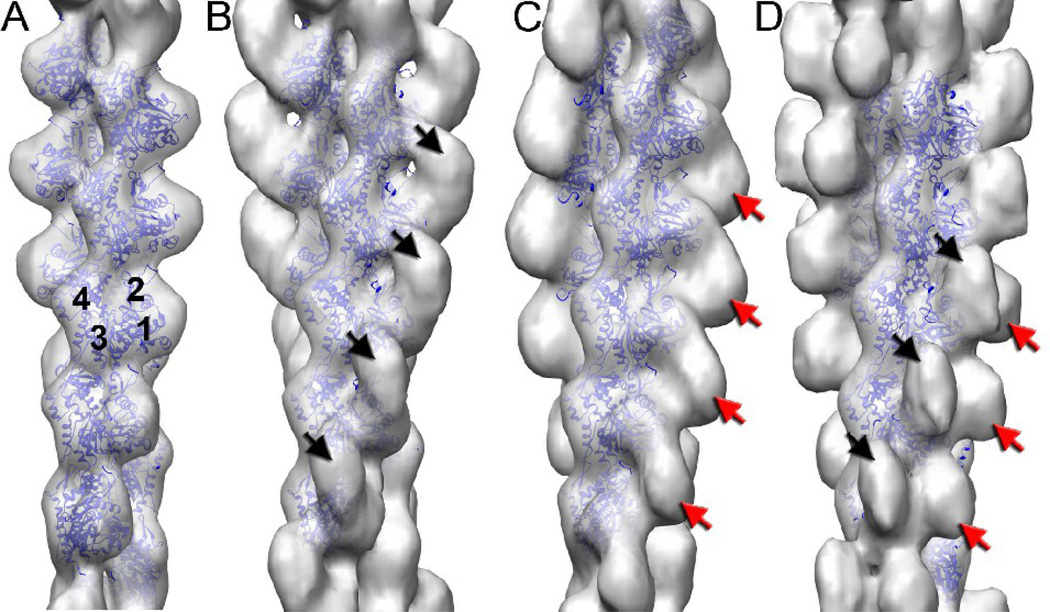
Actin filaments can be seen by eye to be decorated with the C0-C1 fragment (Fig. 1C) in comparison with naked F-actin (Fig. 1B). Extensive bundling and aggregation of the actin filaments occurs when C0-C1 is present, similar to a previous report showing such cross-linking by the C0-C1-m-C2 fragment18. This is a prima facie argument that multiple actin binding sites also exist within the C0-C1 fragment18. Some effort was therefore invested to find single filaments suitable for three-dimensional reconstruction. An overall reconstruction of all segments could not be simply interpreted, suggesting structural heterogeneity. By an iterative process we were able to sort all segments into one of four classes: 1) largely naked F-actin (Fig. 2A), ~ 40%; 2) a compact mass bound to the front of the actin subunit, spanning from Subdomain 1 of one actin subunit to Subdomain 1 of a subunit below it (Fig. 2B), ~ 20%; 3) a compact mass bound to the Subdomain 1–2 side of an actin subunit (Fig. 2C), ~25%; and 4) the additional mass of (2) and (3) present together on the same subunits (Fig. 2D), ~ 15%. Although the reconstruction in Fig. 2A is from segments classified as largely naked, traces of additional bound mass are clearly present.
Figure 2.
Three-dimensional reconstructions of largely naked F-actin (A), and with bound C0-C1 (B–D). The four actin subdomains are labeled (A). The additional density from cMyBP-C bound to F-actin is either on the front face of the actin subunit (B, black arrows) or on the side of the actin subunit containing Subdomains 1 and 2 (C, red arrow). Approximately 15% of the segments gave rise to the reconstruction (D) where both densities are present.
While the additional mass seen in these reconstructions is comparable in size to individual C0 (PDB 2K1M) and C1 (PDB 2V6H) domains19, interpreting which mass corresponds to C0 and which to C1 was not possible due to the similar size of these domains and the limited resolution of the reconstructions (~ 23 A). We therefore incubated F-actin with either the C0 domain alone (Fig. 1D, or the C1 domain alone (Fig. 1E). The electron micrographs suggest that when C0 (Fig. 1D) or C1 (Fig. 1E) are added to actin filaments the filaments become more flexible compared to either pure F-actin (Fig. 1B) or F-actin incubated with the C0-C1 fragment (Fig. 1C). The flexibility of F-actin has been shown previously to be modulated by solution conditions20 or by actin-binding proteins such as cofilin21, which increases the flexibility of F-actin by a factor of four to five. In both of these instances the increased flexibility arises20, 22 from a disordering of Subdomain 2 of actin23, which normally generates the highest radius inter-protomer contact in the filament. As the resistance to bending is dependent upon the fourth power of the radial mass distribution, it can be seen how breaking a high-radius contact can lead to a large increase in flexibility. Three-dimensional reconstructions (below) show that this is also the case for both C0 and C1 when they bind alone.
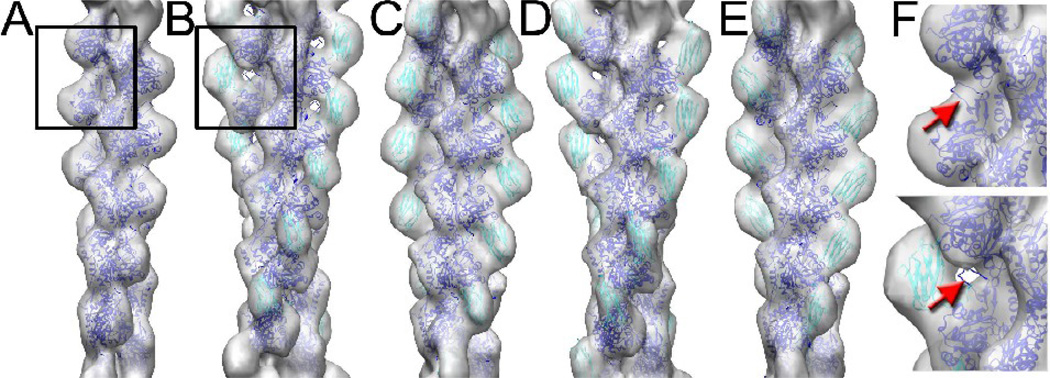
The simplest interpretation of the reconstructions in Fig. 2 with the C0-C1 fragment is that one bound mass is due to C0 and the other C1, and the density observed in Fig. 2D arises when both C0 and C1 are bound simultaneously. Quite surprisingly, we have been able to capture two different binding modes when either C0 (Fig. 3B,C) or C1 (Fig. 3D,E) are present alone implying that each isolated domain can bind to the same or similar sites on F-actin, and can bind to two distinctly different positions. The frequency with which either C0 or C1 binds to these two different sites did not appear significantly different. When using C0-C1 together we observe a third mode (Fig. 2D) where both sites are occupied, which we do not find when either C0 or C1 are alone. A comparison between the reconstruction of naked F-actin and when C0 is bound (Fig. 3F) shows that the density due to the actin Subdomain 2 is largely missing when C0 is present, similar to what was shown previously for cofilin22. Thus, there is a very good concordance between the increased flexibility of the filaments observed by EM (Fig. 1D, E) and the details of the three-dimensional reconstruction.
Figure 3.
Pure F-actin (A) is shown alongside reconstructions of F-actin decorated with C0 alone (B,C) or C1 alone (D,E). Surprisingly, each domain can bind to two different positions on F-actin, and these two sites are the same or very similar for the two domains. A blowup of the regions marked with a box in (A,B) is shown in (F), where density from subdomain-2 of actin is present in naked actin (top, red arrow), but absent when C0 is bound (bottom, red arrow).
We have used atomic models for the actin filament24 and the C0 and C1 domains19 to show that the bound density is the appropriate size expected for such a single domain (Fig. 3). Due to the limited resolution, the orientation of C0 and C1 with respect to each other or actin cannot be determined and therefore we make no detailed interpretation, for example of interacting residues in actin, C0 and C1.
Discussion
The ability of two different cMyBP-C domains, C0 and C1, to interact with similar sites on actin protomers is quite striking, as is the ability of each domain to bind to the same two different positions on F-actin. Our observations of specific binding sites for both C0 and C1 on F-actin conflict with a recent report suggesting that these N-terminal domains interact with actin in a non-specific manner25. Our results can be reconciled with a model derived from small-angle neutron scattering which involved globally averaging all interactions and which were also performed in solution at relatively high protein concentrations16. The position of the C0 domain in the neutron scattering models of mouse C0C2-decorated F-actin aligns reasonably with the front binding mode observed in the EM reconstructions. Interestingly, the next ‘best-fit’ neutron model reflects the position of C0 in the side binding mode, suggesting that both modes were contributing to the previous scattering results.
The C0 and C1 domains are structural homologs (and each is an Ig domain), but such homology does not by itself require conserved interactions. The modular domain structure present in many proteins that bind F-actin was recognized many years ago26, but the detailed understanding of how such domains bind to F-actin is still unfolding. The calponin homology (CH) domains present in the spectrin/α-actinin/fimbrin superfamily of actin-binding proteins provides us with clear examples of how highly conserved structural domains can interact with F-actin differently. The CH domain within the eponymous actin-binding protein calponin has been shown to not bind F-actin at all27, 28, while in a protein such as fimbrin the CH3 domain makes a very different interaction with F-actin than does the CH4 domain29. In α-actinin, only the CH1 domain binds F-actin30, while the structurally conserved CH2 domain is a negative regulator of this interaction (since destabilization of this domain increases the affinity of α-actinin for actin). Thus, the presence of structurally homologous domains in an actin-binding protein tells us nothing about whether they will all interact with F-actin in the same manner, or even whether they will all interact with F-actin.
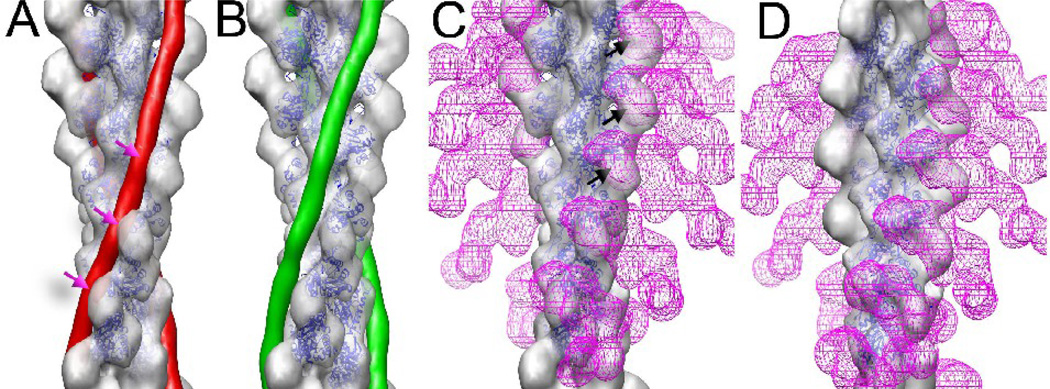
The fact that both C0 and C1 can occupy two distinctly different positions on F-actin is very reminiscent of tropomyosin, where the steric-blocking model4 for striated muscle regulation postulates that tropomyosin shifts its binding site on actin from one that would prevent myosin from attaching to F-actin (the blocked or ‘off’ state) to one that allows myosin to bind (the open or ‘on’ state). As cMyBP-C appears to provide a crucial layer of regulatory control in cardiac muscle, obvious questions arise about the relationship between the binding that we see and interactions of tropomyosin and myosin with F-actin. The binding of either C0 or C1 to the front of the actin subunit (Fig. 4A, ‘front binding mode’) would clash with both thin filament tropomyosin in the off/blocked position (i.e., when the binding of myosin to actin is inhibited) and with myosin head attachment to actin in the rigor state (Fig. 4C). However, there would be no clash between either C0 or C1 and tropomyosin, nor steric interference with myosin head attachment to actin, when C0 or C1 bind in the alternative side binding position (Fig. 4D).
Figure 4.
The binding of C0 and C1 to F-actin can be compared with the binding of tropomyosin46 (A,B) and myosin S133 (C,D). Atomic models for tropomyosin and S1 were filtered to 23 Å resolution for these figures. When tropomyosin is in the off/blocked state (A, red rods) it would sterically clash with a C0 or C1 domain (A, red arrows), while no such clashes exist when tropomyosin is in the on/open state (B, green rods). The binding of C0 or C1 to the front of an actin subunit would sterically clash with the binding of S1 in the rigor state (C, magenta mesh). However, the binding of C0 or C1 to the side of an actin subunit (D) would create no clashes with rigor binding of S1 (D, magenta mesh).
A two state binding model of C0C1 attachment to actin is consistent with experiments31 showing that the N-terminal domains of cMyBP-C can activate myocardial contraction in the absence of Ca2+ while inhibit thin filament sliding in high calcium conditions. In both the EM reconstructions, and previous neutron scattering models, the front binding modes of C0 or C1 would clash with tropomyosin in the off/blocked position. As tropomyosin occupies a dynamic equilibrium between on/off states in low calcium, actin bound C0 or C1 domains could conceivably shift tropomyosin into an on/open state to activate thin filaments, effectively providing a partial decoupling of thin filament activation from calcium concentration. As long as C0C1, C0 or C1 are bound sub-stoichiometrically in the front binding mode, then thin filament activation can occur without a complete steric inhibition of myosin head attachment to actin by the C-domains. In high calcium concentrations tropomyosin predominantly occupies an on/open position, but the C0 or C1 domains in the front binding mode can act as a steric block to inhibit the number of myosin heads that can attach to actin filaments.
The rigor binding of myosin S1 to F-actin (in the absence of nucleotide) is much better understood structurally32, 33 than the weak binding34, 35 in the presence of ATP. In the rigor state (Fig. 4C) myosin S1 would sterically clash with either C0 or C1 bound to the front of actin. However, when C0 or C1 are bound to the side of actin, no clashes are seen with rigor binding (Fig. 4D). These results explain the observation that in the absence of ATP (rigor binding), myosin S1 limited but did not eliminate the binding of cMyBP-C to F-actin36.The side binding of either C0 or C1 to actin would clash with myosin’s interaction with actin’s N-terminus, which has been shown to be important for the weak binding state34, 35, 37–39. Together, these interactions would explain the observation that whole cMyBP-C or the N-terminal domains inhibit actomyosin sliding velocity36, 40. Since cMyBP-C within intact cardiac muscle would be interacting with both myosin and actin, it is very difficult (or impossible) to assign a simple role such as activation or inhibition to the isolated C0 or C1 domains. We expect that cMyBP-C will be involved in an added layer of regulation to cardiac muscle that is not fully understood.
Lastly, the fact that C0 and C1 can bind to the same or very similar sites on each F-actin protomer suggests how MyBP-C can function in skeletal muscle in the absence of a C0 domain. C0 may have arisen by a gene duplication event from an ancestral C1-like domain, and C0 provides an additional layer of regulation in cardiac muscle, even though it makes similar interactions with actin as C1.
Materials and Methods
Protein preparation and electron microscopy
A synthetic gene fragment encoding the N-terminal domains (C0-C1-m-C2-C3-C4; C0C4) of human cMyBP-C (Genpept accession CAA71216.1) was designed incorporating near-optimal Escherichia coli codon usage and synthesised by Genscript Inc (USA) in a pUC57 cloning vector. Bam H1 and Eco R1 restriction endonucleases (unique sites engineered into the 5’ and 3’ ends of the synthetic gene, respectively) were used to isolate the C0C4 gene that was then sub-cloned into the expression plasmid pETM (a derivative of pET28a minus the T7 tag, but includes an in-frame N-terminal polyhistidine tag, HMHHHHHHSSGLVPRGSH). PCR primers were synthesised (Geneworks Ltd, Australia) and high fidelity pfu-DNA polymerase PCR was performed to isolate gene fragments encoding human C0 (amino acids 1-101), C1 (amino acids 151-258) and C0C1 (amino acids 1-258). The gene fragments were sub-cloned back into pETM using the same Bam H1 and Eco R1 restriction sites. The pETM-cMyBP-C variants were individually transformed into E. coli Rosetta2 (pLysRARE; Invitrogen) and all human cMyBP-C protein fragments were expressed in a soluble form and purified using the same protocols previously described16. Due to the susceptibility of the C0 and C1 domains to proteolysis, the His tags were not removed.
Bovine cardiac muscle actin was purchased from Cytoskeleton, Inc. (Denver, Colorado). Lyophilized powder was dissolved in G-buffer (5mM Tris-HCl, pH 7.8, 0.1mM CaCl2, 1mM NaN3, 1mM DTT, 1mM ATP) and dialyzed overnight against the same buffer. Actin was clarified by centrifugation in a TLX-Beckman centrifuge for 1 hour at 85,000 rpm. For sample preparation only the top 2/3 of the supernatant was used. F-actin was polymerized for 1–2 hours in F-buffer (10mM MOPS, pH 7.2, 40mM KCl, 1mM MgCl2, 0.5mM ATP). For EM samples 2–4 µM F-actin was incubated for 1–4 min with 10–16 µM C0C1, or 6–10µM of either C0 or C1 alone, and applied to carbon coated glow discharged grids. Samples were negatively stained with 2% (wt/vol) uranyl acetate. A Tecnai-12 electron microscope (FEI, Inc.) was used at an accelerating voltage of 80kv, at a nominal magnification of 30,000x. Negatives were densitometered with a Nikon Super Coolscan 8000.
Image processing
The SPIDER software package41 was used for most image processing, while the EMAN package 42 was used to extract filament images from micrographs. A Nikon COOLPIX 8000 scanner was used to digitize EM micrographs at a raster of 4.16 Å/pixel. Segments (each 100 pixels long, n=15,877) of F-actin decorated with the C0-C1 fragment were extracted from filament images and reconstructed by the IHRSR method43. The resultant reconstruction showed two additional densities bound to F-actin - one at the front interface between SD1 and SD2 of two adjacent actin protomers, while the other one at the side of SD1. Three model volumes were created by using crystal structures of G-actin44 having an Ig-domain attached to the first site, second site, or to both sites simultaneously. These volumes, along with a naked F-actin volume, were projected onto 100 × 100-pixel images with an azimuthal rotational increment of 4°, and the resultant 360 reference projections (4 × 90) were cross-correlated with the 15,877 filament segments. Each of the four classes was reconstructed independently and the reconstructions are shown in Fig 2. The naked class (n=6,154) yielded a symmetry of 166.5°/27 Å (Fig 2A), the “front” class (n=3,201) yielded a symmetry of 166.6°/27 Å (Fig 2B), the “side” class (n=3,770) converged to 166.9°/27 Å (Fig 2C), while a set of images classified as having both sites occupied (n=2,752) reached a stable solution having 166.4°/27 Å symmetry values (Fig 2D). The same sorting procedure was applied to actin filaments decorated with the C0 (n=7,458), or C1 (n=7,198) fragments. In contrast to the C0-C1 fragment, images sorted as having both sites occupied from the C0 (n=507) or C1 (n=370) sets did not yield interpretable reconstructions. The naked segments extracted from the filaments decorated with C0 and C1 (n=4,613 and n=4,685, respectively) converged to a 166°/27 Å solution, the “front” classes (n=1,087 and n=899, respectively) yielded 166.5°/26.8 Å, while the “side” classes (n=1,251 and n=1,244, respectively) converged to a symmetry of 166°/27 Å. Figures 2, 3 and 4 were produced using the Chimera software45.
Acknowledgments
This work was supported by NIH GM081303 (to E.H.E.) and Australian Research Council Federation Fellowship FF0457488 (to J.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Huxley HE. A personal view of muscle and motility mechanisms. Annu. Rev. Physiol. 1996;58:1–19. doi: 10.1146/annurev.ph.58.030196.000245. [DOI] [PubMed] [Google Scholar]
- 2.Spudich JA, Huxley HE, Finch JT. Regulation of skeletal muscle contraction. II. Structural studies of the interaction of the tropomyosin-troponin complex with actin. J. Mol. Biol. 1972;72:619–632. doi: 10.1016/0022-2836(72)90180-5. [DOI] [PubMed] [Google Scholar]
- 3.Lehman W, Galinska-Rakoczy A, Hatch V, Tobacman LS, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J. Mol. Biol. 2009;388:673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman W, Vibert P, Uman P, Craig R. Steric-blocking by tropomyosin visualized in relaxed vertebrate muscle thin filaments. J. Mol. Biol. 1995;251:191–196. doi: 10.1006/jmbi.1995.0425. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer JF, Gillis TE. Evolution of the regulatory control of vertebrate striated muscle: the roles of troponin I and myosin binding protein-C. Physiol Genomics. 2010;42:406–419. doi: 10.1152/physiolgenomics.00055.2010. [DOI] [PubMed] [Google Scholar]
- 6.James J, Robbins J. Signaling and myosin binding protein C. J. Biol. Chem. 2011 doi: 10.1074/jbc.R110.171801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 8.Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 2001;33:655–670. doi: 10.1006/jmcc.2001.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakley CE, Chamoun J, Brown LJ, Hambly BD. Myosin binding protein-C: enigmatic regulator of cardiac contraction. Int. J. Biochem. Cell Biol. 2007;39:2161–2166. doi: 10.1016/j.biocel.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Luther PK, Winkler H, Taylor K, Zoghbi ME, Craig R, Padron R, Squire JM, Liu J. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11423–11428. doi: 10.1073/pnas.1103216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer JF, Harris SP. Species-specific differences in the Pro-Ala rich region of cardiac myosin binding protein-C. J. Muscle Res. Cell Motil. 2009;30:303–306. doi: 10.1007/s10974-010-9207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer JF, Wong P, Bezold KL, Harris SP. Functional differences between the N-terminal domains of mouse and human myosin binding protein-C. J. Biomed. Biotechnol. 2010:789–798. doi: 10.1155/2010/789798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witt CC, Gerull B, Davies MJ, Centner T, Linke WA, Thierfelder L. Hypercontractile properties of cardiac muscle fibers in a knock-in mouse model of cardiac myosin-binding protein-C. J. Biol. Chem. 2001;276:5353–5359. doi: 10.1074/jbc.M008691200. [DOI] [PubMed] [Google Scholar]
- 14.Kensler RW, Shaffer JF, Harris SP. Binding of the N-terminal fragment C0-C2 of cardiac MyBP-C to cardiac F-actin. J. Struct. Biol. 2010 doi: 10.1016/j.jsb.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: implications for cardiac function. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffries CM, Whitten AE, Harris SP, Trewhella J. Small-angle X-ray scattering reveals the N-terminal domain organization of cardiac myosin binding protein C. J. Mol. Biol. 2008;377:1186–1199. doi: 10.1016/j.jmb.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 17.Kulikovskaya I, McClellan G, Flavigny J, Carrier L, Winegrad S. Effect of MyBP-C binding to actin on contractility in heart muscle. J. Gen. Physiol. 2003;122:761–774. doi: 10.1085/jgp.200308941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J. Biol. Chem. 2009;284:12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govada L, Carpenter L, da Fonseca PC, Helliwell JR, Rizkallah P, Flashman E, Chayen NE, Redwood C, Squire JM. Crystal structure of the C1 domain of cardiac myosin binding protein-C: implications for hypertrophic cardiomyopathy. J. Mol. Biol. 2008;378:387–397. doi: 10.1016/j.jmb.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Orlova A, Egelman EH. A conformational change in the actin subunit can change the flexibility of the actin filament. J. Mol. Biol. 1993;232:334–341. doi: 10.1006/jmbi.1993.1393. [DOI] [PubMed] [Google Scholar]
- 21.McCullough BR, Blanchoin L, Martiel JL, De La Cruz EM. Cofilin increases the bending flexibility of actin filaments: implications for severing and cell mechanics. J. Mol. Biol. 2008;381:550–558. doi: 10.1016/j.jmb.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkin VE, Orlova A, VanLoock MS, Shvetsov A, Reisler E, Egelman EH. ADF/cofilin use an intrinsic mode of F-actin instability to disrupt actin filaments. J. Cell Biol. 2003;163:1057–1066. doi: 10.1083/jcb.200308144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galkin VE, Orlova A, Schroder GF, Egelman EH. Structural polymorphism in F-actin. Nat. Struct. Mol. Biol. 2010;17:1318–1323. doi: 10.1038/nsmb.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467:724–728. doi: 10.1038/nature09372. [DOI] [PubMed] [Google Scholar]
- 25.Rybakova IN, Greaser ML, Moss RL. Myosin Binding Protein C Interaction with Actin: CHARACTERIZATION AND MAPPING OF THE BINDING SITE. J. Biol. Chem. 2011;286:2008–2016. doi: 10.1074/jbc.M110.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsudaira P. Modular organization of actin crosslinking proteins. Trends in Biochemical Sciences. 1991;16:87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- 27.Gimona M, Mital R. The single CH domain of calponin is neither sufficient nor necessary for F-actin binding. J. Cell Sci. 1998;111:1813–1821. doi: 10.1242/jcs.111.13.1813. [DOI] [PubMed] [Google Scholar]
- 28.Galkin VE, Orlova A, Fattoum A, Walsh MP, Egelman EH. The CH-domain of calponin does not determine the modes of calponin binding to F-actin. J. Mol. Biol. 2006;359:478–485. doi: 10.1016/j.jmb.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Galkin VE, Orlova A, Cherepanova O, Lebart MC, Egelman EH. High-resolution cryo-EM structure of the F-actin-fimbrin/plastin ABD2 complex. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1494–1498. doi: 10.1073/pnas.0708667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galkin VE, Orlova A, Salmazo A, Djinovic-Carugo K, Egelman EH. Opening of tandem calponin homology domains regulates their affinity for F-actin. Nat. Struct. Mol. Biol. 2010;17:614–616. doi: 10.1038/nsmb.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herron TJ, Rostkova E, Kunst G, Chaturvedi R, Gautel M, Kentish JC. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ. Res. 2006;98:1290–1298. doi: 10.1161/01.RES.0000222059.54917.ef. [DOI] [PubMed] [Google Scholar]
- 32.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the Actin-Myosin Complex and Its Implications for Muscle Contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 33.Holmes KC, Angert I, Kull FJ, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 34.DasGupta G, Reisler E. Actomyosin Interactions in the Presence of ATP and the N-terminal Segment of Actin. Biochemistry. 1992;31:1836–1841. doi: 10.1021/bi00121a036. [DOI] [PubMed] [Google Scholar]
- 35.DasGupta G, Reisler E. Nucleotide-Induced Changes in the Interaction of Myosin Subfragment 1 with Actin: Detection by Antibodies against the N-terminal Segment of Actin. Biochemistry. 1991;30:9961–9966. doi: 10.1021/bi00105a021. [DOI] [PubMed] [Google Scholar]
- 36.Saber W, Begin KJ, Warshaw DM, VanBuren P. Cardiac myosin binding protein-C modulates actomyosin binding and kinetics in the in vitro motility assay. J. Mol. Cell Cardiol. 2008;44:1053–1061. doi: 10.1016/j.yjmcc.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook RK, Root D, Miller C, Reisler E, Rubenstein PA. Enhanced stimulation of myosin subfragment 1 ATPase activity by addition of negatively charged residues to the yeast actin NH2 terminus. J. Biol. Chem. 1993;268:2410–2415. [PubMed] [Google Scholar]
- 38.DasGupta G, Reisler E. Antibody Against the Amino Terminus of α-Actin Inhibits Actomyosin Interactions in the Presence of ATP. J. Mol. Biol. 1989;207:833–836. doi: 10.1016/0022-2836(89)90249-0. [DOI] [PubMed] [Google Scholar]
- 39.Gu J, Xu S, Yu LC. A model of cross-bridge attachment to actin in the A*M*ATP state based on x-ray diffraction from permeabilized rabbit psoas muscle. Biophys. J. 2002;82:2123–2133. doi: 10.1016/S0006-3495(02)75559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razumova MV, Shaffer JF, Tu AY, Flint GV, Regnier M, Harris SP. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: Evidence for long-lived cross-bridges. J. Biol. Chem. 2006;281:35846–35854. doi: 10.1074/jbc.M606949200. [DOI] [PubMed] [Google Scholar]
- 41.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 42.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 43.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 44.Chik JK, Lindberg U, Schutt CE. The structure of an open state of β-actin at 2.65 Ångstrom resolution. J. Mol. Biol. 1996;263:607–623. doi: 10.1006/jmbi.1996.0602. [DOI] [PubMed] [Google Scholar]
- 45.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 46.Pirani A, Vinogradova MV, Curmi PM, King WA, Fletterick RJ, Craig R, Tobacman LS, Xu C, Hatch V, Lehman W. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J. Mol. Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]