Abstract
Temperature-dependent induction of ecdysteroid deficiency in the ecdysoneless mutant ecd1 adult Drosophila melanogaster results in altered courtship behavior in males. Ecdysteroid deficiency brings about significantly elevated male-male courtship behavior including song production resembling that directed towards females. Supplementation with dietary 20-hydroxyecdysone reduces male-male attraction, but does not change motor activity, courtship patterns or attraction to females. These observations support the hypothesis that reduced levels of ecdysteroids increase the probability that male fruit flies will display courtship behaviors to male stimuli.
Keywords: male-male, ecdysone, neuroactive, courtship, bisexual, preference
Introduction
Courtship behavior in male Drosophila requires the interplay among sex-determination hierarchy mechanisms that regulate sex-specific differentiation of central nervous circuits, perception and integration of attractive sensory cues, and precisely patterned motor output (Villella and Hall, 2008). We investigated whether male courtship behavior is modulated by alteration of ecdysteroid signaling in the adult fly. As demonstrated in various insect species, this family of steroid hormones is involved in developmental processes including regulation of apoptosis, cellular differentiation, ecdysis and metamorphosis (Riddiford, 1993; Cayre et al., 2000), as well as adult physiological processes and behaviors such as memory formation, sleep, feeding, locomotion and age-related division of labor in social hymenopterans (Haskell and Moorhouse, 1963; Beach, 1979; Nemoto and Hara, 2007; Takeuchi et al. 2007; Ishimoto et al., 2009; Ishimoto and Kitamoto, 2010). In addition, previous studies reported the occurrence of male-male courtship in Drosophila melanogaster with reduced intracellular ecdysone receptor activity (Ganter et al., 2007; Dalton et al., 2009). In addition to various isoforms of ecdysone receptor proteins that heterodimerize with ultraspiracle protein to form a functional receptor that translocates to the nucleus after ecdysteroid binding (Yao et al., 1993), Drosophila also contains membrane-bound steroid receptors that mediate non-genomic effects on various cellular processes including reduction of synaptic transmitter release (Li et al., 2001).
The ecdysoneless gene was identified in a mutant screen by its ecdysteroid-deficiency phenotype, such that larvae homozygous for various mutant alleles of ecdysoneless fail to progress through molts (Garen et al., 1977). Flies with mutations in ecdysoneless have greatly reduced levels of ecdysteroid (eg. 2 pg per ecdysoneless1 (ecd1) fly at 29°C, compared with 14 pg for wild-type controls), and larvae provided with a diet supplemented with 20-hydroxyecdysone (20E) can be rescued to successful pupariation (Garen et al., 1977). The function of the Drosophila ecdysoneless gene product has yet to be described, although a human ortholog has been shown to interact with the tumor suppressor p53 (Zhang et al., 2006) and has been implicated in cell cycle control (Kim et al., 2009).
We used a temperature sensitive allele of ecdysoneless, ecd1, to reduce ecdysteroid availability in adult male flies. Since ecdysteroid signaling is necessary for development, this conditional allele allows successful development when animals are maintained at a permissive temperature of 18°C. Upon shift to a restrictive temperature of 29°C, ecdysteroid levels drop to about 13% of normal (Garen et al., 1977). We found that when adult ecd1 males are deprived of normal ecdysteroid levels in this manner, they exhibit significantly altered courtship behavior. Strikingly, ecdysteroid-depleted males court other males, including unilateral wing extension and vibration that produces song patterns similar to those directed towards females.
Materials and Methods
Flies and conditions
Wild-type (Canton-S) and ecd1 / ecd1 (FBgn0000543) flies were obtained from the Bloomington Drosophila Stock Center (stocks #1 and #218, respectively). Flies were maintained on dextrose-yeast-cornmeal medium, 18°C, 50–60% humidity, 12h light:12h dark. To ensure social naïveté in behavioral trials, all flies were isolated in individual vials as pharate adults. Three days after eclosion, male experimental flies were transferred to 29°C, then assayed five days after eclosion. Experimental flies in rescue experiments were fed standard food supplemented with 10−3M 20-hydroxyecdysone (20E: Sigma), control diet consisted of the standard food supplemented with solvent only (ethanol, 0.03% total).
Assays and analysis
After the treatment period at 29°C or 18°C and within 4 hours of subjective dawn, test males were aspirated without anesthesia into 35 mm diameter (1.25 cm3) plastic assay chambers containing a layer of food and decapitated wild-type or ecdysteroid depleted targets of similar age (male in Fig 1, female in Fig 3A, male and female in Fig 3B); video recording was initiated immediately. In Figure 2, a group of 12 intact ecdysone depleted males were placed together in the 35 mm chamber. Target animals were decapitated using carbon dioxide anesthesia and a razor blade, then allowed to recover to a standing position before use. Assays were recorded by overhead video cameras placed in a 29°C incubator. Video recordings were analyzed from minute 10 to minute 20 by trained observers blind to the genotype and experimental treatment of the subjects, the first 10 minutes of the period being excluded from analysis to allow acclimation following transfer. Stopwatches were used to measure the total time each subject spent performing unilateral wing extension, licking, or attempting copulation with the target fly during the 10-minute period. Courtship index was calculated as the percentage of total duration of these behaviors per 600 seconds of assay time. In motor activity assays (Fig 4), flies were placed in courtship chambers not supplied with courtship targets, and the number of times each fly crossed the midline was counted from minute 10 to minute 20. Mann-Whitney (Fig 1), Wilcoxon rank sum test (Fig 3B) or Student t-test (Fig 3A, 4) were used to analyze the results (InStat 3.0 from GraphPad).
Figure 1. Male-male courtship.
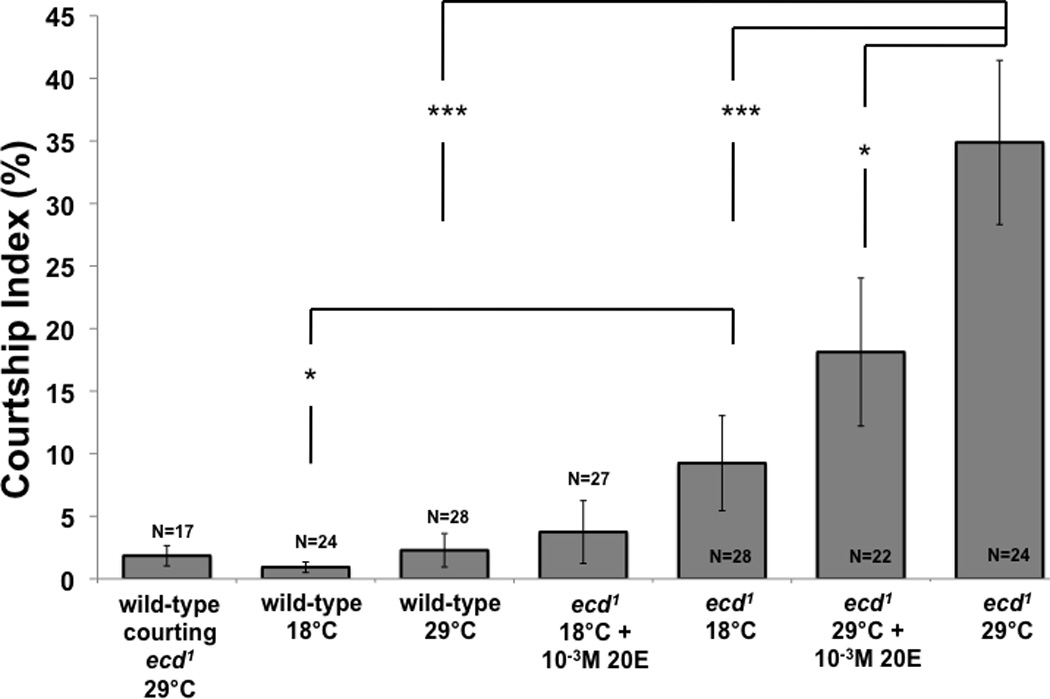
ecd1 males treated at 29°C for 48 hours to reduce ecdysteroid availability show increased male-male courtship. The descriptions underneath each histogram bar describe treatment condition of subject males flies. Socially naive five-day-old ecd1 male subjects (“ecd1 29°C”) court mature decapitated wild-type (Canton-S) target males with significantly longer duration than do either similarly treated wild-type subjects (“wild-type 29°C”) or ecd1 males not heat-treated (“ecd1 18°C”). Male-male courtship levels of wild-type 29°C subjects was not different from that of wild-type 18°C subjects (Mann-Whitney). ecd1 29°C subject males fed diet containing 10−3M 20E during exposure to 29°C show significantly less male-male courtship than identically treated males fed normal diet (* indicates p < 0.05, Mann-Whitney). Wild-type 29°C subject males courted ecd1 29°C objects at the same level at which they court wild type 29°C objects (Mann-Whitney). Courtship Index (see Materials and Methods) was measured for 10 minutes, beginning 10 minutes after male subjects were introduced to the chambers. *** indicates p < 0.001 (Mann-Whitney), whiskers indicate SEM.
Figure 3. A. Attraction to females.
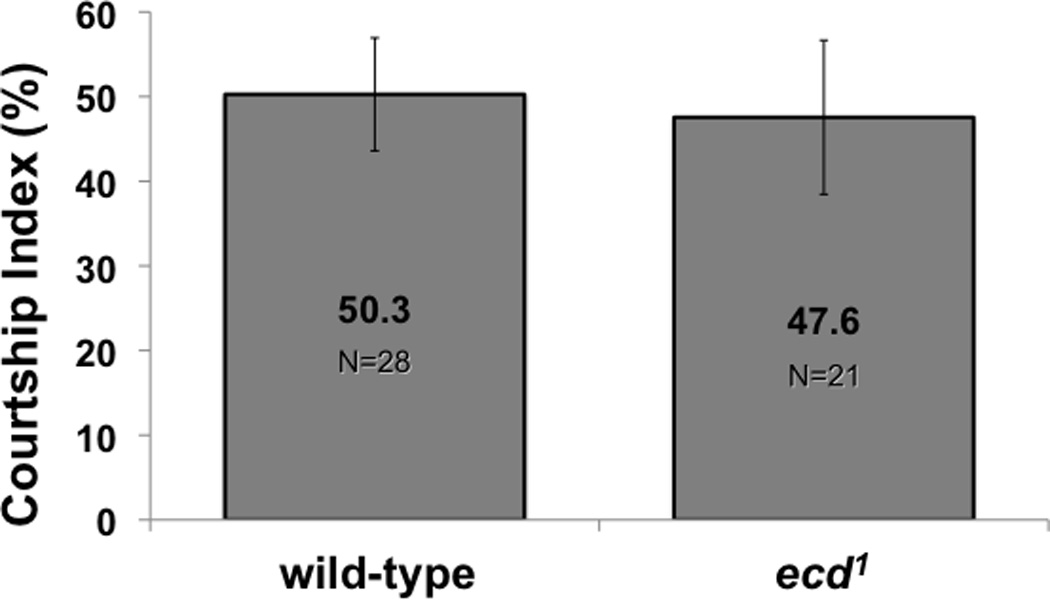
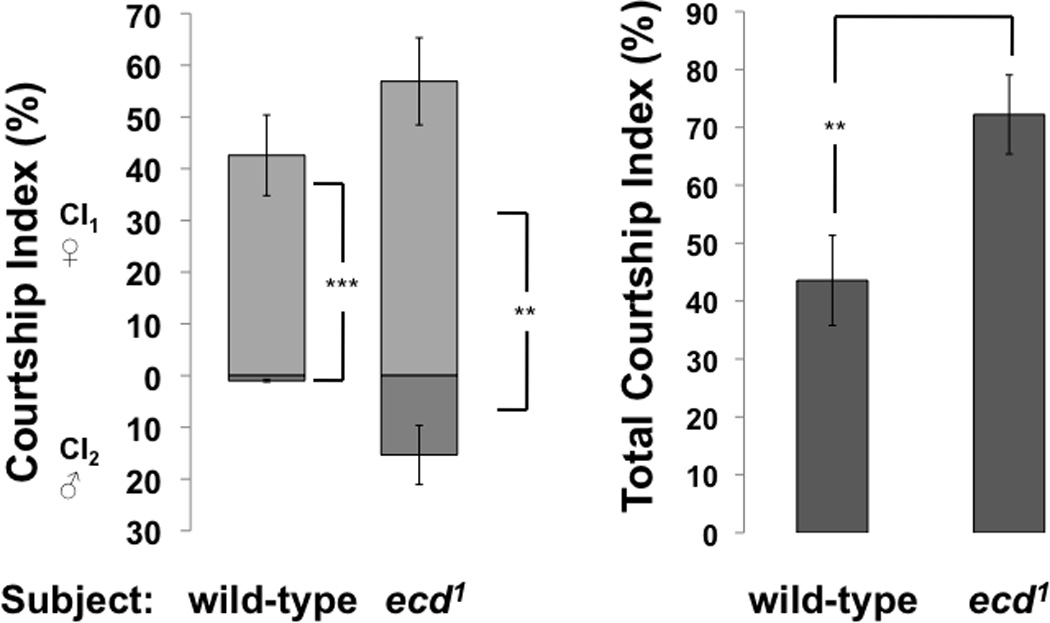
Adult ecd1 males treated at 29°C to reduce ecdysteroid availability court females at levels similar to those of wild-type males. B. Courtship preference. At left, adult ecd1 males treated at 29°C court females (CI1) and males (CI2) when offered a choice of decapitated mature virgin male and female targets in the same experiment. In contrast, wild-type males almost exclusively court the female target. Both wild-type (Canton-S) and ecd1 males significantly prefer to court females over males (compare CI1 with CI2; ** indicates p < 0.01; *** indicates p < 0.001). In these choice experiments, the total duration of courtship to both targets (CI1 + CI2) was significantly higher in ecd1 males than in wild-type (right diagram). The duration of all unilateral wing extension, tapping, licking, and attempting copulation in a ten-minute period was measured. P values are from Wilcoxon Matched Pairs test (left) and t-test (right), whiskers indicate SEM.
Figure 2. Male courtship chains.

ecd1 males treated at 29°C for 48 hours to reduce ecdysteroid availability engage in male-male courtship chains, in which males courts a more anterior male, while simultaneously being courted by a following male.
Figure 4. Motor activity levels.
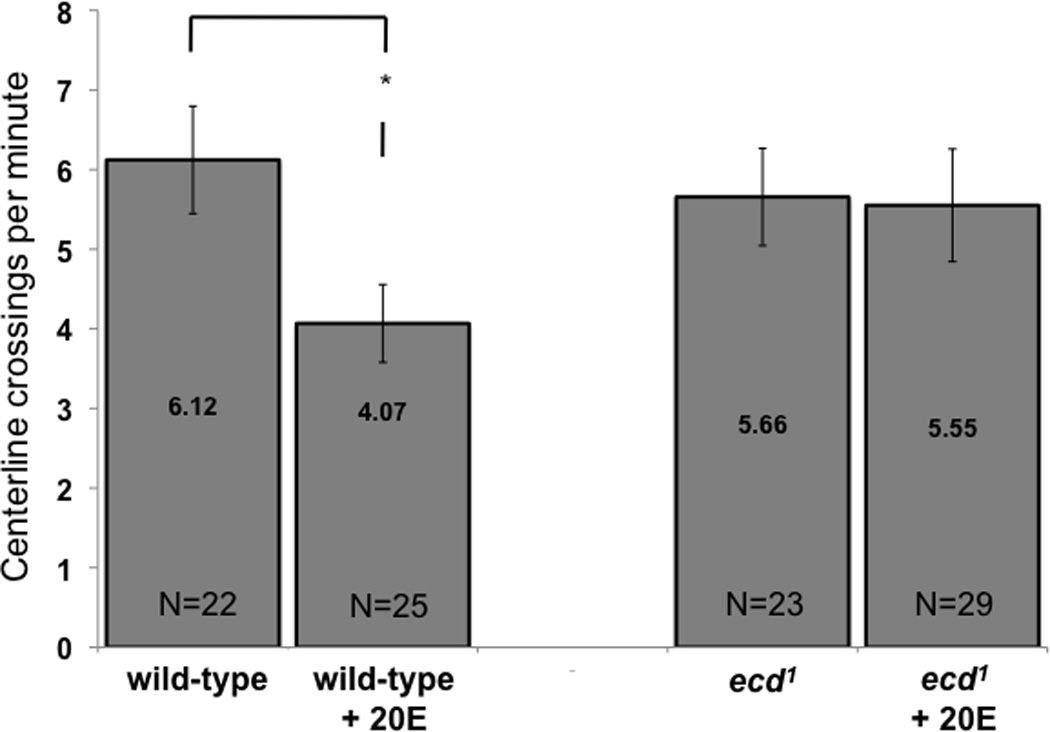
Adult ecd1 males treated at 29°C to reduce ecdysteroid availability show similar levels of spontaneous locomotor activity as wild-type males. Flies were placed in courtship chambers without courtship targets and analyzed for 10 minutes. Activity of wild-type decreased when fed 10−3M 20E during the 48-hour treatment at 29°C (* indicates p < 0.05), but this was not the case with ecd1 males. The number of centerline crossings was counted, and is presented as number of crossings per minute. P values are from Student t-test, values inside bars are means and whiskers indicate SEM.
Courtship songs were continuously recorded during 20-minute interactions of one wild-type or one ecd1 male with one decapitated wild-type female or male target at room temperature (Fig 5). Acoustic signals were recorded with a microphone (Bruel & Kjaer Type 4165), amplified (Bruel & Kjaer Type 2619 and 5935) and directly digitized. The software Audacity 1.3.12beta (http://audacity.sourceforge.net) was used for data acquisition and analysis. Frequency spectra of pulse songs and sine songs were determined by Fourier transformation with a 2048 width Hanning window. Song sequences that contained similar durations of both sine and pulse song were selected for comparison.
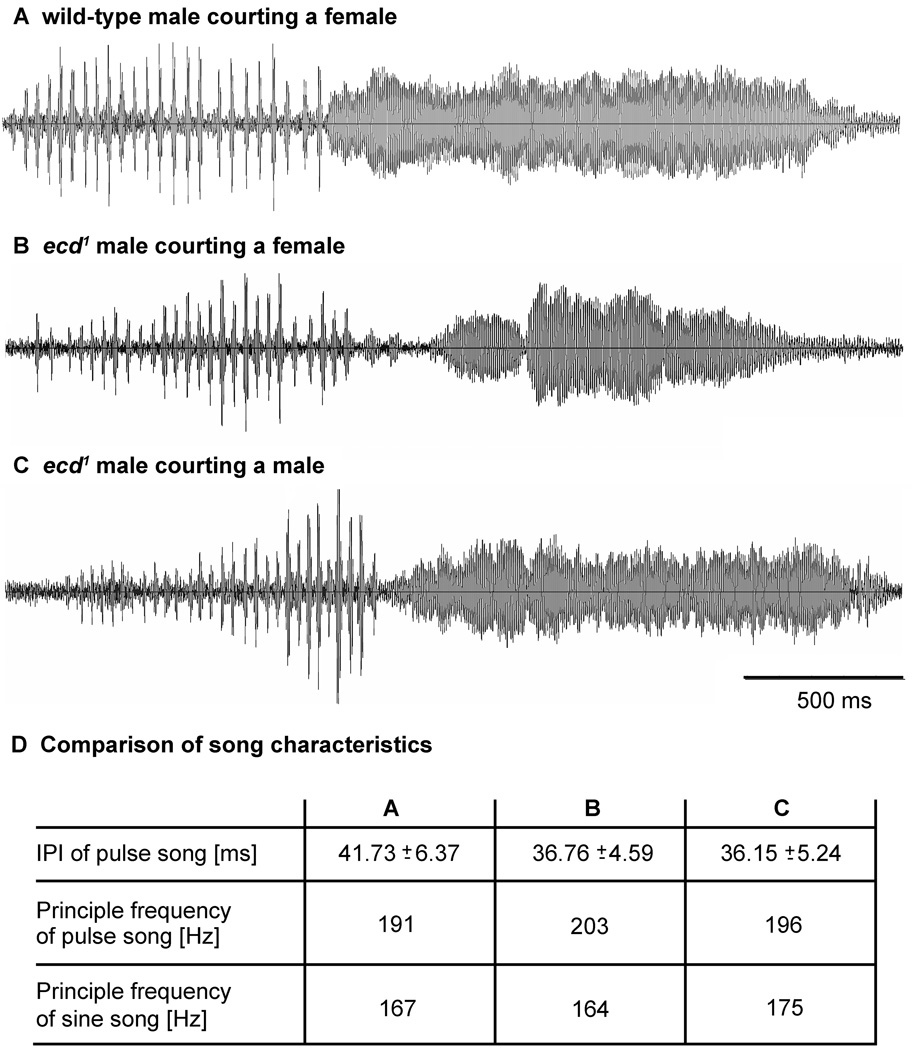
Figure 5. Courtship songs.
Adult ecd1 males treated at 29°C to reduce ecdysteroid availability generate similar courtship song patterns as wild-type males towards both female and male targets. Acoustic signals that contained pulse and sine song elements of similar durations were selected from 20 min. recordings of a wild-type (A) and an ecd1 mutant male (B) courting a decapitated wild-type female and the same ecd1 mutant male courting a decapitated wild-type male (C). D. Acoustic parameters. Major frequency components of pulse song and sine song and inter pulse intervals (IPI) were similar in all recordings displayed in A–C and in songs recorded from other wild-type and ecd1 mutant males under the same conditions (not shown).
Results
Adult ecd1 males shifted from 18°C (the rearing temperature) to 29°C for 48 hours prior to assaying their courtship behavior exhibited significantly higher levels of courtship toward decapitated mature wild-type male targets than did identically treated wild-type subjects (p < 0.0001; Fig 1) and ecd1 males that were not shifted to 29°C (p < 0.0002; Fig 1). This temperature shift did not alter the behavior of wild-type males. The resulting homosexual courtship in ecd1 males included tapping, licking, unilateral wing extension, and attempted copulation, although these components were not individually measured. When ecd1 males were fed 10−3M 20E during the 48 hour 29°C treatment, a reduction in the resulting homosexual courtship was observed (p = 0.0303, Mann-Whitney, Fig 1). Wild-type males fed the same diet did not show any changes in courtship behavior (not shown). When decapitated mature ecdysteroid-depleted ecd1 males treated at 29°C were used as targets, they did not elicit elevated levels of courtship from wild-type males treated at 29°C (Fig 1), indicating that ecd1 males are distinguished from females. ecd1 males maintained at 18°C courted wild-type male targets more intensively than wild-type males at the same temperature. This difference was abolished by feeding these ecd1 males with 20E. Groups of intact ecd1 males formed courtship chains, with each male following and singing to the male ahead of him while at the same time being courted by a following male (Fig 2). Chaining behavior was not observed in similarly treated wild-type males.
Ecdysteroid-depleted ecd1 males courted both female targets and male targets (Fig 3). The attraction of wild-type and ecd1 male subjects to a decapitated female target was similar (Fig 3A). When mature ecd1 males treated at 29°C were offered a choice between decapitated wild-type targets of both sexes in the same assay chamber, they courted the female targets at the same levels observed when using wild-type male subjects (Fig 3B, left panel). At the same time, ecd1 males showed elevated courtship towards the male targets, compared with wild-type control subjects. In the presence of a female target, however, the attraction of the ecd1 male subject to the male target was diminished, in comparison with the levels observed when the male target was presented alone (compare with Fig 1). Wild-type and ecd1 males discriminated significantly between male and female targets (p = 6.4 × 10−5 and 0.007 respectively, Wilcoxon Matched Pairs). We did notice that in this assay, the overall levels of courtship to both targets was significantly higher in ecd1 males than were the total levels of wild-type males (p = 0.009, Student t-test; Fig 3B right panel). This difference resulted from an increase of total courtship activity of ecd1 males in the presence of two courtship targets, while courtship activity towards one or two targets was not different in wild-type males (compare Fig 3A with Fig 3B right panel).
Motor activity of ecdysteroid-depleted ecd1 males was similar to that of wild-type (Fig 4). When ecdysoneless1 and wild-type males were analyzed individually in courtship chambers without courtship targets provided, the centerline crossing rate of ecd1 males was comparable to that of wild-type. When these flies were fed 10−3M 20E during the 48 hour 29°C treatment, activity of ecd1 males was not affected, while activity of wild-type males decreased (p = 0.016, Student t-test).
During courtship, ecd1 males generated sound signals by vibration of one extended wing. Both wild-type and ecd1 mutants produced rather irregular series of pulse song and sine song, the principal acoustic patterns of Drosophila courtship (Fig 5). Analysis of frequency composition of pulse and sine song elements and of inter pulse intervals (IPI) of songs from wild-type males courting a female (Fig 5A), ecd1 males courting a female (Fig 5B) and the same ecd1 males courting a male target (Fig 5C) revealed no differences (Fig 5D), suggesting that ecd1 males are fully capable of producing attractive courtship songs.
Discussion
Ecdysteroid is known to exert influence on the fly nervous system in various ways. By either membrane or nuclear mechanisms or both, ecdysteroid is capable of controlling neuron development including proliferation and apoptosis (Riddiford, 1993; Freeman et al., 1999), cell differentiation (Sprecher and Desplan, 2008), and neurite remodeling (Grueber and Jan, 2004). In addition to its canonical roles in insect development, ecdysteroid function has also been described in adult physiology and behavior, although to a lesser extent. For example ecdysteroid has been shown to influence excitability of Drosophila neurons (Srivastava et al., 2005), and has been implicated in memory and sleep (Ishimoto et al., 2009; Ishimoto and Kitamoto, 2010). It has been reported that reduction of the activity of the intracellular ecdysone receptor leads to male-male courtship (Ganter et al., 2007; Dalton et al., 2009). Using the well-characterized conditional ecdysoneless mutant ecd1, we show in this study that male-male courtship can result from reduction of ecdysteroid levels in adult male flies while details of courtship behavioral components and locomotor activity are not affected. While absolute ecdysteroid levels were not determined in this study, the level of ecdysteroid reduction was sufficient to cause failure of molting and metamorphosis at 29°C (not shown) as described in earlier studies of this mutant (Garen et al., 1977; Gaziova et al., 2004).
ecd1 adult males treated at 29°C to reduce ecdysteroid levels show significantly greater male-male courtship than wild-type controls (Fig 1). The male-male courtship repertoire of temperature shifted ecd1 male subjects includes elements typical of male-female courtship behavior, including orientation, following, unilateral wing extension, production of sine and pulse song, tapping, licking, and attempted copulation, indicating that significant reduction of ecdysteroid level after eclosion neither impair their performance nor the correct sequence of their appearance. Rather, ecdysteroid depletion seems to modulate whether and how frequently courtship behavior is induced by male-specific sensory stimuli. Groups of ecdysteroid-depleted ecd1 males form courtship chains (Fig 2) as does the fruitless1 mutant (Gailey and Hall, 1989), whereas wild-type males do not. Such chains are suggested to be due to the failure of fruitless1 males to recognize inhibitory male cues, and this may be the case with ecd1 males. Supplementation of an ecd1 male’s food with 20E during the shift to restrictive temperature (29°C) reduces his male-male courtship level significantly (Fig 1), supporting the hypothesis that the ecdysteroid deficiency phenotype of ecd1 flies underlies the induction of elevated male-male courtship levels.
While ecdysteroid depleted male flies are attracted to one another such that groups will exhibit chaining behavior (Fig 2), they are not strongly attractive to wild-type males (Fig 1). This result indicates that ecd1 male targets are fully recognized as males by wild-type male subjects and that the reduction of ecdysteroid signaling in this mutant does not alter a male target’s detectable male phenotype. In comparison, certain fruitless mutants, in addition to their courtship of male targets as mentioned above, are also themselves more attractive to wild-type males (Gailey and Hall, 1989).
In the presence of a wild-type female target, ecdysteroid depleted males and wild-type males are observed to court her at comparable levels (Fig 3A). Hence, the increased attraction of ecd1 males to other males is not connected to their reduced attraction to females. By comparison, in some fruitless1 mutants, male-male attraction is connected to a loss of attraction to females, to the point of behavioral sterility (Gailey and Hall, 1989). When offered both a male and a female target at the same time, wild-type and ecd1 male subjects court the female target significantly more than the male target (Fig 3B). Total courtship duration in the ecd1 male was significantly higher than that of the wild-type male (p = 0.009, Student t-test), but duration of courtship of females was similar. Thus, ecdysteroid depletion does not significantly raise or lower the attraction of male flies to females, instead it adds the new element of male-male courtship to its otherwise unaltered courtship repertoire.
Apart from directing courtship toward both female and male targets, courtship behavior of ecd1 males is not different from wild-type (reviewed by Greenspan and Ferveur, 2000). ecd1 mutant males display all behavioral components of courtship, including approach, tapping, licking, unilateral wing extension and copulation. Wing extension and its vibration generated acoustic courtship signals that included pulse song and sine song elements. These songs have been demonstrated to contain species-specific spectro-temporal patterns (Kyriacou and Hall, 1982) and can clearly be distinguished from aggression songs generated during male agonistic interactions (Jonsson et al., 2011). Analysis of the song patterns generated by ecd1 males (Fig 5) revealed similar carrier frequencies and inter pulse intervals as recorded from wild-type males in the same setup (Fig 5D) and reported from earlier studies (Rybak et al., 2002; Wheeler et al., 1988) suggesting that ecdysteroid signaling is not involved in control of song pattern generating neuronal activity. Moreover, female target-directed and male target-directed song patterns of ecd1 males were the same and aggression songs, that are produced by vibration of both wings, lack sine song elements and consist of longer interpulse intervals (Jonsson et al., 2011), were never observed during male-directed interactions. This suggests that ecd1 males consistently recognize other males as potential mating partners and not as competitors.
The male-male courtship results presented here agree with previous studies using ecdysone receptor (EcR) mutants (Ganter et al., 2007, Dalton et al., 2009). In those experiments utilizing assay conditions similar to those reported here, induced ecdysone receptor hypomorphy resulted in a similar homosexual courtship phenotype. The observation of the same courtship phenotype associated with mutations that reduce ecdysteroid signaling from both the ligand availability and receptor aspects of the pathway, and in distinct genetic backgrounds, is another indication that the homosexual courtship observed in each case is in fact caused by disruption of ecdysteroid signaling.
The evidence presented supports the hypothesis that ecdysteroid modulates courtship behavior in Drosophila. In adult males, reduction of the levels of this steroid hormone significantly alters the gender orientation component of courtship behavior without changing the behavioral modules of courtship behavior or attractiveness to wild-type males, and rescue with dietary hormone restores behavior that is more similar to wild-type. Restoration of normal behavior is not complete, however, perhaps because ecdysteroid levels at the critical sites of action remain reduced despite the exogenous 20-hydroxyecdysone supplied. Since individual components of courtship are unaffected and ecd1 males can distinguish males from females, altered levels of ecdysone do not seem to affect pattern-generating circuits in thoraco-abdominal neuromeres and sensory organs involved in the perception of sex-specific signals. The elevated male-male attraction observed in ecdysteroid-depleted males rather indicates that ecdysteroids regulate courtship decision-making in the fly by connecting male-specific cues to courtship initiation instead of to agonistic behavior.
General locomotor activity level does not appear to change with ecdysteroid deficiency (Fig 4), although we note that feeding wild-type males 1mM 20-hydoxyecdysone led to a significant decrease in activity. This treatment did not have the same effect on ecd1 males, perhaps because these males were restored to more normal ecdysteroid levels by the treatment, whereas wild-type males were subjected to ecdysteroid levels that are higher than normal, and such overdose somehow leads to reduced motor activity. Regulation of cell proliferation has been shown to depend on ecdysteroid “threshold” concentrations (Cherbas and Cherbas, 1981; Peel and Milner 1992; Champlin and Truman, 1998) and similar mechanisms may also regulate physiology and behavior of adult flies.
A male-male courtship phenotype resulted when EcR deficiency was specifically induced in neurons that activate the P1 promoter of fruitless (fru-P1; Dalton et al., 2009) agreeing well with the results presented here. In comparison with that study, however, we note an interesting difference. While Dalton and coworkers noticed no increase in male-male courtship when EcR hypomorphy was induced after eclosion, we measured a significant increase in male-male courtship in ecd1 flies shifted to 29°C three days after eclosion, and noticed that longer incubations at that restrictive temperature produce a more dramatic phenotype (not shown), both in the present study and in our previous study using EcR hypmorphy (Ganter et al., 2007). Because the method used in this report produces a hypomorphy in ecdysteroid signaling that is global rather than restricted to fru-P1 neurons, as was the method in Dalton’s work, the discrepancy may suggest that cells other than fru-P1 use ecdysteroid signaling to influence courtship behavior in the adult. In any case, it seems that a certain ecdysteroid-dependent plasticity exists in the mechanisms that control courtship behavior in adult male flies.
It is hypothesized that sex specific patterns of behavior in humans can be influenced in the developing brain by exposure to varying levels of steroid hormones (Robinson and Manning, 2000). Our results illustrate that steroids serve a behavioral role in the fruit fly Drosophila melanogaster, and that study of this genetically tractable model organism may illuminate our understanding of mechanisms at play in the shaping of courtship behavior in other animals.
Conclusions
Our studies indicate that a steroid hormone exerts considerable control over courtship behavior in male fruit flies. Steroids are known to be important modulators of courtship behavior in many animals including fish, birds, and rodents (Bass, 2008; McCarthy and Ball, 2008). Our results support the hypothesis that ecdysteroids modulate courtship behavior in Drosophila melanogaster. Study of this genetically tractable model organism will allow the elucidation of the mechanisms by which steroids influence courtship and other sex-specific behaviors in other animals.
Acknowledgements
The authors thank R. Weston for technical assistance. This work was supported by grants from the National Institutes of Health (NIGMS R15 GM080713-01 and 3R15GM080713-01A1S1 to GG), and by the Dean's and Provost's offices of the College of Arts and Sciences, University of New England, and these funding sources had no scientific involvement. Fly stocks were provided by the Bloomington Drosophila Stock Center at Indiana University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
GK Ganter, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
AE Panaitiu, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
J Desilets, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
J Davis-Heim, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
EA Fisher, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
LCH Tan, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
R Heinrich, Department of Cellular Neurobiology, Institute for Zoology, Georg-August-University, Göttingen, Germany.
EB Buchanan, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
KM Brooks, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
MT Kenney, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
MG Verde, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
J Downey, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
AM Adams, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
JS Grenier, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
S Maddula, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
P Shah, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
K Kincaid, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
Works Cited
- Bass AH. Steroid-dependent plasticity of vocal motor systems: Novel insights from teleost fish. Brain Research Reviews. 2008;57(2):299–308. doi: 10.1016/j.brainresrev.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Beach R. Mosquitoes: biting behavior inhibited by ecdysone. Science. 1979;205:829–831. doi: 10.1126/science.205.4408.829. [DOI] [PubMed] [Google Scholar]
- Cayre M, Strambi C, Strambi A, Charpin P, Ternaux J-P. Dual effect of ecdysone on adult cricket mushroom bodies. European Journal of Neuroscience. 2000;12:633–642. doi: 10.1046/j.1460-9568.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth Manduca sexta. Development. 1998;125:269–277. doi: 10.1242/dev.125.2.269. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Cherbas P. The effects of ecdysteroid hormones on Drosophila cell lines. Advances in Cell Culture. 1981;1:91–124. [Google Scholar]
- Dalton JE, Lebo MS, Sanders LE, Sun F, Arbeitman MN. Ecdysone receptor acts in fruitless- expressing neurons to mediate Drosophila courtship behaviors. Current Biology. 2009;19(17):1447–1452. doi: 10.1016/j.cub.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Dobritsa A, Gaines P, Segraves WA, Carlson JR. The dare gene: steroid hormone production, olfactory behavior, and neural degeneration in Drosophila. Development. 1999;126:4591–4602. doi: 10.1242/dev.126.20.4591. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Hall JC. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Erratum in: Genetics. 1989;122(2):465. doi: 10.1093/genetics/121.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter GK, Merriman J, Salmon M, Walton K, Kravitz EA. EcR deficiency results in male-male courtship in adult flies. Behavior Genetics. 2007;37(3):507–512. doi: 10.1007/s10519-006-9140-1. [DOI] [PubMed] [Google Scholar]
- Garen A, Kauvar L, Lepesant JA. Roles of ecdysone in Drosophila development. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(11):5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131(11):2715–2725. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annual Reviews in Genetics. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan YN. Dendritic development: Lessons from Drosophila and related branches. Current Opinion in Neurobiology. 2004;14(1):74–82. doi: 10.1016/j.conb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Haskell PT, Moorhouse JE. A blood-borne factor influencing the activity of the central nervous system of the desert locust. Nature. 1963;197:56. [Google Scholar]
- Ishimoto H, Kitamoto T. The steroid molting hormone ecdysone regulates sleep in adult Drosophila melanogaster. Genetics. 2010;185(1):269–281. doi: 10.1534/genetics.110.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6381–6386. doi: 10.1073/pnas.0810213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Kravitz EA, Heinrich R. Sound production during agonistic behavior of male Drosophila melanogaster. Fly. 2011;5(1) doi: 10.4161/fly.5.1.13713. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Gurumurthy CB, Naramura M, Zhang Y, Dudley AT, Doglio L, Band H. Role of mammalian ecdysoneless in cell cycle regulation. Journal of Biological Chemistry. 2009;284(39):26402–26410. doi: 10.1074/jbc.M109.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. The function of courtship song rhythms in Drosophila. Animal Behavior. 1982;30:794–801. [Google Scholar]
- Li H, Harrison D, Jones G, Jones D, Cooper RL. Alterations in development, behavior, and physiology in Drosophila larva that have reduced ecdysone production. Journal of Neurophysiology. 2001;85:98–104. doi: 10.1152/jn.2001.85.1.98. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Ball GF. Chapter 7: The Neuroendocrine Control of Sex Specific Behavior in Vertebrates. Lessons from Mammals and Birds. Current Topics in Developmental Biology. 2008;83:213–248. doi: 10.1016/S0070-2153(08)00407-9. [DOI] [PubMed] [Google Scholar]
- Nemoto M, Hara K. Ecdysone receptor expression in developing and adult mushroom bodies of the ant Camponotus japonicus. Development Genes Evolution. 2007;217:619–627. doi: 10.1007/s00427-007-0172-1. [DOI] [PubMed] [Google Scholar]
- Peel DJ, Milner MJ. The response of Drosophila imaginal disc cell lines to ecdysteroids. Roux’s Archive of Developmental Biology. 1992;202:23–35. doi: 10.1007/BF00364594. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3(3):203–209. [PubMed] [Google Scholar]
- Robinson SJ, Manning JT. The ratio of 2nd to 4th digit length and male homosexuality. Evolution and Human Behavior. 2008;21(5):333–345. doi: 10.1016/s1090-5138(00)00052-0. [DOI] [PubMed] [Google Scholar]
- Rybak F, Aubin T, Moulin B, Jallon JM. Acoustic communication in Drosophila melanogaster courtship: Are pulse- and sine-song frequencies important for courtship success? Canadian Journal of Zoology. 2002;80:987–996. [Google Scholar]
- Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454(7203):533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. The Journal of Neuroscience. 2005;25(26):6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Paul RK, Matsuzaka E, Kubo T. EcR-A expression in the brain and ovary of the honeybee (Apis mellifera L.) Zoological Science. 2007;24:596–603. doi: 10.2108/zsj.24.596. [DOI] [PubMed] [Google Scholar]
- Villella A, Hall JC. Chapter 3 Neurogenetics of Courtship and Mating in Drosophila. Advances in Genetics. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Fields WL, Hall JC. Spectral analysis of Drosophila courtship songs: D. melanogaster, D. simulans and their interspecific hybrid. Behavioral Genetics. 1988;18:675–703. doi: 10.1007/BF01066850. [DOI] [PubMed] [Google Scholar]
- Yao T-P, Forman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM. Functional ecdysone receptor is the product of EcR and ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Gurumurthy CB, Kim J, Bhat I, Gao Q, Dimri G, Lee SW, Band H, Band V. The human orthologue of Drosophila ecdysoneless protein interacts with p53 and regulates its function. Cancer Res. 2006;66(14):7167–7175. doi: 10.1158/0008-5472.CAN-06-0722. [DOI] [PubMed] [Google Scholar]