Highlights
► MSC regulatory networks gradually deteriorate with advancing age. ► MSC and HSC jointly share a stem cell niche in the bone marrow. ► MSC specifically interact with immune cells and secrete immunoregulatory molecules. ► Chronic inflammation of the MSC microenvironment leads to adverse manifestations. ► MSC in an aging environment perturb both tissue homeostasis and immunology.
Abstract
In adults, mesenchymal stromal cells contain tissue-specific multipotent stem cells, MSC, which can be found throughout the body. With advancing age, tight controls of regulatory networks, which guide MSC biology, gradually deteriorate. Aberrations within the MSC microenvironment such as chronic inflammation eventually lead to adverse manifestations, such as the accumulation of fat deposits in bone and muscles, impaired healing and fibrosis after severe injury, or altered hematopoiesis and autoimmunity. MSC can also specifically interact with a large variety of immune cells, and in doing so, they secrete cytoprotective and immunoregulatory molecules, which together with intercellular contacts mediate immune modulatory processes. This review comprehends the current knowledge regarding molecular mechanisms and cellular interactions that occur in stem cell niches, which are jointly shared between MSC and hematopoietic stem and progenitor cells, as well as those intracellular interdependences taking place between mesenchymal and a wide variety of hematopoietic progeny in particular T lymphocytes, which eventually perturb tissue homeostasis and immunology at advanced age.
Background
Aging of somatic tissues and organs comes along with a decline of regenerative capacity. Often, tissue homeostasis, regeneration and repair involve the consecutive emergence and parallel integration of new parenchymal cells, which descend from undifferentiated precursors.
Multipotent stromal progenitor cells also known as Mesenchymal Stem Cells (MSC) are pertinent tissue-specific stem cells in adult beings (Box 1). The concept of MSC appears to be particularly interesting since this special type of precursor can bring forth a large spectrum of cell types as diverse as bone, cartilage, tendon, or fat precursor cells. Besides these descendants, also terminally differentiated cells that are considered most common and widespread throughout the body, namely interstitial stromal cells and fibroblasts are reckoned direct offspring of MSC, albeit this assumption still lacks good experimental proofs.
Box 1.
MSC basic properties
| In vitro | In vivo |
|---|---|
| plastic adherence | mural vascular localization |
| clonogenic growth | marker: |
| tri-lineage differentiation | - CD146 |
| - osteogenic | - SCA1 |
| - adipogenic | - PDGFRα |
| - chondrogenic | - CXCL12 |
| surface marker | - nestin |
| - positive: CD73, CD90, CD105 (STRO1, SSEA4, CD56, CD271) | aging markers (ex vivo), |
| - negative:, CD34, CD45, CD11b or CD14, CD19or CD79alpha, HLA-DR | - CD106 |
| immune modulation | - CD295 |
| self renewal capacity | |
| hematopoietic support | |
| immune modulation |
MSC reside in a complex three-dimensional network, which comprises a plethora of other cell types such as, in the case of bone marrow, hematopoietic stem cells (HSC), adipocytes, and endothelial cells, altogether embedded in distinct extracellular matrix, and within this blend, MSC guide differentiation of hematopoietic precursor cells into mature progeny [1••]. MSC appear to exert yet another pertinent function, namely maintaining blood vessel integrity [2,3••]. Linked to these presumptions, it can be envisaged that upon tissue damage and injury, MSC are being activated and/or released from their perivascular niche, in order to support wound healing and tissue regeneration.
Also MSC are considered highly effective therapeutic assets to combat a wide spectrum of diseases: most puzzling in this context is that culture-expanded MSC exert immune modulatory activities inasmuch as graft-versus-host disease can be ablated when infusing high MSC numbers in deathly sick patients [4,5]; currently also the potential of ex vivo expanded MSC for cell-based therapeutic application in multiple sclerosis patients is being explored [6]; and last but not least based on their wide differentiation potential, defined approaches under the premise of regenerative medicine and tissue engineering with the aim of rebuilding damaged or diseased organs and body parts [7], has yielded a virtually endless list of only recently initiated clinical trials (see http://www.trial.gov).
With advancing age, tight control in many aspects of physiology, cell proliferation or signal transduction increasingly deteriorate, and it is reasoned that this ascending deficit may lead to derailed guidance of cell differentiation, which in turn is growing up to adverse effects such as the accumulation of fat deposits in bone and muscles, or impaired healing and fibrosis after severe injury, yet also altered hemopoiesis and autoimmunity.
MSC age
Similarly to somatic cells, stem cells experience lifelong exposure to various threads such as reactive oxygen species (ROS), biological toxins, harmful chemical agents or physical stressors, which taken together may lead to premature senescence of individual cells, or provokes accelerated cell death, yet also puts cells at risk to cellular transformation [8–11]. In this context, it is also worthwhile to assume that stem cells exhibit enhanced cellular repair capacities, or other still unveiled protective measures, which allow them to efficiently restore otherwise irreparable damages.
While extensive research regarding this particular topic has been undertaken for HSC, and distinct age-related changes and potent molecular mechanisms could be deciphered, distinct details about MSC aging taking place in vivo is scarce, simply because we are still lacking consistent knowledge about intrinsic properties in a bodily setting. Conspicuously many characteristic features regarding these stem and progenitor cell types were solely deduced from in vitro observations. Hence evaluations of their actual involvement in biological processes of regeneration and repair is currently undertaken, not considering actual activities and interactions that are effectively pursued by MSC in a well choreographed interplay with closely interacting niche cells. More than that such a cellular conglomerate is sure enough further coordinated through powerful factors permeating a jointly shared microenvironment. Also, most of the available literature is specifically focusing on bone marrow-derived MSC, which certainly restrains full comprehensive understanding of their action in vivo (see Box 1).
A first plain question regarding age-related variations is whether MSC numbers change during adult life span. Synopsis of published work yields conflicting results. This is however largely owned to the problem that there is no agreement on a single standardized protocol for MSC isolation as well as how to proceed with further analysis of primary cell isolates. Another important issue in this context is the often neglected fact that isolates comprise many different cell types, in particular macrophages and various other types of hematopoietic cells, which impact on colony formation in vitro. Moreover heterogeneous cultures clearly affect the differentiation potential of single clones, which makes it troublesome to determine the true degree of multipotency of primary MSC. Considering the proliferative potential with age, the published data are less conflicting because most authors report declining performances in long-term culture. This variance could be referred to the fact that MSC show telomere attrition at high passages and this type of genotoxic stress eventually may contribute to the limited replicative life-span in vitro. However the length of telomeric ends, although being significantly higher in children [12] is maintained at a considerable long length in adult age [13,14,15•]. This suggests that expression of telomerase takes place in MSC in vivo be it at a very low constitutive level, or in a transient fashion thereby maintaining the proper structure of chromosome ends. This clearly shows that changes occurring in MSC in vivo are only insufficiently described by patchy examinations of MSC, which are replicatively aged in culture.
Assuming a fit cell population can withstand accrued damage and the compounding of irreversible molecular changes longer than aged cells, specification by means of differential expression analysis of those aberrations that take place before a cell slithers into the final state of senescence was expected to yield meaningful insights. Working along this line, CD295 (leptin receptor) was found to increase as a function of intrinsic cellular aging [16]. Flow cytometric analysis of this surface marker further discriminated a distinct CD295-bright subpopulation, which interestingly enough also stained positive for annexin V. Conclusively, enhanced CD295 expression marks apoptotic cells. Interestingly, the death rate steadily raises with increasing cellular age. Taken this example into further consideration, it is certainly more valuable to distinguish phenotypic appearances in MSC, which are being isolated from differently aged healthy individuals to unveil those mechanisms, which actually take place in a natural situation instead of solely studying in vitro MSC senescence. Indeed, grossevaluations regarding the increased production of ROS [17], deviating SOD activity [18], whole genome gene expression profiles [14,15•,19,20], and epigenetic signatures [21] have been reported only recently.
Less bone, more fat
Several functional studies have tackled the question of whether age-associated changes would impinge on MSC properties with respect to their inherent regenerative potential. In an immune suppressed rat model for ischemic cardiomyopathy, human MSC from aged donors performed worse [22]. In an attempt to use MSC in cell replacement therapies for neurologic disorders, the neuroectodermal differentiation potential, which can be provoked in vitro in MSC derived from young donors, was completely lost in MSC from old donors [23]. In several studies addressing the pivotal role of MSC supporting hematopoietic stem cells and their progeny, cells derived from young bone marrow were superior over the aged ones [24,25]. As MSC are clinically applied to suppress graft-versus-host-disease, it would be interesting to uncover whether age-associated changes also skew MSC in this regard. To our knowledge no such attempt has been undertaken yet, nor have experimental results addressing these questions been published to date. Again most data are regarding MSC isolated from bone and bone marrow, and as there are many other sites and cellular populations containing mesenchymal progenitor cells such as the muscle, fat, vessel-associated pericytes, or blood, evidently the aforementioned questions also apply here: as of now, no stringent conclusions can be drawn at this point as most questions in this latter context are still unanswered.
As a consequence of their ubiquitous presence, individual MSC may escape later in life from specific fate control and become diverted to bring forth unwanted progeny. In the case of a vascular mural location, it is conceivable that MSC-like cells may become involved in ectopic calcification of arteries and heart valves, which often occurs in the course of diabetes, renal failure or atherosclerosis [26]. Ectopic bone formation is considered a good example for a well established theory in aging biology named antagonistic pleiotropy. Interestingly MSC at pericyte location are undergoing osteogenic differentiation when facing inflammatory conditions [27]. These results suggest that MSC within an aged vascular wall show the propensity to develop along the osteogenic lineage rather than turning into cell types that support the vascular architecture.
Contrastingly in osseous tissues at advanced age, both mass and mineral density of cortical and cancellous bone steadily decreases while at the same time more and more fat cells emerge within bone marrow. The actions of crucial determinants of this ‘adipogenic switch’ are currently being unraveled. The mutual exclusivity of osteoblast and adipocyte cell fates are thought to be determined by factors such as the CCAAT/enhancer binding protein (C/EBP), a trigger for adipogenesis, PPARγ, which promotes adipocyte maturation, or Runx2, an osteoblastic transcriptional mediator. Impaired PPARγ signaling shifts the fate of MSC toward the osteoblast lineage. The wnt pathway can suppress PPARγ, favoring MSC differentiation to osteoblasts. Other transcriptional mediators associated with osteoblast/adipocyte specification include ΔFosB, TAZ, Esr1, Msx2, C/EBPβ, and Id4 [28]. The basic leucine-zipper transcription factor, musculo-aponeurotic fibrosarcoma or Maf appears central to osteoblast lineage commitment with age [29•]. Maf is highly expressed in MSC yet its expression is greatly reduced during aging and in the presence of ROS. This transcriptional modulator is thought to be crucial determinant for skeletal development and for balancing adipogenic versus osteoblastogenic gene expression as Maf+/− mice suffer from reduced bone formation while at the same time experience increasing numbers of adipocytes.
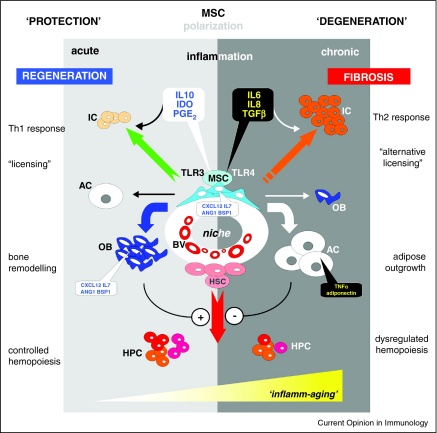
Deviations of the age-associated MSC differentiation capacity was reported by many laboratories inasmuch osteogenic potential of MSC isolated from aged donors appears to decline while the respective adipogenic differentiation performance remained unchanged, or worse, was found to be enhanced. Vascular Cell Adhesion Molecule 1 (VCAM1/CD106) was found increased in mesenchymal progenitor cells that are committed to lineage-specific differentiation and this marker is also upregulated in primary MSC derived from bone of aged donors [15•]. As interferon gamma (IFNγ) or tumor necrosis factor alpha (TNFα) activate VCAM1 expression in MSC, and levels of pro-inflammatory cytokines such as TNFα are increased in older people [30], derailed inflammatory cues may scramble the delicate balance of regulatory networks necessary to govern tissue specific regeneration and remodelling (Figure 1). In contrast to well adjusted, slightly elevated levels of inflammatory stimuli that are backing up wound and bone healing by supporting osteoblastogenesis [31], an inflammatory overshoot, be it acutely or chronically, favors adipogenic differentiation. This is in line with observations that bone loss, also included is the advance of osteoporosis in the course of autoimmune disorders of the bone, is associated if not caused by inflammatory disorders [32,33]. Thus, dominant aberrations within the MSC microenvironment may arise from systemic chronic inflammation, which as mentioned above occurs regularly in elderly persons. It may also be mediated through unbalanced inflammatory and anti-inflammatory networks as a consequence to life-long antigenic burden or age-related diseases. These circumstances are often are often circumscribed by the term ‘inflamm-aging’ (Figure 1).
Figure 1.
-
(i)secrete indoleamine 2,3-dioxygenase (IDO), interleukin 6 (IL6), interleukin 8 (IL8), interleukin 10 (IL10), prostaglandin E-2 (PGE2), transforming growth factor beta (TGFβ) after activation of toll-like receptors 3 or 4 (TLR3, TLR4) to act on immune cells (IC) to trigger specific T helper (Th) cell responses;
-
(ii)to convey under low or transient inflammatory stimuli the differentiation of osteoblasts (OB) while at the same time checking progression of adipogenic cells (AC); contrastingly within a chronically inflamed periphery, the osteogenic fate of MSC progeny is stalled and adipogenic differentiation upholstered; the latter further propagate an inflammatory milieu through further excretion of tumor necrosis factor alpha (TNFα) or adiponectin;
-
(iii)to support the emergence and diversity of hematopoietic precursor cells (HPC) through strong osseous structures in a protected environment while stem cell proximities destined to degenerate by adipose outgrowth hinder the advent of HPC formation.
Deteriorating long-lasting chronic inflammation is frequently observed in biological communication systems at advanced age, for which the frequently used expression ‘inflamm-aging’ has been coined.
MSC scent blood
Hematopoeisis during adulthood takes place in the bone marrow, and one potential HSC niche is congruent with osseous structures, which is the endosteal lining and trabecular cavities of long bones. Osteoblasts were shown to functionally contribute to this confined niche (Figure 1). In mouse, nestin-expressing MSC were found to closely associate with HSC and sympathetic nerve fibers [1••], and the latter regulate HSC mobilization. Comparable to osteoblasts, nestin+ MSC condition their microenvironment with high levels of HSC maintenance factors such as the chemokine CXCL12, angiopoietin 1, interleukin 7, or stem cell factor as well as extracellular matrix such as the bone sialo protein, osteopontin (Figure 1). Depletion of nestin+ cells leads to mobilization of HSC and the homing of transplanted hematopoietic progenitors is greatly reduced. Also treatment with the HSC-mobilizing factor granulocyte colony-stimulating factor stimulates nestin+ MSC to produce high amounts of CXCL12 and other factors, which support HSC stemness and niche retention. These data greatly suggest that MSC are indeed functional entities of the endosteal HSC niche. In conclusion, HSC and MSC share a common niche.
Bone marrow also contains adipocytes and it is likely, albeit highly controversial, that they are indeed MSC descendents. Although often perceived as passive space fillers, only recently an inverse correlation between adipocyte numbers and active hematopoiesis became evident. Notably the major inducer of adipogenesis peroxisome proliferator-activated receptor-γ, PPARγ is a negative regulator of osteoblastogenesis, and conceivably also adipocytes can actually form at the expense of osteoblasts [34]. In mice, which are genetically incapable of forming adipocytes such as the lipoatrophic A-ZIP/F1 ‘fatless’ mouse strain, or that are treated with the PPARγ inhibitor Bisphenol-A-DiGlycidyl-Ether to induce abrogation of adipogenesis, engraftment of transplanted hematopoietic precursors was greatly enhanced. These findings support the notion that adipocytes exert a suppressive influence within the bone marrow microenvironment through secretion of various factors such as adiponectin or TNFα, each of which can impair hematopoietic proliferation through impeding progenitor activity. At the same time, adipocytes appear to positively influence the most primitive HSC, suggesting that they prevent hematopoietic progenitor expansion while preserving the hematopoietic stem cell pool (Figure 1). Conclusively therefore, MSC are the source of two coexisting mature cell types with apparently antagonistic properties on HSCs. Although major questions remain still unanswered, in particular concerning the precise developmental stages that MSCs undergo during differentiation in situ, permissive inflammatory influences appear to govern lineage commitment decisions in vivo. As a consequence of the dysbalance of osteoblast and adipocyte production, the entire hematopoietic system may become awry.
T lymphocytes and natural killer cells are potential sources of proinflammatory cytokines. MSC interact with T cells and many other immune cells thereby promoting dominant modulatory processes. The effects on CD4 and CD8 T cells are best characterized [35]. Independent of whether naïve or antigen-experienced, regardless of their functional state, irrespective of which T-cell receptor expressed, MSC suppress CD4+ and CD8+ cells non-selectively and non-specifically [36,37]. Of note, IFNγ is required as a crucial ‘licensing’ step to emit this suppressive activity, and this observation indicates that MSC not only passively withstand inflammatory stimuli, but also actually decisively respond to inflammatory cues. For instance the expression of indoleamine 2,3-dioxygenase (IDO) is upregulated, which in due course leads to tryptophan depletion in lymphocytes resulting in the blockage of their activity and proliferation. Prostaglandin E2 (PGE2), another mediator with immunosuppressive potential is selectively produced by MSC. It acts in synergy with IDO, and MSC-derived PGE2 is capable to reverse an inflammatory environment into an anti-inflammatory one, altering the cytokine secretion profile of dendritic and T-cell subsets [38]. Owing to the reversible nature of this inhibition, responding cells are not triggered to undergo apoptosis; it appears likely that MSC to all intents and purposes protect from apoptosis, which is in concordance with the notion that the actual duty of MSC is to support tissue repair.
Toll-like receptors (TLR) are expressed on MSC. TLR4 signaling antagonizes MSC immunosuppressive activity by entailing a proinflammatory profile owing to the secretion of IL-6, IL-8, or TGFβ [39]. On the contrary, activation of MSC through TLR3 induces IDO and PGE2 as well as the anti-inflammatory cytokine IL-10 [40••]. Operating along these lines, it was shown that experimental animals suffering from severe sepsis and consequently dying there from, could be rescued through infusion of MSC that reprogrammed macrophages to produce IL10 [41•] (Figure 1). Also the stress-responsive, cytoprotective and immunoregulatory molecule heme oxygenase 1 (HO1) is a key factor in this context. HO1 produced by MSC triggers the formation of specific T regulatory subsets (Tr1 and Th3) and IL-10 production [42]. In the presence of MSC also dendritic precursor cells change their fate and acquire regulatory functions by producing IL10 [43]. Although the regulatory network governing this particular MSC activity is largely unknown to date, in all likelihood IL10 is pivot.
In conclusion, there are good reasons to believe that fate and function of all aforementioned cell classes are tightly interconnected and age-related changes appear to be compensated to a certain degree by complementary modes, in particular maintaining memory T cells as well as plasma cells in a non-proliferative state, which propagates and supports homeostasis of immunological memory [44•]. The notion that MSC are functionally plastic has certainly fundamental implications to understand their physiology and their putative role in pathological processes, as well as during aging.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Conflicts of interest
None to disclose.
Acknowledgements
GL's research is aided by grants gifted by the EC (FP7 – Health VascuBone), the Austrian Research Agency (Laura Bassi Centre for Excellence – DIALIFE) and The Tyrolean Future Fund (Translational Project – Smart Implants).
References
- Mendez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]; MSC could be indentified in mouse owing to nestin expression. In bone this cell population constitutes an essential HSC niche component, as nestin(+) MSCs are spatially associated with HSC and adrenergic nerve fibres, and express HSC maintenance genes. The results presented in this article are indicative of a unique niche in the bone marrow made of heterotypic stem-cell pairs.
- 2.da Silva Meirelles L., Caplan A.I., Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]; Perivascular cells contain multipotential progenitor cells being myogenic in culture and in vivo, yet also exhibit osteogenic, chondrogenic, and adipogenic potentials. Native, noncultured perivascular cells carry MSC-specific surface markers, conclusively blood vessel walls harbor a reserve of progenitor cells closely related to MSC.
- 4.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 5.Tolar J., Le Blanc K., Keating A., Blazar B.R. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman M.S., Bar-Or A., Atkins H.L., Karussis D., Frassoni F., Lazarus H., Scolding N., Slavin S., Le Blanc K., Uccelli A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 7.Caplan A.I. New era of cell-based orthopedic therapies. Tissue Eng Part B Rev. 2009;15:195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandl A., Meyer M., Bechmann V., Nerlich M., Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317:1541-1547. doi: 10.1016/j.yexcr.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Fehrer C., Brunauer R., Laschober G., Unterluggauer H., Reitinger S., Kloss F., Gülly C., Gassner R., Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their life span. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasper G., Mao L., Geissler S., Draycheva A., Trippens J., Kuhnisch J., Tschirschmann M., Kaspar K., Perka C., Duda G.N., Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 11.Lepperdinger G., Brunauer R., Jamnig A., Laschober G., Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43:1018–1023. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Choumerianou D.M., Martimianaki G., Stiakaki E., Kalmanti L., Kalmanti M., Dimitriou H. Comparative study of stemness characteristics of mesenchymal cells from bone marrow of children and adults. Cytotherapy. 2010;12:881–887. doi: 10.3109/14653249.2010.501790. [DOI] [PubMed] [Google Scholar]
- 13.Lund T.C., Kobs A., Blazar B.R., Tolar J. Mesenchymal stromal cells from donors varying widely in age are of equal cellular fitness after in vitro expansion under hypoxic conditions. Cytotherapy. 2011;12:971–981. doi: 10.3109/14653249.2010.509394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner W., Bork S., Horn P., Krunic D., Walenda T., Diehlmann A., Benes V., Blake J., Huber F.-X., Eckstein V. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschober G.T., Brunauer R., Jamnig A., Singh S., Hafen U., Fehrer C., Kloss F., Gassner R., Lepperdinger G. Age-specific changes of mesenchymal stem cells are paralleled by upregulation of CD106 expression as a response to an inflammatory environment. Rejuvenat Res. 2011;14:119–131. doi: 10.1089/rej.2010.1077. [DOI] [PubMed] [Google Scholar]; This work demonstrates that moderate levels of inflammatory stimuli are interpreted by MSCs at a young age as instructive signals for osteoblastogenesis, whereas at old age, an inflammatory milieu can effectively impact on bone remodeling and repair while at the same time uninterrupted adipogenic differentiation leads to adipose upgrowth.
- 16.Laschober G.T., Brunauer R., Jamnig A., Fehrer C., Greiderer B., Lepperdinger G. Leptin receptor/CD295 is upregulated on primary human mesenchymal stem cells of advancing biological age and distinctly marks the subpopulation of dying cells. Exp Gerontol. 2009;44:57–62. doi: 10.1016/j.exger.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kemp K., Gray E., Mallam E., Scolding N., Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. 2010;6:548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 19.Laschober G.T., Ruli D., Hofer E., Muck C., Carmona-Gutierrez D., Ring J., Hutter E., Ruckenstuhl C., Micutkova L., Brunauer R. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2011;9:1084–1097. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaffidi P., Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bork S., Pfister S., Witt H., Horn P., Korn B., Ho A.D., Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan M., Chen W., Liu W., Du G.-Q., Jiang S.-L., Tian W.-C., Sun L., Li R.-K., Tian H. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenat Res. 2010;13:429–438. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 23.Hermann A., List C., Habisch H.-J., Vukicevic V., Ehrhart-Bornstein M., Brenner R., Bernstein P., Fickert S., Storch A. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: limitations for autologous cell replacement strategies. Cytotherapy. 2010;12:17–30. doi: 10.3109/14653240903313941. [DOI] [PubMed] [Google Scholar]
- 24.Wagner W., Horn P., Bork S., Ho A.D. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp Gerontol. 2008;43:974–980. doi: 10.1016/j.exger.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Walenda T., Bork S., Horn P., Wein F., Saffrich R., Diehlmann A., Eckstein V., Ho A.D., Wagner W. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med. 2010;14:337–350. doi: 10.1111/j.1582-4934.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J.H., Yip C.Y., Sone E.D., Simmons C.A. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao J.S., Cheng S.L., Sadhu J., Towler D.A. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCauley L.K. c-Maf and you won’t see fat. J Clin Invest. 2010;120:3440–3442. doi: 10.1172/JCI44786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Nakashima T., Takeda S., Isogai M., Hamada M., Kimura A., Kodama T., Yamaguchi A., Owen M.J., Takahashi S., Takayanagi H. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120:3455–3465. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]; The switch between osteoblastic or adipogenic fates is mediated through the basic leucine-zipper transcription factor Maf. It could be shown that increased oxidative stress – as thought to raise during aging – decreases Maf expression. This report advances our understanding how MSC control fate decisions, which are potentially deteriorated during aging.
- 30.de Gonzalo-Calvo D., Neitzert K., Fernández M., Vega-Naredo I., Caballero B., García-Macía M., Suárez F.M., Rodríguez-Colunga M.J., Solano J.J., Coto-Montes A. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med. 2010;49:733–737. doi: 10.1016/j.freeradbiomed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Duque G., Huang D.C., Macoritto M., Rivas D., Yang X.F., Ste-Marie L.G., Kremer R. Autocrine regulation of interferon {gamma} in mesenchymal stem cells plays a role in early osteoblastogenesis. Stem Cells. 2009;27:550-558. doi: 10.1634/stemcells.2008-0886. [DOI] [PubMed] [Google Scholar]
- 32.Hardy R., Cooper M.S. Bone loss in inflammatory disorders. J Endocrinol. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 33.Mohanty S.T., Kottam L., Gambardella A., Nicklin M.J., Coulton L., Hughes D., Wilson A.G., Croucher P.I., Bellantuono I. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R149. doi: 10.1186/ar3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada I., Kouzmenko A.P., Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets. 2009;13:593–603. doi: 10.1517/14728220902915310. [DOI] [PubMed] [Google Scholar]
- 35.Dazzi F., Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. 2011;24:49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Ghannam S., Pene J., Torcy-Moquet G., Jorgensen C., Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 37.Prigione I., Benvenuto F., Bocca P., Battistini L., Uccelli A., Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 38.Trento C., Dazzi F. Mesenchymal stem cells and innate tolerance: biology and clinical applications. Swiss Med Wkly. 2010;140:w13121. doi: 10.4414/smw.2010.13121. [DOI] [PubMed] [Google Scholar]
- 39.Liotta F., Angeli R., Cosmi L., Fili L., Manuelli C., Frosali F., Mazzinghi B., Maggi L., Pasini A., Lisi V. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes that specific toll-like receptor signaling of MSC affects immune modulation. Polarization of MSC after TLR4-priming produces pro-inflammatory mediators, while TLR3 triggers the expression of immunosuppressive factors.
- Nemeth K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrate the potency of infused MSC to efficiently treat sepsis in an experimental mouse model. The effect brought about by macrophages producing high amounts interleukin-10.
- 42.Mougiakakos D., Jitschin R., Johansson C.C., Okita R., Kiessling R., Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K., Hauser A.E., Nakayama T., Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]; Comprehensive overview on regulatory network enforcing immunological memory through the action of stromal cells.