Abstract
The mechanisms underlying the chronic neurodegeneration that occurs in Parkinson’s disease (PD) are unknown. One emerging hypothesis is that neural systems deteriorate and eventually degenerate due to a primary failure of either extrinsic neurotrophic support or the intrinsic cellular pathways that mediate such support. One of the cellular pathways that has been often identified in mediating neurotrophic effects is that of PI3K/Akt signaling. In addition, recent observations have suggested a primary failure of PI3K/Akt signaling in animal models and in PD patients. Therefore, to explore the possible role of endogenous Akt signaling in maintaining the viability and functionality of substantia nigra (SN) dopamine neurons, one of the principal systems affected in PD, we have used an adeno-associated viral vector to transduce them with a dominant negative (DN) form of Akt, the pleckstrin homology (PH) domain alone (DN(PH)-Akt). In addition, we have examined the effect of DN(PH)-Akt in murine models of two risk factors for human PD: advanced age and increased expression of α-synuclein. We find that transduction of these neurons in normal adult mice has no effect on any aspect of their morphology at 4 or 7 weeks. However, in both aged mice and in transgenic mice with increased expression of human α-synuclein we observe decreased phenotypic expression of the catecholamine synthetic enzyme tyrosine hydroxylase (TH) in dopaminergic axons and terminals in the striatum. In aged transgenic α-synuclein over-expressing mice this reduction was 2-fold as great. We conclude that the two principal risk factors for human PD, advanced age and increased expression of α-synuclein, reveal a dependence of dopaminergic neurons on endogenous Akt signaling for maintenance of axonal phenotype.
Keywords: axon, Akt, substantia nigra, dopamine, Parkinson’s disease, synuclein
Introduction
Although there presently exist many effective therapies to relieve the motor symptoms of Parkinson’s disease (PD), its underlying pathogenesis remains unknown. This lack of knowledge is an impediment to the development of therapies that would hopefully forestall not only the progression of motor impairments, but also the numerous and disabling non-motor manifestations of the disease. Many important and promising hypotheses for the underlying pathogenesis of PD have been put forward, and they are not mutually exclusive (Schnabel 2010;Lin and Beal 2006). One concept has been that the neurodegeneration that is characteristic of the disease at some point in its course involves the abnormal, uncontrolled activation of programmed cell death pathways (reviewed in (Burke 2008). While the precise role that these pathways may play in the disease remains yet unknown, the fundamental concept that neuron death may be due to activation of intrinsic cellular mechanisms has given rise to other closely related hypotheses. One related concept has proposed that rather than neuron death being due primarily to an activation of cell death pathways, it may instead be due to a primary failure of the molecular pathways that are essential for survival, such that neuron death occurs by default.
This hypothesis, that of failure of survival signaling, has been given some support by observations made in rodents in which critical survival-mediating genes for dopamine neurons have been deleted. Pascual and colleagues have shown that deletion of glial cell line-derived neurotrophic factor (GDNF) in adult mice results in a delayed and progressive degeneration of dopamine neurons (Pascual et al. 2008). Similarly, Kramer and colleagues have shown that selective deletion of the Ret tyrosine kinase, which mediates GDNF signaling, in dopamine neurons leads to a late-onset adult neurodegeneration (Kramer et al. 2007). Genetic deletion of the transcription factor Nurr1, which is essential for the development of dopamine neurons, in adulthood results in a slowly progressive loss of striatal dopaminergic markers (Kadkhodaei et al. 2009).
A signaling pathway that has frequently been demonstrated to be important in mediating survival effects on dopamine neurons by diverse neurotrophic factors is that of phosphoinositide-3-kinase (PI3K) activation of Akt/PKB (Pong et al. 1998;Soler et al. 1999;Besset et al. 2000;De Vita et al. 2000;Chen et al. 2001;Sawada et al. 2000;Coulpier and Ibanez 2004). We have shown in a developmental context in vivo that abrogation of intrinsic dopaminergic Akt signaling by use of viral vector transduction of a dominant negative mutant results in augmentation of developmental apoptosis, neuron atrophy, and diminished striatal innervation (Ries et al. 2009). The possibility that failure of Akt signaling may underlie dopamine neuron degeneration in PD has been suggested on the basis of studies of the mechanisms of neurotoxins in tissue culture and rodent models (Malagelada et al. 2008;Malagelada et al. 2010), genetic models of PD (Rieker et al. 2011;Aleyasin et al. 2010), and post-mortem studies demonstrating diminished Akt phosphorylation in PD brain (Malagelada et al. 2008;Timmons et al. 2009)(reviewed in (Greene et al. 2011)). Therefore to investigate the possible role of Akt signaling in maintaining the viability of adult dopamine neurons we have herein made use of a well-characterized dominant negative (DN) mutant of Akt, consisting of its plekstrin homology (PH) domain alone. The efficacy and specificity of this mutant have previously been well-characterized in vitro (Dudek et al. 1997;Songyang et al. 1997;Wang et al. 2003), and we have previously demonstrated that it is also effective in dopamine neurons in vivo (Ries et al. 2009). We have elected to utilize this dominant negative approach to blockade of Akt signaling rather than genetic deletion approaches, for the reason that three isoforms of Akt, derived from distinct genes, are expressed in SN (Ries et al. 2006), making conditional deletion in adulthood a formidable challenge.
In order to identify a possible role for Akt in the support of dopamine neurons in adult mice under conditions that may bear relevance to human PD, we have incorporated into our studies models of the two most clearly defined risk factors for the human disease: advanced age (Bower et al. 2000) and augmented expression of α-synuclein (Singleton et al. 2003;Pals et al. 2004).
Methods
Animals
Adult (8 week) male C57BL/6 mice weighing ~25 g were obtained from Charles River Laboratories (Wilmington, MA). Aged C57BL/6 male mice (≥22 months old) were obtained through the National Institute on Aging (Bethesda, MD) (Harlan Sprague Dawley, Inc., Frederick, MD).
Adult α-synuclein (A53T) transgenic mice were also used in these studies. These animals selectively express the mutant variant of α-synuclein under the tyrosine hydroxylase promoter (TH-hαSYN(A53T); (Matsuoka et al. 2001)) and they were back-crossed onto the murine synuclein knockout mouse (SYN(-/-); (Dauer et al. 2002)) to produce a transgenic mouse that only expressed human α–synuclein in regions that also express tyrosine hydroxylase (SYN(-/-):TH-hαSYN(A53T)).
Generation of recombinant adeno-associated virus (AAV)
A plasmid encoding the pleckstrin homology (PH) domain of mouse Akt1 (dominant negative Akt, DN(PH)-Akt) was kindly provided by Dr Thomas Franke (Songyang et al. 1997), modified to incorporate a FLAG-encoding sequence at the 3′-end, and inserted into an AAV1 packaging construct as previously described (Olson et al. 2006;Ries et al. 2006). The titer was 4.6E12 viral genomes/ml. For use as controls, enhanced green fluorescent protein and yellow fluorescent protein were subcloned into the same viral backbone, and viral stocks were used at titers of 2.6-4.6E12 and 2E12, respectively.
Intra-nigral injection of AAVs
Mice were anesthetized with ketamine/xylazine solution and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). The tip of 5.0-μl syringe (Agilent, Santa Clara, CA) needle (26S) was inserted to stereotaxic coordinates: AP, −0.35 cm; ML, +0.11 cm; and DV, −0.37 cm, relative to bregma. Viral vector suspension in a volume of 2.0 μl was injected at 0.1 μl/min over 20 min. After a wait of 5 min, the needle was slowly withdrawn. Successful transduction in the SNpc was confirmed histologically by immunolabeling for FLAG. This injection procedures was approved by the Columbia University Animal Care and Use Committee.
Immunohistochemical procedures
Immunostaining for tyrosine hydroxylase (TH) was performed as described previously (Ries et al. 2006). Mice were perfused intracardially with 0.9% NaCl followed by 4.0% paraformaldehyde in 0.1 M phosphate buffer (PB) at 4 or 7 weeks after viral injection. The brain was carefully removed and blocked into midbrain and forebrain regions. The region containing the midbrain was postfixed for 1 week in the same fixative, cryoprotected in 20% sucrose overnight, and then rapidly frozen by immersion in isopentane on dry ice. A complete set of serial sections was taken through the SN at 30 μm, and every fourth section was processed free-floating for immunostaining. After washes with phosphate-buffered saline (PBS), sections were incubated in rabbit anti-TH antibody (Calbiochem, La Jolla, CA) at 1:750 for 48 hr. Sections were then treated with biotinylated protein A and avidin-biotinylated horseradish peroxidase complexes (ABC; Vector Labs, Burlingame, CA), followed by incubation with diaminobenzidine-HCl (DAB). After immunoperoxidase staining, sections were thionin counterstained. The forebrain region containing the striatum was postfixed for 48 h, frozen without cryoprotection, and then processed as described previously (Ries et al. 2006).
Immunostaining for FLAG was performed at 4 weeks following AAV injection. Mice were perfused as described above, and the brains were postfixed for 24 h. After cryoprotection, brains were frozen and sectioned through the SN at 30 μm. Sections were initially treated with Mouse-on-Mouse Blocking Reagent (Vector Labs) and then processed free-floating with a mouse monoclonal anti-FLAG antibody at 1:1000 (Sigma, St. Louis, MO). Sections were incubated with biotinylated anti-mouse IgG (Vector Labs), followed by ABC, and then incubated with DAB. For human α-synuclein immunostaining, mice were perfused and sectioned in the same way. Sections were processed free-floating with a mouse monoclonal anti-Hu-α-Syn antibody at 1:500 (Invitrogen, Camarillo, CA).
For immunostaining of astrocytes and microglia, sections were incubated in rabbit anti-glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA) or ionized calcium binding adaptor molecule 1 (Iba-1; Wako-Chem, Osaka, Japan), respectively, at 1:1000 for 24 hr. Sections were then visualized as described in TH immunostaining above.
Quantitative morphologic analysis: Determination of the number of TH-positive neurons in the SN by stereology and of their size
The SN on both sides of each mouse brain was analyzed. For each section, the entire SN was identified as the region of interest. Using StereoInvestigator software (MicroBrightField, Inc, Williston, VT), a fractionator probe was established for each section. The number of TH-positive neurons in each counting frame was then determined by focusing down through the section, using 100× objective under oil, as required by the optical disector method. Our criterion for counting an individual TH-positive neuron was the presence of its nucleus either within the counting frame, or touching the right or top frame lines (green), but not touching the left or bottom lines (red). The total number of TH-positive neurons for each side of the SN was then determined by the StereoInvestigator program (Ries et al. 2006). The cross-sectional area of TH-positive neurons in the SN was also determined by use of the StereoInvestigator program by outlining the neuron soma and proximal portions of dendrites, under 100X.
Optical density analysis of striatal TH-positive fibers
The optical density analysis was performed with an Imaging Research (St. Catherine, Ontario) Analytical Imaging Station under blinded conditions on coded slides. The region of whole striatum was determined as previously described (Chen et al. 2008). In a separate analysis, optical density of dorso-lateral striatum was determined by outlining that region. We procedurally defined ‘dorso-lateral striatum’ with the Imaging Research program by establishing a line between the dorso-medial apex of the striatum and the lateral-most convex point of the striatum, and by tracing along the inner boundary of the external capsule as shown in Figures 3C and 4B. We utilized this oblique line to define a restricted dorso-lateral region of the striatum because this region corresponds to one defined by the anatomical organization of: (1) cortico-striatal afferent projections (reviewed and illustrated in (Voorn et al. 2004)); (2) dopaminergic A9 projections to the striatum (Gerfen et al. 1987); (3) striatal projections to the SN (Maurin et al. 1999); and (4) selected intrinsic striatal markers, including cholinergic neuropil (Burke and Karanas 1990) and NGF receptor binding (Altar et al. 1991). TH-positive fiber optical density of the whole or dorsal-lateral striatum on the side of the brain injected with AAV was expressed as a percentage of optical density on the contralateral non-injected side .
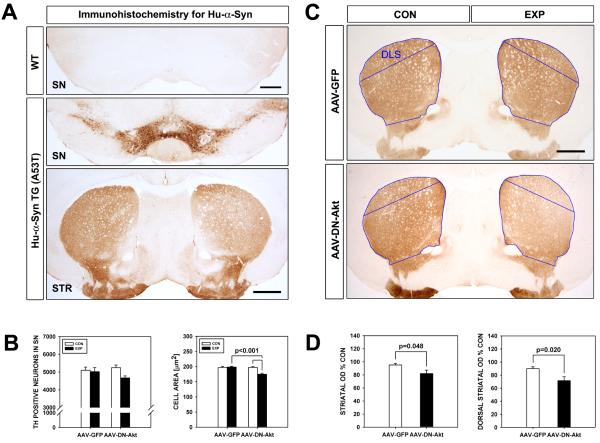
Fig. 3.
Effects of DN(PH)-Akt on the nigro-striatal dopaminergic system in SYN(-/-):TH-hαSYN(A53T) mice. A: Immunostaining for human α-synuclein reveals an absence of staining in wildtype mice. In SYN(-/-):TH-hαSYN(A53T) mice, intense immunoperoxidase staining is observed in the SNpc and in the striatum, as would be predicted due to regulation of transgene expression by the TH promoter. B: At 7 weeks following injection of SYN(-/-):TH-hαSYN(A53T) mice, DN(PH)-Akt has no effect on the number of TH-positive dopaminergic neurons in the SN, determined by stereologic counts (left graph), in comparison to AAV GFP-injected controls (n = 6, both groups). DN(PH)-Akt does, however, have a small (11%) but significant effect on TH-positive neuron size (n = 120 neurons, each experimental condition; p < 0.001, ANOVA; Tukey post hoc analysis as shown) (right graph). C: Representative coronal sections of the striatum immunoperoxidase stained for TH demonstrate a visually apparent decrease in the density of staining at 7 weeks following intranigral injection of AAV DN(PH)-Akt in a SYN(-/-):TH-hαSYN(A53T) mouse. There is a gradient in the diminution of staining, with a more visible loss dorsolaterally and a less visible loss ventromedially. D: Quantification of the optical density of TH immunoperoxidase staining reveals a significant 14% decrease over the whole striatum following AAV DN(PH)-Akt (p = 0.048, t-test) and a 20% decrease over the dorsal striatum (p = 0.02, t-test), indicated by the blue outlines (DLS: dorso-lateral striatum).
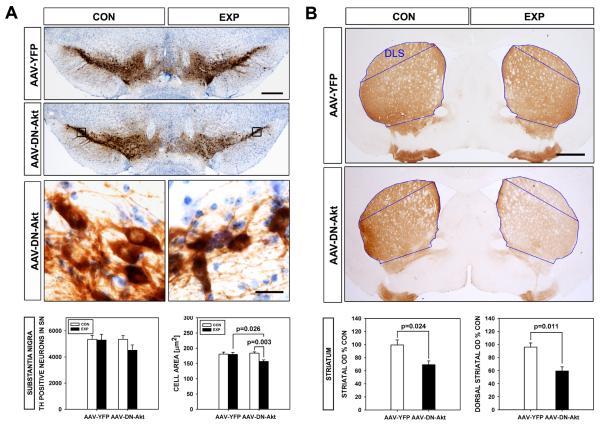
Fig. 4.
Effects of DN(PH)-Akt on the nigro-striatal dopaminergic system in aged SYN(-/-):TH-hαSYN(A53T) mice. A: Immunoperoxidase staining for TH in coronal sections of the mesencephalon shows diminished intensity of staining on the side of injection of AAV DN(PH)-Akt in aged SYN(-/-):TH-hαSYN(A53T) mice (Bar = 250 μm). Quantitative morphologic analysis reveals that this difference appears to be due to a trend for a decrease in the number of TH-positive neurons (by 16%) that does not reach significance (AAV YFP n = 3; AAV DN(PH)-Akt n = 4; p = 0.3, ANOVA), as shown by the stereologic counts, in addition to a significant decrease (13%) in their size (n = 60-80 neurons, all conditions; p = 0.002, ANOVA; Tukey post hoc analysis as shown). Representative higher power micrographs of the regions bounded by black rectangles illustrate the atrophy of TH-positive neurons following treatment with DN(PH)-Akt (Bar = 25 μm) in comparison to the contralateral non-injected control side. B: Representative coronal sections of the striatum immunoperoxidase stained for TH demonstrate a visually distinct decrease in the density of staining at 7 weeks following intranigral injection of AAV DN(PH)-Akt in an aged SYN(-/-):TH-hαSYN(A53T) mouse. The decreased staining is uniform throughout the striatum. Quantification of the optical density of TH immunoperoxidase staining reveals a significant 30% decrease over the whole striatum following AAV DN(PH)-Akt (p = 0.024, t-test) and a 38% decrease over the dorsal striatum (p = 0.011, t-test), indicated by the blue outlines (DLS: dorso-lateral striatum).
Statistical analysis
The difference between two groups was analyzed by the t test. Multiple comparisons among groups were performed by one-way ANOVA and Tukey’s post hoc analysis. All statistical analyses were performed using SigmaStat software (SPSS Science, Chicago, IL).
Results
Intra-nigral injection of AAV DN(PH)-Akt resulted in effective transduction of dopaminergic neurons of the SNpc, as detected by double immunofluorescence straining for TH and FLAG (Fig. 1). At 4 or 7 weeks following AAV injection in normal adult male C57BL/6 mice there was no effect detected on SN TH-positive neuron number or size, or striatal density of TH immunostaining (Fig. 2A and the Table).
Fig. 1.
Transduction of TH-positive neurons of the SN with AAV DN(PH)-Akt. Representative sections from three different rostro-caudal planes , obtained from a single mouse, are shown. Sections have been stained by immunofluorescence to demonstrate both TH (red) and the FLAG epitope (green) expressed by the transgene. The upper row of sections represents a single rostral plane (Plane (−)3.08 relative to bregma (Paxinos and Franklin 2001)); the middle row represents a central plane (Plane (−)3.40); the bottom row represents a caudal plane (Plane (−)3.64). It can bee seen that in the caudal-most plane ((−)3.64), which is adjacent to the site of AAV injection, virtually 100% of TH-positive neurons have been transduced. In central and rostral planes, while double-labeling is present, it is far less. In the panels depicting the central and rostral planes, a single double-labeled neuron, marked by a white arrow, is shown in the insets.
Fig. 2.
Effects of DN(PH)-Akt on the nigro-striatal dopaminergic system in normal adult and aged C57BL/6 mice. A: Transduction of the SNpc by intra-nigral injection of AAV DN(PH)-Akt is demonstrated in a representative coronal section of the mesencephalon obtained from a normal adult mouse immunostained for the FLAG epitope at 4 weeks following injection. Brown chromogen deposition is observed throughout the SNpc on the injected experimental side (EXP), but not on the non-injected, control (CON) side (Bar = 250 μm). TH immunostaining of an adjacent section, with thionin counterstain, is shown in the lower panel. The adjacent location of the FLAG- and TH-stained sections is indicated by the similar appearance of distinct landmarks, the fasciculus retroflexus (FR) and the medial terminal nucleus (MT). There is no acute toxicity to the SN, indicated by an intact population of TH-positive neurons in the SNpc on the injected (EXP) side and confirmed by stereologic counts (TABLE). B: At 7 weeks following injection of an aged mouse, DN(PH)-Akt has no effect on the number of TH-positive dopaminergic neurons in the SN, determined by stereologic counts (upper left graph; bars depict the mean ± standard error of the mean in each graph), in comparison to AAV GFP-injected controls (n = 5, both groups). DN(PH)-Akt does, however, have a small (9%) but significant effect on TH-positive neuron size (n = 50 neurons, each experimental condition; p = 0.002, ANOVA; Tukey post hoc analysis as shown) (upper right graph). At 7 weeks following injection, DN(PH)-Akt has a tendency to decrease the density of TH-immunoperoxidase staining of the whole striatum that is not quite significant (p = 0.052, t-test). However, a significant effect is observed in the dorsolateral region of the striatum, where the density of TH immunostaining is reduced by 17% (n = 5 AAV-GFP, 9 AAV DN(PH)-Akt; p = 0.008, t-test) (lower right graph).
TABLE.
Effects of DN(PH)-Akt on the mesencephalic dopaminergic system in normal adult mice
| TH NEURONS (mean ± SEM) (n) |
TH NEURON SIZE (micron2) (mean ± SEM) (n) |
TH OPTICAL DENSITY: TOTAL (E % C) (mean ± SEM) (n) |
TH OPTICAL DENSITY: DORSAL (E % C) (mean ± SEM) (n) |
|
|---|---|---|---|---|
| 4 | 7 | 7 | 7 | |
| GFP | 4625 ± 279 (7) | 192 ± 4.1 (50) | 98.8 ± 3.4 (6) | 101.4 ± 3.9 (5) |
| DN(PH)-AKT | 3914 ± 294 (7) | 183 ± 4.2 (50) | 94.7 ± 3.3 (4) | 93.7 ± 6.8 (4) |
AAV: adeno-associated virus; PIT: post-injection time; SEM: standard error of the mean.
In contrast, modest effects were observed in aged C57BL/6 mice. Although there was no effect on TH-positive neuron number, there was a modest (9%) but significant (p=0.002, ANOVA) decrease in their size, determined as cross-sectional area (Fig. 2B). In addition, there was a modest reduction (17%) in the density of TH-immunoperoxidase staining of dorsal striatal dopaminergic innervation (Fig. 2B).
Intra-nigral injection of AAV DN(PH)-Akt in adult SYN(-/-):TH-hαSYN(A53T) mice had no effect on the number of SN dopaminergic neurons, but, as was the case for aged mice, it induced modest (11%) neuronal atrophy (Fig. 3B). In the SYN(-/-):TH-hαSYN(A53T) mice, DN(PH)-Akt had more striking, and visually apparent, effects on striatal dopaminergic innervation (Fig. 3C). It reduced total striatal TH optical density by 14% (p=0.048, t-test) and dorsal optical density by 20% (P=0.02, t-test) (Fig. 3D).
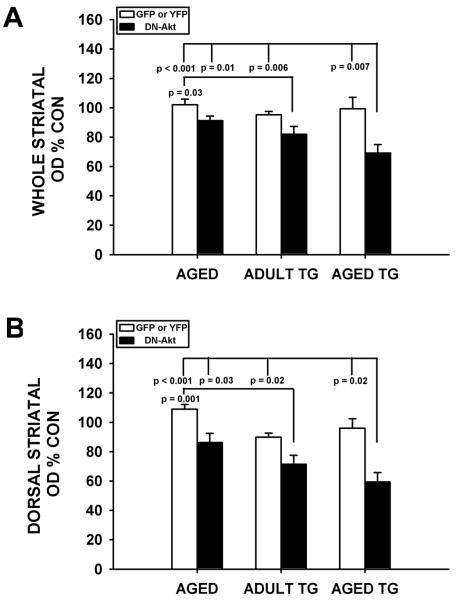
In aged SYN(-/-):TH-hαSYN(A53T) mice, while the effects of DN(PH)-Akt on TH-positive neurons were similar to those observed in aged wildtype C57BL/6 mice and adult SYN(-/-):TH-hαSYN(A53T) mice (Fig. 4A), the effects on striatal dopaminergic innervation were much more pronounced and visually apparent (Fig. 4B). In the aged SYN(-/-):TH-hαSYN(A53T) mice, whole striatal TH staining density was reduced by 30% (p= 0.02, t-test) and dorsal staining density by 38% (p= 0.01, t-test) (Fig. 4B). An analysis of striatal TH optical density data derived from the three separate studies on aged, adult SYN(-/-):TH-hαSYN(A53T) and aged SYN(-/-):TH-hαSYN(A53T) mice demonstrated that the combination of advanced age and TH-hαSYN(A53T) transgene expression resulted in a two-fold greater effect of DN(PH)-Akt to reduce staining density (Fig. 5).
Fig. 5.
Comparison of the effects of age, α-synuclein overexpression and the two factors combined on striatal TH immunoperoxidase staining. A: Each bar represents whole striatal TH optical density on the side of AAV injection as a percent of the optical density on the contralateral non-injected control. The white bars represent the AAV GFP or YFP conditions for each of the three separate experiments, and these values are all close to 100%, as expected. The black bars in each of the three experiments represent the AAV DN(PH)-Akt condition. A significant effect of DN(PH)-Akt to decrease optical density is observed (p < 0.001, ANOVA; Tukey post hoc analysis as shown). In the post-hoc analysis of this comparison, while age alone shows a trend for an effect (an 11% decrease), it does not achieve significance in comparison to any control AAV condition. Transgenic α-synuclein overexpression alone shows a greater effect (a 14% decrease) that is significant in comparison to the AAV GFP condition in the experiment with aged mice (p = 0.03). The two factors combined show a 2-fold larger effect than either single factor alone (a 30% decrease), and this effect is significant in comparison to all AAV control injections. B: An analysis applied to the dorsal striatum alone yields similar results. While age and transgenic α-synuclein overexpression alone both show effects on staining density (by 21% in both cases), the effect of the two factors combined was greater by almost 2-fold (a 38% decrease) (p < 0.001, ANOVA; Tukey post hoc analysis as shown).
We examined striatal sections that showed diminished TH-immunoperoxidase staining from both aged wildtype and aged SYN(-/-):TH-hαSYN(A53T) mice that had been treated with AAV DN(PH)-Akt for the presence of degenerative features at the single fiber level. We specifically sought the presence of axon spheroids that are characteristic of axon degeneration induced by a variety of insults (El-Khodor and Burke 2002;Ries et al. 2008;Li et al. 2009), but none were observed. In addition, we sought evidence of striatal gliosis and an inflammatory cellular infiltrate by performing immunohistochemistry for GFAP and the ionized calcium binding adaptor molecule 1 (Iba-1), respectively, but neither was observed. We therefore conclude that although we identified a loss of axonal TH expression phenotype following treatment with DN(PH)-Akt, we did not identify degenerative changes.
Discussion
Akt has over one hundred known substrates and has potent and diverse effects on cellular function and growth (Manning and Cantley 2007). In addition to regulating general cellular functions, it has effects that are unique to neurons, including induction of axon growth (Namikawa et al. 2000;Park et al. 2008), control of synaptic strength (Wang et al. 2003), and dendritic growth (Kwon et al. 2006). The possibility that endogenous Akt may play a role in the biology of mesencephalic dopamine neurons is supported by the observations that mRNA expression of Akt isoforms 1, 2, and 3 can be identified in the SNpc, and phospho-Akt (Ser437) expression occurs specifically within these neurons (Ries et al. 2006). Of the three isoforms, Akt1 is the most abundant in rat mesencephalon, and interestingly, its mRNA expression is maintained at high levels even in the mature brain, unlike that of Akt2 and -3, in which expression decreases sharply after development (Ries et al. 2006), suggesting that Akt1 may play a vital role in these neurons in maturity.
During postnatal development, the dependence of mesencephalic dopamine neurons on Akt signaling can be demonstrated by transduction with AAV DN(PH)-Akt. Expression of this dominant negative mutant results in the induction of apoptosis, with fewer neurons surviving into adulthood, diminished neuron size, and decreased innervation of the striatum (Ries et al. 2009). In the context of postnatal development, this latter effect is interpreted as being due to diminished axon growth. In normal adult C57BL/6 mice, transduction of SN dopamine neurons with this same mutant does not induce any of these effects, even at 7 weeks following injection (Table). These observations are in keeping with many others demonstrating that the nature of the dependence of SN dopamine neurons on neurotrophic support undergoes a fundamental change between the postnatal period and adulthood; while abrupt deprivation of trophic support during development typically induces acute and pronounced effects, deprivation during adulthood induces effects that are delayed and gradual if they occur at all. For example, whereas acute blockade of striatal target-derived GDNF during postnatal development produces a prompt induction of apoptosis (Oo et al. 2003), acute genetic deletion of GDNF in adulthood only induces a delayed and gradual loss of dopamine neurons (Pascual et al. 2008). In view of observations suggesting a failure of Akt signaling in PD, a chronic disease, and models thereof (Malagelada et al. 2008;Timmons et al. 2009), we elected to examine the effects of the two most clearly established risk factors for the human disease, increased age (Bower et al. 2000) (and reviewed in (Lees et al. 2009)) and increased synuclein expression (Singleton et al. 2003;Pals et al. 2004) (and reviewed in (Cookson and Bandmann 2010)) on the vulnerability of SN dopamine neurons to diminished Akt signaling. Each of these factors, when modeled in mice, either alone or in combination, revealed effects of diminished Akt signaling, induced by AAV DN(PH)-Akt, on the morphology of SN dopamine neurons. Just as we had observed in the postnatal period (Ries et al. 2009), transduction of dopamine neurons induced a modest reduction in their size in each of the three experimental conditions: 9% in aged mice; 11% in SYN(-/-):TH-hαSYN(A53T) mice; and 13% in aged SYN(-/-):TH-hαSYN(A53T) mice. These changes were all comparable to the decrease of 11% a observed postnatally (Ries et al. 2009). However, unlike what was observed postnatally, transduction with DN(PH)-Akt did not, even in the presence of these risk factors, induce a loss of dopamine neuron number.
The most pronounced morphologic change induced by DN(PH)-Akt among the three experimental conditions was a reduction in the density of TH immunoreactivity localized to dopaminergic axons and terminals within the target striatum. Interestingly, this same effect was observed for the two independent risk factors, age and level of synuclein expression, and the combination of the two accentuated this effect by about 2-fold. Several assessments for evidence of actual neurodegeneration, including examination of single fiber morphology, and efforts to identify both gliosis and cellular evidence of inflammation, were unrevealing. These negative results lead us to conclude that in the presence of increased age, or increased synuclein expression, or both, suppression of Akt signaling results in a diminished expression of phenotype by dopaminergic axons, but not degenerative loss of either neuron cell bodies or their axons. Interestingly, this effect on axons resembles that induced by Nurr1 ablation in adult mice (Kadkhodaei et al. 2009). Similar to what we had observed for suppression of Akt signaling during development, deletion of Nurr1 during development resulted in rapid degeneration of dopamine neurons. However, selective genetic deletion of Nurr1 in adult mice by use of AAV Cre did not induce neurodegeneration, but rather a progressive loss of the TH-expression phenotype in both neuron cell bodies and axons (Kadkhodaei et al. 2009). Further studies will be required to determine whether other aspects of the dopaminergic phenotype are affected by DN(PH)-Akt and the functional consequences of such effects.
Although the existence of three genetically distinct isoforms of Akt necessitated the use of a dominant negative approach that would be effective in suppressing the activity of all of them, this approach nevertheless has limitations that lead us to suggest that we are likely to be underestimating the role of endogenous Akt. While transduction with AAV DN(PH)-Akt was effective, as shown in Fig. 1, transgene expression did not occur in all dopaminergic neurons, especially in planes rostral to the injection site. In addition, while we are able to detect cellular expression of the mutant transgene by immunostaining for FLAG, this assessment is of course not quantitative, and it is not possible to know the proportion of neurons in which levels of expression were sufficient to abrogate endogenous Akt signaling. Furthermore, FLAG expression was detected at a single point in time. It is entirely possible that the cell may respond to the presence of the foreign transgene in ways that limit the persistence of its expression. And finally, even if we assume that we have achieved a complete and lasting abrogation of Akt signaling, there may be compensatory changes in the status of other, closely related AGC kinases such that normal, physiologic dependence on Akt signaling is masked. For example, serum-and glucose-induced kinase is another member of the AGC family (Hanada et al. 2004) that is expressed in SN dopamine neurons and has neurotrophic signaling effects on them (Chen et al. 2009) (and submitted). We do not know the mechanisms underlying the ability of advanced age to reveal a dependence of dopamine neurons on Akt signaling. There are so many known consequences of increased age that speculation becomes difficult, but one major possibility is that there may be a gradual failure of these postulated cellular compensatory responses.
It is equally difficult to conjecture about the underlying mechanisms whereby augmented synuclein expression reveals an increased dependency of dopamine neurons on endogenous Akt signaling, because little is yet known of the normal function of synuclein (Cookson and Bandmann 2010). The leading hypothesis for its role in causing neurodegeneration suggests that, based on its propensity to self-aggregate, it forms oligomeric species that are injurious to the cell. In spite of its many other identified roles in cellular function, Akt does not have a defined, direct role in the processing of toxic protein species, so it is difficult to envision how a decrease in Akt signaling may predispose to the toxic effects of α-synuclein overexpression. Alternatively, it is possible that the converse relationship is more important; augmented synuclein expression may either create a greater demand for Akt signaling, or inhibits its function, or interfere with an ability to compensate for its loss, such that a greater sensitivity to the DN(PH)-Akt mutant is revealed.
While much remains to be learned about the relationships between age, level of synuclein expression and endogenous Akt signaling in the pathogenesis of PD, our findings nevertheless support the concepts proposed by others that failure of Akt survival signaling may be an important underlying feature of the disease (Malagelada et al. 2008;Timmons et al. 2009;Rieker et al. 2011;Greene et al. 2011). If such is the case, then it may be possible to achieve clinical benefits, and perhaps even forestall progression, by the use of therapeutic approaches to augment Akt signaling.
Highlights.
Parkinson’s disease (PD) may be due to a failure of survival signaling by PI3K/Akt
To test this we transduced dopamine neurons with dominant negative Akt, DN(PH)-Akt
In aged mice DN(PH)-Akt leads to a loss of dopaminergic phenotype in axons
This effect is additive in aged α-synuclein transgenic mice
We conclude that dopaminergic axonal phenotype is dependent on Akt signaling
This dependence is revealed in the presence of two principal risk factors for PD
Acknowledgements
This work was supported by NIH NS26836 and NS38370, the Parkinson’s Disease Foundation, and the Parkinson’s Alliance (REB).
Abbreviations
- AAV
adeno-associated virus
- DAB
diaminobenzidine-HCl
- DN
dominant negative
- GDNF
glial cell line-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- PH
pleckstrin homology
- PI3K
phosphoinositide-3-kinase
- SN
substantia nigra
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Dugich-Djordjevic M, Armanini M, Bakhit C. Medial-to-lateral gradient of neostriatal NGF receptors: relationship to cholinergic neurons and NGF-like immunoreactivity. J Neurosci. 1991;11:828–836. doi: 10.1523/JNEUROSCI.11-03-00828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besset V, Scott RP, Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson’s disease. Mov Disord. 2000;15:819–825. doi: 10.1002/1531-8257(200009)15:5<819::aid-mds1009>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Burke RE. Programmed cell death and new discoveries in the genetics of parkinsonism. J Neurochem. 2008;104:875–890. doi: 10.1111/j.1471-4159.2007.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Karanas AL. Demonstration of a medial to lateral gradient in the density of cholinergic neuropil in the rat striatum. Neurosci Lett. 1990;108:58–64. doi: 10.1016/0304-3940(90)90706-f. [DOI] [PubMed] [Google Scholar]
- Chen X, Kareva T, Kholodilov N, Burke RE. SGK has neurotrophic and neuroprotective effects on the nigrostriatal dopaminergic system in vivo. Abstr Soc Neurosci. 2009 [Google Scholar]
- Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, Kholodilov N, Burke RE. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci. 2008;28:672–680. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chai Y, Cao L, Huang A, Cui R, Lu C, He C. Glial cell line-derived neurotrophic factor promotes survival and induces differentiation through the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathway respectively in PC12 cells. Neuroscience. 2001;104:593–598. doi: 10.1016/s0306-4522(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Bandmann O. Parkinson’s disease: insights from pathways. Hum Mol Genet. 2010;19:R21–R27. doi: 10.1093/hmg/ddq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier M, Ibanez CF. Retrograde propagation of GDNF-mediated signals in sympathetic neurons. Mol Cell Neurosci. 2004;27:132–139. doi: 10.1016/j.mcn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Dauer WT, Kholodilov N, Vila M, Trillat A, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke RE, Hen R. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2000;60:3727–3731. [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. J Comp Neurol. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Levy O, Malagelada C. Akt as a Victim, Villain and Potential Hero in Parkinson’s Disease Pathophysiology and Treatment. Cell Mol Neurobiol. 2011 doi: 10.1007/s10571-011-9671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, Chambon P, Lindqvist E, Larsson NG, Olson L, Bjorklund A, Ichinose H, Perlmann T. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Greene LA. RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28:14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- Maurin Y, Banrezes B, Menetrey A, Mailly P, Deniau JM. Three-dimensional distribution of nigrostriatal neurons in the rat: relation to the topography of striatonigral projections. Neuroscience. 1999;91:891–909. doi: 10.1016/s0306-4522(98)00681-2. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Oo TF, Kholodilov N, Burke RE. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal GDNF in vivo. J Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pals P, Lincoln S, Manning J, Heckman M, Skipper L, Hulihan M, Van den BM, De Pooter T, Cras P, Crook J, Van Broeckhoven C, Farrer MJ. alpha-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol. 2004;56:591–595. doi: 10.1002/ana.20268. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
- Pong K, Xu RY, Baron WF, Louis JC, Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J Neurochem. 1998;71:1912–1919. doi: 10.1046/j.1471-4159.1998.71051912.x. [DOI] [PubMed] [Google Scholar]
- Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, Neumann M, Yuan X, Grummt I, Schutz G, Parlato R. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Cheng HC, Baohan A, Kareva T, Oo TF, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Regulation of the postnatal development of dopamine neurons of the substantia nigra in vivo by Akt/protein kinase B. J Neurochem. 2009;110:23–33. doi: 10.1111/j.1471-4159.2009.06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/PKB: Trophic effects in murine models of Parkinson’s Disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Silva RM, Oo TF, Cheng HC, Rzhetskaya M, Kholodilov N, Flavell RA, Kuan CY, Rakic P, Burke RE. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J Neurochem. 2008;107:1578–1588. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, Shimohama S. Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. J Neurochem. 2000;74:1175–1184. doi: 10.1046/j.1471-4159.2000.741175.x. [DOI] [PubMed] [Google Scholar]
- Schnabel J. Secrets of the shaking palsy. Nature. 2010;466:S2–S5. doi: 10.1038/466S2b. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons S, Coakley MF, Moloney AM, O’ Neill C. Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett. 2009;467:30–35. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]