Abstract
Objective
Elevated trait anger (TRANG; heightened propensity to experience anger) is associated with greater pain responsiveness, possibly via associations with deficient endogenous opioid analgesia. This study tested whether acute anger arousal moderates the impact of TRANG on endogenous opioid analgesia.
Methods
94 chronic low back pain participants (LBP) and 85 healthy controls received opioid blockade (8mg naloxone) or placebo in randomized, counterbalanced order in separate sessions. Participants were randomly assigned to undergo either a 5-minute anger recall interview (ARI) or neutral control interview (NCI) across both drug conditions. Immediately following the assigned interview, participants engaged sequentially in finger pressure and ischemic forearm pain tasks. Opioid blockade effects were derived (blockade minus placebo condition pain ratings) to index opioid antinociceptive function.
Results
Placebo condition TRANG × Interview interactions (p’s<.05) indicated that TRANG was hyperalgesic only in the context of acute anger arousal (ARI condition; p’s<.05). Blockade effect analyses suggested these hyperalgesic effects were related to deficient opioid analgesia. Significant TRANG × Interview interactions (p’s<.05) for both pain tasks indicated that elevated TRANG was associated with smaller blockade effects (less endogenous opioid analgesia) only in the ARI condition (p’s<.05). Results for ischemic task VAS intensity blockade effects suggested that associations between TRANG and impaired opioid function were most evident in LBP participants when experiencing anger (Type × Interview × TRANG Interaction; p<.05).
Conclusions
Results indicate that hyperalgesic effects of TRANG are most prominent when acute anger is aroused, and suggest that endogenous opioid mechanisms contribute.
Keywords: Trait Anger, Anger Arousal, Anger Recall, Endogenous Opioids, Acute Pain, Chronic Pain
Introduction
Elevated trait anger (dispositional propensity to experience anger) and trait anger-out (dispositional tendency to regulate anger via expressive strategies) are both associated with enhanced acute pain responsiveness and greater chronic pain intensity (1-8). Mechanisms underlying the hyperalgesia associated with anger-related variables are not well understood, although prior work suggests that endogenous opioid systems contribute (1,2,9-11). This opioid hypothesis is based on functional imaging work documenting that activity in a brain network including rostral anterior cingulate cortex, anterior insula, orbitofrontal cortex, amygdala, and periaqueductal gray underlies not only pain experience, but also anger and its regulation (12). Activity in these brain regions has been shown to be modulated by endogenous opioid activity (13-15). Given the inhibitory function of opioids (reduced arousal, pain, and negative affect), impaired opioid inhibitory activity might result in a phenotype reflecting greater pain responsiveness, greater propensity to experience anger (trait anger), and more outwardly expressive regulation of that anger (anger-out).
We have previously used opioid blockade methodology to explore the role of endogenous opioids in the pain-related effects of both state anger arousal and trait anger-related variables (1,2,9-11,16). We recently reported that state anger induced via an interpersonal harassment manipulation may produce endogenous opioid-mediated analgesia (16). Results of several studies further suggest that elevated anger-out is associated with enhanced pain responsiveness in part due to impaired opioid analgesic function (1,2,9-11). Relatively little work has examined whether pain-related effects of trait anger may also be related to endogenous opioid mechanisms. In one study, individuals low in trait anger displayed increased acute pain responses following opioid blockade compared to no such increases among individuals high in trait anger, consistent with impaired endogenous opioid analgesia in the latter (1). Opioid blockade also impaired cardiovascular recovery following exposure to acute pain in low trait anger participants, but not those high in trait anger, again suggesting opioid inhibitory deficits in the latter group (17).
A key methodological issue in the studies above is that opioid-related hyperalgesic effects of trait anger have not been examined in the presence of anger arousal. It has been argued that the health-related consequences of affect-related traits cannot be understood without considering how they interact with affective states (18). For example, considering that elevated trait anger reflects an increased propensity to become angry, the pain-related effects of this trait may be most apparent under conditions in which an angry state has actually been aroused. The current study sought to examine possible trait × state interactions between trait anger and acute anger arousal (state) as they influence endogenous opioid analgesia and responses to acute pain. If high trait anger is associated with deficient endogenous opioid analgesia, then arousal of anger could trigger opioid analgesia selectively only among individuals low in trait anger, thus magnifying differences in pain responsiveness between those high and low on this trait. We expected, therefore, that elevated trait anger would be associated with lower endogenous opioid analgesia during acute pain, and that this effect would be exaggerated following acute arousal of anger.
Method
Design
A double-blind, placebo-controlled crossover design was used employing an opioid blockade methodology as in our past anger-related work (1,2,9,10). Order of drug administration was randomized and counterbalanced. Given the hypothesis that the pain-related effects of trait anger would be most prominent in the context of actual anger arousal, participants were randomly assigned to one of two experimental conditions designed to elicit anger or a neutral emotional response, respectively: Anger Recall Interview (ARI) or Neutral Control Interview (NCI). The purpose of this manipulation was to permit examination of the effects of trait anger with and without acute anger arousal on the outcomes of interest (opioid blockade effects). Participants remained in the assigned interview condition across both drug conditions, permitting examination of within-subject changes in pain responses under opioid blockade in the given interview condition. Thus, drug condition was a within-subject variable in this study, reflecting the change in acute pain intensity and unpleasantness measures across drug conditions. Between-subject independent variables were Participant Type (Healthy versus Chronic Low Back Pain), Interview condition (Anger Recall versus Neutral Control), and trait anger status. While a fully within-subject design (i.e., not only drug condition but also Interview condition) would have been statistically more powerful and would have strengthened conclusion regarding Interview effects, this design was not used due to the pragmatic difficulties of conducting four sessions per participant and concerns regarding habituation to experimental procedures that might offset any advantages of this design. All data collection took place over a period of 3 years and 7 months, 2006-2009.
Participants
Participants included 94 individuals with chronic low back pain (LBP) and 85 healthy pain-free controls (Healthy). Both Healthy and LBP participants were included in the study to address the possibility that anger/opioid links might differ as a function of chronic pain status, given previous findings that persistent pain may itself potentially increase (19) or decrease endogenous opioid analgesia (e.g.,[20-24]). A priori directional hypotheses regarding the influence of chronic pain status on the effects of interest were not possible given the limited data available to inform such hypotheses. All participants were recruited through on-line advertisements on the Vanderbilt e-mail recruitment system or advertisements in local print media. General criteria for participation included age between 18-55; no history of cardiovascular disease, hypertension, liver or kidney disorders, posttraumatic stress disorder, diabetes, seizure disorder, or opiate dependence; no use of anti-hypertensive medications; and no daily use of opioid analgesics. As in our past studies (1,2,10), additional inclusion criteria for the LBP group were chronic daily low back pain of at least 3 months duration with an average past month severity of at least 3/10. Within the LBP subgroup, 80% had consulted a physician for their back pain complaints, with 37.3% reporting a diagnosis of disc-related problems. All participants were asked to avoid use of as-needed opioid analgesics for three days prior to each study session (confirmed via urine opiate screen). Potential participants who were pregnant (determined by urine pregnancy screens) were excluded. All participants were asked to refrain from use of any analgesic or anti-inflammatory medications (e.g., acetaminophen, ibuprofen, etc.) for 12 hours prior to study participation, and to avoid use of caffeine for 3 hours prior to each study session. No participants in either group were taking neuroleptic medications. Three Healthy participants and 6 LBP participants were taking antidepressants; this difference was not significant (phi = 0.07, p=.39). Exclusion of these participants from analyses did not change the pattern of results reported below.
Characteristics of both study subgroups are summarized in Table 1. Although both groups were largely of non-Hispanic white ethnicity/race, Healthy controls were significantly more often of non-Hispanic ethnicity than were LBP participants. Neither race nor ethnicity significantly differed across assigned interview conditions (p’s>.30). While gender distribution across groups was not significantly different, LBP participants were significantly older than Healthy participants, although the magnitude of this difference was not large. Both groups were quite similar in terms of TRANG scores. However, LBP participants had significantly higher scores on the State Trait Anxiety Inventory (25) and Beck Depression Inventory (26). Both groups were nonetheless in the non-depressed range on the latter measure.
Table 1.
Participant characteristics by participant type.
| Measure | Healthy Controls (n=85) |
LBP (n=94) |
|---|---|---|
| Sex (% female) | 65.9 | 54.3 |
| Race†: | ||
| White | 85.9 | 71.3 |
| African-American | 9.4 | 20.2 |
| Ethnicity*: | ||
| Non-Hispanic | 97.6 | 90.4 |
| Age (years)** | 30.7±8.70 | 36.5±9.73 |
| VAS Chronic Pain Intensity (0-100) | 48.5±17.08 | |
| Pain Duration (median, in months) | 67.9 | |
| TRANG | 15.8±3.55 | 16.6±4.39 |
| BDI** | 3.4±3.70 | 7.3±6.09 |
| STAI** | 33.0±7.27 | 36.8±9.42 |
p<.10
p < .05
p < .003
Note: Summary statistics are presented as percentages or means (± SD).
Measures
The Trait Anger Scale (TRANG;[27]), the Beck Depression Inventory (BDI;[26]), and the trait form of the State Trait Anxiety Inventory (STAI;[25]) were completed by all participants. The TRANG is a validated measure of dispositional tendency to experience anger (27). TRANG scores in part reflect the frequency over time that angry episodes are experienced (27), with high scorers describing themselves as “hot-headed”, quick tempered, and dealing aggressively with frustration. Potential scores on the TRANG scale range from 10-40. The BDI and STAI were included to statistically control for effects of general negative affect on links among trait anger, pain and endogenous opioid analgesia.
Participants completed the McGill Pain Questionnaire-Short Form (MPQ;[28]) to describe the acute pain experienced during the laboratory pain tasks. The MPQ provides separate subscales assessing the sensory (MPQ-S) and affective (MPQ-A) dimensions of pain. A visual analog scale (VAS) measure of overall pain intensity is included on the MPQ (anchored with “No Pain” and “Worst Possible Pain”). A parallel VAS measure of pain unpleasantness (anchored with “Not Unpleasant at All” and “Most Unpleasant Possible”) was added for this study.
As a manipulation check on affective responses to the ARI/NCI manipulation, participants were asked to rate their emotional state using four subscales of the Emotional Assessment Scale (29) as in our past anger/opioid work (10). This measure consisted of twelve 100mm VAS items each presenting an emotional stem (three each for Anger, Fear, Anxiety, and Joy) which was rated from “Not at All” to “The Most Possible” to describe current emotional state. For example, anger stems were “Angry,” “Mad,” and “Annoyed.” The items for each core emotion were summed, leaving a potential range of 0-300 for each. The current study examined changes in anger elicited by the ARI/NCI, and to address emotional specificity of the ARI/NCI, also examined comparable changes in anxiety.
Also as a manipulation check on anger-related arousal in response to the ARI/NCI manipulation, blood pressure (BP) was assessed at baseline and during the assigned interview using a Dinamap Compact-T automated oscillometric blood pressure cuff placed on the dominant arm (Johnson & Johnson, Inc).
Opioid Blockade Agent
The opioid blockade agent used in this study was naloxone, a nonselective opioid receptor antagonist with a brief half life (1.1 hours). A 20ml dose of normal saline or an 8mg dose of naloxone (in 20ml saline vehicle) was infused via an automated infusion pump over a 10-minute period through a venous cannula. Order of drug administration was randomized and counterbalanced. The naloxone dosage used was adequate to block all opioid receptor subtypes (30).
Semi-Structured Interviews
Both the Anger Recall Interview (ARI) and Neutral Control Interview (NCI) were 5 min in duration, and all were conducted by the same trained interviewer. The ARI was originally developed based on work by Dimsdale et al. (31), and is described in our prior work (e.g.,[32]). ARI instructions to participants were: “I’d like you to think of a recent event that made you very angry or even furious. Usually anger-provoking events involve other people. Can you recall a recent event that involved a stranger, co-worker, friend, family member, doctor, case manager, health care worker, anyone, in which you became extremely angry? Think back to that event. Try to put yourself back there: what was happening, what you were thinking, what everyone was doing, and what was being said. Imagine you are back in that moment. Now, tell me everything that happened.” During the ARI, the interviewer guided participants to concentrate on their angry feelings through probes (e.g., “how did that make you feel?”) and reflecting back the feelings (e.g., “you must have been furious”). The interviewer avoided becoming hostile or argumentative, but indirectly challenged participants by expressing confusion and asking questions.
Participants assigned to the NCI condition received the following instructions: “We are interested in your diet. We would therefore like to interview you briefly, for five minutes, about the foods you commonly eat. I may ask you questions periodically to get you to clarify a response or provide additional information. Please describe to me the types of foods - including meats, vegetables, and fruits - you typically eat. You can describe these in any order you wish.” Interactions between the participant and experimenter in the NCI were non-challenging, and were intended simply to foster a similar level of experimenter interaction as occurred in the ARI.
Participants randomly assigned to the ARI condition were slightly older (ARI = 34.9±10.25 years; NCI = 32.6±8.90 years; t(178) = 1.61, p=.01). TRANG, BDI, and STAI scores did not differ across interview conditions (all p’s>.10). Gender ratio was not significantly different across interview conditions (ARI = 62.6% female, NCI = 57.3% female; p=.47), nor was participant type (ARI = 48.2% Controls, NCI = 52.8% controls; p=.51).
Experimental Acute Pain Induction
Participants underwent two experimental acute pain tasks. First was a one-minute finger pressure (FP) pain task using a modified Forgione-Barber finger pressure pain stimulator that applied 2000 grams of pressure to the dorsal surface of the second phalanx of the index finger of the dominant hand (33). Next was a forearm ischemic (ISC) pain task based on procedures described by Maurset et al. (34). Participants were first asked to raise their dominant forearm over their head for 30 seconds followed by two minutes of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength (as determined prior to beginning the laboratory procedures). Immediately following this, a BP cuff was inflated on the participant’s dominant bicep to 200 mmHg. The cuff remained inflated until participants indicated that their pain tolerance had been reached, up to a maximum of 5 minutes (due to ethical requirements).
Procedure
All procedures were performed at the Vanderbilt General Clinical Research Center, and were approved by the university Institutional Review Board. After providing written informed consent, participants engaged in two laboratory sessions (one under placebo and one under opioid blockade) one week apart at the same time of day to control for circadian rhythms. Immediately prior to the laboratory procedures, participants provided demographic information and completed the psychometric questionnaires. Then, participants assigned to the ARI condition were asked to identify two preferably recent incidents involving other individuals in which they had become “very angry” or “furious.” After providing a brief description, with additional prompting as necessary, to insure that both incidents were generally comparable and met study requirements, participants proceeded to the laboratory portion of the study. These two identified anger incidents provided the basis for the ARI across the two drug conditions.
Participants remained seated upright in a comfortable chair throughout all laboratory procedures. During each laboratory session, participants initially completed a 10-min seated rest period, followed by a series of five BP determinations at 1-min intervals. Participants were next asked to rate their emotional state using the VAS emotion rating scale described above. A registered nurse under physician supervision then placed an indwelling venous cannula in the participant’s non-dominant arm, followed by a 30-minute resting adaptation period. The appropriate study drug was then infused over a 10-minute period and the cannula removed. After a 10-minute rest following infusion to allow peak opioid blockade activity to be achieved, participants engaged in the assigned interview condition (ARI or NCI). BP was assessed at 1-min intervals throughout both interview conditions as a manipulation check for anger-related physiological arousal.
After completing the assigned interview manipulation, participants again completed VAS emotional state ratings as a manipulation check, and the Dinamap BP cuff was removed. Participants then immediately underwent the FP pain task. Immediately upon cessation of the FP task, participants completed the MPQ to describe the acute pain experienced during this task. Participants then engaged in the ISC pain task and immediately afterwards rated the acute pain experienced using the MPQ. Because of the high proportion of participants reaching the 5-min tolerance limit (more than 60% of participants in both drug conditions), valid analyses of ISC task tolerance were not possible. ISC pain threshold (time from task onset to first report that the task felt “painful,” in sec) was also obtained. Placebo condition and blockade effect analyses for ISC threshold were all nonsignificant (p’s>.10), and are not detailed further in the interests of space.
Statistical Analysis
All analyses were conducted using the PASW 18 statistical package (SPSS, Inc., Chicago, IL). Primary analyses consisted of two sets of hierarchical regressions, the first focusing on placebo condition pain ratings and the second focused on endogenous opioid analgesic responses. To index opioid analgesic activity, opioid blockade effects were derived reflecting the difference between placebo condition and naloxone condition acute pain ratings, as in our prior work (1,2,9,10). Raw pain rating values for each drug condition used in calculating opioid blockade effects are presented in Table 2. Blockade effects were derived such that positive values reflected increased pain in the naloxone condition relative to placebo, and thus, positive blockade effects indicated evidence for endogenous opioid analgesia. For example, a participant with a VAS intensity rating of 50/100 under placebo who reported a rating of 70/100 under naloxone would have a blockade effect value of 20, indicating greater endogenous opioid analgesia than a participant with a blockade effect value of 5. In both sets of regressions, independent predictors were Participant Type (dummy coded as Healthy=1 and LBP=2), Interview (dummy coded as ARI=1 and NCI=2), and TRANG (as a continuous measure). Main effects were entered jointly first, followed by the 2-way (multiplicative) interactions of these variables in the next step, and the 3-way interaction (Type × Interview × TRANG) in the last step. Conceptually, trait anger is considered a type of general negative affect, as are depression and anxiety. Not surprisingly, TRANG scores correlated significantly with both BDI (r=0.38, p<.001) and STAI scores (r=0.56, p<.001). To permit more specific examination of opioid analgesic responses as they relate to trait anger itself (as opposed to shared variance with general negative affect), all analyses entered both BDI and STAI scores into the first step of the hierarchical regression models as control variables. A significance criterion of p<.05 was used in all analyses, and correlation coefficients (r) are presented to indicate the magnitude of the reported associations.
Table 2.
Unadjusted mean (± SD) pain ratings by interview condition and participant type across drug conditions.
| Healthy Controls | LBP | |||||||
|---|---|---|---|---|---|---|---|---|
| ARI (n=41) | NCI (n=44) | ARI (n=50) | NCI (n=44) | |||||
| Variable | Placebo | Naloxone | Placebo | Naloxone | Placebo | Naloxone | Placebo | Naloxone |
| FP – MPQ-S | 13.1±5.64 | 12.7±5.85 | 10.1±6.38 | 10.0±5.64 | 12.9±6.11 | 13.1±6.12 | 10.7±5.87 | 10.7±5.39 |
| FP – MPQ-A | 1.3±1.75 | 1.4±1.62 | 1.0±1.72 | 0.9±1.70 | 2.6±2.47 | 2.1±2.25 | 1.1±1.30 | 1.2±1.40 |
| FP – VAS Intensity | 55.0±22.07 | 53.1±22.74 | 45.7±23.39 | 44.4±20.30 | 60.7±22.33 | 59.4±23.82 | 51.0±22.14 | 55.2±23.12 |
| FP – VAS Unpleasantness | 59.1±21.98 | 58.4±24.82 | 50.0±24.37 | 48.1±19.81 | 65.3±23.86 | 66.2±24.12 | 54.3±23.18 | 58.8±25.62 |
| ISC – MPQ-S | 11.4±6.26 | 12.6±7.48 | 8.1±6.26 | 8.1±6.00 | 11.3±6.89 | 11.9±7.03 | 10.2±5.25 | 9.3±4.82 |
| ISC – MPQ-A | 2.2±2.48 | 2.1±2.71 | 1.5±1.96 | 1.4±2.02 | 2.7±2.84 | 2.2±2.47 | 1.2±1.19 | 1.2±1.63 |
| ISC – VAS Intensity | 50.5±24.73 | 51.3±25.11 | 41.2±27.25 | 41.3±25.92 | 51.4±29.82 | 49.4±26.28 | 48.5±22.51 | 44.4±25.86 |
| ISC – VAS Unpleasantness | 59.0±24.46 | 63.0±23.19 | 50.0±26.02 | 52.3±26.49 | 56.1±30.94 | 57.6±28.3 | 51.4±22.26 | 47.0±26.48 |
Note: Comparisons of pain ratings between drug conditions within interview condition and participant type are all nonsignificant.
Preliminary analyses indicated that gender did not exert significant effects on any blockade effect variables, with the exception of ISC MPQ-A blockade effects. Women exhibited smaller blockade effects (lower opioid analgesia) on this variable than did men. Controlling for gender in the analysis of ISC MPQ-A blockade effects left the pattern of findings reported below entirely unchanged.
Results
Manipulation Check on Anger Induction
Placebo condition changes in BP and self-reported anger from baseline to the assigned interview were examined across ARI and NCI conditions as a manipulation check. Mean systolic BP reactivity [Placebo ARI: Δ 12.2±11.7 mmHg, Placebo NCI: Δ 2.0±8.37 mmHg; t(179) = 6.77, p<.001; r=0.45] and diastolic BP reactivity [Placebo ARI: Δ 8.9±7.06 mmHg, Placebo NCI: Δ 3.5±5.50 mmHg; t(179) = 5.72, p<.001; r=0.39] were significantly greater in the ARI than in the NCI conditions. As expected, visual analog scale ratings of anger during the assigned interview increased significantly more [t(174) = 8.37, p<.001; r=0.53] in the ARI condition (Placebo Δ 54.5±62.04) than in the NCI condition (Placebo Δ −9.5±34.0). In contrast, self-reported anxiety decreased slightly and to a similar degree in both conditions (Placebo ARI: Δ −9.2±35.04, Placebo NCI: Δ −18.2±41.87; p=.13). These results confirm that compared to emotionally neutral speech (the NCI), the ARI produced significantly greater emotion-specific increases in self-reported anger and physiological arousal consistent with anger. Examination of the manipulation check variables above across drug conditions did not reveal any significant Drug × Interview interactions (p’s>.42).
Trait Anger and Placebo Condition Pain Responses
Finger Pressure Pain Task
For the FP task, regression analyses of placebo condition MPQ-S ratings revealed a significant TRANG × Interview interaction (t = −2.10, p=.04; r=0.16). Simple effect analyses indicated that this interaction resulted from a significant positive association between TRANG and MPQ-S ratings in the ARI condition (beta = 0.35; t = 2.73, p=.008; r=0.28), with a nonsignificant association in the NCI condition (beta = −0.15; t = −1.28, p=.20; r=0.14). Placebo condition MPQ-A ratings produced a similar pattern, revealing a significant TRANG × Interview interaction (t = −2.53, p=.02; r=0.19). As for MPQ-S ratings, there was a significant positive association between TRANG and placebo condition MPQ-A ratings in the ARI condition (beta = 0.34; t = 2.71, p=.008; r=0.27), with a nonsignificant association in the opposite direction in the NCI condition (beta = −0.22; t = −1.93, p=.06; r=0.20). The only other notable effect in this analysis was a Participant Type × Interview interaction (t = −1.95, p=.05; r=0.14), reflecting a significant association between the anger arousal manipulation (ARI) and elevated MPQ-A ratings in the LBP subsample (beta = −0.34; t = −3.48, p<.001; r=0.34) that was absent in the Healthy subsample (p>.10).
Although examination of placebo condition FP VAS intensity did not reveal a significant TRANG × Interview interaction (p>.10), a significant main effect for Interview was found, with pain ratings higher in the ARI condition (beta = −0.18; t = −2.55, p=.02; r=0.19). For VAS unpleasantness, the TRANG × Interview interaction did not reach statistical significance (t = −1.79, p=.08; r=0.13). However, a significant positive association between TRANG and VAS unpleasantness was noted in the ARI condition (beta = 0.26; t = 2.01, p=.04; r=0.21) that was absent in the NCI condition (beta = −0.17; t = −1.48, p=.15; r=0.16). The VAS unpleasantness main effect for Interview was also significant, with higher unpleasantness ratings in the ARI condition (beta = −0.18; t = −2.56, p=.02; r=0.19).
Ischemic Pain Task
Findings for placebo condition pain ratings on the ISC task were generally similar to findings for the FP task. For placebo MPQ-S ratings, a significant TRANG × Interview interaction was observed (t = −2.09, p=.04; r=0.15). This interaction resulted from a nonsignificant positive association between TRANG and MPQ-S rating in the ARI condition (beta = 0.23; t = 1.74, p=.08; r=0.18), with a nonsignificant inverse association in the NCI condition (beta = −0.20; t = −1.75, p=.08; r=0.13). The TRANG × Interview interaction for MPQ-A ratings revealed a similar pattern, in which higher TRANG was associated with significantly higher MPQ-A ratings in the ARI condition (beta = 0.27; t = 2.06, p=.04; r=0.21), whereas in the NCI condition a nonsignificant association in the opposite direction was noted (beta = −0.22; t = −1.89, p=.07; r=0.20; TRANG × Interview interaction t = −1.85, p=.07; r=0.14). All other main and interaction effects in the MPQ-S and MPQ-A analyses above, and all effects in analyses of placebo condition ISC VAS intensity and unpleasantness were nonsignificant (p’s>.12).
Trait Anger and Opioid Blockade Effects
Finger Pressure Pain Task
A significant TRANG × Interview interaction on MPQ-A blockade effects for the FP task was observed (t = 1.95, p=.04; r=0.14). Follow-up simple effects analyses indicated that this significant interaction was due to nonsignificant associations between TRANG and MPQ-A blockade effects that were in opposite directions in the ARI condition (beta = −0.19; t = −1.46, p=.14; r=0.15) and the NCI condition (beta = 0.12; t = 0.94, p=.34; r=0.10). Similar analyses of MPQ-S blockade effects revealed that higher TRANG was associated with significantly smaller MPQ-S blockade effects in the ARI condition (i.e., less endogenous opioid analgesia; beta = −0.32; t = −2.42, p=.02; r=0.25), whereas this association was nonsignificant in the NCI condition (beta = 0.04; t = 0.34, p=.74; r=0.04; TRANG × Interview interaction t = 1.82, p=.07; r=0.13).
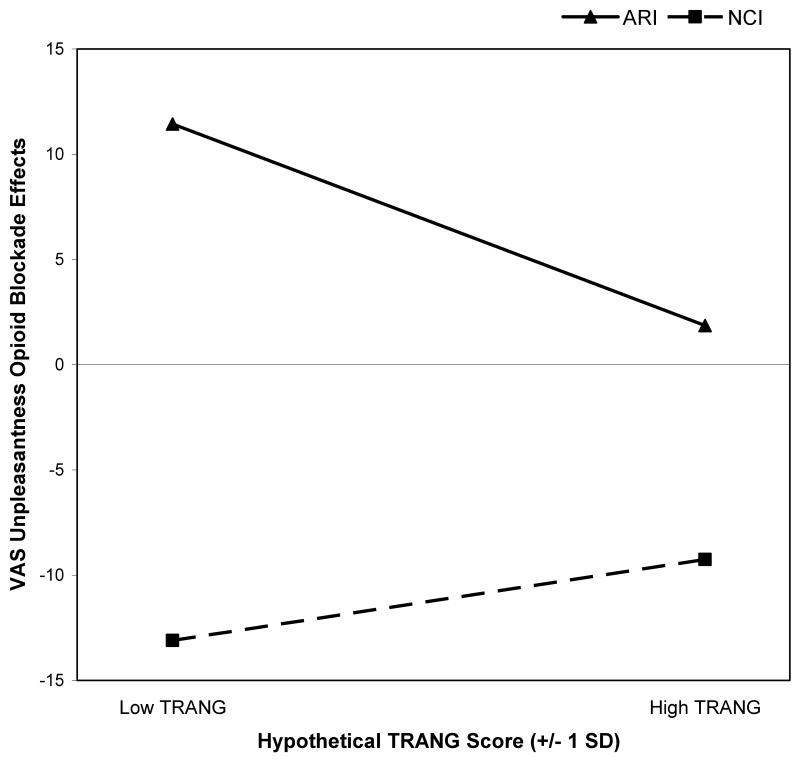
Although analyses of FP VAS intensity blockade effects did not reveal any significant effects (p’s>.10), analyses of VAS unpleasantness did, paralleling the pattern observed above. A significant TRANG × Interview interaction was observed (t = −2.71, p=.02; r=0.20), reflecting an inverse association between TRANG and VAS unpleasantness blockade effects approaching significance in the ARI condition (beta = −0.26; t = −1.94, p=.06; r=0.20) that was absent in the NCI condition (beta = 0.08; t = 0.70, p=.49; r=0.07). As an illustrative example, this interaction observed for FP task VAS unpleasantness blockade effects is portrayed graphically in Figure 1 in the manner recommended by Aiken and West (35). Specifically, the regression equations computed for ARI and NCI conditions were solved for hypothetical low and high TRANG values (−1 SD and + 1 SD from the mean TRANG score), with blockade effects values plotted for each.
Figure 1.
Effects of trait anger (TRANG) on finger pressure task visual analog scale (VAS) unpleasantness opioid blockade effects across anger recall interview (ARI) and neutral control interview (NCI) conditions. TRANG values plotted are hypothetical values representing one standard deviation (SD) below and above the sample mean. Larger positive blockade effects indicate greater endogenous opioid analgesia.
VAS unpleasantness blockade effect analyses also revealed a significant TRANG × Participant Type interaction (t = −2.71, p=.008; r=0.20). This interaction resulted from a significant positive association between TRANG and VAS unpleasantness blockade effects in Healthy controls (beta = 0.24; t = 1.97, p=.05; r=0.22), with an opposing and significant inverse association in LBP participants (beta = −0.37; t = −2.95, p=.004; r=0.30). Other main and interaction effects in these analyses were nonsignificant (p’s>.29).
Main effects of BDI and STAI were not significant predictors of opioid blockade effects for any FP task pain ratings (all p’s > .07) It should be noted that effects of BDI and STAI were controlled statistically in all of the analyses above, and therefore significant TRANG effects reported were independent of any influence of these non-anger negative affects.
Ischemic Pain Task
Analyses of ISC task blockade effects also revealed several significant findings. For MPQ-S blockade effects, a significant TRANG × Interview interaction was observed (t = 2.86, p<.005; r=0.21). While higher TRANG scores were associated with significantly smaller blockade effects (i.e., less opioid analgesia) in the ARI condition (beta = −0.29; t = −2.12, p=.04; r=0.22), TRANG was associated with significantly larger MPQ-S blockade effects in the NCI condition (beta = 0.28; t = 2.38, p=.03; r=0.25).
A significant TRANG × Interview interaction was also noted for MPQ-A blockade effects (t = 2.39, p=.02; r=0.18). In the ARI condition, greater TRANG was associated with significantly smaller MPQ-A blockade effects (beta = −0.36; t = −2.78, p=.007; r=0.28), whereas in the NCI condition, TRANG was linked to significantly larger blockade effects (beta = 0.29; t = 2.49, p=.02; r=0.26). All other main and interaction effects in ISC blockade effect analyses for MPQ-S and MPQ-A were nonsignificant.
Analyses of ISC task VAS intensity blockade effects revealed a significant 3-way TRANG × Interview × Participant Type interaction (t = 2.37, p=.02; r=0.17). Examination of component 2-way interactions by subject type indicated that the Interview × TRANG interaction was nonsignificant in Healthy controls (p=.34), but significant among the LBP participants (t = 2.75, p=.007; r=0.28). The latter two-way interaction was due to a significant inverse association between TRANG and blockade effects in the ARI condition (beta = −0.50; t = −2.73, p=.009; r=0.36), with no significant relationship in the NCI condition (beta = 0.22; t = 1.24, p=.23; r=0.18). Thus, associations between greater TRANG and reduced opioid analgesia were most prominent in LBP participants under conditions of anger arousal. Analyses of VAS unpleasantness failed to reveal any significant effects (p’s>.14). Main effects for BDI and STAI were nonsignificant in all ISC blockade effect analyses (p’s>.15).
Discussion
Anger-related traits affect both acute and chronic pain responses, but underlying mechanisms remain to be fully elucidated. Prior work suggests that deficient endogenous opioid analgesia may contribute (1,2,9-11). Little is known about how these trait variables interact with situational (state) anger arousal to affect endogenous opioid antinociceptive function. In light of theoretical arguments emphasizing the importance of trait × state interactions vis-à-vis health outcomes (18) and recent findings demonstrating that acute anger arousal can trigger opioid-mediated analgesia (16), we hypothesized that state anger arousal would moderate the opioid-related effects of trait anger on acute pain responses.
Manipulation check analyses confirmed the ARI was effective in arousing acute anger. Results further indicated that this anger arousal moderated the impact of trait anger on placebo condition pain responses. Across the two acute pain stimuli and both sensory and affective pain measures, elevated trait anger was associated with significantly greater acute pain responsiveness only in the context of acute anger arousal (the ARI condition). This trait × state interaction parallels previous findings for other anger-related variables. For example, the hyperalgesic impact of trait anger-out is in some cases stronger, or may only be evident, when state anger is aroused (36,37). Similarly, chronic low back pain patients high in anger-out exhibit increased lumbar paraspinal muscle reactivity only when state anger is actually experienced and suppressed (38). These findings however stand in contrast to other work (e.g.,[1]) indicating positive associations between trait anger and acute pain responses even in the absence of acute anger arousal. One notable difference from this past work was that in the current study, subjects in the “non-anger arousal” condition discussed their diet with an interviewer for 5 minutes immediately prior to undergoing the pain tasks rather than simply resting quietly as in the other study (1). Whether and how effects of distraction and attention, physiological effects of speech, or some other aspect of the current study procedures influenced the present findings cannot be conclusively determined. The fact that positive correlations between trait anger and acute pain responsiveness were noted in the current study only in the context of anger arousal but that these were observed for both acute pain tasks and for measures of both sensory and affective pain suggest it is unlikely that the current results are entirely spurious. Nonetheless, replication is necessary to draw more definitive conclusions regarding the nature of anger-related trait × state interactions on pain outcomes.
The primary aim of the current study was to explore possible interactions between trait anger and acute anger arousal on endogenous opioid analgesia. Findings supported the hypothesis that the opioid-related effects of trait anger depend on whether or not acute anger is aroused. Under conditions of acute anger arousal (the ARI condition), elevated trait anger was associated with less endogenous opioid analgesia on both acute pain tasks. When placebo condition and opioid blockade effect results are considered jointly, the overall pattern of findings in this study suggests the following. First, there was little association between trait anger and acute pain responses in the absence of state anger, as noted above. Second, a positive association between trait anger and pain responsiveness emerged only in the context of anger arousal. Third, the observed hyperalgesic effects of trait anger are likely related to parallel links observed between elevated trait anger and impaired endogenous opioid analgesia that also emerged only in the context of anger arousal. We speculate that the latter effects may be due to an anger-related triggering of endogenous opioid analgesia among people relatively low in trait anger that is absent among people high in trait anger (whose opioid systems may be impaired), thereby exaggerating differences in pain responses between the two groups. Similar triggering of opioid analgesia by acute anger arousal (via harassment) has been found in our prior work (16).
Across multiple measures and both acute pain tasks, greater trait anger in the context of acute anger arousal was associated with diminished endogenous opioid analgesia. One unexpected finding was that higher levels of trait anger were associated with greater opioid analgesic activity following the neutral control interview (no anger arousal), at least for ISC task MPQ-Sensory and MPQ-Affective subscales, in contrast to similar past work (1). This unexpected pattern was not observed for any of the FP task blockade effects. Thus, while there was consistent evidence that anger arousal elicited an inverse association between trait anger and opioid analgesic dysfunction, the direction of these effects in the absence of anger arousal is somewhat less certain. Methodological issues (e.g., presence of a diet interview prior to the pain tasks) may be relevant, and further exploration of this issue is merited to better understand the nature and determinants of the observed moderation effects.
Findings of the current study did not consistently suggest that links between trait anger and endogenous opioid analgesia differed as a function of chronic pain status, a finding similar to some previous work (1,11). One exception in this regard was for ISC task unpleasantness, which revealed that trait anger/opioid links were evident only in LBP participants, and only under conditions of state anger arousal. In a related vein, an association between elevated trait anger and diminished endogenous opioid analgesia (on FP task unpleasantness) was observed only in LBP participants. More generally, results for affective pain responses to the FP task indicated that the ARI itself (regardless of trait anger levels) produced hyperalgesia selectively in LBP participants. Although these findings should be interpreted with caution until replicated, they are consistent with the idea that individuals with chronic pain might be particularly susceptible to opioid system dysfunction, as suggested by some prior work (e.g.,[19-24]). Given the ubiquity of anger in the chronic pain population (7), it may be interesting to explore whether interventions designed to reduce anger in chronic pain patients might as a consequence improve functioning of endogenous opioid analgesic systems.
All analyses in this study controlled statistically for the effects of non-anger negative affect, including both depression (BDI) and anxiety (STAI). Therefore, results summarized above do not appear to be due to general negative affect (assessed using trait-like measures rather than measures of acute emotional state), but more specifically to a propensity to experience anger. That is, elevated trait anger is associated with reduced endogenous opioid analgesia in the context of acute anger independent of any overlap with general negative affect. This finding is consistent with our prior work demonstrating that elevated anger-out is associated with lower endogenous opioid analgesia independent of general negative affect (2,9,11).
Several potential study limitations are noted. Given that participants assigned to the ARI condition participated in two sessions under different drug conditions, there is no way of conclusively demonstrating (unconfounded by drug effects) that the memories recalled across the two drug conditions were similar in terms of the degree of anger elicited. Despite this limitation, the fact that changes in self-reported anger from baseline to the ARI were not significantly different across the two drug conditions (p>.10) supports the general equivalence of anger arousal across the two study sessions. In addition, the trained individual conducting all ARI interviews sought to insure that the two anger-provoking events selected for the ARI prior to the laboratory portion of the study were approximately comparable. Given that drug administration order was randomly determined, differences in anger intensity across the two sessions should not be confounded with drug condition even if such differences were systematic (e.g., if most intense anger memories were always selected first).
Another limitation also relates to the emotion induction manipulation in the current study. Trait × state interactions for induced non-anger negative affects (e.g., sadness) were not evaluated. It is possible that the trait × state interactions reported in this study were not specific to state anger arousal, but rather, might also have been observed with other negative affect states. Our prior work demonstrating significant pain-relevant interactions selectively between trait anger-out and recall-based state anger arousal but not recall-based sadness suggests that results in the current study are most likely specific to the arousal of anger (32,39,40).
An additional potential limitation relates to the blockade effect measures themselves. These measures did not indicate that opioid blockade resulted in significant changes in pain responses in the overall sample. While this might raise questions about the efficacy of the opioid blockade manipulation for examining endogenous opioid analgesia, the study explicitly hypothesized a priori interactions proposing that increases in pain responses due to opioid blockade (blockade effects) would be selective to certain participant subgroups, and this was in fact the case. Therefore, individual difference variables may be the crucial determining factor as to whether endogenous opioid analgesia is elicited. It is clear, nonetheless, that the hypothesized opioid effects were not observed consistently across every measure examined in this study for the two laboratory pain tasks. Replication of the findings of this study are necessary to permit more definitive conclusions.
Finally, given prior work showing that endogenous opioid analgesia may differ by gender, although not always in consistent directions (41,42), the lack of full exploration of all relevant gender interactions in the current study is a potential weakness. Main effects of gender were examined in preliminary analyses, and were with one exception, not related significantly to opioid blockade effects. Given the already complex design of the study (2 Interview Conditions × 2 Participant Types × TRANG), exploration of all relevant interactions would have required examination of numerous 3-way interactions as well as 4-way interactions, with inadequate cell sizes for reliable interpretation.
In summary, this study found that the hyperalgesic effects of a strong dispositional tendency to experience anger are significantly more prominent in the context of acute anger arousal. These hyperalgesic effects of elevated trait anger appear to be due in part to deficient endogenous opioid analgesia, most evident when anger is being experienced. The impact of these anger-related opioid deficits may be stronger in individuals experiencing chronic pain. Potential clinical implications of these findings remain to be explored.
Acknowledgments
Support: This research was supported by NIH Grant R01-NS050578 and Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH.
Acronyms
- TRANG
Trait Anger
- ARI
Anger Recall Interview
- NCI
Neutral Control Interview
- LBP
Chronic Low Back Pain
- BDI
Beck Depression Inventory
- STAI
Trait form of the State Trait Anxiety Inventory
- MPQ-S and MPQ-A
McGill Pain Questionnaire – Short Form Sensory and Affective subscales
- BP
Blood Pressure
- FP
Finger Pressure
- ISC
Ischemic
Footnotes
The authors have no conflicts of interest to report.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- [2].Bruehl S, Chung OY, Burns JW, Biridepalli S. The association between anger expression and chronic pain intensity: evidence for partial mediation by endogenous opioid dysfunction. Pain. 2003;106:317–324. doi: 10.1016/S0304-3959(03)00319-1. [DOI] [PubMed] [Google Scholar]
- [3].Conant LL. Psychological variables associated with pain perception among individuals with chronic spinal cord injury pain. J Clin Psych Med Settings. 1998;5:71–90. [Google Scholar]
- [4].Gaskin ME, Greene AF, Robinson ME, Geisser ME. Negative affect and the experience of chronic pain. J Psychsom Res. 1992;36:707–713. doi: 10.1016/0022-3999(92)90128-o. [DOI] [PubMed] [Google Scholar]
- [5].Hirsh AT, George SZ, Riley JL, 3rd, Robinson ME. An evaluation of the measurement of pain catastrophizing by the coping strategies questionnaire. Eur J Pain. 2007;11:75–81. doi: 10.1016/j.ejpain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- [6].Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J Behav Med. 1994;17:57–67. doi: 10.1007/BF01856882. [DOI] [PubMed] [Google Scholar]
- [7].Okifuji A, Turk D, Curran SL. Anger in chronic pain: investigations of anger targets and intensity. J Psychosom Res. 1999;47:1–12. doi: 10.1016/s0022-3999(99)00006-9. [DOI] [PubMed] [Google Scholar]
- [8].Tschannen TA, Duckro PN, Margolis RB, Tomazic TJ. The relationship of anger, depression, and perceived disability among headache patients. Headache. 1992;32:501–503. doi: 10.1111/j.1526-4610.1992.hed3210501.x. [DOI] [PubMed] [Google Scholar]
- [9].Bruehl S, al’Absi M, France CR, France J, Harju A, Burns JW, Chung OY. Anger management style and endogenous opioid function: is gender a moderator? J Behav Med. 2007;30:209–219. doi: 10.1007/s10865-007-9099-2. [DOI] [PubMed] [Google Scholar]
- [10].Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- [11].Bruehl S, Chung OY, Burns JW, Diedrich L. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci Biobehav Rev. 2009;33:475–491. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- [14].Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- [16].Burns JW, Bruehl S, Chung OY, Magid E, Chont M, Goodlad JK, Gilliam W, Matsuura J, Somar K. Endogenous opioids may buffer effects of anger arousal on sensitivity to subsequent pain. Pain. 2009;146:276–282. doi: 10.1016/j.pain.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bruehl S, Chung OY, Burns JW. Trait anger and blood pressure recovery following acute pain: evidence for opioid-mediated effects. Int J Behav Med. 2006;13:138–146. doi: 10.1207/s15327558ijbm1302_5. [DOI] [PubMed] [Google Scholar]
- [18].Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. J Pers Soc Psych. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- [19].Bruehl S, Chung OY, Chont M. Chronic pain-related changes in endogenous opioid analgesia: a case report. Pain. 2010 Jan;148(1):167–71. doi: 10.1016/j.pain.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bruehl S, McCubbin JA, Harden RN. Theoretical review: Altered pain regulatory systems in chronic pain. Neurosci Biobehav Rev. 1999;23:877–890. doi: 10.1016/s0149-7634(99)00039-1. [DOI] [PubMed] [Google Scholar]
- [21].Bruehl S, Chung OY. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124:287–294. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- [22].Fukui T, Hameroff SR, Gandolfi AJ. Alpha-1-acid glycoprotein and beta-endorphin alterations in chronic pain patients. Anesthes. 1984;60:494–496. doi: 10.1097/00000542-198405000-00022. [DOI] [PubMed] [Google Scholar]
- [23].Maixner W, Sigurdsson A, Fillingim RB, Lundeen T, Booker DK. Regulation of acute and chronic orofacial pain. In: Fricton JR, Dubner R, editors. Orofacial Pain and Temporomandibular Disorders. Raven Press; New York: 1995. [Google Scholar]
- [24].Puig MM, Laorden ML, Miralles FS, Olaso MJ. Endorphin levels in cerebrospinal fluid of patients with postoperative and chronic pain. J Anesthes. 1982;57:1–4. doi: 10.1097/00000542-198207000-00001. [DOI] [PubMed] [Google Scholar]
- [25].Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- [26].Beck AT, Ward CH, Mendelson M, Mock JE, Erbough JK. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- [27].Spielberger CD, Jacobs G, Russell S, Crane R. Assessment of anger: The State-Trait Anger Scale. In: Butcher JN, Spielberger CD, editors. Advances in Personality Assessment. Volume 2. LEA; Hillsdale, NJ: 1983. pp. 161–189. [Google Scholar]
- [28].Melzack R. The short form of the McGill pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- [29].Carlson CR, Collins FL, Stewart JF, Porzelius J, Nitz JA, Lind CO. The assessment of emotional reactivity: a scale development and validation study. J Psychopath Behav Assess. 1989;11:313–325. [Google Scholar]
- [30].Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–159. [PubMed] [Google Scholar]
- [31].Dimsdale JE, Stern MJ, Dillon E. The stress interview as a tool for examining physiological reactivity. Psychosom Med. 1988;50:64–71. doi: 10.1097/00006842-198801000-00008. [DOI] [PubMed] [Google Scholar]
- [32].Burns JW. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion. 2006;6:309–319. doi: 10.1037/1528-3542.6.2.309. [DOI] [PubMed] [Google Scholar]
- [33].Forgione AG, Barber TX. A strain gauge pain stimulator. Psychophys. 1971;8:102–106. doi: 10.1111/j.1469-8986.1971.tb00441.x. [DOI] [PubMed] [Google Scholar]
- [34].Maurset A, Skoglung LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1992;13:643–647. [PubMed] [Google Scholar]
- [35].Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Thousand Oaks, CA: 1991. [Google Scholar]
- [36].Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: Evidence for a Trait × Situation model. Ann Behav Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- [37].Burns JW, Quartana PJ, Bruehl S. Anger management style moderates effects of emotion suppression during initial stress on pain and cardiovascular responses during subsequent pain-induction. Ann Behav Med. 2007;34:154–165. doi: 10.1007/BF02872670. [DOI] [PubMed] [Google Scholar]
- [38].Burns JW, Holly A, Quartana P, Wolff B, Gray E, Bruehl S. Trait anger management style moderates effects of actual (“state”) anger regulation on symptom-specific reactivity and recovery among chronic low back pain patients. Psychosom Med. 2008;70:898–905. doi: 10.1097/PSY.0b013e3181835cb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burns JW, Bruehl S, Quartana P. Anger management style and hostility among chronic pain patients: effects on symptom-specific physiological reactivity during anger- and sadness-recall interviews. Psychosom Med. 2006;68:786–793. doi: 10.1097/01.psy.0000238211.89198.e4. [DOI] [PubMed] [Google Scholar]
- [40].Burns JW, Kubilus A, Bruehl S. Emotion-induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–18. doi: 10.1016/s0304-3959(03)00298-7. [DOI] [PubMed] [Google Scholar]
- [41].Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007;132(Suppl 1):S77–85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- [42].Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]