Abstract
Feline leukemia virus (FeLV) was not detected in Florida pumas (Puma concolor coryi) in almost 20 yr of surveillance; however, the finding of two FeLV antigen-positive pumas during the 2002–2003 capture season led to an investigation of FeLV in the population. Between January 1990 and April 2007, the proportion of pumas testing FeLV antibody positive increased, with antibody-positive pumas concentrated in the northern portion of puma range. Five of 131 (4%) pumas sampled between July 2000 and April 2007 were viremic, with all cases clustered in Okaloacoochee Slough (OKS). Clinical signs and clinical pathology at capture were absent or included lymphadenopathy, moderate-to-severe anemia, and lymphopenia. All viremic pumas died; causes of death were septicemia (n=2), intraspecific aggression (n=2), and anemia/dehydration (n=1). Outcome after FeLV exposure in pumas was similar to that in domestic cats, with evidence of regressive, latent, and persistent infections. Management of the epizootic included vaccination, and as of April 2007, 52 free-ranging pumas had received one or more inoculations. Vaccinations were concentrated in OKS and in a band between OKS and the remainder of the puma population. There have been no new cases since July 2004; however, the potential for reintroduction of the virus remains.
Keywords: Feline leukemia virus, Florida panther, infectious disease, Puma concolor coryi, retrovirus, vaccination
INTRODUCTION
The Florida puma (Puma concolor coryi) is an endangered subspecies whose range was contiguous with other puma populations (Young and Goldman, 1946). By the late 20th century, however, habitat destruction, exploitation, and human population growth had reduced the Florida puma population to an isolated remnant numbering an estimated 20 to 30 individuals (Nowak and McBride, 1974). With protection and management, including the translocation of eight pumas (Puma concolor couguar) from Texas in 1995 as part of a genetic restoration program (Seal, 1994), the population rebounded to at least 87 by 2003 (McBride, 2003). Consequently, this greater density may have increased both exposure to domestic animals and the risks of infectious disease transmission. Herein, we describe an epizootic of feline leukemia virus (FeLV) and its management in the free-ranging Florida puma population.
Feline leukemia virus is a retrovirus of domestic cats (Felis silvestris catus). In Florida, the prevalence of antigenemia among feral cats is <4% (Lee et al., 2002). Transmission is usually by direct contact, and outcome after exposure depends on several host and viral factors. Most infections are self-limiting in domestic cats, being eliminated shortly after exposure (regressive infection) or progressing to latent infections before containment (Hardy, 1980; Torres et al., 2005). Although domestic cats developing regressive or latent infections may be transiently viremic postexposure, they are not considered important to the maintenance of the disease in domestic cats. In approximately one third of exposed cats, viremia is persistent and eventually results in clinical syndromes of immunosuppression, anemia, neoplasia, or a combination. Mortality among persistently infected domestic cats is approximately fivefold that of uninfected cats, and 83% die within 3.5 yr (McClelland et al., 1980).
Feline leukemia virus in nondomestic felids is rare. Reported infections in captive nondomestic felids include a leopard cat (Felis bengalensis) (Rasheed and Gardner, 1981), puma (Meric, 1984), clouded leopard (Neofelis nebulosa) (Citino, 1986), bobcat (Lynx rufus) (Sleeman et al., 2001), and several cheetahs (Acinonyx jubatus) (Briggs and Ott, 1986; Marker et al., 2003). Despite extensive testing for FeLV in free-ranging felid populations, published reports of FeLV infections in the wild have been limited to three pumas (P. concolor) (Rickard and Foreyt, 1992; Jessup et al., 1993) and a sand cat (Felis margarita) (Ostrowski et al., 2003). Ten to 24% of European wildcats (Felis silvestris silvestris) also were FeLV antigen positive (Daniels et al., 1999; Fromont et al., 2000), although interbreeding with domestic cats occurs in this subspecies frequently (French et al., 1988). Most infections in nondomestic felids were self-limiting. In a survey of North American zoos, seven of 11 (64%) nondomestic felids that tested FeLV positive initially were negative when retested. The remaining four felids were not retested, and they did not develop clinical signs of FeLV (Kennedy-Stoskopf, 1999). Persistent infections were seen in a free-ranging and a captive puma, a bobcat, and cheetahs, and clinical pathology and necropsy findings included anemia, lymphopenia, other cytopenias, lymphadenopathy, septicemia, opportunistic infections, and lymphoma (Meric, 1984; Briggs and Ott, 1986; Jessup et al., 1993; Sleeman et al., 2001).
Management of FeLV in domestic cat populations includes vaccination, test-removal, or both. Inactivated whole-virus vaccines require an initial (prime) inoculation followed by booster in 3–6 wk. In challenge studies, whole-virus vaccines such as Fel-O-Vax Lvk® and Fevaxyn FeLV® provided from 86 to 100% protection (reviewed by Sparkes, 1997; Torres et al., 2005); however, protection after a single inoculation is likely low. Few studies have examined duration of immunity beyond 1 yr, although a recombinant FeLV vaccine was shown to be protective over 3 yr (Hofmann-Lehmann et al., 1995). Vaccination has been used to prevent FeLV in captive nondomestic felids; however, to our knowledge FeLV vaccination of free-ranging nondomestic felids has not been reported.
In Florida pumas, routine FeLV enzyme-linked immunosorbent assay (ELISA) antigen testing was negative between 1978 and November 2002 (Roelke et al., 1993; Florida Fish and Wildlife Conservation Commission [FWC], unpubl. data); however, during the 2002–2003 capture season, two pumas tested antigen positive. These findings led to an investigation of FeLV in the puma population and efforts to manage the epizootic.
MATERIALS AND METHODS
Florida puma capture, handling, and necropsy
Free-ranging pumas were captured by the FWC and National Park Service (NPS) between 1 July 2000 and 1 April 2007 in southern peninsular Florida (south of 28°N) as described by McCown et al. (1990) and McBride and McBride (2007). Pumas were immobilized with ketamine HCl (Congaree Veterinary Pharmacy, Cayce, South Carolina, USA) combined with α-2 agonists tiletamine HCl/zolazepam HCl (Telazol®, Fort Dodge Animal Health [FDAH], Fort Dodge, Iowa, USA), midazolam HCl (Abbott Laboratories, North Chicago, Illinois, USA), or both. All animals underwent physical examination. Approximately 70–140 ml of blood was collected in serum separator, ethylenediaminetetraacetic acid (EDTA), Na-heparin, and ACD blood tubes (BD Biosciences, Franklin Lakes, New Jersey, USA). Blood smears were made from whole blood at capture or with EDTA whole blood approximately 6–24 hr after collection. Puma ages were either known (handled as neonates), or they were estimated from tooth wear and gum line recession. Neonates were handled as described by Land et al. (1998).
Pumas >4 mo old were vaccinated subcutaneously against feline viral rhinotracheitis, feline calicivirus, feline panleukopenia virus (Fel-O-Vax PCT [FDAH], 1 ml, lower left leg), and rabies (Rabvac™ 3 [FDAH], 1 ml, lower right leg). Beginning June 2003, captive and free-ranging pumas also were vaccinated against FeLV (Fel-O-Vax Lv-K [FDAH] or Fevaxyn FeLV, Schering-Plough Animal Health Corporation, Omaha, Nebraska, USA, 2 ml, lower left leg). Between November 2003 and April 2004, higher risk pumas (males, pumas in or near Okaloacoochee Slough [OKS]) were boosted (2 ml) intramuscularly (IM) by dart 3–16 wk after initial inoculation. Thereafter, pumas were boosted at recapture. Captured adult and juvenile pumas were fitted with a very high frequency (VHF) or VHF/global positioning system radiocollar and located three times weekly.
Pumas were necropsied at the University of Florida College of Veterinary Medicine (UF-CVM, Gainesville, Florida, USA), Disney’s Animal Kingdom (Celebration, Florida, USA), or the Wildlife Research Laboratory (FWC, Gainesville, Florida, USA). Organ tissues and fluids (blood, urine, and aqueous humor) were collected at necropsy. Blood was centrifuged at 2,000 rpm for 10 min, and the supernatant was decanted. Representative tissues were placed in 10% neutral buffered formalin. Fixed tissues were embedded in paraffin, sectioned at 5–6 μm, and stained with hematoxylin and eosin. All tissues not analyzed immediately were archived at −20 or −70 C.
Diagnostics
Feline leukemia virus ELISA antibody optical densities (ODs) were performed at Hansen Veterinary Immunology (Dixon, California, USA) using techniques described by Lutz et al. (1980); ODs of <0.25 were considered negative, 0.25 to <0.35 were low positive, 0.35 to <0.5 were medium positive, and ≥0.500 were high positive. For statistical analyses, ODs ≥0.25 were considered positive. Antigen ELISAs (ViraCHEK® FeLV, Synbiotics Animal Health, San Diego, California, USA) were performed at the New York State Diagnostic Laboratory (Cornell University, Ithaca, New York, USA). Adsorbing reagents were used to remove heterophile antibody. Beginning November 2003, EDTA whole blood from captured pumas was tested in the field using a rapid immunochromatic assay (SNAP Combo, IDEXX Laboratories, West-brook, Maine, USA). The SNAP Combo also was used to test fluids collected from necropsied pumas, including blood collected from the thoracic cavity, heart, vessels, and marrow cavity, and aqueous humor. Blood smears from ELISA antigen-positive pumas were also tested by immunofluorescence assay (IFA) at the National Veterinary Laboratory (Franklin Lakes, New Jersey, USA) using techniques described by Hardy et al. (1973).
Conventional polymerase chain reaction (PCR) to detect provirus was performed at the Laboratory of Genomic Diversity (National Cancer Institute [NCI], Frederick, Maryland, USA) on tissues collected from pumas at capture and necropsy. Methods and results are described by Brown et al. (2008). Viral isolation and subgroup analysis were performed at the Center for Retrovirus Research (The Ohio State University, Columbus, Ohio, USA). Plasma from Florida Puma (FP) 109 and FP115 was inoculated directly onto HT1080 (human fibroblastoid), H927 (feline fibroblastoid), FEA (feline fibroblastoid), and primary feline peripheral blood mononuclear cells (PBMCs) and tested by ELISA SNAP at 2 and 4 wk. Peripheral blood mononuclear cells from FP123 and FP122 were cultured in lymphocyte medium (RPMI 1640 medium with 10% fetal bovine serum, 1 mM l-glutamine, and antibiotics) with 7 μg/ml concanavalin A and 20 U/ml interleukin-2. The inoculated cells then were cocultured with 3201 (feline lymphoblastoid), Molt 4 (human lymphoblastoid), and domestic cat PBMCs. Cultures were tested weekly for FeLV p27 by ELISA.
Pumas were tested for feline immunodeficiency virus (FIV) antibodies by Western blot using Petaluma antigen (New York State Diagnostic Laboratory), a three-antigen (fca, pco, and ple) chemiluminescence method described by Troyer et al. (2005), or both.
Criteria for defining outcomes after exposure
Possible outcomes after exposure to FeLV in pumas were classified as regressive, latent, or persistent infections based on test results, duration of antigenemia, and presence of clinical disease. Pumas with previous regressive infections had a positive ELISA antibody OD, but they were PCR and ELISA antigen negative. Latently infected pumas retained provirus in leukocytes at a level sufficient to be detectable by conventional PCR, but they were ELISA antigen negative. Persistently infected pumas were ELISA antigen positive and were IFA positive, were antigenemic for ≥16 wk, and/or developed FeLV-related diseases.
Statistics
Proportion of pumas positive for FeLV antibodies was calculated as the percentage with ELISA ODs >0.25. Three binary categorical predictors were considered: gender, FIV status, and location (north or south of Interstate highway 75 [ca. 28.05°N]) (Fig. 1). Two predictors were treated as continuous variables: age (in months/12) and sample date (in days/365.25 since 1 January 1960). Logistic regression using Egret® software (Cytel Software Corporation, Cambridge, Massachusetts, USA) was performed to investigate ELISA antibody status as a binary response variable. Odds ratios and their 95% confidence limits were calculated for one state of the binary predictors in comparison with the other state. For the continuous predictors, the odds ratios represented the change in odds of positive FeLV per unit change in the predictor. Significant (P<0.05) difference from 1.0 was determined for the odds ratios by the Wald test. To account for correlation among replicate outcomes from individuals with multiple test results, puma identity was modeled as a random effect within the logistic regression model and evaluated by a likelihood ratio test. Significant predictors emerging from univariate analyses (location and date) showed no significant interaction. Odds ratios are presented from a bivariate logistic model, with location and date as additive predictors.
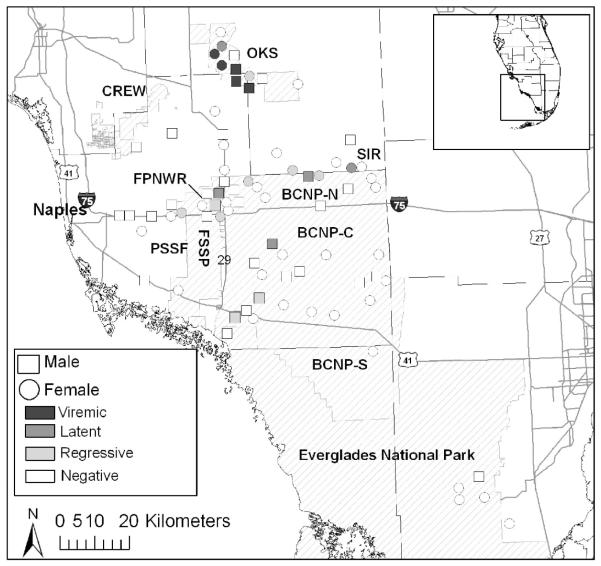
Figure 1.
Distribution of free-ranging Florida pumas (Puma concolor coryi) sampled in South Florida between 1 July 2000 and 30 June 2005 not vaccinated previously against feline leukemia virus (FeLV). Putative classification of FeLV infections are based on enzyme-linked immunosorbent assay (ELISA) antibody, polymerase chain reaction (PCR; Brown et al., 2008), ELISA antigen, and immunofluorescence assay results and clinical findings. Transient infections were positive only for FeLV antibodies, and latent infections were PCR positive but antigen negative. OKS = Okaloacoochee Slough; CREW = Corkscrew Regional Ecosystem Watershed; FPNWR = Florida Panther National Wildlife Refuge; BCNP = Big Cypress National Preserve (N = north; C = central; S = south); SIR = Big Cypress Seminole Indian Reservation; PSSF = Picayune Strand State Forest; FSSP = Fakahatchee Strand State Forest.
Vaccine trial
While in captivity at White Oak Conservation Center (WOCC, Yulee, Florida, USA), three Florida pumas and three Texas pumas were vaccinated against FeLV, monitored for adverse reactions, and sampled for antibody response. Texas pumas were females approximately 10 yr old, and they were removed from the wild after completion of a genetic restoration project. Florida pumas were in captivity after being orphaned (FP113 and FP114 were siblings). Kittens (one male, two females) were 1 to 1.25 yr old when vaccinated. Pumas were chemically immobilized, examined, sampled, and primed with Fel-O-Vax LvK (2 ml IM); the procedure was repeated 3–6 wk later. FeLV ELISA antibody ODs were determined for serum collected at initial inoculation (all pumas), booster (all pumas), and 15 days after the first booster (Texas pumas). Pumas were not challenged with live FeLV. Two adult pumas were similarly primed and boosted with Fevaxyn-FeLV while in captivity recovering from injuries. These pumas were monitored for adverse reactions, but FeLV antibody ODs were not determined. The vaccine trial was approved by the WOCC Institutional Animal Care and Use Committee.
RESULTS
Diagnostics
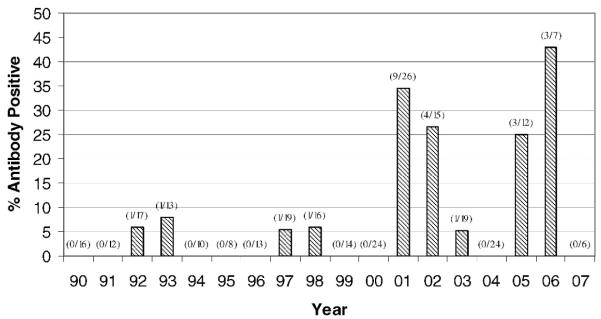
Results of FeLV diagnostics and putative classification of FeLV infections in Florida pumas are presented in Table 1. ELISA antibody ODs were determined for 143 pumas (not vaccinated previously) sampled on 270 occasions between January 1990 and April 2007; 24 (9%) total samples from 23 (16%) individuals were positive. The proportion of positive antibody ODs increased significantly with time (odds ratio=1.26, 95% confidence interval [CI]=1.11–1.42, P<0.001) (Fig. 2) and among pumas sampled north of Interstate highway 75 compared with south (odds ratio=6.65, 95% CI=2.07–21.4, P=0.001). No positive ODs were found in the extreme southern portion of puma range (Fig. 1). The probability of having a positive antibody OD was not affected significantly by age, gender, or FIV status for the complete data set or whether analysis was limited to pumas sampled north of Interstate highway 75 and after the suspected onset of the epizootic (2001 and beyond). Of pumas sampled on multiple occasions, six had positive ODs initially, but they were negative when resampled 9 mo to 3 yr later (Table 1).
Table 1.
Putative classification of feline leukemia virus (FeLV) infections in Florida pumas (Puma concolor coryi) based on enzyme-linked immunosorbent assay (ELISA) antibody, polymerase chain reaction (PCR), ELISA antigen, and immunofluorescence assay (IFA) results and clinical findings 1990 to April 2007.
| Classification | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Puma no. | Date | Gender | Age (yr) | Event | ELISA antibody |
PCRa | ELISA antigen | IFA | Viral culture | FeLV- related disease |
FIVb status |
| Regressive Infection | |||||||||||
| FP11 | 8 January 1998 | ♀ | 16 | Capture | (Pc) 0.251 | NDd | Ne,f | ND | ND | No | N |
| FP12 | 4 January 1993 | ♂ | 12 | Capture | (P) 0.28 | N | Nf | ND | ND | No | ND |
| FP36 | 8 January 1992 | ♀ | 5.5 | Capture (P) | 0.251 | N | Nf | ND | ND | No | P |
| 22 February 1994 | 7.5 | Capture | (N) 0.249g | ND | Nf | ND | ND | No | P | ||
| FP65 | 20 November 1997 |
♂ | 1 | Capture | (P) 0.362 | N | Nf | ND | ND | No | N |
| 8 March 1999 | 3.4 | Capture | (N) 0 | ND | Nf | ND | ND | No | E | ||
| FP67 | 22 April 2002 | ♀ | 4.8 | Capture | (P) 0.26 | N | N | ND | ND | No | N |
| FP69 | 3 January 2005 | ♀ | 7.8 | Capture | (P) 0.252 | N | N | ND | ND | No | P |
| FP78 | 14 December 2001 |
♀ | 4 | Capture | (P) 0.454 | N | N | ND | ND | No | P |
| FP82 | 6 December 2002 | ♀ | 6 | Capture | (P) 0.262 | N | N | ND | ND | No | N |
| FP99 | 26 January 2001 | ♂ | 0.9 | Capture | (P) 0.296 | N | N | ND | ND | No | E |
| 6 November 2001 | 1.7 | Capture | (P) 0.426 | ND | N | ND | ND | No | P | ||
| FP104 | 2 April 2001 | ♂ | 0.6 | Capture | (P) 0.292 | N | N | ND | ND | No | N |
| 13 December 2002 |
2.1 | Capture | (N) 0.132 | ND | N | ND | ND | No | P | ||
| FP107 | 1 November 2001 | ♀ | 1.6 | Capture | (P) 0.324 | N | N | ND | ND | No | P |
| 6 December 2004 | 4.7 | Capture | (N) 0.230 | ND | N | ND | ND | No | P | ||
| FP108 | 3 November 2001 | ♂ | 0.9 | Capture | (P) 0.273 | N | N | ND | ND | No | P |
| FP140 | 14 November 2005 |
♀ | 3.5 | Capture | (P) 0.298 | N | N | ND | ND | No | P |
| FP141 | 30 November 2005 |
♂ | 3.5 | Capture | (P) 0.297 | ND | N | ND | ND | No | P |
| FP143 | 9 January 2006 | ♂ | 1.5 | Capture | (P) 0.467 | ND | N | ND | ND | No | N |
| FP145 | 16 February 2006 | ♀ | 1.5 | Capture | (P) 0.347 | ND | N | ND | ND | No | P |
| FP146 | 27 February 2006 | ♂ | 3 | Capture | (P) 0.267 | ND | N | ND | ND | No | P |
| UCFP43 | 29 August 2001 | ♂ | 2.5 | Capture | (P) 0.277 | N | N | ND | ND | No | N |
| Latent infection | |||||||||||
| FP96 | 1 November 2001 | ♂ | 1.6 | Capture | (P) 0.337 | P | N | ND | ND | No | P |
| 29 May 2002 | 1.8 | Necropsy | ND | P | N | ND | ND | No | ND | ||
| FP100 | 31 January 2001 | ♂ | 4 | Capture | (P) 0.3 | P | N | ND | ND | No | N |
| 6 January 2004 | 7 | Capture | (N) 0.155 | P | N | ND | ND | No | N | ||
| FP110 | 13 February 2002 | ♀ | 1.1 | Capture | (P) 0.256 | N | N | ND | ND | No | N |
| 25 November 2002 |
2 | Capture | N | P | N | ND | ND | No | N | ||
| FP118 | 5 March 2003 | ♀ | 0.9 | Capture | N | P | N | ND | ND | No | N |
| 5 April 2003 | 1 | Necropsy | ND | ND | N | ND | ND | No | ND | ||
| FP119 | 2 April 2003 | ♂ | 1 | Capture | N | P | N | ND | ND | No | P |
| 17 November 2004 |
2.6 | Capture | N | N | N | ND | ND | N/D | ND | ||
| Persistent infection | |||||||||||
| FP115 | 26 November 2002 |
♀ | 4.5 | Capture | (P) 0.499 | P | P | Inconclu- sive |
P | No | P |
| 18 May 2003 | 5 | Necropsy | ND | P | P | P | P | Yes | ND | ||
| FP122 | 30 January 2004 | ♀ | 2.3 | Capture | N | P | P | P | P | Yes | N |
| 14 February 2004 | 2.4 | Necropsy | ND | P | P | ND | P | Yes | ND | ||
| FP123 | 2 February 2004 | ♂ | 3.5 | Capture | N | P | P | P | P | No | P |
| 15 March 2004 | 4 | Necropsy | ND | P | P | ND | ND | Unknown | ND | ||
| FP132 | 17 March 2004 | ♂ | 3 | Capture | N | N | N | ND | ND | No | N |
| 22 July 2004 | 3.3 | Necropsy | ND | P | P | P | ND | Yes | ND | ||
| Transient viremia or persistent infection | |||||||||||
| FP109 | 10 February 2002 | ♂ | 10 | Capture | N | ND | N | ND | ND | No | N |
| 24 January 2003 | 11 | Capture | (P) 0.546 | P | P | Inconclu- sive |
P | Yes | N | ||
| 26 February 2003 | 11.1 | Necropsy | N/D | ND | Unknown | ND | N/D | Unknown | ND | ||
Data from Brown et al. (2008).
FIV = feline immunodeficiency virus.
P = positive.
ND = not determined.
N = negative.
Florida Fish and Wildlife Conservation Commission (unpubl. data).
Optical density continued to decline to 0.179 by last sampling March 1997.
Figure 2.
Percentage by year of free-ranging pumas sampled in Florida 1990 to 2007 testing positive for feline leukemia virus antibodies by enzyme-linked immunosorbent assay.
Before July 2000, all pumas sampled (117 individuals sampled on 256 occasions) were ELISA antigen negative based on published (Roelke et al., 1993) and unpublished data (FWC) and retrospective testing. During the study period, 142 pumas were tested on 225 occasions for FeLV antigen by ELISA; the proportion of antigenemic (viremic) pumas ≥3 mo of age, not vaccinated previously for FeLV, and sampled during the study period, was 4% (5/131). All viremic pumas were captured in OKS, and they had overlapping home ranges. The proportion of pumas with viremia in OKS (Fig. 1) during the outbreak (approximately January 2001 to June 2005) was 46% (5 of 11). Average age of viremic pumas (three males, two females) was 4.85 yr (SD ±3.5, range 2.25–11 yr). FeLV antigen was detected by SNAP test in thoracic, splenic, and venous blood, and aqueous humor collected from viremic pumas at necropsy, even in severely autolyzed carcasses.
Feline leukemia virus was cultured from four viremic pumas (FP132 was not cultured). Of the human and feline cell lines inoculated, only primary domestic cat PBMCs, Molt 4 cells, and 3201 feline lymphoid cells became positive at 1–2 wk and remained positive for 4 wk. These cell lines had continuous production of FeLV p27 as well as significant giant cell formation (Molt 4 cells) and cell death (3201 cells). Negative cultures were maintained for up to 6 wk. Growth in feline cells was consistent with subgroup A virus. The ability of the sample from FP122 to grow in human cells (which would have indicated the presence of subgroup B, C, or both in addition to subgroup A) may be an artifact due to prior coculture of the puma cells with domestic cat PBMCs.
During the study period, 47% of pumas tested were positive for FIV antibodies by Western blot, ELISA, or both. Three of five (60%) FeLV antigen-positive pumas also tested positive for FIV.
Clinical findings
Physical exam, selected complete blood count parameters, and significant necropsy findings for viremic pumas are listed in Table 2. Suspected causes of death for the five viremic pumas were septicemia (n=2), intraspecific aggression (n=2), and anemia/dehydration (n=1). Time from first antigen-positive sample collection to death averaged 9.25 wk (SD ±10.3, range 2–24.6 wk) in pumas viremic at capture (FP109, FP115, FP122, and FP123). Time from suspected exposure to death for one puma (FP132) was 18 wk. The case-fatality rate for pumas with evidence of exposure to FeLV was 13% (3/23 exposed [positive for FeLV antibodies]) to 22% (5/23) depending on the inclusion or exclusion respectively of two viremic pumas dying from intraspecific aggression. This rate, however, may be altered artificially because of false positives or failure of some exposed pumas to generate, or maintain, antibody levels sufficient to test positive by ELISA.
Table 2.
Selected clinical pathology, physical examination, and necropsy findings in viremic Florida pumas (Puma concolor coryi).
| Live-capture |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical pathologya | Necropsy | ||||||||||||
| FeLV ELISA antigen |
|||||||||||||
| Puma no. | Age (yr) |
Sex | Date | Physical exam | HCTb (%) |
RBCc × 106/μl |
Lymphocytes | Cytology | Date | FeLV ELISA antigen |
Pathology | Other microbiology/ virologyd |
|
| FP115 | 4.5 | ♀ | November 2002 |
WNLe | + | 28.4 | 6.85 | 736 | May 2004 | + | Septicemia, suppurative lymphadenopathy, bronchointerstitial pneumonia |
Heavy growth Escherichia coli |
|
| FP109 | 11 | ♂ | January 2003 |
Lymphade- nopathy |
+ | 23.8 | 4.91 | 490 | Lymphoid hyperplasia on FNAf of peripheral lymph node |
February 2003 |
Unknown | Bite wounds, scavenging, severe osteoarthritis left coxofemoral joint |
Not performed due to severe autolysis |
| FP122 | 2.25 | ♀ | January 2004 |
Lymphade- nopathy, muscle wasting |
+ | 22.5 | 4.18 | 2,550 | Large immature mononuclear cells with prominent nucleoli, 10 nucleated RBCs/HPFg on blood smear |
February 2004 |
+ | Severe pallor, muscle wasting, severe dehy- dration, lymphadenop- athy, hypercellular bone marrow with >90% hematopoietic cells |
Negative |
| FP123 | 4 | ♂ | February 2004 |
WNL | + | 42.5 | 8.75 | 884 | Large immature mononuclear cells with prominent nucleoli on blood smear |
March 2004 |
+ | Bite wounds | Not performed due to severe autolysis |
| FP132 | 3 | ♂ | March 2004 |
WNL | − | NA | NA | NA | July 2004 | + | Severe pallor, moderate dehydration, abscesses, lymphadenopathy, sep- ticemia, suppurative lymphadenopathy, bronchointerstitial pneumonia, hypercel- lular bone marrow with >90% hematopoietic cells |
Heavy growth β-hemolytic Streptococcus sp. |
|
| Normalh (SD) |
36.4i (5.3) |
7.64 (1.0) |
3,400 (1,700) |
||||||||||
Complete blood counts and serum biochemistry parameters were determined by Antech® Diagnostics (Smyrna, Georgia, USA). Blood smears were examined at the University of Florida College of Veterinary Medicine for hemoparasites, white blood cell differential counts, and red blood cell morphology.
HCT = hematocrit.
RBC = red blood cell count.
Necropsied pumas were tested for rabies virus by direct fluorescent antibody at the Jacksonville Central Laboratory (Jacksonville, Florida, USA). Viral isolation and real-time and conventional PCR for canine distemper virus, pseudorabies virus, flaviviruses, and alphaviruses were performed at the Southeastern Cooperative Wildlife Disease Study (Athens, Georgia, USA) on brain, heart, and other tissues collected from pumas dying of unknown causes.
WNL = within normal limits.
FNA = fine-needle aspirate.
HPF = high-power field.
Value is reported in Dunbar et al. (1997) as packed cell volume.
Vaccination
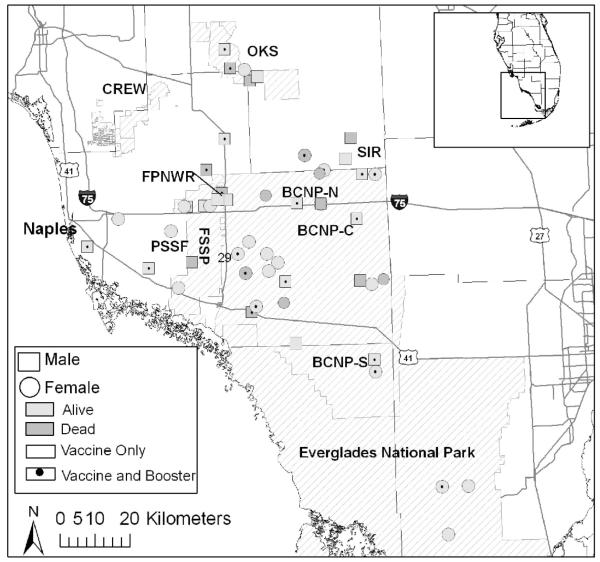
During the vaccine trial, no adverse reactions were observed after initial inoculation or booster, and most pumas developed an antibody response. On 20 August 2003, the three vaccine trial Florida pumas were released into the Florida Panther National Wildlife Refuge (FPNWR; FP113 and FP114) and private lands east of Immokalee (FP116). Vaccination of free-ranging pumas began in November 2003, and as of 1 April 2007, 52 free-ranging FeLV-negative pumas had received at least one inoculation; of these pumas, 26 were boosted. Distribution of FeLV vaccinated pumas is presented in Fig. 3. One puma (FP132) was primed approximately 1–2 days after suspected exposure to FeLV and boosted 4 wk later, but nevertheless it became persistently infected. No other vaccinated pumas have become infected.
Figure 3.
Distribution of free-ranging pumas receiving at least one inoculation against feline leukemia virus in South Florida between August 2003 and April 2007. Darker shading depicts pumas that were dead as of April 2007. OKS = Okaloacoochee Slough; CREW = Corkscrew Regional Ecosystem Watershed; FPNWR = Florida Panther National Wildlife Refuge; BCNP = Big Cypress National Preserve (N = north; C = central; S = south); SIR = Big Cypress Seminole Indian Reservation; PSSF = Picayune Strand State Forest; FSSP = Fakahatchee Strand State Forest.
DISCUSSION
We investigated and managed an epizootic of FeLV in free-ranging Florida pumas. Diagnostic tests developed for use in domestic cats were used to diagnose and help infer the pathogenesis of the disease. Although diagnostic tests validated for domestic animals but used on wildlife must be interpreted with caution (Hietala and Gardner, 1999), the test results in this study were consistent biologically and seemed to be suitable for use in pumas.
The outcome in pumas after exposure to FeLV seems to be similar to that in domestic cats, with pumas showing evidence of regressive, latent, or persistent infections (Table 1). These classifications serve to simplify and categorize our results; in reality, FeLV exposure more likely results in a continuum of possible outcomes from failure of viral replication to persistent infection and death (Torres et al., 2005). Furthermore, premature deaths, severe autolysis, limitations of diagnostic tests, and limited ability to resample pumas while living precluded complete determination of disease progression in all cases. Based on positive ELISA antibody ODs but antigen- and PCR-negative test results, many pumas exposed to the virus are able to clear the infection. Assuming a similar pathogenesis in domestic cats, pumas in this category would have cleared the infection within weeks to months of exposure. The majority of domestic cats in this category are considered refractory to reinfection (Charreyre and Pedersen, 1991). Puma FP109 may have had a regressive infection when captured in January 2003; at capture, he was anemic, lymphopenic, and had peripheral lymphadenopathy. Levy (1999) and Citino (1986) described similar signs in domestic cats and a clouded leopard, respectively, with transient viremias. Puma FP109 also had a high ELISA antibody OD. Antibodies detectable by ELISA occur shortly after infection in domestic cats (Lutz et al., 1980), and high antibody ODs in domestic cats are a good prognostic indicator for recovery (Hofmann-Lehmann et al., 2001). Poor carcass quality and an inconclusive FeLV IFA precluded further assessment of FP109’s FeLV status.
Pumas classified as latently infected presumably failed to control viral replication until later in the course of infection. Based on telemetry data (FWC, unpubl. data), at least one female (FP110) with evidence of a latent infection survived exposure to at least two FeLV-positive males without developing persistent viremia. This female continues to survive, reproduce, and remain nonantigenemic almost 5 yr after diagnosis. No latently infected pumas are known to have progressed to a persistent infection.
Persistent infections were diagnosed in four pumas of which three were thought to have had FeLV-related disease conditions. Persistently infected pumas had relatively low antibody ODs, suggesting a muted humoral response to infection. In domestic cats, low ELISA antibody ODs are characteristic of persistent infections (Hofmann-Lehmann et al., 2001). Non-neoplastic diseases, including secondary infections and anemia, were most commonly associated with FeLV infection in domestic cats (Reinacher, 1989). Septicemia, resulting from opportunistic bacterial infections, is thought to have killed two FeLV-infected pumas (FP115 and FP132). Nonregenerative anemias were seen in FP109 and FP122 when captured and may have been the cause of death in FP122. Progression of clinical disease seemed to be rapid in persistently infected pumas. Although 50% of viremic domestic cats die within 6 mo of diagnosis (Jarrett, 1983), adult cats experience a longer induction period and less severe disease compared with younger age groups (Hoover et al., 1976). All viremic pumas were adults, and although the time of infection is unknown in most infected pumas, the average time from positive sample collection to mortality was just over 9 wk. Lack of supportive care (as in captive or domestic cats) and presumably increased exposure to pathogens may play a role in this apparently more rapid clinical course.
In domestic cats, the most significant factor affecting outcome after exposure is thought to be host age (Hoover et al., 1976), although genotype, immunocompetence, coinfection with FIV, route of exposure, virus burden, and strain of virus also may be important (Hoover et al., 1980; Grindem et al., 1989; Hoover and Mullins, 1991; Rojko and Kociba, 1991). Nevertheless, the factors affecting the outcome after exposure to FeLV in domestic cats remain largely unknown (Hofmann-Lehmann et al., 2001). With the average age of viremic pumas approaching 5 yr, maturity did not seem to protect against infection. In addition, genetic variation (based on expected heterozygosity [He] at selected microsatellite DNA loci; Roelke, unpubl. data) within the puma population did not seem to influence significantly the outcome after exposure. Although some infected pumas had very low genetic variation (likely the result of inbreeding and genetic drift), at least two had He values much greater than the average for the population. Similarly, ancestral admixture did not seem to play a role because both intergrade and canonical Florida pumas developed FeLV and clinical disease (Brown et al., 2008; Johnson, unpubl. data). Both retroviruses (FIV and FeLV) have overlapping host cell tropism. In domestic cats, coinfection with FIV results in synergism of immunosuppression and severe clinical disease (Pedersen et al., 1990). The effect of pre-existing FIV infection in Florida pumas is unknown; however, some FIV-infected pumas were capable of resisting persistent infection after FeLV exposure. Conversely, persistent FeLV infections occurred in the absence of FIV infection. Finally, the pathogenicity of the FeLV strain infecting pumas may play a role in the apparent greater impact of FeLV on pumas. Based on genotyping, the strain isolated from pumas seems to be related to a virulent domestic cat strain (Brown et al., 2008).
The source of infection in pumas is unknown; however, in reports of FeLV infection in nondomestic felids, the authors speculated or provided direct evidence that infected domestic cats were the source. Kennedy-Stoskopf (1999) speculated that consumption of FeLV-infected domestic cats by nondomestic felids would be an effective way to transmit the virus, and domestic cat remains have been found in the stomachs of necropsied pumas from California (Jessup et al., 1993) and in two Florida pumas. Exposure of Florida pumas to domestic cats may be increasing as growing puma and human populations expand the urban-wildland interface. The transmission of FeLV from a domestic cat to a puma is probably a rare event, but once the species barrier was crossed, the virus was likely spread puma to puma. In domestic cats, prolonged exposure usually is necessary for transmission; however, we suspect that FP132 was infected after an aggressive encounter with a viremic puma (FP123). Although FP132 was FeLV antigen negative when handled 1–2 days after the suspected encounter, he developed persistent FeLV infection and died within 5 mo. It is also possible that FP132 was infected after capture or was exposed before the encounter with FP123 but was not yet viremic. Although FeLV is present in the semen of domestic cats, venereal transmission is not considered important (Hoover and Mullins, 1991). Nevertheless, the presence of infection in female pumas suggests that transmission also may occur during courtship and mating. Higher puma densities may facilitate puma-to-puma transmission. The population has more than tripled since the early 1990s (McBride, unpubl. data), whereas puma habitat has been reduced.
Based on ELISA antibodies, PCR results, and viral sequencing (Brown et al., 2008), the FeLV epizootic may have begun on the FPNWR in early 2001. Only one of five pumas sampled in FPNWR in early 2001 was positive for FeLV antibodies; however, all four pumas captured there in late 2001 were antibody positive. Based on telemetry data, three of these subadult pumas formed a loosely associated group between August and December 2001, possibly facilitating exposure if any were viremic at the time. Indeed, one of these pumas (FP96) had a latent infection at necropsy in early 2002 (death due to intrapecific aggression). Although no pumas from FPNWR tested antigen positive at capture or necropsy, we speculate that one or more unknown viremic pumas may have spread the infection to other regions, including OKS. The finding by Brown et al. (2008) that all persistently and latently infected pumas were infected with the same strain of virus supports this hypothesis. Alternatively, there may have been separate introductions of the same virus. Regardless of the source, FeLV was likely introduced into OKS in 2002 resulting in persistent infections in at least four pumas. Since July 2004, however, none of 84 pumas examined tested FeLV antigen positive, indicating that the epizootic may be over. Several factors may have contributed to this finding: 1) the rapid progression of disease may have limited the number of exposure events, 2) pumas are solitary generally, 3) some individuals are refractory to infection, 4) the Florida puma population is small and thus less likely to sustain an FeLV epizootic (Fromont et al., 1998), and 5) some pumas were vaccinated against FeLV.
Because of the unprecedented nature of this epizootic and serologic evidence of significant exposure without persistent infection, management of FeLV in the puma population was conservative initially. Few reported adverse effects in FeLV-vaccinated captive nondomestic felids combined with the results of the vaccine trial indicated FeLV vaccination was safe for free-ranging pumas; however, the efficacy of vaccination in free-ranging nondomestic felids is unknown. In domestic cats, inactivated whole-virus FeLV vaccines can be highly effective, although the need for boosters limits their usefulness in free-ranging populations. Management of FeLV in free-ranging pumas began in August 2003 with the release of three vaccinated subadults used in the vaccine trial. Thereafter, free-ranging pumas were primed at capture, and depending on FeLV risk, some were boosted by dart or at recapture. Assuming a minimum population size of 87 (McBride, 2003), the largest percentage of the living population receiving at least one inoculation during the epizootic was 23% (from 9 April to 28 July 2004); the largest percentage primed and boosted was 13% (same time period). Using computer models, Lubkin et al. (1996) estimated that from 23% to 72% of a closed domestic cat population with a FeLV prevalence of 10% must be vaccinated effectively each year to eliminate infection. Because vaccination efforts targeted the northern portion of puma range, the percentage vaccinated in these areas was likely much greater (Fig. 3). Haydon et al. (2006) used modeling to demonstrate that the impact of infectious disease outbreaks on endangered populations can be curtailed by concentrating vaccinations in habitat corridors. This targeted vaccination can be enhanced by concurrently vaccinating the core population. A similar strategy was used for pumas; vaccinations were concentrated initially in a band between OKS and the remainder of the population, followed by vaccination throughout their range. Test-removal of infected individuals, although proven to be beneficial in closed domestic cat populations, initially was not part of FeLV management in pumas because of perceived risks for social structure disruption and increased intraspecific aggression. Nevertheless, test-removal is now included in the Florida puma FeLV management plan.
Historically, the lack of antigen-positive animals and absence of clustered FeLV cases suggested that FeLV was not maintained in free-ranging nondomestic felid populations (Kennedy-Stoskopf, 1999). The finding, however, of five viremic pumas over 2 yr suggests that the virus had, at least temporarily, become established in the puma population. Florida pumas consist of a single small population; thus, they are at greater risk for extinction resulting from a catastrophic disease epizootic (Beier et al., 2003). Therefore, Florida pumas should continue to be monitored for and vaccinated against FeLV, and new epizootics should be managed aggressively. Managers of other free-ranging nondomestic felid populations similarly should monitor for FeLV antigen and consider vaccination, test-removal, or both should FeLV be introduced.
ACKNOWLEDGMENTS
We are deeply indebted to FWC, NPS, and US Fish and Wildlife Service biologists and veterinarians who assisted in fieldwork and collected samples over the past 30 yr. Faculty at UF-CVM provided diagnostic and editorial support and included D. Forrester, J. Levy, M. Sunquist, R. Alleman, C. Crawford, C. Buergelt, and B. Homer. We very much appreciate the diagnostic support of S. Hansen and W. Hardy, Jr., and the technical assistance of W. Johnson and A. Roca. This project was funded in part by FWC through the Federal Endangered Species Project E-1 and the Florida Panther Research and Management Trust Fund and with federal funds from the NCI, National Institutes of Health (NIH), under contract N01-CO-12400. This Research was supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research. Funding was also provided by the SeaWorld and Busch Gardens Conservation Fund. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
LITERATURE CITED
- Beier P, Vaughan MR, Conroy MJ, Quigley H. Final report. Florida Fish and Wildlife Conservation Commission; Tallahassee, Florida: 2003. An analysis of scientific literature related to the Florida panther; p. 202. [Google Scholar]
- Briggs MB, Ott RL. Feline leukemia virus infection in a captive cheetah and the clinical and antibody response of six captive cheetahs to vaccination with a subunit feline leukemia virus vaccine. Journal of the American Veterinary Medical Association. 1986;189:1197–1199. [PubMed] [Google Scholar]
- Brown M, Cunningham M, Roca AL, Troyer J, Johnson W, O’brien SJ. Genetic characterization of feline leukemia virus (FeLV) in the free-ranging Florida panther population. Emerging Infectious Diseases. 2008;14:252–259. doi: 10.3201/eid1402.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charreyre C, Pedersen NC. Study of feline leukemia virus immunity. Journal of the American Veterinary Medical Association. 1991;199:1316–1324. [PubMed] [Google Scholar]
- Citino SB. Transient FeLV viremia in a clouded leopard. Journal of Zoo and Wildlife Medicine. 1986;17:5–7. [Google Scholar]
- Daniels MJ, Golder MC, Jarrett O, Macdonald DW. Feline viruses in wildcats in Scotland. Journal of Wildlife Diseases. 1999;35:121–124. doi: 10.7589/0090-3558-35.1.121. [DOI] [PubMed] [Google Scholar]
- Dunbar MR, Nol P, Linda SB. Hematologic and serum biochemical reference intervals for Florida panthers. Journal of Wildlife Diseases. 1997;33:783–789. doi: 10.7589/0090-3558-33.4.783. [DOI] [PubMed] [Google Scholar]
- French DD, Corbett LK, N Easterbee. Morphological discriminants of Scottish wildcats (Felis sylvestris), domestic cats (F catus) and their hybrids. Journal of Zoology. 1988;214:235–259. [Google Scholar]
- Fromont E, Pontier D, Langlais M. Dynamics of a feline retrovirus (FeLV) in host populations with variable spatial structure. Proceedings of the Royal Society of London B. 1998;265:1097–1104. doi: 10.1098/rspb.1998.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont E, Sager A, Léger F, Bourguemeister F, Jouquelet E, Stahl P, Pontier D, Artois M. Prevalence and pathogenicity of retroviruses in wildcats in France. Veterinary Record. 2000;146:317–319. doi: 10.1136/vr.146.11.317. [DOI] [PubMed] [Google Scholar]
- Grindem CB, Corbett WT, Ammerman BE, Tomkins MT. Seroepidemiologic survey of feline immunodeficiency virus infection in cats of Wake County, North Carolina. Journal of the American Veterinary Medical Association. 1989;194:226–228. [PubMed] [Google Scholar]
- Hardy WD. The virology, immunology and epidemiology of the feline leukemia virus. In: Hardy WD Jr., Essex M, Mcclelland A, editors. Feline leukemia virus. Elsevier/North-Holland; New York, New York: 1980. pp. 33–78. [Google Scholar]
- Hardy WD, Jr., Old LJ, Hess PW, Essex M, Cotter SM. Horizontal transmission of feline leukemia virus. Nature. 1973;244:266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Haydon DT, Randall DA, Matthews L, Knobel DL, Tallents LA, Gravenor MB, Williams SD, Pollinger JP, Cleaveland S, Woolhouse MEJ, Sillero-Zubiri C, Marino J, Macdonald DW, Laurenson MK. Low-coverage vaccination strategies for the conservation of endangered species. Nature. 2006;443:692–695. doi: 10.1038/nature05177. [DOI] [PubMed] [Google Scholar]
- Hietala SK, Gardner IA. Validity ofusing diagnostic tests that are approved for use in domestic animals for nondomestic species. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. W. B. Saunders; Philadelphia, Pennsylvania: 1999. pp. 55–58. [Google Scholar]
- Hofmann-Lehmann R, Holznagel E, Ossent P, Reinacher M, Lutz H. Recombinant FeLV vaccine: long-term protection and effect on course and outcome of FIV infection. Veterinary Immunology and Immunopathology. 1995;46:127–137. doi: 10.1016/0165-2427(94)07012-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. Feline leukemia provirus load during the course of experimental infection and in naturally infected cats. Journal of General Virology. 2001;82:1589–1596. doi: 10.1099/0022-1317-82-7-1589. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Mullins JI. Feline leukemia virus infection and disease. Journal of American Veterinary Medical Association. 1991;199:1287–1297. [PubMed] [Google Scholar]
- Hoover EA, Olsen RG, Hardy WD, Jr., Schaller JP, Mathes LE. Felineleukemia virus infection: age-related variation in response of cats to experimental infection. Journal of the National Cancer Institute. 1976;57:365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Rojko JL, Olsen RG. Factors influencing host resistance to feline leukemia virus. In: Olsen RG, editor. Feline leukemia. CRC Press; Boca Raton, Florida: 1980. pp. 69–76. [Google Scholar]
- Jarrett O. Recent advances in the epidemiology of feline leukaemia virus. Veterinary Annual. 1983;23:287–293. [Google Scholar]
- Jessup DA, Pettan C, Lowenstine LJ, Pederson NC. Feline leukemia virus infection and renal spirochetosis in a free-ranging cougar (Felis concolor) Journal of Zoo and Wildlife Medicine. 1993;24:73–79. [Google Scholar]
- Kennedy-Stoskopf S. Emerging viral infections in large cats. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. W. B. Saunders; Philadelphia, Pennsylvania: 1999. pp. 401–410. [Google Scholar]
- Land ED, Garman DR, Holt GA. Monitoring female Florida panthers via cellular telephone. Wildlife Society Bulletin. 1998;26:29–31. [Google Scholar]
- Lee IT, Levy JK, Gorman SP, Crawford PC, Slater MR. Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in un-owned free-roaming cats. Journal of the American Veterinary Medical Association. 2002;220:620–622. doi: 10.2460/javma.2002.220.620. [DOI] [PubMed] [Google Scholar]
- Levy JK. FeLV and non-neoplastic FeLV-related disease. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. 5th Edition W. B. Saunders; Philadelphia, Pennsylvania: 1999. pp. 424–432. [Google Scholar]
- Lubkin SR, Romatowski J, Zhu M, Kulesa PM, White KAJ. Evaluation of feline leukemia virus control measures. Journal of Theoretical Biology. 1996;178:53–60. doi: 10.1006/jtbi.1996.0006. [DOI] [PubMed] [Google Scholar]
- Lutz H, Pedersen N, Higgins J, Hubscher U, Troy FA, Theilen GH. Humoral immune reactivity to feline leukemia virus and associated antigens in cats naturally infected with feline leukemia virus. Cancer Research. 1980;40:3642–3651. [PubMed] [Google Scholar]
- Marker L, Munson L, Basson PA, Quackenbush S. Multicentric T-cell lymphoma associated with feline leukemia virus infection in a captive Namibian cheetah (Acinonyx jubatus) Journal of Wildlife Diseases. 2003;39:690–695. doi: 10.7589/0090-3558-39.3.690. [DOI] [PubMed] [Google Scholar]
- Mcbride RT. The documented panther population (DPP) and its current distribution from July 1, 2002 to June 30, 2003. Livestock Protection Company; Alpine, Texas: 2003. p. 11. [Google Scholar]
- Mcbride RT, Jr., Mcbride RT. Safe and selective capture technique for jaguars in the Paraguayan Chaco. Southwestern Naturalist. 2007;52:570–577. [Google Scholar]
- Mcclelland AJ, Hardy WD, Zuckerman EE. Prognosis of healthy feline leukemia virus infected cats. In: Hardy WD, Essex M, McClelland AJ, editors. Feline leukemia virus. Elsevier/North-Holland; New York, New York: 1980. pp. 121–126. [Google Scholar]
- Mccown JW, Maehr DS, Roboski J. A portable cushion as a wildlife capture aid. Wildlife Society Bulletin. 1990;18:34–36. [Google Scholar]
- Meric SM. Suspected feline leukemia virus infection and pancytopenia in a western cougar. Journal of the American Veterinary Medical Association. 1984;185:1390–1391. [PubMed] [Google Scholar]
- Nowak RM, Mcbride R. World Wildlife Fund Yearbook 1973–1974. Danbury Press; Danbury, Connecticut: 1974. Status surveyof the Florida panther; pp. 112–113. [Google Scholar]
- Ostrowski S, Van Vuuren M, Lenain DM, Durand A. A serologic survey of wild felids from central west Saudi Arabia. Journal of Wildlife Diseases. 2003;39:696–701. doi: 10.7589/0090-3558-39.3.696. [DOI] [PubMed] [Google Scholar]
- Pedersen NC, Torten M, Rideout B, Sparger E, Tonachini T, Luciw PA, Ackley C, Levy N, Yamamoto J. Feline leukemia virus infection as a potentiating cofactor for the primary and secondary stages of experimentally induced feline immunodeficiency virus infection. Journal of Virology. 1990;64:598–606. doi: 10.1128/jvi.64.2.598-606.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Gardner MB. Isolation of feline leukemia virus from a leopard cat cell line and search for retrovirus in wild Felidae. Journal of the National Cancer Institute. 1981;67:929–933. [PubMed] [Google Scholar]
- Reinacher M. Diseases associated with spontaneous feline leukemia virus (FeLV) infection in cats. Veterinary Immunology and Immunopathology. 1989;21:85–95. doi: 10.1016/0165-2427(89)90132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard LG, Foreyt WJ. Gastroin-testinal parasites of cougars (Felis concolor) in Washington and the first report of Ollulanus tricuspis in a sylvatic felid from North America. Journal of Wildlife Diseases. 1992;28:130–133. doi: 10.7589/0090-3558-28.1.130. [DOI] [PubMed] [Google Scholar]
- Roelke ME, Forrester DJ, Jacobson ER, Kolias GV, Scott FW, Barr MC, Evermann JF, Pirtle EC. Seroprevalence of infectious disease agents in free-ranging Florida panthers (Felis concolor coryi) Journal of Wildlife Diseases. 1993;29:36–49. doi: 10.7589/0090-3558-29.1.36. [DOI] [PubMed] [Google Scholar]
- Rojko JL, Kociba GJ. Pathogenesis of infection by the feline leukemia virus. Journal of the American Veterinary Medical Association. 1991;199:1305–1310. [PubMed] [Google Scholar]
- Seal US. Report to the Florida Game and Fresh Water Fish Commission. Conservation Breeding Specialist Group, SSC/IUCN; White Oak Conservation Center, Yulee, Florida: 1994. A plan for genetic restoration and management of the Florida panther (Felis concolor coryi) p. 23. [Google Scholar]
- Sleeman JM, Keane JM, Johnson JS, Brown RJ, Woude SV. Feline leukemiavirus in a captive bobcat. Journal of Wildlife Diseases. 2001;37:194–200. doi: 10.7589/0090-3558-37.1.194. [DOI] [PubMed] [Google Scholar]
- Sparkes AH. Feline leukaemia virus: Areview of immunity and vaccination. Journal of Small Animal Practice. 1997;38:187–194. doi: 10.1111/j.1748-5827.1997.tb03339.x. [DOI] [PubMed] [Google Scholar]
- Torres AN, Mathiason CK, Hoover EA. Re-examination of feline leukemia virus: Host relationships using real-time PCR. Virology. 2005;332:272–283. doi: 10.1016/j.virol.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Troyer JL, Pecon-Slattery YJ, Roelke ME, Johnson W, Vandewoude S, Vazquez-Salat N, Brown M, Frank L, Woodroffe R, Winterbach C, Winterbach H, Hemson G, Bush M, Alexander KA, Revilla E, O’brien SJ. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. Journal of Virology. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SP, Goldman EA. The puma, mysterious American cat. Dover Publications, Inc.; New York, New York: 1946. p. 358. [Google Scholar]