Abstract
N-(5-(2-(5-Chloro-2-methoxyphenylamino)thiazol-4-yl)-4-methylthiazol-2-yl)pivalamide 1 (compound 15Jf) was found previously to correct defective cellular processing of the cystic fibrosis protein ΔF508-CFTR. Eight C4′-C5 C,C-bond-controlling bithiazole analogs of 1 were designed, synthesized, and evaluated to establish that constraining rotation about the bithiazole-tethering has a significant effect on corrector activity. For example, constraining the C4′-C5 bithiazole tether in the s-cis conformation [N-(2-(5-chloro-2-methoxyphenyl-amino)-7,8-dihydro-6H-cyclohepta[1,2-d:3,4-d′]bithiazole-2′-yl)pivalamide; 29] results in improved corrector activity. Heteroatom placement in the bithaizole core is also critical as evidenced by the decisive loss of corrector activity with s-cis constrained N-(2-(5-chloro-2-methoxyphenylamino)-5,6-dihydro-4H-cyclohepta[1,2-d:3,4-d′]bithiazole-2′-yl)pivalamide 33. In addition, computational models were utilized to examine the conformational preferences for select model systems. Following our analysis, the “s-cis locked” cycloheptathiazolothiazole 29 was found to be the most potent bithiazole corrector, with an IC50 of ~450 nM.
Introduction
Cystic fibrosis (CF), an inherited disease that afflicts ~1 in 2,500 Caucasian individuals,1 is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. The CFTR gene encodes a cAMP-regulated chloride channel expressed at the apical membrane of epithelial cells in various tissues (lung, pancreas, testes, and others2,3) with the primary cause of mortality being chronic lung infection and deterioration of lung function. ΔF508-CFTR, the most common CF-producing mutation, has a phenylalanine deletion at residue 508 of CFTR and is present in at least one allele of ~90% of CF patients.1 ΔF508-CFTR is misfolded, retained at the endoplasmic reticulum (ER), and rapidly degraded.4 Despite the multiplicity of cellular defects associated with the ΔF508 mutation, small-molecule therapy of CF caused by the ΔF508 mutation is thought to have considerable promise.5,6 Such therapy may require compounds with two complementary modes of action – a ‘corrector’ to facilitate ΔF508-CFTR folding and plasma membrane targeting, and a ‘potentiator’ to improve ΔF508-CFTR chloride channel function. However, a highly effective corrector that restores normal folding of ΔF508-CFTR may obviate the need for a separate potentiator. Nanomolar-potency ΔF508-CFTR potentiators have already been identified and characterized.6
The complex, multistep nature of protein folding and trafficking presents a significant challenge in identifying potent, selective correctors of defective ΔF508-CFTR cellular processing. We previously reported the identification and characterization of ΔF508-CFTR correctors by screening a collection of 150,000 diverse small molecules utilizing Fischer rat thyroid (FRT) epithelial cells co-expressing ΔF508-CFTR and the halide-sensitive fluorescent protein YFP-H148Q/I152L. 7 ΔF508-CFTR–facilitated iodide influx was determined for each test compound by the kinetics of decreasing YFP fluorescence following addition of extracellular iodide in the presence of the potentiators genistein8 and forskolin.6,9
Analyses of the specificity, cellular mechanism, and efficacy in human CF cells of four chemical classes of active compounds identified from the screen established methyl-bithiazoles10 as most promising for further development. A subsequent synthesis and screening study of 148 methylbithiazole analogs focused on the peripheral amide and aniline substructures (e.g., blue substructures in the generalized methylbithiazole depicted in Chart 1) established initial structure activity relationship (SAR) data for this class of correctors with methylbithiazole corrector 1 (compound 15Jf in our previous study) having the greatest corrector efficacy.11 The purpose of the study here was to explore the bithiazole core structure of 1 (red substructure in 1; Chart 1) to establish requisite structural features of the bis-heterocyclic portion of bithiazole ΔF508-CFTR correctors.
Chart 1.
Small molecule bithiazole correctors of ΔF508-CFTR.11
Results and Discussion
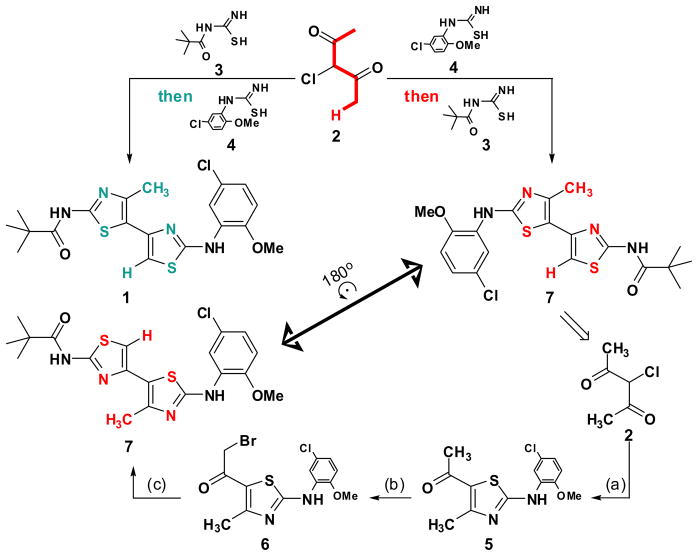
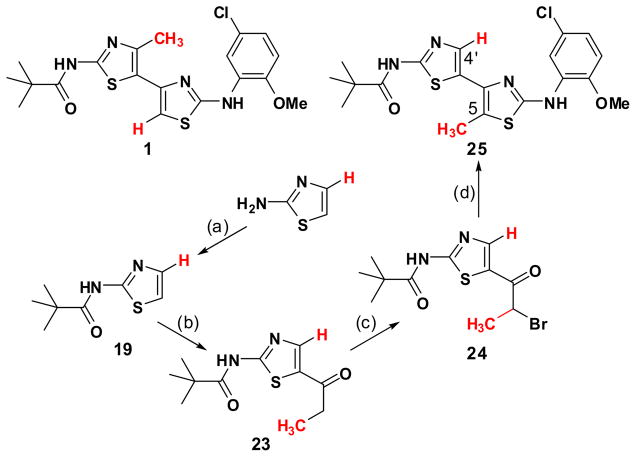
The first objective was to determine if the bithiazole substructure of 1 plays a crucial role in ΔF508-CFTR corrector activity or if it simply orchestrates the proper 3-dimensional placement of the flanking pivalamide and 5-chloro-2-methoxyaniline substructures. To accomplish this, the C2 symmetry of 3-chloropentane-2,4-dione (2) 12 was divergently exploited to prepare, from this one starting material, both 111 and 7 as detailed in Scheme 1. The preparation of corrector 1 was accomplished by condensation of chlorodiketone 2 with N-pivaloylcarbamimidothioic acid (3) 13 to give a 1-(thiazol-5-yl)ethanone intermediate.11 Bromination 14 alpha to the carbonyl of this thiazole and subsequent condensation with N-(5-chloro-2-methoxyphenyl)carbamimidothioic acid (4) delivers 1. By transposing the thiazole formation order, analog 7 is obtained from the same starting material (2) as 1. That is, condensation of 2 first with 4 followed by α-bromination and subsequent condensation with the equivalent of 3 (e.g., thiourea condensation followed by N-acylation with pivaloyl chloride) delivers transposed bithiazole 7 where the bithiazole core has been inverted relative to the topography set by the appended pivalamide and 5-chloro-2-methoxyaniline substructures (compare blue substructures in 1 with red substructures in bithiazole transposed 7 in Scheme 1).
Scheme 1.
Synthesis of 7.a
a Reagents: (a) 4, EtOH, reflux; (b) pyridinium tribromide, 33% w.t. in HOAc, room temperature; (c) i. thiourea, EtOH, reflux; ii. pivaloyl chloride, TEA, CH3CN, THF, reflux.
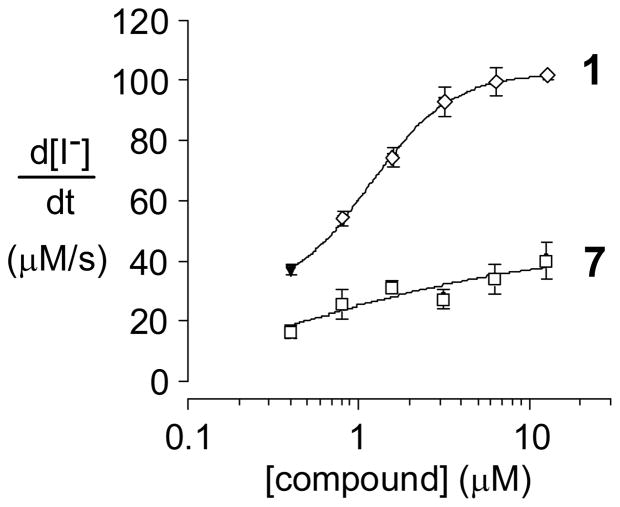
Figure 1 shows that this bithiazole transposition in 7 results in near complete loss of ΔF508-CFTR corrector activity as assayed in FRT epithelial cells stably coexpressing human ΔF508-CFTR and the high-sensitivity halide-sensing fluorescent protein YFP-H148Q/I152L as described previously.6b Since the conformational biases of 1 and 7 should be nearly identical, this dramatic change in corrector activity has three important implications: (i) proper 3-dimensional display of the pivalamide and 5-chloro-2-methoxyaniline substructures is insufficient for corrector activity; (ii) the substituted bithiazole core is a significant contributor to the activity of 1; and (iii) while the target of 1 remains unknown, the remarkable activity differences for these two quite similar bithiazoles suggests that the activity of 1 may be the consequence of a specific ΔF508-CFTR binding event.
Figure 1.
Concentration-activity profiles of 1 and 7.
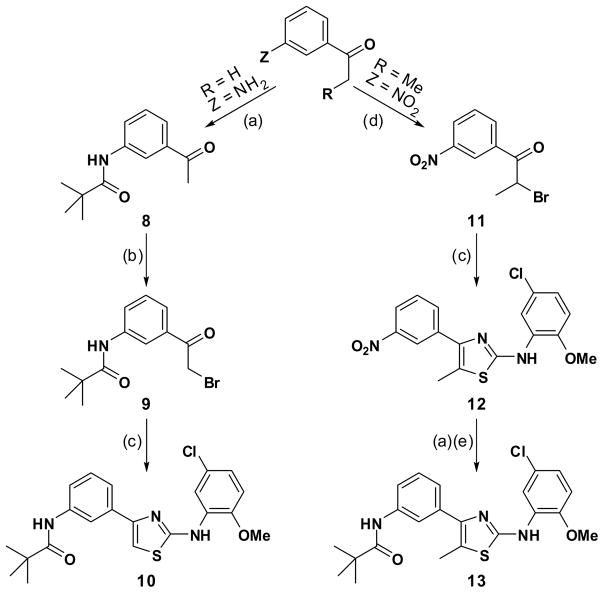
To initially explore the implications of (ii) above, the next objective was to partially modify the bithiazole core structure by replacing one of the thiazole rings with a phenyl ring. The chemistry to accomplish this objective is detailed in Scheme 2 and starts with 1-(3-aminophenyl)ethanone. N-Acylation of the aniline moiety with pivaloyl chloride was followed by bromination alpha to the carbonyl. Subsequent condensation of this bromoacetophenone with 4 delivered the 4-phenylthiazole analog 10. Starting with 1-(3-nitrophenyl)propan-1-one, a sequence consisting of bromination, thiazole formation, Sn(II)-mediated nitro reduction 15 and subsequent N-acylation delivers the 5-methylthiazole analog 13. ΔF508-CFTR assay results for 10 and 13 reveal that each of these 4-phenylthiazole compounds have no ΔF508-CFTR corrector activity (see Figure S1 in Supporting Information).
Scheme 2.
Synthesis of 4-phenylthiazole analogs 10 and 13.a
a Reagents: (a) pivaloyl chloride, TEA, CHCl3, 0 °C; (b) pyridinium tribromide, 33% w.t. HBr in HOAc, room temperature; (c) 4, EtOH, reflux; (d) Br2, HOAc; (e) SnCl2•2 H2O, MeOH.
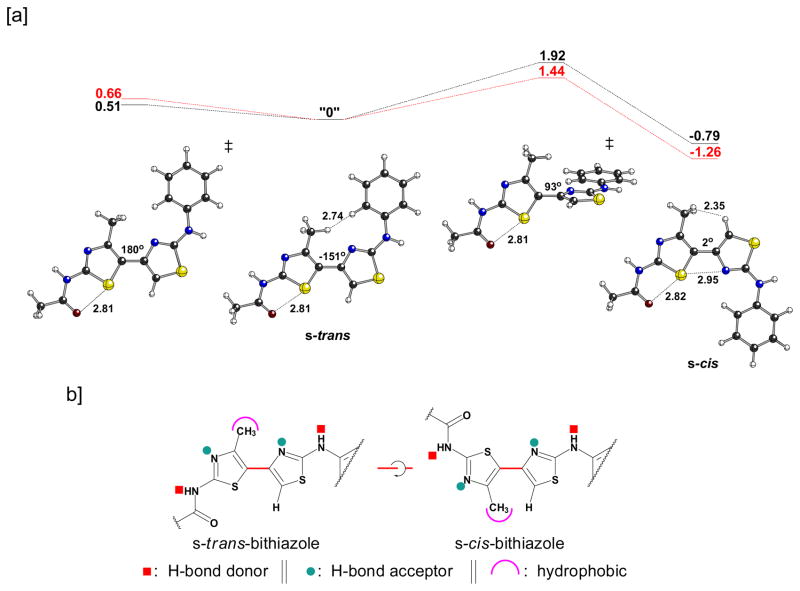
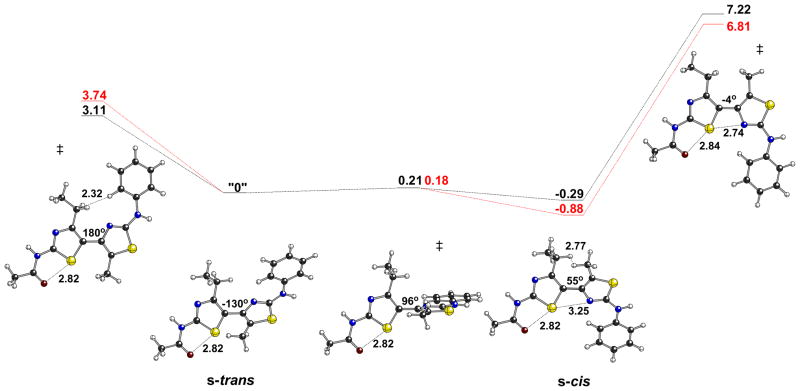
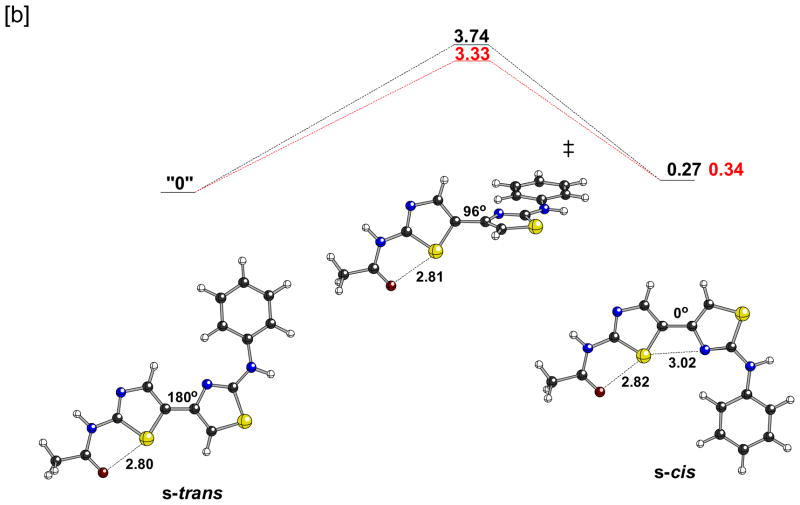
The lack of corrector activity for 7, 10, and 13 is consistent with the bithiazole core being an important determinant of the corrector activity of 1. We therefore designed bithiazole analogs that would probe structural and conformational features of this central bis-heterocycle. One aspect of the 4′-methyl-4,5′-bithiazole moiety that could greatly affect its activity involves the dihedral angle of the thiazole-tethering C(4)–C(5′) bond (see Chart 1 for the numbering scheme). As depicted in Figure 2a, the 4′-methyl-4,5′-bithiazole system can adopt two approximately planar conformations: a conformation where the C(4′)-CH3 substituent is s-trans to the C(5)-H, and a conformation where the C(4′)-CH3 substituent is s-cis to the C(5)-H. Interestingly, based on quantum chemical calculations (Figure 2a), the s-cis conformer is actually slightly lower in energy (by ~1 kcal/mol), despite the potential steric clash between the C(4′)-CH3 and the C(5)-H. This appears to be the result of an attractive S•••N interaction. Although there are precedents for S•••X interactions,16 we did not initially appreciate their relevance to our bithiazole systems. The relevance of such interactions to the conformations of thiazole-heterocycle systems was brought to our attention by Dr. Michael Bartberger (Amgen; personal communication to D.J.T. in 2007). Note that the preferred conformation of the amide group in 1 (that shown in Figure 2a) also displays an S•••X interaction, in this case between a thiazole S and the amide carbonyl O. 17 The s-trans conformer is also twisted from planarity, although planarization of this structure (Figure 2a, left) is associated with a very small energetic penalty.18 The barrier for conversion of the s-cis to the s-trans conformation is only 2–3 kcal/mol, so these structures are expected to interconvert freely in solution. However, as depicted in Figure 2b, the structural profiles of the s-trans and s-cis bithiazole conformations are quite distinct from one another in how they present bithiazole structural features such as H-bond donors, H-bond acceptors, and the hydrophobic C(4′)-CH3. Two questions arise: does s-trans/s-cis conformational interplay influence the activity of 1 and, if so, would providing a conformational bias to this feature lead to improved activity?
Figure 2.
[a] Structures and associated energiesa for a model of 1. From left to right, the structures shown are: the transition state structure for interconversion of enantiomeric s-trans conformations, one enantiomeric s-trans minimum, the transition state structure for interconversion of the s-trans and s-cis minima, and the s-cis minimum. [b] Structural consequences of a 180° rotation about the C(4)–C(5′) bithiazole bond.
aB3LYP/6-31+G(d,p), see Computational Methods section for details; selected distances shown in Å; dihedral angles shown are for the central C-C-C-C substructure; energies in kcal/mol relative to the s-trans minimum; gas-phase values in black, single point calculations in water in red.
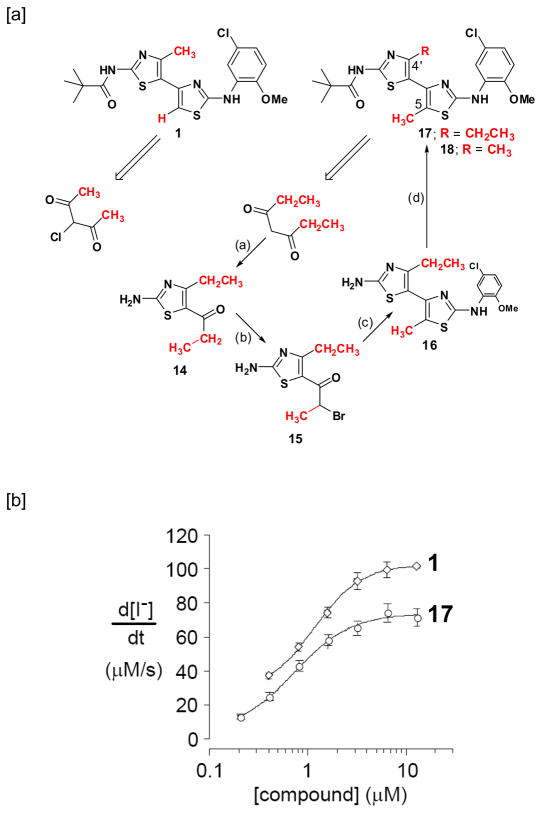
It was initially reasoned that increasing the steric bulk of the C(5)-substituent on the bithiazole core would effectively preclude access to the s-cis conformation. The C(4′), C(5) dimethyl analog 18 (see Scheme 3[a]) would have been our preferred target for this study because the presumed hydrophobic methyl pocket requirements of 1 would have been unperturbed. That target preference was, however, offset by retro-synthetic considerations. Just as symmetrical 2 is an ideal precursor to 1 because the first thiazole-forming condensation can occur redundantly on either carbonyl, 4-chloroheptane-3,5-dione is an ideal precursor to 17 – the C(4′)-CH2CH3/C(5)-CH3 analog of 1. A similar retro-synthetic analysis of the C(4′)-CH3/C(5)-CH3 analog of 18 points to unsymmetrical 3-chlorohexane-2,4-dione as the starting material, but 18 encounters a vexing carbonyl selectivity issue in the first thiazole-forming condensation reaction. For this reason, bithiazole 17 was selected as an initial probe of the s-trans/s-cis conformation questions. Its synthesis – with the pivalamide and 5-chloro-2-methoxyaniline substructures fixed as in 1 – was accomplished in four steps as outlined in Scheme 3[a]. Corrector activity assay revealed that 17, while not as active as 1, is a better corrector than 7, 10 and 13, which supports the notion that the substituted bithiazole core is a critical contributor to the corrector activity of 1 (see Scheme 3[b]). A conformational profile for 17 is shown in Figure 3. Note that, despite the potential steric clash that was engineered into 17, the s-cis conformer is again slightly lower in energy than the s-trans conformer. The s-cis minimum is now significantly distorted from planarity (twisted by ~55°), however, presumably representing a compromise between the favorable S•••N interaction and steric repulsion between the ethyl and methyl groups. The s-trans minimum is also more twisted than it was for 1. Planarization of either of these structures is associated with a significant energetic penalty (Figure 3, far left and far right). Thus, if an approximately planar conformation, be it s-trans or s-cis, is required for binding, then the penalty associated with achieving such a conformation (3–7 kcal/mol) may account for the slightly reduced activity of 17 relative to 1.
Scheme 3.
Synthesis and activity of 17.a
[a] a Reagents: (a) i. Br2, EtOH; ii. thiourea, EtOH, room temperature; (b) Br2, HOAc; (c) 4, EtOH, reflux; (d) pivaloyl chloride, DCM, TEA, room temperature. [b] Concentration-activity analysis of 1 and 17 (mean ± S.E., n = 4).
Figure 3.
Structures and associated energiesa for a model of 17. From left to right, the structures shown are: the transition state structure for interconversion of enantiomeric s-trans conformations, one enantiomeric s-trans minimum, the transition state structure for interconversion of the s-trans and s-cis minima, one enantiomeric s-cis minimum, and the transition state structure for interconversion of enantiomeric s-cis conformations.
aB3LYP/6-31+G(d,p), see Computational Methods section for details; selected distances shown in Å; dihedral angles shown are for the central C-C-C-C substructure; energies in kcal/mol relative to the s-trans minimum; gas-phase values in black, single point calculations in water in red.
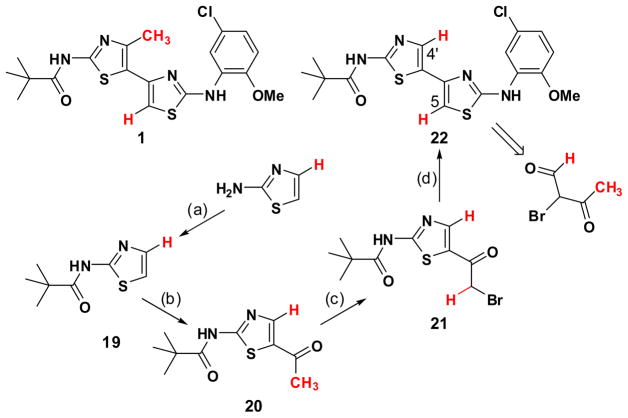
Since 17 is not as active as 1, bithiazole analog 22, in which the C(4′)-CH3 of 1 is replaced with a C(4′)-H to remove this impediment to achieving a planar conformation, was synthesized to determine if such an analog would improve corrector activity. Retro-synthetic analysis of 22 (see Scheme 4) does not point back to a symmetric dicarbonyl starting material. Rather, the retrosynthetic precursor in this strategy would be 2-halo-3-oxobutanal, which is known as its bromo analog. Indeed, 2-bromo-3-oxobutanal has been employed in the regioselective preparation of imidazole, 19 oxazole, 20 and thiazole 21 heterocycles. However, as an alternate option, a quite different route to 5-substituted-2-aminothiazoles starting from commercial 2-aminothiazole has been reported by Katritzky.22 In that work, 2-aminothiazole is protected as its N,N-bis(trimethylsilyl) derivative and subsequently regioselective C(5)-lithiated. This latter route was selected for our work because this strategy could be readily used to introduce various groups at the C(5) position of the 2-aminothiazole ring. The C(4′), C(5)-unsubstituted analog 22 was obtained in five steps from 2-aminothiazole as outlined in Scheme 4.
Scheme 4.
Synthesis of C(4′), C(5)-unsubstituted analog 22.a
a Reagents: (a) pivaloyl chloride, DCM, TEA, room temperature; (b) i. LDA, THF, then acetaldehyde; ii. MnO2, CHCl3; (c) pyridinium tribromide, 33% w.t. HBr in HOAc; room temperature; (d) 4, EtOH, reflux.
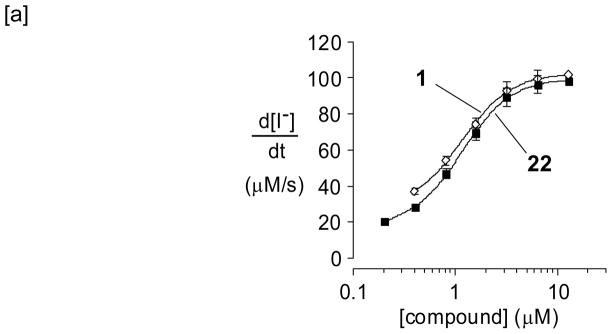
Figure 4a shows that 22 has a comparable IC50 to 1. The s-trans and s-cis minima for bithiazole 22 are both planar and extremely close in energy (Figure 4b), providing circumstantial evidence that an approximately planar conformation of 22 is likely the active conformation.
Figure 4.
[a] Concentration-activity profiles of 1 and 22; P = pivalamide and A = 5-chloro-2-methoxyaniline. [b] Structures and associated energiesa for a model of 22. From left to right, the structures shown are: the s-trans minimum, the transition state structure for interconversion of the s-trans and s-cis minima, and the s-cis minimum.
aB3LYP/6-31+G(d,p), see Computational Methods section for details; selected distances shown in Å; dihedral angles shown are for the central C-C-C-C substructure; energies in kcal/mol relative to the s-trans minimum; gas-phase values in black, single point calculations in water in red.
Following from these insights with corrector 22, we next investigated bithiazole methyl placement with analog 25 (see Scheme 5). This compound is the C(4′)/C(5) methyl–hydrogen interchange analog of 1. By utilizing a modification of Katritzky’s 2-aminothiazole protection/C(5)-lithiation strategy employed in Scheme 4 but replacing acetaldehyde with propionaldehyde, the methyl–hydrogen transposed bithiazole 25 was obtained in five steps from 2-aminothiazole.
Scheme 5.
Synthesis of 25.a
a Reagents: (a) pivaloyl chloride, DCM, TEA, room temperature; (b) i. LDA, THF, then propionaldehyde; ii. MnO2, CHCl3; (c) pyridinium tribromide, 33% w.t. HBr in HOAc; room temperature; (d) 4, EtOH, reflux.
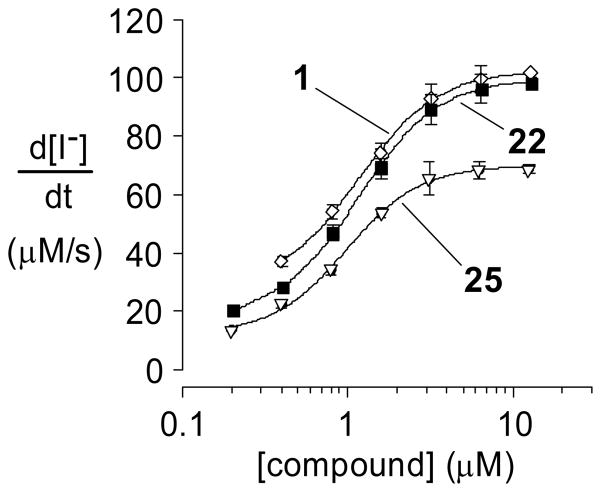
Figure 5 shows the corrector activity data of 25 compared with 1 and 22. These data support the notion that methyl placement is important. Indeed, that 1 is a more effective corrector than 25 suggests that a C(4′)-CH3 better addresses a hydrophobic binding pocket than does a C(5)-CH3 placement.
Figure 5.
Concentration-activity profile of 25 compared to 1 and 22.
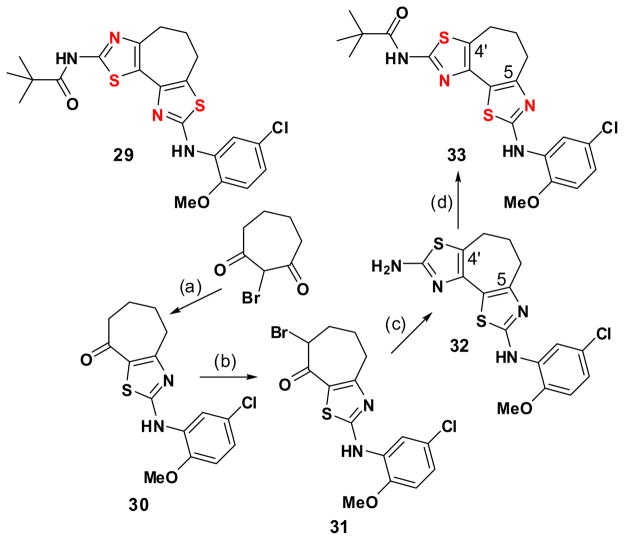
The structure-activity relationships above support the notion that a planar bithiazole conformation is required for bithiazole ΔF508-CFTR corrector activity. While both 22 and 1 accommodate that requirement, neither constrains the bithiazole moiety to be planar nor do they predispose it in either the s-cis or s-trans conformation. To address these issues, the synthesis of conformationally locked analogs was undertaken, beginning with a compound constrained to be s-cis. Of the structural options considered, cycloheptathiazolothiazole 29 appeared to be the best s-cis alternative because (a) the corresponding cyclohexa-analog would be susceptible to aromatization (an event that would reduce solubility) and (b) precursors to the cycloocta-analog would be more difficult to prepare. Our route to 29 starts with cycloheptane-1,3-dione and is outlined in Scheme 6. Cycloheptathiazolothiazole 33, the N S transposed isomer of 29, was also prepared. The route to 33 is related to that used for the synthesis of 29 except that the 2-(5-chloro-2-methoxyanilino)thiazole heterocycle is now introduced first followed then by the 2-(N-pivalamido)thiazole heterocycle (Scheme 7) by analogy with the strategy outlined in Scheme 1.
Scheme 6.
Synthesis of cycloheptathiazolothiazole analog 29.a
a Reagents: (a) Br2, CCl4/H2O, room temperature; (b) thiourea, EtOH, room temperature; (c) Br2 in HOAc, room temperature; (d) 4, EtOH, reflux; (e) pivaloyl chloride, DCM, TEA, room temperature.
Scheme 7.
Synthesis of cycloheptathiazolothiazole analog 33.a
a Reagents: (a) 4, EtOH, reflux; (b) Br2 in HOAc, room temperature; (c) thiourea, EtOH, reflux; (d) pivaloyl chloride, DCM, TEA, room temperature.
The ΔF508-CFTR corrector activity data for cycloheptathiazolothiazoles 29 and 33 are shown relative to 22 and 1 in Figure 6a. Given that 29 is an effective corrector and cannot adopt an s-trans conformation, we conclude that an s-cis conformation is required for bithiazole activity. Moreover, the fact that bithiazole 17 is a relatively ineffective ΔF508-CFTR corrector supports the contention that an approximately planar s-cis conformation is ideal for maximizing activity. Our calculations indicate that the preferred conformation for 29 involves essentially coplanar thiazole rings (Figure 6b), although twisting from planarity by up to 25° is associated with a penalty of just over 1 kcal/mol (see Figure S2 in Supporting Information).
Figure 6.
[a] The concentration-activity profile of corrector 29 relative to 1, 22, and 33. [b] Structurea of the conformational minimum for a model of 29.
aB3LYP/6-31+G(d,p), see Computational Methods section for details; selected distances shown in Å; dihedral angles shown are for the central C-C-C-C substructure; energies in kcal/mol relative to the s-trans minimum; gas-phase values in black, single point calculations in water in red.
Correctors 29, 22 and 1 can readily adopt a conformation with coplanar thiazole rings and, consequently, each of these has strong ΔF508-CFTR corrector activity. The fact that s-cis-locked corrector 29 is the most active of these compounds is likely a result of removal of the entropy penalty expected upon binding by the conformationally flexible 1 and 22 by conformational preorganization in 29.
Bithiazole 33, the N S transposed isomer of 29, is inactive even though it can place the pivalamide and 5-chloro-2-methoxyaniline substructures in similar orientations relative to the most active compound, corrector 29. These data are consistent with proper placement of the four bithiazole heteroatoms being an important structural determinant in bithiazole ΔF508-CFTR corrector activity.
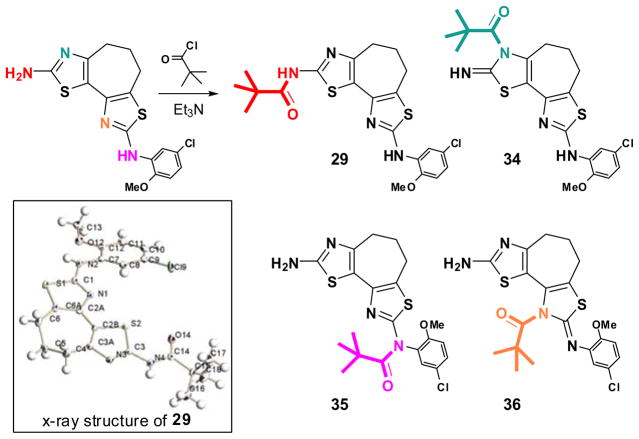
The final question regards the structure of the most potent activator cycloheptathiazolothiazole 29; specifically, did the N-acylation with pivaloyl chloride occur at the 2-amino position to give 29 or did this N-acylation occur to give one of the potential products 34–36 (see Chart 2)? Spectroscopic analysis of this reaction product proved difficult to unambiguously establish which product had been formed. Therefore, X-ray quality crystals were obtained and crystallographic analysis established that the sole product of this reaction was, indeed, 29.
Chart 2.
Chemoselective N-acylation of the cycloheptathiazolothiazole heterocycle.
Conclusions
In conclusion, a systematic analysis of lead bithiazole core ΔF508-CFTR corrector 1 has been reported. Loss of corrector activity with analogs 7, 10, and 13 is consistent with the bithiazole substructure playing a central role in the activity of 1. Conformational analysis of the thiazole-tethering C(4)–C(5′) bond suggested that two distinctly different conformations – one where the C(4′)-CH3 substituent is s-trans to the C(5)-H and another where the C(4′)-CH3 substituent is s-cis to the C(5)-H – are available to the 4′-methyl-4,5′-bithiazole moiety of 1. Activity data for bithiazole analogs 17, 22 and 25, as well as the s-cis-locked analog 29 and its transposed counterpart 33 indicate that an approximately planar s-cis bithiazole conformation, with proper placement of the four bithiazole heteroatoms, is likely the active presentation of 1.
Experimental
Δ508-CFTR corrector activity assay
FRT epithelial cells stably coexpressing human ΔF508-CFTR and the high-sensitivity halide-sensing fluorescent protein YFP-H148Q/I152L7a were used as described previously.7b Cells were grown at 37° C (95% air/5% CO2) for 24 h and then incubated for 16 – 20 h with 50 μL of medium containing the test compound. At the time of the assay, cells were washed with PBS and then incubated with PBS containing forskolin (20 μM) and genistein (50 μM) for 20 min. Measurements were carried out using FLUOstar fluorescence plate readers (Optima; BMG LABTECH Gmbh), each equipped with 500 ± 10 nm excitation and 535 ± 15 nm emission filters (Chroma Technology Corp.). Each well was assayed individually for I− influx by recording fluorescence continuously (200 ms per point) for 2 s (baseline) and then for 12 s after rapid (<1 second) addition of 165 μL PBS in which 137 mM Cl− was replaced by I−. I− influx rate was computed by fitting the final 11.5 s of the data to an exponential for extrapolation of initial slope and normalizing for background-subtracted initial fluorescence. All experiments contained negative control (DMSO vehicle) and positive control [N-(2-(5-chloro-2-methoxyphenylamino)-4′-methyl-4,5′-bithiazol-2′-yl)benzamide].
1-{2-[(5-Chloro-2-methoxyphenyl)amino]-4-methyl-1,3-thiazol-5-yl}ethanone 5
A mixture containing 2 (0.67 g, 5 mmol) and 4 (1.08 g, 5 mmol) in absolute ethanol (25 mL) was refluxed for 24 h. Upon cooling the reaction mixture in an ice bath, the product precipitated and was collected by filtration and washed with cold ethanol to afford 5 as a yellow-brown solid (0.82 g, 55%). 1H NMR (600 MHz, DMSO-d6): δ 10.17 (s, 1H), 8.41 (br s, 1H), 7.00–7.03 (m, 2H), 3.82 (s, 3H), 2.50 (s, 3H), 2.37 (s, 3H). 13C NMR (150 MHz, DMSO-d6): δ 189.5, 165.1, 155.9, 147.4, 130.2, 124.1, 123.3, 122.4, 118.9, 112.4, 56.1, 29.8, 18.5. MS m/z (ESI) 296.99 (M + H)+.
2-Bromo-1-{2-[(5-chloro-2-methoxyphenyl)amino]-4-methyl-1,3-thiazol-5-yl}ethanone 6
To a solution of 5 (0.15 g, 0.5 mmol) in HBr/HOAc (33% wt HBr in HOAc; 2.5 mL) was added pyridinium tribromide (0.18 g, 0.55 mmol). The reaction mixture was stirred at room temperature for 24 h and poured onto ice-water. The precipitated product was collected by filtration, washed with cold water, and dried to afford 6 (0.19 g, 99%). Rf = 0.714 in Hexane:EtOAc::1:1. 1H NMR (600 MHz, DMSO-d6): δ 8.38 (d, J = 2.4 Hz, 1H), 7.07–7.12 (m, 2H), 4.58 (s, 2H), 3.86 (s, 3H), 2.57 (s, 3H). 13C NMR (150 MHz, DMSO-d6): δ 183.1, 166.1, 158.1, 147.8, 129.8, 124.1, 123.1, 119.6, 119.4, 112.7, 56.2, 36.0, 18.7. MS m/z (ESI) 376.92 (M + H)+.
N-(4-(2-(5-Chloro-2-methoxyphenylamineo)-4-methylthiazole-5-yl)thiazole-2-yl)pival-amide 7
An absolute ethanol (8 mL) solution of 6 (0.19 g, 0.5 mmol) and thiourea (0.04 g, 0.5 mmol) was refluxed for 24 h. Upon completion of reaction, the solvent was removed by evaporation under reduced pressure and the residue was washed with chloroform and dried to give a pale grey solid (0.15 g, 85%) which was used in the next step without purification.
Pivaloyl chloride (0.10 g, 0.85 mmol) was added dropwise to a CH3CN/THF (1:1::vol:vol; 20 mL) solution of the crude material from above [N2′-(5-chloro-2-methoxyphenyl)-4′-methyl-4,5′-bithiazole-2,2′-diamine; 0.15 g, 0.43 mmol] and triethylamine (0.09 g, 0.86 mmol) at room temperature. To effect starting material dissolution, the reaction mixture was warmed to reflux for 20 h. The reaction mixture was concentrated under vacuum and the resulting residue was washed with chloroform and filtered. The filtrate was washed with water and dried (Na2SO4). Filtration followed by solvent removal under vacuum produced a residue that was subjected to preparative HPLC purification (UV detector: 224 nm; Eluents: H2O (A), CH3CN (B); Gradients: 0–1 min: 90% A, 1–13 min: 90%-40% A, 13–18 min: 40%-0% A, 18–21 min: 0% A, 21–21.5 min: 0%–90% A, 21.5–25 min: 90% A). The product 7 was obtained as white needles (0.06 g, 34%). 1H NMR (300 MHz, CDCl3): δ 9.00 (s, 1H), 7.41 (d, J = 2.1 Hz, 1H), 7.23–7.27 (m, 2H), 6.93 (s, 1H), 6.90 (s, 1H), 3.89 (s, 3H), 2.55 (s, 3H), 1.34 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 176.6, 167.5, 158.4, 151.7, 139.4, 134.4, 128.5, 127.8, 125.8, 123.6, 113.8, 113.3, 109.7, 56.5, 39.5, 27.4, 14.0. HRMS m/z (ESI) calcd. for C19H21ClN4O2S2 (M + H)+ 437.0867, found 437.0867.
N-(3-Acetylphenyl)pivalamide 8
To a stirred solution of 3-aminoacetophenone (1.0 g, 7.4 mmol) in chloroform cooled to 0 °C was added triethylamine dropwise (1.44 g, 14.2 mmol). The mixture was stirred for 15 min at this temperature and then pivaloyl chloride (0.83 g, 7.4 mmol) was added dropwise and the mixture was stirred overnight. Water was added and the aqueous layer was extracted with DCM. The collected organic extracts were washed with sat. aq. NaHCO3 and brine, dried over anhydrous MgSO4, and filtered. The solvent was evaporated under reduced pressure to afford 8 as a white solid (1.29 g, 80%). Mp 130–133 °C. 1H NMR (600 MHz, CDCl3): δ 8.07 (t, J = 1.8 Hz, 1H), 7.90-7.88 (m, 1H), 7.76 (br s, 1H), 7.65-7.63 (m, 1H), 7.37(t, J = 1.8 Hz 1H), 2.57 (s, 3H), 1.31(s, 9H). 13C NMR (150 MHz, CDCl3): δ 198.2, 177.2, 138.8, 137.7, 129.3, 124.9, 124.1, 119.7, 39.8, 27.6, 26.8.
N-(3-(2-Bromoacetyl)phenyl)pivalamide 9
To a stirred solution of N-(3-acetylphenyl)pivalamide 8 (0.30 g, 1.37 mmol) in 33% HBr in HOAc (5 mL) was added pyridinium tribromide (0.48 g, 1.51 mmol) and the mixture was stirred at room temperature for 24 h then poured into ice-cold water. The organic layer was extracted with DCM, washed with sat. aq. NaHCO3 and brine, dried over anhydrous MgSO4, and filtered. Evaporation of the solvent afforded 9 (0.32 g, 78 %) which was used in the next step without further purification. 1H NMR (600 MHz, CDCl3): δ 8.08 (t, J = 1.8 Hz, 1H), 7.94 (br s, 1H), 7.82 (dt, J = 8.4, 1.2 Hz, 1H), 7.60 (dd, J = 7.2 Hz, 1.2 Hz, 1H), 7.32 (t, J = 7.8 Hz, 1H), 4.30 (s, 2H), 1.27 (s, 9H). 13C NMR (150 MHz, CDCl3): δ 191.2, 177.3, 139.0, 134.4, 129.3, 125.8, 124.4, 120.4, 39.7, 31.4, 27.5. MS m/z (ESI) 298.06 [M + H]+, 300.02 [(M +2)+ H]+.
N-(3-(2-(5-Chloro-2-methoxyphenylamino)thiazol-4-yl)phenyl)pivalamide 10
A mixture of 9 (0.32 g, 1.06 mmol) and 4 (0.23 g, 1.06 mmol) in ethanol was refluxed for 48 h. The solvent was evaporated and the residue was purified by preparative HPLC to afford 10 as a white-grey solid (0.32 g, 73 %). Mp 137–138 °C. 1H NMR (300 MHz, CDCl3): δ 7.99 (s, 1H), 7.82 (m, 1H), 7.67 (s, 1H), 7.48 (d, J = 2.4 Hz, 1H), 7.42 (m, 1H), 7.25 (m, 1H), 6.93 (d, J = 8.7 Hz, 1H), 6.73 (s, 1H), 3.89 (s, 3H), 1.34 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 177.4, 169.6, 151.2, 142.8, 139.3, 129.2, 128.2 127.7, 126.0, 123.4, 2 × 121.3, 117.7, 113.2, 100.2, 56.3, 39.9, 27.6. HRMS m/z (ESI) calcd. for C21H22ClN3O2S (M + H)+ 416.1194, found 416.1193.
2-Bromo-1-(3-nitrophenyl)propan-1-one 11
To a stirred solution of 1-(3-nitrophenyl)propan-1-one (1.41 g, 7.90 mmol) in acetic acid (20 mL) was added bromine dropwise (1.27 g, 7.93 mmol) and the mixture was stirred at room temperature for 24 h. The mixture was poured into ice-cold water and the organic layer was extracted with DCM. The organic extract was washed with sat. aq. NaHCO3 and brine, dried over anhydrous MgSO4, and filtered. Evaporation of the solvent under reduced pressure afforded 11 as a white solid23 which was used in the next step without further purification (1.78 g, 87%). 1H NMR matches literature data.23
N-(5-Chloro-2-methoxyphenyl)-5-methyl-4-(3-nitrophenyl)thiazol-2-amine 12
A mixture of 11 (0.50 g, 1.94 mmol) and 4 (0.42 g, 1.94 mmol) in ethanol was refluxed for 24 h. The mixture was cooled to room temperature and the precipitate was collected by filtration to afford 12 as a yellow solid (0.57 g, 78%). Mp decomposition at 185 °C. 1H NMR (300 MHz, DMSO-d6): δ 8.64 (m, 1H), 8.50 (m, 1H), 8.15 (m, 2H), 7.73 (m, 1H), 6.96 (m, 2H), 4.84 (br s, 1H), 3.87 (s, 3H), 2.48 (s, 3H). 13C NMR (150 MHz, DMSO-d6): δ 159.4, 148.0, 146.4, 141.9, 136.4, 131.2, 124.3, 120.5, 133.6 130.0, 122.1, 121.6, 120.4, 116.9, 111.8, 56.0, 11.9. MS m/z (ESI) 376.06 [M + H]+.
N-(3-(2-(5-Chloro-2-methoxyphenylamino)-5-methylthiazol-4-yl)phenyl)-pivalamide 13
A mixture of 12 (0.25 g, 0.66 mmol) and SnCl2.2H2O (1.35 g, 6.0 mmol) in methanol was refluxed for 48 h. Evaporation of the solvent under reduced pressure afforded 4-(3-aminophenyl)-N-(5-chloro-2-methoxyphenyl)-5-methylthiazol-2-amine as a solid which was dissolved in chloroform and cooled in an ice bath to 0 °C. Triethylamine (93 μL, 0.66 mmol) was added and the mixture was stirred for 15 min at the same temperature. Pivaloyl chloride (0.08 g, 0.66 mmol) was then added dropwise at 0 °C and the mixture was stirred overnight at which point the mixture was poured onto ice-cold water and the aqueous layer was extracted with DCM. The collected organic extract was washed with sat. aq. NaHCO3 and brine, dried over anhydrous MgSO4, and filtered. Evaporation of the solvent under reduced pressure afforded 13 as a yellowish white solid (0.21 g, 75%). Mp decomposition at 195 °C. 1H NMR (300 MHz, CDCl3): δ 7.83 (s, 1H), 7.76 (s, 1H), 7.45 (m, 1H), 7.38 (d, J = 2.40 Hz, 1H), 7.33 (s, 1H), 7.27 (m, 1H), 6.93 (d, J = 9.0 Hz, 1H), 3.89 (s, 3H), 2.42 (s, 3H), 1.33 (s, 9H). 13C NMR (150 MHz, CDCl3): δ 177.4, 168.4, 151.5, 139.1, 129.9, 127.8, 125.9, 124.3, 123.6, 121.3, 120.3, 118.5, 114.5, 113.3, 111.4, 111.0, 56.4, 39.9, 27.7, 12.5. HRMS m/z (ESI) calcd. for C22H24ClN3O2S (M + H)+ 430.1351, found 430.1345.
1-(2-Amino-4-ethylthiazol-5-yl)propan-1-one 1424
An abs. ethanol solution of bromine (453 μL, 8.82 mmol) was added dropwise at room temperature to an abs. ethanol solution of 3,5-heptanedione (1.13 g, 8.82 mmol). The resulting reaction mixture was refluxed overnight at which point the ethanol was evaporated in vacuo and the residue was triturated with a small quantity of DCM. The DCM was evaporated to dryness under reduced pressure and the residue was treated with cold acetone. The resulting brown solid was collected by filtration and rinsed with cold acetone to afford 14 as a white powder (1.3 g, 80%). Mp decomposition at 133 °C. 1H NMR matches literature data.23
1-(2-Amino-4-ethylthiazol-5-yl)-2-bromopropan-1-one 15
Compound 14 (0.53 g, 1.99 mmol; HBr form) in acetic acid (2 mL) was treated dropwise with bromine (103 μL, 1.99 mmol), and the reaction mixture was stirred at room temperature for 3 h. The white precipitate was collected by filtration and washed with cold acetone to yield 15 as a white powder (0.59 g, 86%). Mp decomposition at 122 °C. 1H NMR (600 MHz, DMSO-d6): δ 4.99 (q, J = 6 Hz, 1H), 2.88 (q, J = 6 Hz, 2H), 1.69 (d, J = 6 Hz, 3H), 1.16(t, J = 6 Hz, 3H). 13C NMR (150 MHz, DMSO-d6): δ 185.5, 170.7, 115.5×2, 47.6, 47.5, 24.0, 20.8, 13.5. MS m/z (ESI) 263.00 [M + H]+, 264.96 [(M +2)+ H]+.
4-(2-Amino-4-ethylthiazol-5-yl)-N-(5-chloro-2-methoxyphenyl)-5-methylthiazol-2-amine 16
Compound 15 (0.56 g, 1.64 mmol) was dissolved in abs. ethanol (15 mL) and 4 (0.36 g, 1.64 mmol) was added at room temperature. The resulting suspension was stirred at reflux for 2 h. After removal of ethanol in vacuo, the solid was collected by filtration and washed with cold ethanol to yield 16 as a yellow powder (0.75 g, 99%). Mp decomposition at 133 °C. 1H NMR (600 MHz, CDCl3): δ 8.96 (br s, 2H), 7.79 (d, J = 2.4 Hz, 1H), 7.01 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 6.82(d, J = 8.4 Hz, 1H), 3.87 (s, 3H), 2.66 (q, J = 7.6 Hz, 2H), 2.29 (s, 3H), 1.33 (t, J = 7.6 Hz, 3H). 13C NMR (150 MHz, DMSO-d6): δ 169.6, 162.5, 148.0, 140.2, 128.8, 126.1, 124.5, 123.2, 119.1, 112.1, 56.5, 21.2, 18.6, 13.1. MS m/z (ESI) 380.97 [M + H]+.
N-(5-(2-(5-Chloro-2-methoxyphenylamino)-5-methylthiazol-4-yl)-4-ethylthiazol-2-yl)pivalamide 17
To a suspension of 16 (0.50 g, 1.1 mmol) in DCM (55 mL) was added TEA (395 μL, 2.84 mmol). Pivaloyl chloride (174 μL, 1.42 mmol) was then added to the suspension in one portion and the reaction mixture was stirred at room temperature for 10 min at which time TLC indicated reaction completion. The reaction mixture was washed with cold water and extracted with DCM (2x). The organic layer was dried over anhydrous sodium sulfate and filtered and DCM was removed in vacuo. The resulting solid was purified by silica gel column chromatography (hexane:ethyl acetate = 4:1eluent) to yield 17 as a light orange powder (0.34 g, 66%). Mp decomposition at 192 °C. 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 2.8 Hz, 1H), 7.13 (dd, J = 8.8 Hz, 2.8 Hz, 1H), 6.88 (d, J = 8.8 Hz, 1H), 3.90 (s, 3H), 2.79 (q, J = 7.6 Hz, 2H), 2.30 (s, 3H), 1.39 (s, 9H), 1.34 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 178.6, 164.6, 164.2, 163.8, 162.2, 149.1, 144.9, 128.9, 125.5, 120.5, 113.8, 112.3, 104.9, 56.3, 40.1, 26.7, 21.4, 13.5, 12.2. HRMS m/z (ESI) calcd. for C21H25ClN4O2S2 (M + H)+ 465.1180, found 465.1181.
N-(5-Acetylthiazol-2-yl)pivalamide 20
Freshly distilled diisopropylamine (5.98 mL, 42.70 mmol) was dissolved in dry THF (30 mL) and cooled to −78 °C under nitrogen. This solution was treated dropwise with 2.5 M n-BuLi in hexane (17.1 mL, 42.70 mmol) and stirred for 30 min. A solution of 1925 (3.58 g, 19.41 mmol) in anhydrous THF (20 mL) was then added dropwise to this LDA solution and stirred for 30 min at −78 °C at which time acetaldehyde (3.59 mL, 64.05 mmol) was added dropwise. The resulting mixture was stirred overnight as it warmed to ambient temperature. The reaction was quenched by dropwise addition of water, diluted with DCM (3x the THF volume), washed with water and dried over anhydrous sodium sulfate and filtered. After removal of solvents, the resulting crude material was used in the next step without purification.
This crude material (0.80 g, 3.50 mmol) was dissolved in CHCl3 (35 mL), manganese dioxide (9 g, 104 mmol) was added, and the resulting mixture was stirred at room temperature overnight. Filtration of the reaction mixture through a pad of celite and chloroform removal gave crude product which was purified by silica gel column chromatography (hexane:ethyl acetate = 4:1 eluent) to yield 20 as a white powder (0.42 g, 53%). 1H NMR (300 MHz, CDCl3): δ 10.0 (br s, 1H), 7.98 (s, 1H), 2.48 (s, 3H), 1.29 (s, 9H).
N-(5-(2-Bromoacetyl)thiazol-2-yl)pivalamide 21
Compound 20 (0.26 g, 1.15 mmol) was dissolved in 33% HBr in HOAc (100 mL), pyridinium tribromide (0.37 g, 1.15 mmol) was added, and the reaction was stirred at room temperature overnight. The reaction mixture was poured onto ice water and the solid was collected by filtration to yield 21 which was in the next step without purification (0.34 g, 97%). 1H NMR (400 MHz, CDCl3): δ 8.12 (s, 1H), 4.27 (s, 2H), 1.36 (s, 9H).
N-(2-(5-Chloro-2-methoxyphenylamino)-4,5′-bithiazol-2′-yl)pivalamide 22
A suspension of 21 (0.73 g, 2.3 mmol) and N-(5-chloro-2-methoxyphenyl)thiourea (0.73 g, 2.53 mmol) in EtOH (25 mL) was refuxed for 30 min. Upon cooling, the product was collected by filtration and washed with cold ethanol to yield 22 as a pale yellow solid (0.40 g, 84%). Mp decomposition at 216 °C. 1H NMR (600 MHz, DMSO-d6): δ 11.86 (br s, 1H) 9.93 (br s, 1H), 8.64 (d, J = 2.4 Hz, 1H), 7.83 (s, 1H), 7.18 (s, 1H), 7.02 (d, J = 8.4 Hz, 1H), 6.98(dd, J = 8.4 Hz, 2.4 Hz, 1H), 3.86 (s, 3H), 1.25 (s, 9H). 13C NMR (150 MHz, DMSO-d6): δ 176.7, 162.1, 158.9, 146.6, 140.2, 136.4, 130.5, 124.7, 124.2, 121.3, 117.3, 112.1, 91.0, 56.1, 38.8, 26.6. HRMS m/z (ESI) calcd. for C18H19ClN4O2S2 (M + H)+ 423.0711, found 423.0713.
N-(5-Propionylthiazol-2-yl)pivalamide 23
Following the protocol outlined for 20 gave 23 as an off white powder (0.78 g, 52%). Mp decomposition at 126 °C. 1H NMR (400 MHz, CDCl3), δ 9.23 (br s, 1H), 8.03 (s,1H), 2.88 (q, J = 8 Hz, 2H), 1.34 (s, 9H), 1.23 (t, J = 8 Hz, 3H). MS (ESI) m/z 241.07 [M+1]+.
N-(5-(2-Bromopropanoyl)thiazol-2-yl)pivalamide 24
Following the protocol outlined for 21 gave 24 (0.73 g, 71%) as an off white powder. Mp decomposition at 192 °C. 1H NMR (400 MHz, CDCl3): δ 8.25 (s, 1H), 5.02 (q, J = 6.8 Hz, 1H), 1.87 (d, J = 6.8 Hz, 3H), 1.35 (s, 9H). MS m/z (ESI) 319.04 [M + H]+, 321.00 [(M +2)+ H]+.
N-(2-(5-Chloro-2-methoxyphenylamino)-5-methyl-4,5′-bithiazol-2′-yl)pivalamide 25
Following the protocol outlined for 22 gave 25. Mp decomposition at 221 °C. 1H NMR (600 MHz, DMSO-d6): δ 11.79 (br s, 1H), 9.73 (br s, 1H), 8.57 (d, J = 3 Hz, 1H), 7.67 (s, 1H), 7.01 (d, J = 8.4 Hz, 1H), 6.96 (dd, J = 8.4 Hz, 3 Hz, 1H), 3.86 (s, 3H), 2.43 (s, 3H), 1.26 (s, 9H). 13C NMR (150 MHz, DMSO-d6): δ 176.5, 159.6, 157.9, 146.5, 136.7, 134.0, 131.2, 126.4, 124.3, 120.6, 117.0, 116.9, 112.0, 56.1, 38.8, 26.6, 11.7. HRMS m/z (ESI) calcd. for C19H21ClN4O2S2 (M + H)+ 437.0867, found 437.0868.
2-Amino-6,7-dihydro-4H-cyclohepta[d]thiazol-8(5H)-one 26
Following the procedure reported by Ragan,26a afforded cycloheptane-1,3-dione as clear and colorless oil. IR cm−1: 2949, 2870, 1716, 1696; (Lit. 1716, 1693); b.p. 70 °C at 0.3 mmHg; 1H NMR matched literature data.25
To a 0 °C biphasic mixture of cycloheptane-1,3-dione (5.7 g, 45.17 mmol) in CCl4/DI water (1:1; 150 mL) was added (dropwise) Br2 (2.55 mL, 49.7 mmol) in CCl4 (75 mL). The mixture was stirred at 0 °C for 1 h, extracted with DCM, and the organic layer was collected. DCM was removed under reduced pressure at room temperature to afford 2-bromocycloheptane-1,3-diones which was used to the next step without further purification.
To a solution of 2-bromocycloheptane-1,3-dione (45.17 mmol) in abs. EtOH (100 mL) was added thiourea (3.61 g, 47.43 mmol). The reaction mixture was stirred at room temperature overnight at which point the EtOH was removed under reduced pressure and the resulting dark orange residue was triturated with DCM. The residue was recrystalized from EtOH to afford 26 as an off white solid (6 g, 50% overall crude yield from cycloheptane-1,3-dione). 1H NMR (300 MHz, DMSO-d6): δ 8.87 (br s, 2H), 2.87 (t, J = 6 Hz, 2H), 2.64 (t, J = 6 Hz, 2H), 1.89-1.85 (m, 2H), 1.81-1.77 (m, 2H);
2-Amino-7-bromo-6,7-dihydro-4H-cyclohepta[d]thiazol-8(5H)-one 27
Compound 26 (1.96 g, 7.45 mmol; HBr salt form) in glacial acetic acid (70 mL) was treated dropwise with Br2 (421 uL, 8.2 mmol). The reaction mixture was stirred at room temperature for 30 min. The crude product was collected by filtration, washed with cold acetone, and dried to yield 27 which was used in the next step without purification (1.98 g, 78%). H NMR (600 MHz, DMSO-d6): δ 8.71 (br s, 2H), 5.11 (dd, J = 6.9 Hz, 3.3 Hz, 1H), 3.08-2.90 (m, 2H), 2.47-2.39 (m, 1H), 2.28-2.20 (m, 1H), 2.14 (dd, J = 10.1 Hz, 2.3 Hz, 1H), 1.98 -1.90 (m, 1H). MS (ESI) m/z [M+H]+ 260.92; [(M+2)+H]+ 262.88.
N2-(5-chloro-2-methoxyphenyl)-7,8-dihydro-6H-cyclohepta[1,2-d:3,4-d′]bithiazole-2,2′-diamine 28
An abs. ethanol (50 mL) suspension of 27 (1.73 g, 6.64 mmol) and 4 (2.11 g, 7.3 mmol) was heated at reflux overnight. EtOH was removed under reduced pressure and the residue was recrystalized from EtOH to yield 28 (2.1 g, 84%). 1H NMR (400 MHz, DMSO-d6): δ 9.81 (s, 1H), 9.19 (s, 2H), 8.44 (d, J = 2.4 Hz, 1H), 7.00 (d, J = 8.4 Hz, 1H), 6.92 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 3.83 (s, 3H), 2.91-2.87 (m, 4H), 1.98 (m, 2H). 13C NMR (100 MHz, DMSO-d6): δ 167.6, 160.8, 147.0, 135.5, 131.7, 124.9, 122.0, 121.3, 117.2, 114.5, 112.6, 56.7, 28.9, 25.9, 21.9, 19.2. MS (ESI) m/z 378.88 [M+1]+.
N-(2-(5-Chloro-2-methoxyphenylamino)-7,8-dihydro-6H-cyclohepta[1,2-d:3,4-d′]bithi-azole-2′-yl)pivalamide 29
Compound 28 (1.73 g, 3.75 mmol) and dry DCM (40 mL) under N2 was treated sequentially with TEA (1.32 mL, 9.38 mmol) and 2,2-dimethylpropionyl chloride (598 μL, 4.86 mmol). The suspension became light brown within 2 min and DCM was removed in vacuo at room temperature. The residue was purified by flash chromatographic column (hexane:ethyl acetate = 4:1 eluent) to afford 29 (1.46 g. 84%) with 99.0% purity. Mp decomposition at 197 °C. 1H NMR (400 MHz, DMSO-d6): δ 11.60 (s, 1H), 9.65 (s, 1H), 8.58 (d, J = 0.8 Hz, 1H), 6.99-6.92 (m, 2H), 3.84 (s, 3H), 3.01 (t, J = 4 Hz, 2H), 1.91 (t, J = 4 Hz, 2H),1.99 (m, 2H), 1.21 (s, 9H). 13C NMR (100 MHz, DMSO-d6): δ 176.9, 160.1, 156.2, 146.9, 146.8, 138.1, 132.0, 125.0, 121.8, 120.9, 120.7, 117.2, 112.4, 56.7, 33.0, 27.3, 26.8, 23.0. HRMS m/z (ESI) calcd. for C21H23ClN4O2S2 (M + H)+ 463.1024, found 463.1019.
2-(5-Chloro-2-methoxyphenylamino)-4,5,6,7-tetrahydrocyclohepta[d]thiazol-8-one 30
Following the procedure described for 26 by condensing 5-chloro-2-methoxyphenylthiourea gave 30 (light brown solid; 26% for two steps). 1H NMR (600 MHz, DMSO-d6): δ 7.81 (d, J = 2.4 Hz, 1H), 7.75 (dd, J = 9 Hz, 2.4 Hz, 1H), 7.42 (d, J = 9 Hz, 1H), 3.83 (s, 3H), 2.84-2.75 (m, 2H), 2.58-2.52 (m, 1H), 2.33-2.28 (m, 1H), 1.90-1.74 (m, 4H). 13C NMR (150 MHz, DMSO-d6): δ 194.4, 169.8, 154.5, 148.9, 134.1, 130.2, 125.7, 122.6, 121.7, 116.3, 57.6, 42.9, 30.5, 24.4, 21.5.
7-Bromo-2-(5-chloro-2-methoxyphenylamino)-4,5,6,7-tetrahydrocyclohepta[d]thiazol-8-one 31
Followed the procedure described for 27 gave 31 (white powder; 70% yield). MS(ESI) m/z [M+H]+ 400.87; [(M+2)+H]+402.90.
N2-(5-Chloro-2-methoxyphenyl)-5,6-dihydro-4H-cyclohepta[1,2-d:3,4-d′]bithiazole-2,2′-diamine 32
Following the procedure outlined for 28 by condensing with thiourea afforded 32 as a white powder (32%). 1H NMR (600 MHz, DMSO-d6): δ 9.48 (br s, 1H), 7.89-7.84 (m, 2H), 7.81 (d, J = 2.4 Hz, 1H), 7.72 (dd, J = 9 Hz, 2.4 Hz, 1H), 7.40 (d, J = 9 Hz, 1H), 3.84 (s, 3H), 2.81-2.73 (m, 2H), 2.47-2.42 (m, 1H), 2.28-2.23 (m, 1H), 1.93-1.88 (m, 2H). 13C NMR (150 MHz, DMSO-d6): δ 166.7, 165.9, 154.0, 134.4, 134.0, 133.0, 129.6, 124.9, 121.7, 119.4, 115.4, 113.8, 56.8, 28.2, 25.3, 21.6. MS (ESI) m/z 379.01 [M+1]+.
N-(2-(5-Chloro-2-methoxyphenylamino)-5,6-dihydro-4H-cyclohepta[1,2-d:3,4-d′]bithi-azole-2′-yl)pivalamide 33
Following the procedure outlined for 29 gave 33 as a yellow powder (36%). 1H NMR (600 MHz, DMSO-d6): δ 7.64 (d, J = 2.4 Hz, 1H), 7.62 (dd, J = 7.2 Hz, 2.4 Hz, 1H), 7.35 (d, J = 7.2 Hz, 1H), 7.05 (br s, 2H), 3.82 (s, 3H), 2.87-2.85 (m, 2H), 2.68-2.64 (m, 1H), 2.48-2.44 (m, 1H), 2.02-1.96 (m, 2H), 1.02 (s, 9H). 13C NMR (150 MHz, DMSO-d6): δ 187.3, 167.0, 165.8, 154.3, 137.4, 133.1, 131.1, 130.3, 127.3, 124.4, 118.5, 115.4, 114.7, 57.0, 40.7, 29.0, 28.3, 26.5, 22.8. HRMS m/z (ESI) calcd. for C21H23ClN4O2S2 (M + H)+ 463.1024, found 463.1039.
Computational Methods
Model systems of 1, 17, 22, and 29 with the t-butyl group replaced by a methyl group and the methoxy and chloro groups on the aniline replaced by hydrogens were utilized. Preliminary calculations (see Figure S3 in Supporting Information) suggested that the presence of the methoxy and chloro functional groups does not significantly affect the relative energies of the two aniline conformers. In all cases, the lowest energy aniline and amide conformers (which are also consistent with the crystal structure of 29, see Chart 2) were utilized.
All calculations were performed with the GAUSSIAN0327 software suite. Geometries were optimized without symmetry constraints using the B3LYP/6-31+G(d,p) method.28 All stationary points were characterized as either minima or transition state structures via frequency calculations, and the reported energies include unscaled zero-point energy (ZPE) corrections. Single point calculations in water were completed utilizing the CPCM solvation model and UAKS radii.29 Structural diagrams were produced using Ball & Stick version 4.0.30 See Supporting Information for coordinates of all computed structures and details on additional model systems.
Supplementary Material
Acknowledgments
The authors thank the Tara K. Telford Fund for Cystic Fibrosis Research at UC Davis, the National Institutes of Health (DK072517 and GM076151), and the National Science Foundation [CHE-0614756, CHE-0443516, CHE-0449845, CHE-9808183 (NMR spectrometers) and CHE-030089 (computer time from the Pittsburgh Supercomputer Center)] for their generous support. We thank Dr. Michael Bartberger (Amgen) for helpful insights.
Footnotes
X-ray crystallographic data of N-(2-(5-chloro-2-methoxyphenylamino)-7,8-dihydro-6H-cyclohepta[1,2-d:3,4-d′]bithiazole-2′-yl)pivalamide (29; C21H23ClN4O2S2) were submitted to the Cambridge Crystallographic Data Centre (deposition number CCDC687310).
Abbreviations. CFTR: Cystic Fibrosis Transmembrance Conductance Regulator. SAR: structure activity relationship. CF: Cystic fibrosis. ER: endoplasmic reticulum. FRT: Fischer rat thyroid. cAMP: cyclic adenosine monophosphate. YFP: Yellow Fluorescent Protein.
Supporting Information Available: Figures S1–S5; spectral data of compounds 5, 6, 7, 8, 9, 10, 11, 12, 13, 15, 16, 17, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32 and 33; computational data; X-ray crystallographic data for 29. This material is available free of charge via the Internet at http://pubs.acs.org.
References & Notes
- 1.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations — correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.(a) Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]; (b) Lukacs GL, Mohamed A, Kartner N, Chang XB, Riordan JR, Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (ΔF508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167–S173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]; (d) Du K, Sharma M, Lukacs GL. The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 5.(a) Carlile GW, Robert R, Zhang D, Teske KA, Luo Y, Hanrahan JW, Thomas DY. Correctors of protein trafficking defects identified by a novel high-throughput screening assay. Chem Bio Chem. 2007;8:1012–1020. doi: 10.1002/cbic.200700027. [DOI] [PubMed] [Google Scholar]; (b) Becq F. On the discovery and development of CFTR chloride channel activators. Cur Pharm Des. 2006;12:471–484. doi: 10.2174/138161206775474459. [DOI] [PubMed] [Google Scholar]; (c) Van Goor F, Straley KS, Cao D, Gonzalez J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PDJ, Negulescu P. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol. 2006;290:L1117–1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, Galietta LJV, Verkman AS. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J Biol Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 7.(a) Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]; (b) Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJV, Verkman AS. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]; (b) Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, Amaral MD. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang T-C, Horie M, Nairn AC, Gadsby DC. Role of GTP-binding proteins in the regulation of cardiac chloride conductance. J Gen Physiol. 1992;99:465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikelj D, Urleb U. Product class 17: thiazoles. Sci Synth. 2002;11:627–833. [Google Scholar]
- 11.Yoo CL, Yu GJ, Yang B, Robins LI, Verkman AS, Kurth MJ. 4′-Methyl-4,5′-bithiazole-based correctors of defective ΔF508-CFTR cellular processing. Bioorg Med Chem Lett. 2008;18:2610–2614. doi: 10.1016/j.bmcl.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Ahmed Z, Langer P. Synthesis of functionalized diaryl ethers by [3 + 3] cyclization of 1,3-bis(silyl enol ethers) with 2-aryloxy-3-(silyloxy)alk-2-en-1-ones. Synlett. 2006:3361–3363. [Google Scholar]; (b) Hayakawa M, Kaizawa H, Kawaguchi KI, Ishikawa N, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto SI, Raynaud FI, Waterfield MD, Parker P, Workman P. Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110α inhibitors. Bioorg Med Chem. 2007;15:403–412. doi: 10.1016/j.bmc.2006.09.047. [DOI] [PubMed] [Google Scholar]; (c) El-Gazzar ABA, Hussein HAR, Aly AS. Synthesis and reactions of polynuclear heterocycles: azolothienopyrimidines and thienothiazolopyrimidines. Phosphorus, Sulfur Silicon Relat Elem. 2006;181:2771–2784. [Google Scholar]; (d) McInnes C, Wang S, Anderson S, O’Boyle J, Jackson W, Kontopidis G, Meades C, Mezna M, Thomas M, Wood G, Lane DP, Fischer PM. Structural Determinants of CDK4 Inhibition and Design of Selective ATP Competitive Inhibitors. Chem Biol. 2004;11:525–534. doi: 10.1016/j.chembiol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Prepared by analogy with methodology reported in: Boyer C, Finazzi G, Laurent P, Haas A, Blancou H. Synthesis and photosynthetic inhibition activity of substituted 5-[bis(trifluoromethyl)methyl]-2-aminothiazoles. J Fluorine Chem. 2006;127:1522–1527.
- 14.Sayed SM, Raslan MA, Khalil MA, Dawood KM. Synthesis and reactivity of cyanomethyl 2-amino-4-methylthiazolyl ketone. A facile synthesis of novel pyrazolo[5,1-c]-1,2,4-triazine, 1,2,4-triazolo[5,1-c]-1,2,4-triazine, 1,2,4-triazino[4,3-a]benzimidazole, pyridazin-6-imine and 6-oxopyridazinone derivatives. Heteroat Chem. 1999;10:385–390. [Google Scholar]
- 15.Yoo CL, Fettinger JC, Kurth MJ. Stannous chloride in alcohol: A one-pot conversion of 2-nitro-N-arylbenzamides to 2,3-dihydro-1H-quinazoline-4-ones. J Org Chem. 2005;70:6941–6943. doi: 10.1021/jo050450f. [DOI] [PubMed] [Google Scholar]
- 16.See, for example: Angyan J, Poirier RA, Kucsman A, Csizmadia IG. Bonding between nonbonded sulfur and oxygen atoms in selected organic molecules (a quantum chemical study) J Am Chem Soc. 1987;109:2237–2245.Meyer E, Joussef AC, Gallardo H, Bortoluzzi AJ, Longo RL. 1,5-type nonbonded O…S and S…S interactions in (acylimino) and (thioacylimino)benzothiazoline systems. Crystal structures and theoretical calculations. Tetrahedron. 2003;59:10187–10193.Iwaoka M, Takemoto S, Okada M, Tomoda S. Weak nonbonded S…X (X = O, N, and S) interactions in proteins. Statistical and theoretical studies. Bull Chem Soc Jpn. 2002;75:1611–1625.Pomerantz M. Planar 2,2′-bithiophenes with 3,3′- and 3,3′,4,4′-substituents. A computational study. Tetrahedron Lett. 2003;44:1563–1565.Rábai J, Kapovits I, Jalsovszky I, Argay Gy, Fülöp V, Kálmán A, Koritsánszky T. Molecular structures of cyclic sulfilimines without and with intramolecular sulfur-oxygen interaction: an X-ray study. J Mol Struct. 1996;382:13–21.Kucsman Á, Kapovits I, Párkányi L, Kálmán A. Conformation of diaryl sulphides with intramolecular sulphur(II)-oxygen interaction: an X-ray study of methyl 2-(4-nitrophenylthio)benzoate and 2-diazoacetyl-4′-nitrodiphenyl sulphide. J Mol Struct. 1986;140:141–150.
- 17.In 1, the computed gas-phase penalty associated with a 180° rotation about the Cthiazole-Namide bond is approximately 9 kcal/mol. This likely reflects both the loss of the favorable S•••O interaction and the addition of an unfavorable O•••N interaction.
- 18.One might expect that the twisted nature of the s-trans structure results from a C(4′)-CH3 steric interaction with a phenyl hydrogen (see Figure 2a). However, rotation of the phenylamine group to the alternate conformation (which can occur with a barrier of approximately 4–6 kcal/mol) results in a structure that is slightly higher in energy and slightly more twisted (see Computational Methods section and Figures S3 and S4 in the Supporting Information).
- 19.Lipinski CA, Blizniak TE, Craig RH. An improved preparation and use of 2-bromoacetoacetaldehyde in a new synthesis of 2-substituted-4-acetylimidazoles. J Org Chem. 1984;49:566–570. [Google Scholar]
- 20.Matulenko Mark A, Lee Chih-Hung, Jiang Meiqun, Frey Robin R, Cowart Marlon D, Bayburt Erol K, DiDomenico Stanley, Gfesser Gregory A, Gomtsyan Arthur, Zheng Guo Zhu, McKie Jeffery A, Stewart Andrew O, Yu Haixia, Kohlhaas Kathy L, Alexander Karen M, McGaraughty Steve, Wismer Carol T, Mikusa Joseph, Marsh Kennan C, Snyder Ronald D, Diehl Marilyn S, Kowaluk Elizabeth A, Jarvis Michael F, Bhagwat Shripad S. 5-(3-Bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-ylamine: structure-activity relationships of 7-substituted heteroaryl analogs as non-nucleoside adenosine kinase inhibitors. Bioorg Med Chem. 2005;13:3705–3720. doi: 10.1016/j.bmc.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Kochetkov NK, Nifant’ev EE, Molodtsov NV. Bromination of -oxo acetals. Zh Obshchei Khimii. 1959;29:2330–2337. [Google Scholar]
- 22.(a) Katritzky AR, Laurenzo KS, Relyea DI. The preparation and fungicidal activity of a series of thiazolyl- and isothiazolyldiarylcarbinols. Can J Chem. 1988;66:1617–1624. [Google Scholar]; (b) Chen YL, Cherry K, Corman ML, Ebbinghaus CF, Gamlath CB, Liston D, Martin BA, Oborski CE, Sahagan BG. Thiazole - diamides as potent γ-secretase inhibitors. Bioorg Med Chem Lett. 2007;17:5518–5522. doi: 10.1016/j.bmcl.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Van der Mey M, Bommele KM, Boss H, Hatzelmann A, Van Slingerland M, Sterk GJ, Timmerman H. Synthesis and Structure-Activity Relationships of cis-Tetrahydrophthalazinone/Pyridazinone Hybrids: A Novel Series of Potent Dual PDE3/PDE4 Inhibitory Agents. J Med Chem. 2003;46:2008–2016. doi: 10.1021/jm030776l. [DOI] [PubMed] [Google Scholar]
- 24.Kreutzberger A, Schimmelpfennig H. Antiviral drugs. XVIII. 2-Aminothizaoles by cleavage of the S-S bond of disulfidodicarbamidine. Arch Pharm (Weinheim, Ger) 1981;314:385–391. doi: 10.1002/ardp.19813140502. [DOI] [PubMed] [Google Scholar]
- 25.Schiavi B, Ahond A, Al-Mourabit A, Pupat C, Chiaroni A, Gaspard C, Potier P. Synthesis of 5-deazathiogirolines: analogs of a natural antitumor agent. Tetrahedron. 2002;58:4201–4215. [Google Scholar]
- 26.(a) Ragan JA, Makowski TW, Am Ende DJ, Clifford PJ, Young GR, Conrad AK, Eisenbeis SA. A Practical Synthesis of 1,3-cycloheptanedione. Org Process Res Dev. 1998;2:379–381. [Google Scholar]; (b) Bhushan V, Chandrasekaran S. A convenient synthesis of cycloheptane-1,3-dione. Synth Commun. 1984;14:339–345. [Google Scholar]
- 27.Frisch MJ, et al. Gaussian03, revision D01. Gaussian, Inc; Pittsburgh, PA: 2003. (full reference in Supporting Information).
- 28.(a) Becke AD. Density–functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]; (b) Becke AD. A new mixing of Hartree-Fock and local-density-functional theories. J Chem Phys. 1993;98:1372–1377. [Google Scholar]; (c) Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (d) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem. 1994;98:11623–11627. [Google Scholar]
- 29.(a) Barone V, Cossi MJ. Quantum calculation of molecular energy gradients in solution by a conductor solvent model. J Phys Chem A. 1998;102:1995–2001. [Google Scholar]; (b) Barone B, Cossi M, Tomasi J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem. 1998;19:404–417. [Google Scholar]; (c) Takano Y, Houk KN. Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J Chem Theor Comput. 2005;1:70–77. doi: 10.1021/ct049977a. [DOI] [PubMed] [Google Scholar]
- 30.Müller N, Falk A, Gsaller G. Ball & Stick V.4.0a12, molecular graphics application for MacOS computers. Johannes Kepler University; Linz: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.