Abstract
The crucial ‘flaw’ in the existing treatment paradigm for Non-small Cell Lung Cancer (NSCLC) is the ‘one size fits all approach’. Consequently, adjuvant chemotherapy is given to all patients to benefit a minority and, in the metastatic setting doublet chemotherapy only provides modest improvements in response rates and survival. A personalized approach of treatment selection is therefore desperately needed.
Genetic information is stored in the chemical structure of DNA. To maintain the structural integrity of DNA, an intricate network of DNA repair systems have evolved. One of these is the nucleotide excision repair (NER), a highly versatile and sophisticated DNA damage removal pathway. We show here that this DNA repair mechanism is instrumental in defining prognosis and response to treatment. ERCC1, one of the proteins in this pathway, is measured to assess its functional status of the NER pathway. In patients with early stage NSCLC, low ERCC1 predicts for relapse and selects for patients who will benefit from adjuvant cisplatin-based chemotherapy. Conversely, ERCC1 positive resected patients have a better intrinsic prognosis and are not likely to benefit from platinum based chemotherapy. In a phase II trial in metastatic disease, we show that by tailoring chemotherapy using ERCC1 and RRM1 we can obtain one-year survival of 60% (versus approximately 36% in historical controls) and response rates of 42% (versus 25% in historical controls). This approach is currently being validated in a prospective phase III trial. In the future, assessment of NER function may play a central role in NSCLC treatment decision making.
Keywords: Lung Cancer, Nuclear Excision Repair, ERCC1, Chemotherapy, Cisplatin, Adjuvant and Metastatic
INTRODUCTION
Genetic information, the blueprint of life, is stored in the chemical structure of DNA. Maintaining the structural integrity of DNA is therefore crucial. Consequently, early in evolution, intricate networks of DNA repair systems have evolved to preserve DNA (genomic) integrity. One of these is the nucleotide excision repair (NER), a highly versatile and sophisticated DNA damage removal pathway (Hoeijmakers, 1993a).
The NER pathway counteracts the deleterious effects of a multitude of insults. These DNA damaging insults are caused by chemicals (particularly, cyclobutane pyrimidine dimers (CPDs)), radiation (especially the ubiquitous shortwave UV component of sunlight), and several environmental toxins (Hoeijmakers, 1993b). Within the wide spectrum of possible NER lesions, significant distortion of DNA helices appears to be a common denominator. Defects in NER underlie the extreme photosensitivity and predisposition to skin cancer observed with the prototypic syndrome xeroderma pigmentosum (XP) (Hoeijmakers, 1993b).
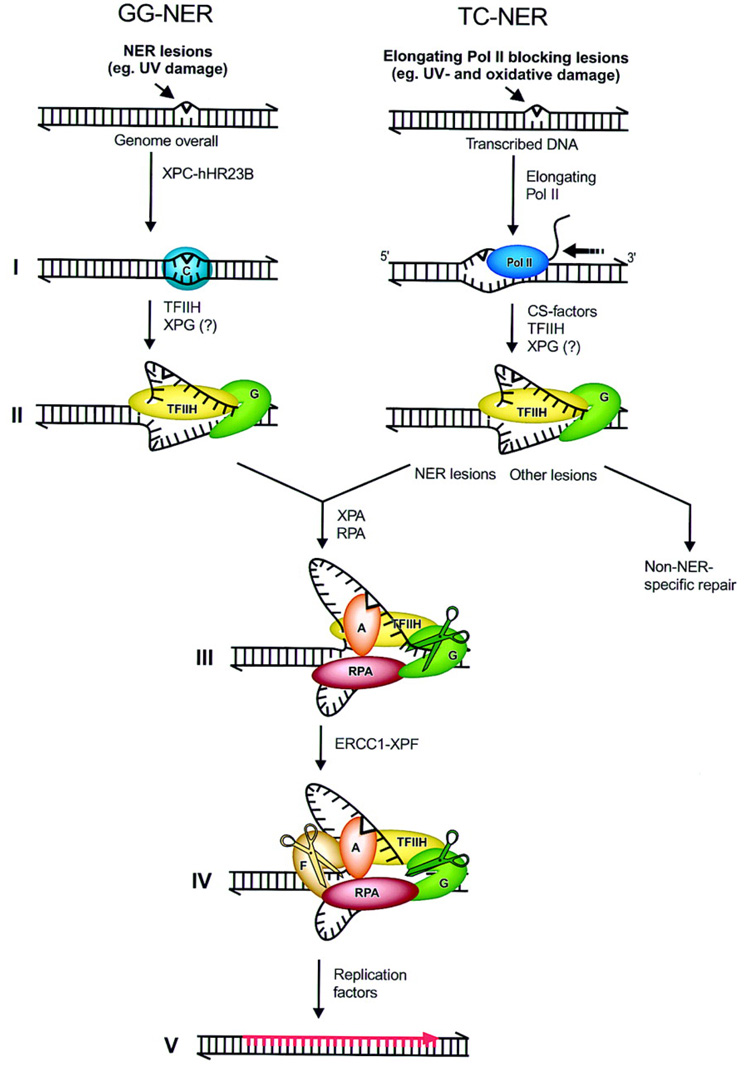
Two distinct types of NER reactions can be identified: repair of damage or lesions over the entire genome, referred to as global genome NER (GG-NER), and repair of transcription-blocking lesions present in transcribed DNA strands, hence called transcription-coupled NER (TC-NER) (Houtsmuller et al., 1999). In TC-NER, damage is detected by the elongating RNA polymerase II complex when it encounters a lesion (See Figure 1) (de Laat, Jaspers, & Hoeijmakers, 1999). Cockayne syndrome (CS), is associated with a specific defect in transcription-coupled repair. CS is characterized by photosensitivity, severe neurological and developmental defects and premature aging. (de Laat et al., 1999).
Figure 1.
Molecular model for the incision stage of NER. (1) XPC-hHR23B senses DNA helix-distorting nuclear excision repair (NER) lesions in global genome NER (GG-NER) leading to conformational alterations of the DNA. In transcription-coupled repair (TC-NER) lesions are detected by elongating RNA Pol II (2) (Left) XPC-hHR23B at lesion attracts TFIIH [and possibly XPG]. TFIIH creates a 10- to 20- nucleotide opened DNA complex around the lesion by virtue of its helicases XPB and XPD; this step requires ATP. (3) XPA and RPA stabilize the 10- to 20-nucleotide opening and position other factors. XPA binds to the damaged nucleotides, RPA to the undamaged DNA strand. XPG stabilizes the fully opened complex. (4) XPG, positioned by TFIIH and RPA, makes the 3′ incision. ERCC1-XPF, positioned by RPA and XPA, makes the second incision 5′ of the lesion. (4) Dual incision is followed by gap-filling DNA synthesis and ligation. Drawn contacts between molecules reflect reported protein-protein interactions (Reproduced from de Laat et al. (de Laat et al., 1999) with permission).
The DNA repair mechanism that was evolved to maintain the integrity of genomic DNA may play a fundamental role in carcinogenesis and may be instrumental in defining the prognosis and response to treatment, in established human tumors. In this review we will outline recently generated prognostic and predictive information, in patients with NSCLC, after a brief review of the biology of the NER mechanism.
MOLECULAR MECHANISMS OF NUCLEAR EXCISION REPAIR
In the last decade, all the key NER factors have been cloned and the details of the 'cut-and-paste' reaction have been worked out in vitro from purified components (de Laat et al., 1999). The reaction is initiated by the damage sensor and repair recruitment factor, XPC and is outlined in Figure 1. XPC complexes with hHR23B to initiate the repair in the TC-NER reaction. Once the damaged site, and consequently the site to be excised, has been identified, the general transcription factor complex TFIIH, containing the XPB (called ERCC3 in mice) and XPD (ERCC2) helicases, mediates strand separation at the site of the lesion. In the GG-NER reaction, TFIIH can initiate the reaction with or without the aid of XPC-hHR23B complex. XPA is then recruited which verifies the damage in an open DNA conformation and is critical to the assembly of the remainder of the repair machinery. Replication protein A (RPA) then stabilizes the opened DNA complex and positions the XPG (ERCC5), which is an endonuclease that excises the 3’ end of the DNA segment. ERCC1 then complexes with XPF (ERCC4) to form an endonuclease which is responsible for the DNA excision around the 5’ end of the lesion (Houtsmuller et al., 1999). The damage-containing oligonucleotide, typically 24–32 nucleotides in length, is then removed. Replication factors are then activated which fill in the gap and the repair process is now complete.
Recent studies have clarified the role of NER in the natural history and response to treatment of patients with NSCLC (Lord et al., 2002; Olaussen et al., 2006; Simon, et al., 2005). The functional status of NER is evaluated in clinical specimens by measuring ERCC1. The generally small size of most tumor biopsies precludes simultaneously measuring several proteins of the NER pathway,. Hence, owing to practical constraints, either ERCC1 mRNA expression (measured by reverse-transcriptase polymerase chain reaction (RT-PCR)) (Simon et al., 2005), or quantitative ERCC1 protein estimation (measured by immunohistochemistry (IHC)) (Olaussen et al., 2006) is used to assess ERCC1 levels in the tumors sampled and is presumed to represent the functioning of the entire NER pathway. In the studies outlined below, we show that intra-tumoral ERCC1 can serve both as a prognostic and predictive marker in patients with NSCLC.
ERCC1 AS A PREDICTOR OF RESPONSE TO CHEMOTHERAPY IN PATIENTS WITH ADVANCED NSCLC
The current standard of treatment for patients with incurable metastatic NSCLC is a doublet chemotherapy regimen. The choice of agents is generally based on convenience, side effect profiles, and the treating physician’s experience and personal preference. The principal agents used are cisplatin or carboplatin, docetaxel or paclitaxel, gemcitabine, and vinorelbine. The reported response rate to such therapy is 17–37%, the median overall survival ranges from 6.7–11.3 months, the 1-year and 2-year survival rates are 31–46% and 9–21%, respectively (Fossella et al., 2003; Gridelli et al., 2000; Kelly et al., 2001; Kosmidis et al., 2002; Rosell et al., 2002; Schiller et al., 2002; Smit et al., 2003). After patients fail initial therapy, response to further systemic treatment is approximately 10% for single agent therapy (Fossella et al., 2000; Hanna et al., 2004; Shepherd et al., 2000). Thus, resistance to systemic therapy does not appear to be an all-or-none phenomenon, but rather a function of the molecular characteristics of the individual tumor. In fact, specific molecular characteristics highly associated with tumor response to a group of therapeutic agents used in NSCLC, epidermal growth factor receptor tyrosine kinase inhibitors, has recently been reported (Lynch et al., 2004) (Paez et al., 2004).
The platinum compounds, specifically cisplatin and carboplatin, are heavy metal complexes that form adducts and covalent cross-links between DNA double strands and thus effectively blocks DNA replication and transcription. These adducts and cross-links are repaired by a complex of molecules in the NER pathway and as outlined above, the ERCC1 gene encodes a protein that is part of this complex. In gastric cancer, ERCC1 mRNA level is inversely associated with response and survival as an independent function of cisplatin efficacy (Metzger et al., 1998). Normally a poor responder to chemotherapy, patients with gastric cancer that did not over express ERCC1 mRNA, had a median survival that had not been reached after 39 months of follow up but was predicted to be more than 24 months. On the other hand, patients with increased ERCC1 expression had a median survival of 5.4 months. This difference was statistically significant (P= 0.034). Similar observations have been reported in ovarian (Dabholkar et al., 1992), esophageal (Metzger, PM, & al, 2001), colorectal (Shirota et al., 2001), NSCLC (Lord et al., 2002) and more recently, breast cancer (Bewick, Conlon, & Lafrenie, 2006).
Lord et al (Lord et al., 2002) correlated response and survival with the level of ERCC1 expression in 56 patients with advanced (stage IIIb or IV) NSCLC treated as part of a multicenter randomized trial with gemcitabine 1250 mg/m² days 1 and 8 plus cisplatin 100 mg/m² on day 1 every 3 weeks. mRNA was isolated from paraffin-embedded pretreatment primary tumor specimens, and relative expression levels of ERCC1/β-actin were measured using a quantitative reverse transcription-PCR (Taqman) system. ERCC1 expression was detectable in all tumors. There were no significant differences in ERCC1 levels by gender, age, performance status, weight loss, or tumor stage. The overall response rate was 44.7%. Median overall survival was significantly longer in patients with low ERCC1 expression tumors (61.6 weeks; 95% confidence interval (CI), 42.4–80.7 weeks) compared to patients with high expression tumors (20.4 weeks, 95% CI, 6.9–33.9 weeks) (P= 0.046). ERCC1 expression levels were significantly higher in squamous cell carcinomas (median, 8.6) compared with adenocarcinomas (median, 5.2; P = 0.015, Mann-Whitney test).
MOLECULAR ANALYSES DIRECTED INDIVIDUALIZED THERAPY TRIALS IN PATIENTS WITH ADVANCED NSCLC
We conducted a prospective phase II clinical trial to test the feasibility and efficacy of molecular analysis-directed individualized therapy (MADe IT) in patients with advanced NSCLC (Simon et al., 2007). We hypothesized that selection of dual agent chemotherapy based on tumoral ERCC1 and RRM1 expression would be feasible and beneficial for patients with advanced NSCLC.
RRM1 is the regulatory subunit of ribonucleotide reductase, an essential metabolic enzyme required for cellular proliferation, that catalyzes the reduction of ribonucleoside diphosphates to the corresponding deoxyribonucleotides (Bepler et al., 2004) (Stubbe, 1998). RRM1 is the molecular target of gemcitabine (2’-2’difluorodeoxycytidine) which is an antimetabolite with activity in several malignancies, and is one of the most efficacious agents in NSCLC (T. Le Chevalier et al., 2005) (Edelman, 2003).
Patients were required to have a dedicated tumor biopsy for determination of ERCC1 and RRM1 gene expression by real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Sixty-six patients underwent the required biopsy. Gene expression analysis could not be performed in 5 patients because of inadequacy of the biopsy specimens. One patient withdrew consent prior to analysis. Thus, gene expression was successful in 92% (60/65) of patients. The time elapsed from tumor biopsy to gene analysis was 1–28 days (median 6 days). Of the 55 patients with available gene expression data that were eligible for treatment, two never received the assigned treatment because of natural disasters in Florida during the summer of 2004.
Dual agent chemotherapy consisting of carboplatin, gemcitabine, docetaxel, and vinorelbine was selected based on gene expression. Predetermined values for ERCC1 and RRM1 were used for decisions regarding usage of the drugs gemcitabine and carboplatin. If RRM1 was equal to or below the value of 16.5, gemcitabine was used in the treatment doublet; if ERCC1 was equal to or below the value of 8.7, carboplatin was used in the treatment doublet. These levels were selected based on our previously published experience with expression analysis in patients (Bepler et al., 2006; Simon et al., 2005) (Bepler et al., 2004) and were expressed as unit less ratio between the gene of interest and the house keeping gene18SrRNA.
This strategy resulted in four possible gene expression strata with the following doublet therapies: The low RRM1 and low ERCC1 group was treated with gemcitabine and carboplatin (GC group). The low RRM1 and high ERCC1 group was treated with gemcitabine and docetaxel (GD group). The high RRM1 and low ERCC1 group was treated with docetaxel and carboplatin (DC group). The high RRM1 and high ERCC1 group was treated with vinorelbine and docetaxel every 28 days (DV group). (Please see Table 1)
Table 1.
Strategy used to customize chemotherapy based on molecular markers ERCC1 and RRM1. Patients with low ERCC1 were treated with carboplatin. Patients with low RRM1 was treated with gemcitabine. Patients with high ERCC1 was treated with docetaxel and patients with high RRM1 was treated with docetaxel or navelbine. Please see text for details.
| Molecular Marker | ERCC1 Low | ERCC1 High |
|---|---|---|
| RRM1 Low | Carboplatin + Gemcitabine | Docetaxel + Gemcitabine |
| RRM1 High | Carboplatin + Docetaxel | Docetaxel + Navelbine |
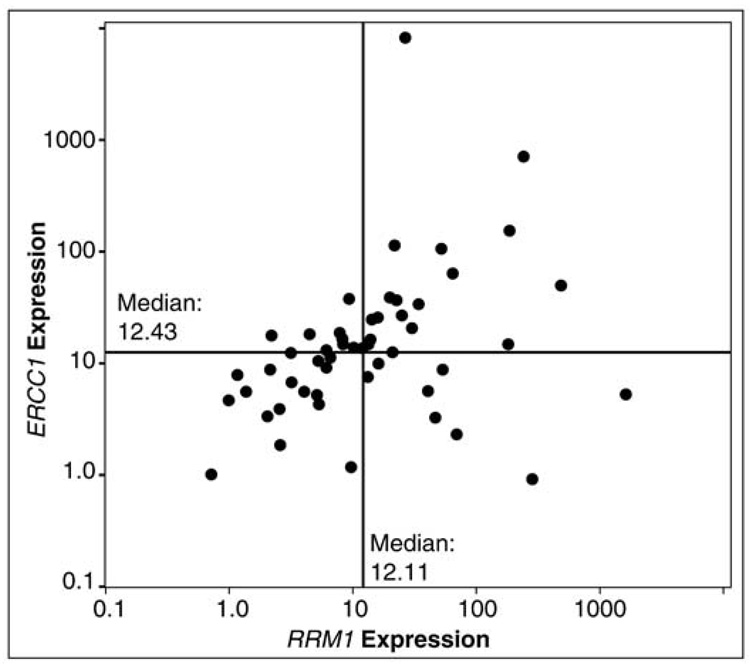
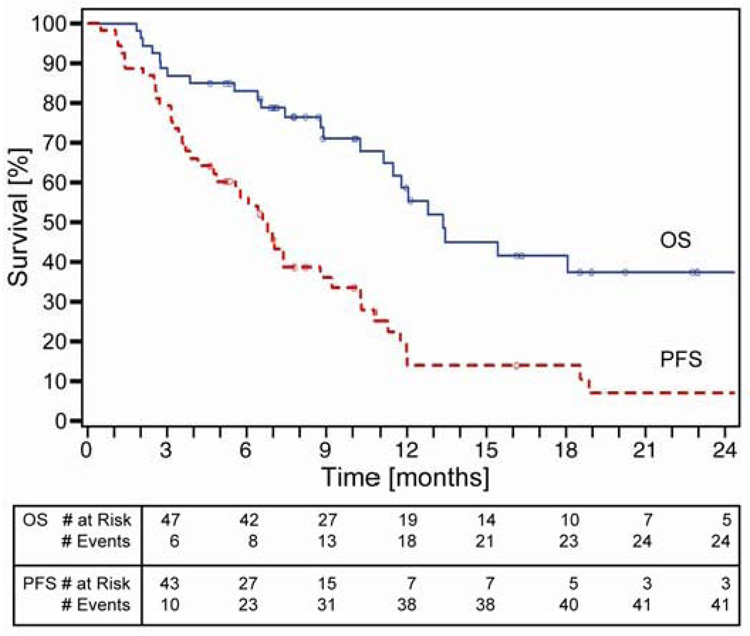
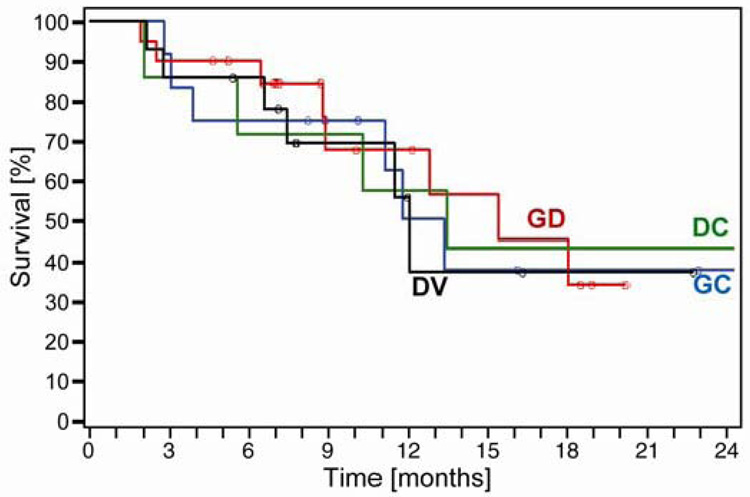
Disease response was sequentially assessed after every two cycles by computed tomography (CT) of the chest, upper abdomen, and other areas as indicated. The primary endpoint was best disease response. Secondary endpoints were overall (OS) and progression-free survival (PFS). In this trial we show that; 1) collection of tumor specimens with gene expression analysis was feasible and 2) RRM1 expression ranged from 0–1,637, ERCC1 expression ranged from 1–8,103, and their expression was directly correlated (Spearman’s rho=0.46, p<0.01) (See Figure 2). Response rate was 42%. Overall and progression-free survivals were 59% and 14% at 12 months with medians of 13.3 and 6.6 months, respectively (Figure 3). As expected, OS did not differ by assigned chemotherapy and there were no statistically significant differences between any of the arms suggesting once customized chemotherapy is utilized survival does not differ (Figure 4).
Figure 2.
Scatter plot of RRM1 and ERCC1 expression in 53 patients with advanced NSCLC showing that the ERCC1 and RRM1 expressions were directly correlated (Spearman’s rho=0.46, p<0.01) (Simon et al., 2007).
Figure 3.
Overall and progression-free survival of 53 patients with advanced NSCLC treated with chemotherapy based on expression of the genes RRM1 and ERCC1 (as outlined in table 1). Overall and progression-free survivals were 59% and 14% at 12 months with medians of 13.3 and 6.6 months, respectively (Simon et al., 2007).
Figure 4.
Overall survival by assigned chemotherapy. GC, gemcitabine and carboplatin; GD, gemcitabine and docetaxel; DC, docetaxel and carboplatin; DV, docetaxel and vinorelbine. There were no statistically significant differences between any of the arms (Simon et al., 2007).
For comparison, in our previous NSCLC phase II efficacy trial, which used the same criteria for participant selection and traditional dual-agent chemotherapy (carboplatin + gemcitabine) for previously untreated advanced-stage NSCLC patients, we achieved a response rate of 24% (vs. 42%), median OS time of 6.7 months (vs. 13.3 months), and median PFS time of 4.9 months (vs. 6.6 months) (Chiappori et al., 2003). The trial accrued a total of 40 patients from October, 2001 to January, 2003; and all were treated by the same multidisciplinary team of physicians. Therefore, our single institution experience using unselected chemotherapy appear inferior to the results reported in the customized chemotherapy trial and this implies that, therapeutic decision making based on ERCC1 and RRM1 gene expression for patients with advanced NSCLC is feasible and may improve response rates and survival.
Conducting a clinical trial along similar lines, Rosell et al. (Rosell et al., 2005) reported an ERCC1 mRNA-tailored randomized phase III trial. NSCLC patients with stage IV disease were randomized in a 1:2 fashion to a control arm where they were treated with cisplatin and docetaxel or a genotypic arm, where if the ERCC1 mRNA expression is high then the patient were treated with gemcitabine and docetaxel and if the ERCC1 expression is low then they are treated with cisplatin and docetaxel (N=300). There was a statistically significant improvement in response rate, which was the primary endpoint, in the genotypic arm compared to the control arm, however there were no statistically significant differences in overall survival. However, this study is still interpreted as a positive trial since there was a statistically significant improvement in response, which was the primary endpoint of this trial.
Ceppi et al (Ceppi et al., 2006), in a retrospective analyses, examined formalin-fixed, paraffin-embedded bronchoscopic/fine needle aspiration biopsies obtained from 70 patients with advanced NSCLC that were collected to investigate the expression of ERCC1 and RRM1 by real-time PCR. Sufficient amounts of messenger RNA (mRNA) were successfully extracted from 61 (87%) specimens, reverse transcribed and amplified with intron-spanning primers. Forty-one patients had stage IV disease and 43 received cisplatin/gemcitabine chemotherapy. Median survival time in patients with low ERCC1 was significantly longer (17.3 versus 10.9, P= 0.0032 log-rank test) as well as in patients with low RRM1 (13.9 versus 10.9, P= 0.039 log-rank test). Concomitant low expression levels of ERCC1 and RRM1 (n = 33) were predictive of a better outcome (14.9 versus 10.0, P= 0.0345, log-rank test). Among cisplatin-treated patients, a low ERCC1 level was highly predictive of a longer survival (23.0 versus 12.4, P= 0.0001 log-rank test). On multivariate analysis, performance status, response to chemotherapy, presence of weight loss and ERCC1 were independent prognostic factors for survival. The authors concluded that ERCC1 and RRM1 expression impacts survival of NSCLC patients treated with cisplatin/gemcitabine chemotherapy.
Evidence for improved response rates and survival with molecular analysis tailored chemotherapy in advanced stage NSCLC, is therefore mounting. A retrospective trial hints at an improvement in both response rates and survival. A prospective phase III trial showed an improvement in response rate, but not survival. A prospective single institution phase II trial implied an improvement both in response rates and survival, over historical controls.
Several issues must be addressed before a general implementation of the gene expression-based therapeutic approach can be recommended. First, the general feasibility of performing core needle biopsies of patients’ tumors with immediate freezing, laser capture microdissection, and subsequent sophisticated gene expression analysis appear limited, given the required infrastructure. Thus, the development of a more generally applicable methodology, based on technology familiar to clinical laboratories and pathologists, such as IHC or objective quantitative in situ protein analyses (AQUA) (McCabe, Dolled-Filhart, Camp, & Rimm, 2005). AQUA, a quantitative method measuring in situ protein level, is currently being investigated and under development by our group. Second, this method must be validated in a phase III randomized design. At our center, a soon to open trial will randomize patients to either the customization arm where they will receive chemotherapy using the customized approach outlined above, or a non-customized arm where all patients will receive chemotherapy with carboplatin and gemcitabine. This phase III randomized trial will be the crucial test of the benefit of the customization approach. Third, the incorporation of specific targeted agents in to the customization algorithm becomes imperative, given the recently reported improvement in survival with the addition of bevacizumab to chemotherapy in select patients eligible to receive bevacizumab (Hanna et al., 2006). Our center is currently evaluating ways of developing customized approaches to incorporate targeted agents to the currently pure chemotherapy algorithm.
PROGNOSTIC SIGNIFICANCE OF ERCC1 IN PATIENTS WITH EARLY-STAGE NSCLC
Published reports indicate that NSCLCs with extensive genomic alterations as determined by DNA ploidy, microsatellite instability, and allele loss have a more malignant phenotype with increased growth rates and higher propensity for metastatic dissemination (Bepler et al., 2002; Bepler et al., 2006; Bepler et al., 2004). Lung cancers display a spectrum of genetic alterations that ranges from few, yet biologically crucial, aberrations to extensive genomic damage. The extent of this damage is likely the result of the type and quantity of carcinogen exposure as well as the intrinsic ability of cells to repair this damage. Cells with extensive damage that have escaped from the physiologic pro-apoptotic surveillance may have a proliferative advantage and more malignant phenotypic behavior compared to cells with less extensive damage. Expression levels of the DNA damage repair gene ERCC1, may be representative of a cell’s intrinsic DNA damage repair ability, and may thus serve as a biomarker of the extent of accumulated intra-tumoral DNA damage.
To test this hypothesis, we evaluated the effect of ERCC1 expression on survival in NSCLC patients that underwent surgical resection for cure. Resected tumor specimens from 51 patients with NSCLC who underwent surgical resection were immediately frozen in liquid nitrogen (Simon et al., 2005). Total RNA was extracted, reverse transcribed, and amplified with intron-spanning primers. Quantification for ERCC1 was done using the Taqman procedure, and gene expression was expressed as a ratio of 18SrRNA (a housekeeping gene used as internal reference) and therefore as unit less.
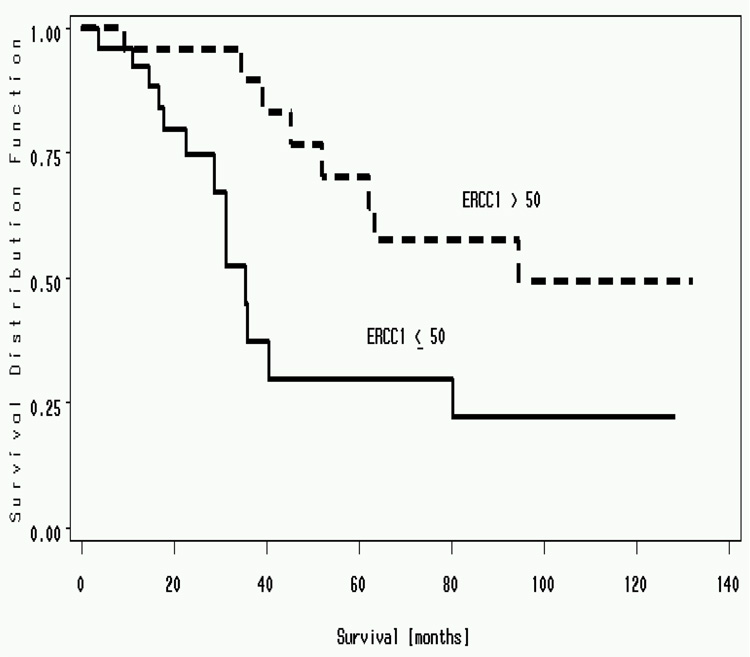
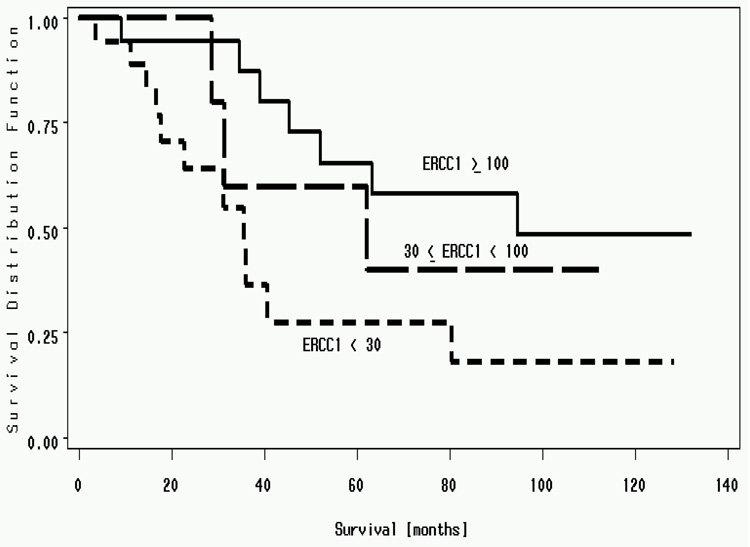
ERCC1 expression ranged from 4.96 to 2008. The median value was 54.76. An ERCC1 value of 50 was used to dichotomize the cohort. There was a statistically significant difference in median survival for patients with ERCC1 expression above 50 (94.6 months) compared to below 50 (35.5 months)(P=0.01) (Figure 5). To demonstrate a linear relationship between ERCC1 expression and survival, we split the cohort in to three groups, ERCC1 expression < 30, 30 to 100 and >100. Median survivals of patients with ERCC1; less than 30 was 35.5 months, 30 to 100 was 62.1 months and more than 100 was 94.6 months (P value = 0.03) (two-sided Log Rank Test) (Figure 6). High ERCC1 expression was an independent predictor for longer survival on multivariate analysis. Hence resected NSCLC patients with high ERCC1 expression (> 50) have a better survival when compared to patients with low ERCC1 expression (<50) (Simon et al., 2005).
Figure 5.
Median survival of resected patients with NSCLC dichotomized based on ERCC1. Patients with ERCC1 of more than 50 had a median survival of 94.6 months vs. patients with median survival of less than 50 had a median survival of 35.5 months (P=.01) (Simon et al., 2005)
Figure 6.
Median survivals of resected patients with NSCLC. Patients with ERCC1; of less than 30 had a median survival of 35.5 months, 30 to 100 had a median survival of 62.1 months and >100 had a median survival of 94.6 months. (P value = 0.03) (Simon et al., 2005).
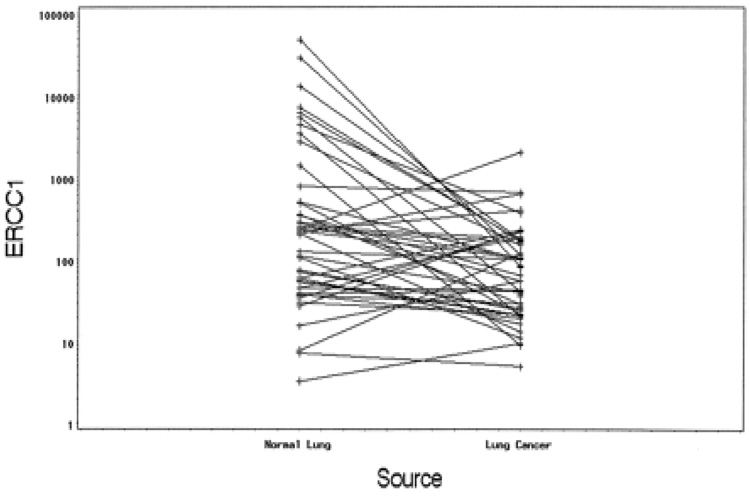
We also compared the ERCC1 expression of the resected tumors with that of the adjacent lung and found no correlation (Simon et al., 2005). This suggests that NER capability of a tumor is an intrinsic tumor characteristic and is not dependent on the NER capability of the organ it arises from. This necessitates the intra-tumoral measurement of ERCC1 for prognostication or prediction. Please see figure 7.
Figure 7.
No correlation was found between ERCC1 expression of lung cancers and adjacent normal lung (P = 0.094) (Simon et al., 2005).
The above results have important implications in the management of patients with early stage and resectable NSCLC. Patients with completely resected NSCLC with high ERCC1 not only have a lower incidence of relapse but are also less likely to benefit from adjuvant cisplatin-based chemotherapy owing to the ability of ERCC1 to confer resistance to platinum. The contrary would be true for patients with low intratumoral ERCC1, i.e. they would be more likely to relapse but also more likely to benefit from adjuvant cisplatin-based chemotherapy. Olaussen et al (Olaussen et al., 2006) validated this hypothesis when they used immunohistochemical (IHC) analysis to determine the expression of the ERCC1 protein in resected specimens of NSCLC patients enrolled in the International Adjuvant Lung Cancer Trial (IALT) (Arriagada et al., 2004).
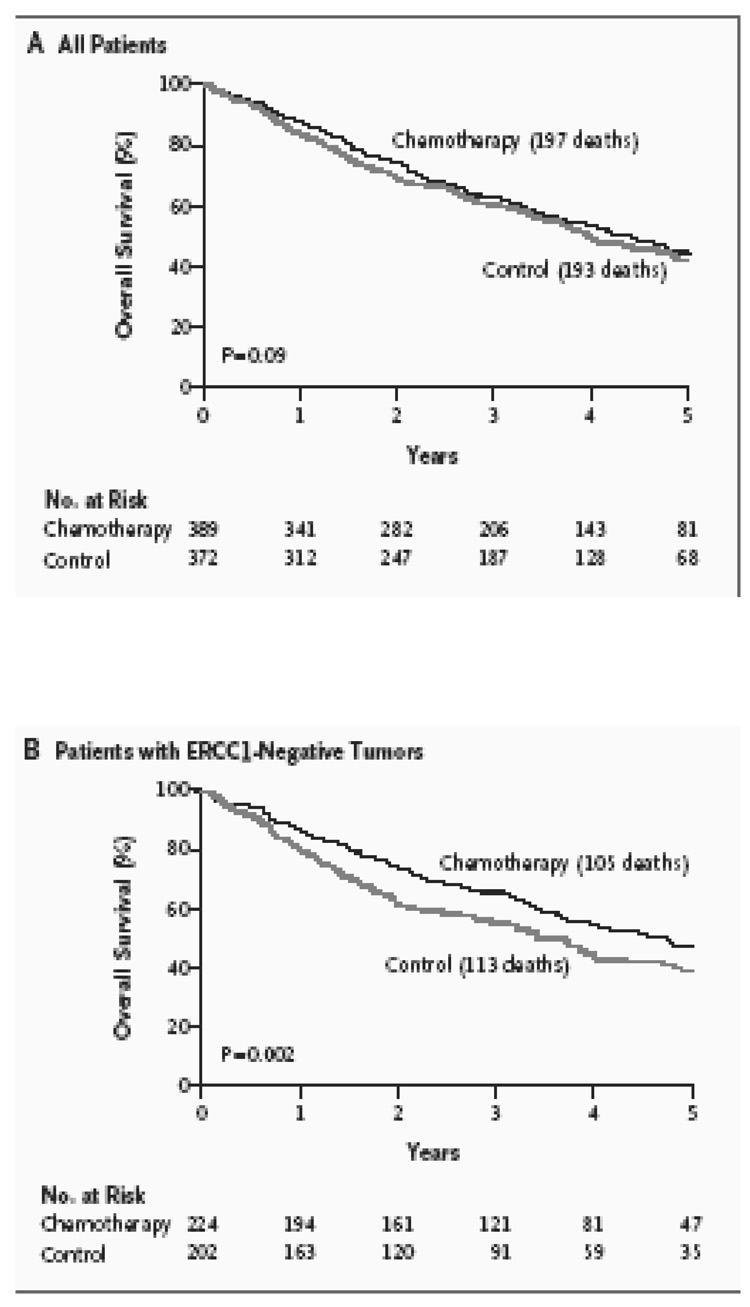
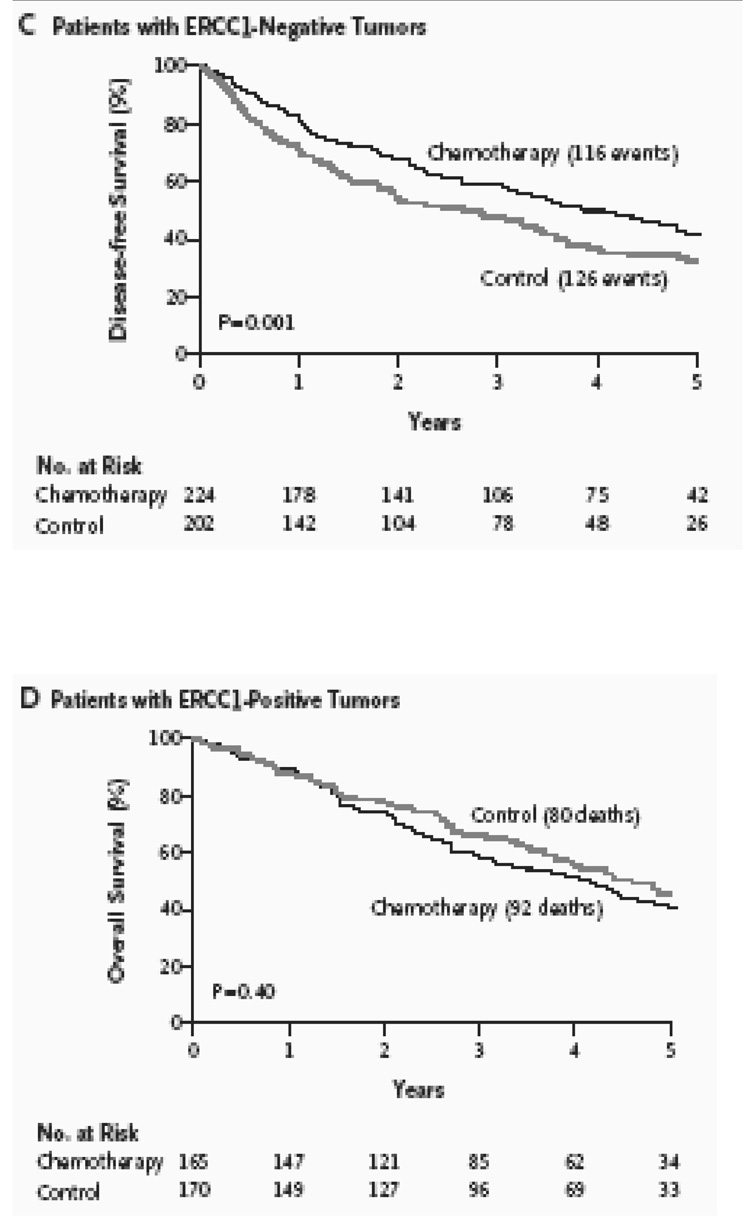
The IALT is a phase III trial that randomized completely resected patients with stage I to III NSCLC to either observation or four cycles of cisplatin based chemotherapy (Le Chevalier et al., 2003). The chemotherapy arm demonstrated a statistically significant, 4.2% improvement in five-year survival. The randomized design whereby half the patients received cisplatin-based chemotherapy and the other half did not allowed a comparison of the effect of adjuvant cisplatin-based chemotherapy on survival, according to ERCC1 expression. Out of the 1,897 patients enrolled in the IALT trial, 761 patients had tumors that were available for ERCC1 analyses. Among 761 tumors, ERCC1 expression was positive in 335 (44%) and negative in 426 (56%). Adjuvant chemotherapy, as compared with observation, significantly prolonged survival among patients with ERCC1-negative tumors (adjusted hazard ratio for death, 0.65; 95% confidence interval [CI], 0.50 to 0.86; P=0.002) but not among patients with ERCC1-positive tumors (adjusted hazard ratio for death, 1.14; 95% CI, 0.84 to 1.55; P=0.40). Among patients who did not receive adjuvant chemotherapy, those with ERCC1-positive tumors survived longer than those with ERCC1-negative tumors (adjusted hazard ratio for death, 0.66; 95% CI, 0.49 to 0.90; P=0.009). Therefore, in this retrospective analysis of a large phase III randomized trial, patients with completely resected NSCLC and ERCC1-negative tumors appear to benefit from adjuvant cisplatin-based chemotherapy. Patients with ERCC1 positive tumors had an innately better prognosis but further improvement was not seen with cisplatin based adjuvant chemotherapy. Please see Figure 8.
Figure 8.
Survival figures in the IALT retrospective analyses conducted by Olaussen et al. A) There were no statistically significant differences in overall survival in the 761 patients for whom ERCC1 IHC analyses was possible. B) Overall Survival was improved in patients with ERCC1 negative tumors who received chemotherapy versus control. (P=0.002) C) Disease Free Survival was also improved in patients with ERCC1 negative tumors who received chemotherapy versus control. (P= 0.001) D) Patients with ERCC1 positive tumors did not benefit from cisplatin-based chemotherapy over controls. (P= 0.40). Please see text for details (Olaussen et al., 2006).
In summary, ERCC1’s role in the clinical management of resectable NSCLC has become increasingly clear. Patients with low ERCC1 expression have poor survival yet their tumors are sensitive to platinum-based therapy, therefore identifying a group of patients that would derive the most benefit from treatment with platinum-based chemotherapy in the neo-adjuvant or adjuvant settings. Conversely, patients with high ERCC1 protein or mRNA expression has a better intrinsic prognosis with a lower probability or relapse and little or no benefit from the traditionally-used cisplatin-based adjuvant chemotherapy. It remains to be determined whether this high ERCC1 group will benefit from a nonplatinum doublet chemotherapy regimen used adjuvantly. Future adjuvant trials are planned that will dichotomize patients based on the ERCC1 status and tailor the chemotherapy regimen according to the ERCC1 and RRM1 expression.
ROLE OF ERCC1 POLYMORPHISMS IN LUNG CANCER CARCINOGENESIS
In humans, NER is the major pathway responsible for repairing DNA adducts induced by smokingrelated carcinogens, such as benzo[a]pyrene diol epoxide. Environmental carcinogens contained in air pollution, such as polycyclic aromatic hydrocarbons, aromatic amines or N-nitroso compounds, predominantly form DNA adducts but can also generate inter-strand cross-links and reactive oxygen species. If unrepaired, such lesions also increase the risk of somatic mutations and the induction of carcinogenesis.
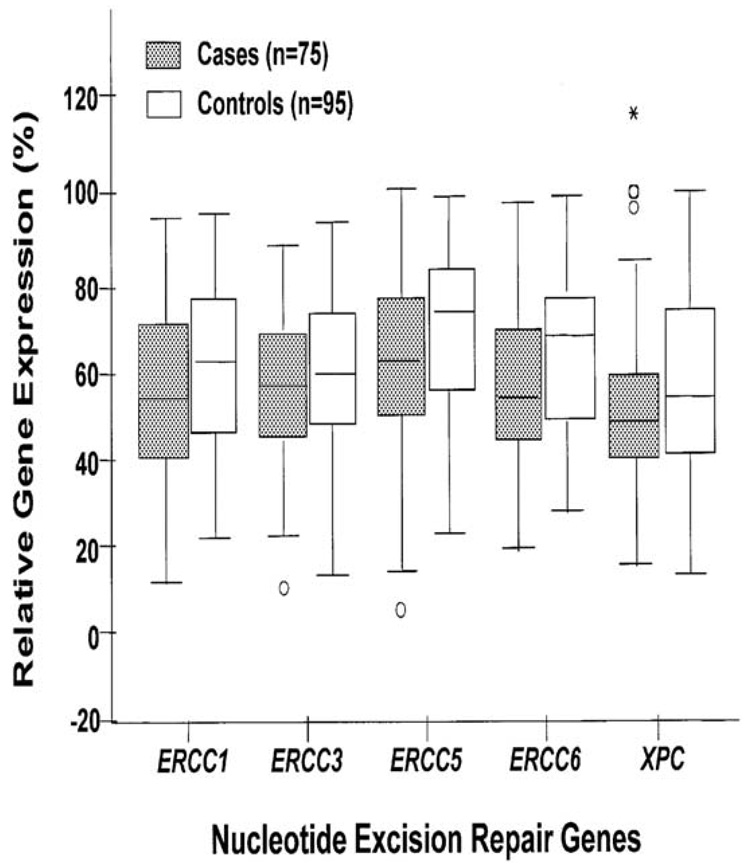
ERCC1 and other NER genes may not only determine prognosis and response to treatment, baseline expression of these gene may also determine cancer risk. Cheng et al. (Cheng, Spitz, Hong, & Wei, 2000) measured the relative expression levels of five NER genes [ERCC1, XPB/ERCC3, XPG/ERCC5, CSB/ERCC6 and XPC] in phytohemagglutinin-stimulated peripheral lymphocytes obtained from 75 lung cancer patients and 95 controls using a newly developed multiplex RT-PCR assay. Cases and controls were matched for age, sex, ethnicity and tobacco use. A 12.2 and 12.5% decrease in the baseline expression levels of XPG/ERCC5 and CSB/ERCC6, respectively, in cases compared with controls was observed. These differences were statistically significant (P < 0.01). When the median expression level in the controls was used as the cut-off point, lung cancer patients were significantly more likely than the controls to have reduced expression levels of XPG/ERCC5 [odds ratio (OR), 2.32; 95% confidence interval (CI), 1.22–4.43] and CSB/ERCC6 (OR, 2.49; 95% CI, 1.28–4.84). There was also a dose-response relationship between reduced expression levels and increased lung cancer risk (trend test: P < 0.01). These results suggest that individuals whose expression levels of XPG/ERCC5 and CSB/ERCC6 are reduced may be at a higher risk for developing lung cancer (Figure 9). Specific ERCC1 polymorphisms may increase cancer risk as well and this is a topic of active investigation in several malignancies including lung cancer.
Figure 9.
Lung cancer cases showed lower ERCC5 and ERCC6 gene expression in comparison with matched controls (P< 0.01) (Cheng et al., 2000).
Our individual propensity to develop lung cancer after exposure to cigarette and environmental carcinogens may depend on our ability to repair the DNA damage induced by the carcinogens. Lower the ability to repair, higher the risk for carcinogenesis. This fact may have future implications for chemoprevention.
CONCLUSIONS AND FUTURE PROSPECTS
NER function plays a central role in the carcinogenesis, prognosis, and response to treatment in patients with NSCLC. It may determine who gets lung cancer once exposure to carcinogens has occurred. In patients with early stage NSCLC, low intratumoral ERCC1 expression (or ERCC1 negative patients by IHC) predicts for relapse and selects for patients who will benefit from adjuvant cisplatin-based chemotherapy. Conversely, ERCC1 positive patients have a better intrinsic prognosis and is not likely to benefit from additional platinum based chemotherapy. Currently several trials are being designed or are underway that tailors adjuvant chemotherapy based on ERCC1.
In advanced stage disease, ERCC1 predicts for platinum sensitivity enabling us to tailor chemotherapy based on an individual patient’s molecular profile. Accumulating evidence suggests that such tailored chemotherapy approaches may improve responses and survival over randomly selected chemotherapy. This approach is currently being validated in a prospective phase III trial. We are also working on developing tailored approaches to incorporate both targeted agents and chemotherapy in the treatment of patients with advanced NSCLC.
To ensure wider applicability of the customization approach to treatment decision-making and chemotherapy selection, techniques are being developed to make quantitative assessment of ERCC1 or RRM1 feasible in paraffin fixed biopsy specimens. IHC analyses (Olaussen et al., 2006), mRNA expression (Ceppi et al., 2006) and quantitative in situ protein analyses (AQUA) (McCabe et al., 2005) have been successfully performed in retrospective studies. Prospective studies with ERCC1 protein or mRNA analyses in paraffin fixes studies are currently underway.
In conclusion, assessment of NER function, particularly ERCC1 may play a central role in NSCLC treatment decision making.
References
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Bepler G, G A, McIntyre LN, Beck AF, Chervinsky DS, Kim YC, Pitterle DM, Hyland A. Prognostic Significance of Molecular genetic aberrations on chromosome segment 11p15.5 in non-small cell lung cancer. J Clin Oncol. 2002;20(5):1353–1360. doi: 10.1200/JCO.2002.20.5.1353. [DOI] [PubMed] [Google Scholar]
- Bepler G, Kusmartseva I, Sharma S, Gautam A, Cantor A, Sharma A, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- Bepler G, Sharma S, Cantor A, Gautam A, Haura E, Simon G, et al. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22(10):1878–1885. doi: 10.1200/JCO.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Bewick MA, Conlon MS, Lafrenie RM. Polymorphisms in XRCC1, XRCC3, and CCND1 and survival after treatment for metastatic breast cancer. J Clin Oncol. 2006;24(36):5645–5651. doi: 10.1200/JCO.2006.05.9923. [DOI] [PubMed] [Google Scholar]
- Ceppi P, Volante M, Novello S, Rapa I, Danenberg K, Danenberg P, et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol. 2006;17(12):1818–1825. doi: 10.1093/annonc/mdl300. [DOI] [PubMed] [Google Scholar]
- Cheng L, Spitz MR, Hong WK, Wei Q. Reduced expression levels of nucleotide excision repair genes in lung cancer: a case-control analysis. Carcinogenesis. 2000;21(8):1527–1530. [PubMed] [Google Scholar]
- Chiappori A, Simon G, Rocha Lima C, Williams C, Haura E, Vaughn J, et al. Phase II study of sequential gemcitabine (G) and carboplatin (C) followed by docetaxel (D) as first-line chemotherapy for patients with advanced non-small cell lung cancer. Lung Cancer. 2003;41(2):S225. doi: 10.1159/000086979. [DOI] [PubMed] [Google Scholar]
- Dabholkar M, Bostick-Bruton F, Weber C, Bohr VA, Egwuagu C, Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992;84(19):1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- de Laat WL, Jaspers NG, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13(7):768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- Edelman MJ. Gemcitabine and carboplatin regimens in advanced non-small-cell lung cancer: focus on randomized phase III trials. Clin Lung Cancer. 2003;4 Suppl 2:S40–S44. doi: 10.3816/clc.2003.s.002. [DOI] [PubMed] [Google Scholar]
- Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21(16):3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18(12):2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Frontini L, Perrone F, Gallo C, Gulisano M, Cigolari S, et al. Gemcitabine plus vinorelbine in advanced non-small cell lung cancer: a phase II study of three different doses. Gem Vin Investigators. Br J Cancer. 2000;83(6):707–714. doi: 10.1054/bjoc.2000.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N, Bunn PA, Jr, Langer C, Einhorn L, Guthrie T, Jr, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. Nucleotide excision repair I: from E. coli to yeast. Trends Genet. 1993a;9(5):173–177. doi: 10.1016/0168-9525(93)90164-d. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993b;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Houtsmuller AB, Rademakers S, Nigg AL, Hoogstraten D, Hoeijmakers JH, Vermeulen W. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science. 1999;284(5416):958–961. doi: 10.1126/science.284.5416.958. [DOI] [PubMed] [Google Scholar]
- Kelly K, Crowley J, Bunn PA, Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--smallcell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- Kosmidis P, Mylonakis N, Nicolaides C, Kalophonos C, Samantas E, Boukovinas J, et al. Paclitaxel plus carboplatin versus gemcitabine plus paclitaxel in advanced non-small-cell lung cancer: a phase III randomized trial. J Clin Oncol. 2002;20(17):3578–3585. doi: 10.1200/JCO.2002.12.112. [DOI] [PubMed] [Google Scholar]
- Le Chevalier T, Scagliotti G, Natale R, Danson S, Rosell R, Stahel R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a metaanalysis of survival outcomes. Lung Cancer. 2005;47(1):69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Le Chevalier T. (f. t. I. I.). Results of the Randomized Adjuvant Lung Cancer Trial (IALT): cisplatin-based chemotherapy (CT) vs. no CT in 1867 Patients with resected non-small cell lung cancer (NSCLC); Proc ASCO; 2003. p. 2. [Google Scholar]
- Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 Expression Correlates with Prolonged Survival after Cisplatin plus Gemcitabine Chemotherapy in Non-Small Cell Lung Cancer. Clin Cancer Res. 2002;8(7):2286–2291. [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97(24):1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16(1):309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- Metzger R, S PM, B Se, et al. Quantitative ERCC1 RNA expression identifies non-response to cis-platinum based neoadjuvant radiochemotherapy for esophageal cancer; Proc ASCO 2001; 2001. p. 130a. [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Rosell R, Cobo M, Isla D, Massuti B, Montes A, Paz-Ares M, et al. ERCC1 mRNA-based randomized Phase III trial of docetaxel (doc) doublets with cisplatin (cis) or gemcitabine (gem) in stage IV non-small clell lung cancer (NSCLC) patients (p); Proc ASCO; 2005. p. 621s. [Google Scholar]
- Rosell R, Gatzemeier U, Betticher DC, Keppler U, Macha HN, Pirker R, et al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: a cooperative multinational trial. Ann Oncol. 2002;13(10):1539–1549. doi: 10.1093/annonc/mdf332. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19(23):4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- Simon GR, Sharma A, Li X, Hazelton T, Walsh F, Williams C, et al. Feasibility and Efficacy of Molecular Analysis-Directed Individualized Therapy in Advanced Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2007 doi: 10.1200/JCO.2006.08.2099. In Press. [DOI] [PubMed] [Google Scholar]
- Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005;127(3):978–983. doi: 10.1378/chest.127.3.978. [DOI] [PubMed] [Google Scholar]
- Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group--EORTC 08975. J Clin Oncol. 2003;21(21):3909–3917. doi: 10.1200/JCO.2003.03.195. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Ribonucleotide reductases in the twenty-first century. Proc Natl Acad Sci U S A. 1998;95(6):2723–2724. doi: 10.1073/pnas.95.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]