Abstract
Previous studies have demonstrated that all-trans retinoic acid (RA) increases collagen production and decreases matrix metalloproteinase (MMP) activity in organ-cultured human skin. Decreased MMP activity is associated with up-regulation of tissue inhibitor of metalloproteinase-1 (TIMP-1). These changes are accompanied by a hyperplastic response in the epidermis. Here we show that a synthetic picolinic ester-substituted retinoid (designated as MDI 301) has comparable effects to those of RA in regard to these activities. What makes these findings of interest is that RA also stimulates elaboration of several pro-inflammatory cytokines and up-regulates leukocyte adhesion molecules in organ-cultured skin. MDI 301 does not induce such changes or is much less active. In a past study we showed that while RA was irritating to the skin of topically treated hairless mice, MDI 301 was essentially non-irritating under the same conditions [Varani et al. (2003) Arch. Dermatol Res 295:255–262]. Taken in conjunction with the findings from the past study, the present data suggest that MDI 301 will be similar to RA in capacity to repair damaged skin, but will be effective under conditions that are not irritating. These findings, thus, suggest that retinoid efficacy and clinically relevant irritancy are not inextricably linked. Potential for efficacy under conditions in which irritation is not observed is a strong rationale for further development of MDI 301 as a skin-repair agent.
Keywords: All trans retinoic acid, MDI 301, Skin, Type-I procollagen, Matrix metalloproteinase, Tissue inhibitor of metalloproteinases, Cytokines, Leukocyte adhesion molecules
Introduction
Topical application of all-trans retinoic acid (RA) improves the appearance of skin damaged as a consequence of chronic exposure to ultraviolet radiation from the sun (photoaging) [17, 33]. Retinoid use also improves the appearance of chronologically aged skin [16]. In both photoaging and natural aging, there is a loss of intact collagen and an increase in fragmented collagen in the skin [10, 26, 32]. RA treatment reverses the increased expression of connective tissue-degrading matrix metalloproteinases (MMPs) that are responsible for collagen degradation in the skin [7, 8] and concomitantly stimulates new collagen synthesis [13, 32]. These cellular events lead to changes in connective tissue structure that are visible at the histological level and, presumably, underlie the improved appearance. This has been convincingly demonstrated in both clinical studies and experimental animal models [16–19, 33]. Given the ability of RA to reduce collagen damage and induce new collagen synthesis, it is not surprising that topical retinoid use not only improves the appearance of damaged skin but also results in better function. Specifically, a number of past studies have demonstrated that RA-pretreatment improves healing of wounds that subsequently occur in skin that has been damaged as a result of aging/photoaging or as a consequence of diabetes or chronic corticosteroid use [2, 20, 25, 34, 36].
A consequence of topical retinoid use is skin irritation. Irritated skin is characterized by redness, dryness and flaking at the treated site. At the histological level, one sees a perivascular accumulation of mononuclear cells, with neutrophils and monocyes scattered throughout the dermis and occasional micro-abscesses in the dermis or epidermis [12]. All-trans retinol (vitamin A), the parent compound of RA, tends to be less irritating than RA in most cases, but even with this agent, significant irritation is observed in many individuals [15]. Likewise, skin irritation is also a complication with synthetic agents currently on the market [3]. Irritation is a major cause of non-compliance among retinoid users. In addition, excessive irritation may counteract the beneficial effects of topical use.
It has been assumed in the past that retinoid efficacy and retinoid irritation could not be separated. Recent studies, however, cast doubt on this. We demonstrated in a recent study that an ester-substituted derivative of 9-cis RA (termed MDI 301) was as efficacious as RA or 9-cis RA in stimulating epidermal hyperplasia and dermal thickening when applied topically to the skin of hairless mice [28]. Unlike RA, the synthetic agent appeared to be completely non-irritating to the rodent skin following topical use. Although hairless mice are commonly used as model for retinoid responses in skin, whether or not MDI 301 will be as effective as RA in repairing aged or photoaged human skin can only be established with human skin, itself. As a way to begin addressing this issue, MDI 301 and RA were compared for effects on human skin cells (dermal fibroblasts and epidermal keratinocytes) in monolayer culture and for effects on human skin in organ culture. The data presented here demonstrate that in both monolayer and organ culture models, MDI 301 was able to stimulate events that underlie skin repair. Of interest, MDI-301 did not up-regulate pro-inflammatory cytokines including interleukin-1β (IL-1β), IL-6, IL-8, or macrophage chemotactic peptide-1 (MCP-1) while these cytokines were induced by RA. Concomitantly, MDI 301 was less effective than RA in inducing expression of intercellular adhesion molecule-1 (ICAM-1) and E-Selectin in the microvasculature of organ-cultured skin.
Materials and methods
Retinoids
MDI 301 is a 9-cis RA derivative in which the terminal carboxylic acid group has been replaced by a picolinic ester. MDI 301 was synthesized as described in the original patent (US Patent 5,837,728; Molecular Design International; Memphis, TN) and our previous report [28]. RA was purchased from Sigma Chemical Company (St. Louis, MO). Both retinoids were stored at −80°C protected from light. For use in cell culture and organ culture, the two retinoids were prepared in DMSO at 20 mg/ml, aliquoted and frozen at −80°C protected from light. The DMSO stocks were stable for at least 1 year. At the time of use, the retinoids were diluted directly in culture medium at a final working solution of no greater than 5 µg/ml. At this concentration, the final amount of DMSO in the medium was 0.025 µl/ml. At this level, DMSO had no detectable effects on cell function.
Human skin organ culture
Replicate 2-mm punch biopsies were obtained from hip skin of volunteers less than 70 years of age. The participation of human subjects in this study was approved by the University of Michigan Institutional Review Board and all subjects provided written informed consent prior to their inclusion in the study. The punch biopsies were incubated in wells of a 24 well dish (one tissue piece per 250 µl of culture medium). Culture medium consisted of Keratinocyte Basal Medium (KBM) (Cambrex Biologicals, Walkersville, MD). KBM is a modification of MCDB-153 medium. For our purposes, it was supplemented with calcium chloride to a final Ca2+ concentration of 1.4 mM. One well was left as control while the others were treated with RA or MDI 301 (0.5–1 µg/ml). Fresh culture medium was provided at 2-day intervals. Organ culture-conditioned medium was saved for assessment of type-I procollagen, MMPs (MMP-2 and -9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) as described below. The same culture fluid was also analyzed for IL-1β, IL-6, IL-8 and MCP-1 as described below. At the end of the incubation period (normally at day-8), organ-cultured tissue was fixed in 10% buffered formalin and embedded in paraffin. Five-µm thick sections were cut and stained with hematoxylin and eosin. Representative sections of each biopsy were selected for histological evaluation and photographed. Epidermal thickness measurements were made from these tissue sections. In certain experiments, tissue was frozen in Optimal Cutting Temperature (OCT) medium and used for immunohistology. Human skin in organ culture has been extensively used in the past to help understand how RA affects skin structure and function. The organ culture protocol used here is virtually identical to that described in a past report [30].
MMP production
Substrate embedded Enzymography (zymography) was used in these studies to assess levels of latent and active MMP-2 and MMP-9 in organ culture and cell culture fluids. As described previously [21], SDS-PAGE gels were prepared with the incorporation of gelatin (1 mg/ml) at the time of casting. After electrophoresis and overnight incubation, zones of hydrolysis were identified and quantified. Values for latent and active MMP-2 and MMP-9 bands were obtained.
Type-I procollagen
Culture fluids were assayed for type-I procollagen by enzyme-linked immunosorbant assay (ELISA) (Pan Vera Corp., Madison, WS) as described previously [21]. Type-I procollagen has the terminal peptide sequences that are present at synthesis and, therefore, is a measure of ongoing collagen synthesis.
TIMP-1 production
Culture fluids were assayed for TIMP-1 by ELISA using a commercially available assay kit (R&D Systems, Mpls. MN) [21].
Cytokines
Levels of IL-1β, IL-6, IL-8 and MCP-1 were quantified. Individual ELISA kits were used for IL-6, IL-8 and MCP-1 while IL-1β was quantified as part of a multiplex assay (BioSource International Inc., Camarillo, CA).
Immunohistology
Organ-cultured tissue was fixed in OCT medium after two days of incubation. The frozen tissue was sectioned and stained for expression of ICAM-1 or E-Selectin. The antibody to ICAM-1 was a mouse monoclonal IgG1 antibody (HA58) from BD-Pharmingen (San Jose, CA). The antibody to E-Selectin was also a mouse monoclonal IgG1 antibody (clone 7A9) obtained from the American Type Culture Collection (ATCC). The sections were stained by the immunoperoxidase method and the reaction product was visualized using diaminobenzadine as the chromogenic substrate. Immunostained sections were examined by light microscopy.
Human epidermal keratinocytes and human dermal fibroblasts in monolayer culture
Normal human epidermal keratinocytes and normal human dermal fibroblasts were isolated from 2-mm punch biopsies of skin as described previously [30]. Primary and early passage keratinocytes were maintained in Keratinocyte Growth Medium (KGM) (Cambrex Inc.). KGM consists of the same basal medium as KBM but is further supplemented with a mixture of growth factors including 0.1 ng/ml EGF, 0.5 µg/ml insulin, and 2% bovine pituitary extract. In addition to using low-passage keratinocytes, we also used the HaCat line of immortalized human epidermal keratinocytes in some experiments [4]. The immortalized keratinocytes were handled exactly as low-passage keratinocytes.
Fibroblasts were obtained from the same tissue and grown in monolayer culture using Dulbecco’s Modified Minimal Essential Medium supplemented with nonessential amino acids and 10% fetal bovine serum (DMEM-FBS). Both keratinocytes and fibroblasts were maintained at 37°C in an atmosphere of 95% air and 5% CO2. Cells were subcultured by exposure to trypsin/ethylenediamine tetraacetic acid (EDTA) and used at passage 2–3.
Keratinocyte adhesion assay
Keratinocytes adhesion was assessed as described previously [29]. Briefly, cells were plated in wells of a 24-well dish at 5 × 104 cells per well in growth medium and treated with RA or MDI 301. At the end of the incubation period, cells were exposed to a solution of 0.05% trypsin/0.02% EDTA and incubated for 15 min. At the end of the incubation period, non-attached cells from each well were harvested and counted. Fresh trypsin/EDTA solution was added and the cells re-incubated until all had detached. Cells recovered in the final incubation were also counted. The percentage of cells detached at the intermediate time-point was determined from these values.
Fibroblast survival assay
Fibroblast survival was assessed as described previously [31]. Briefly, cells were seeded at 5 × 104 cells per well in DMEM-FBS (24-well plate) and allowed to attach overnight. Following this, cells were washed and put into assay buffer (KBM). KBM consists of the same basal medium as KGM but is not supplemented with growth factors. Cells were treated with different concentrations of MDI 301 (or RA), and cell numbers were assessed three days later. For counting, cells were released from the substrate with trypsin/EDTA and enumerated using a particle counter (Coulter Electronics, Hialeah, FL).
Results
Histological features of retinoid-treated human skin in organ culture: comparison of RA and MDI 301
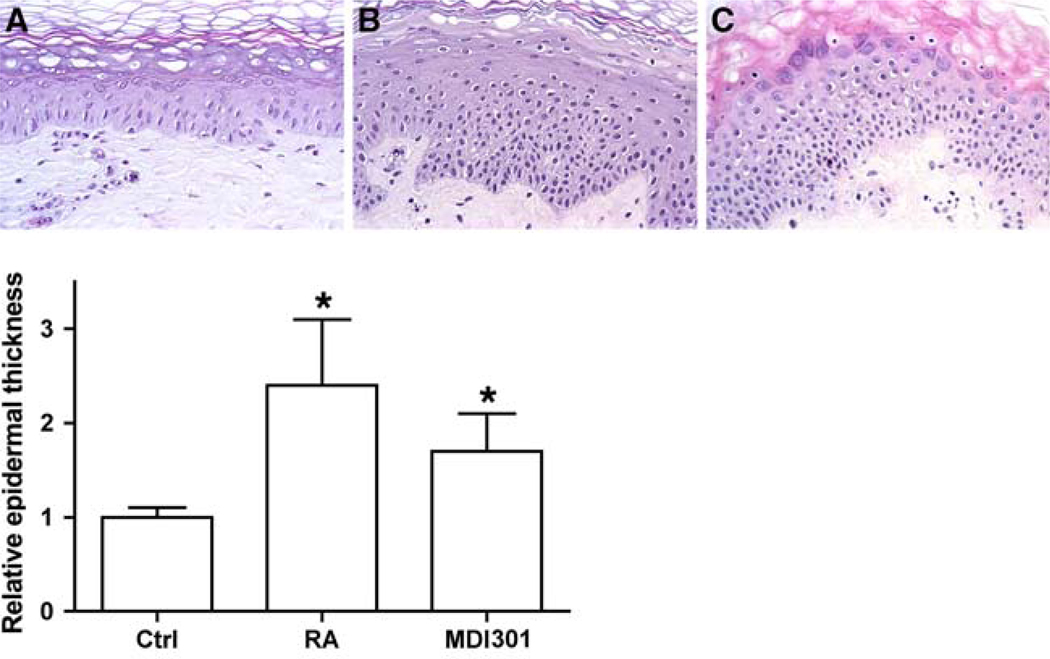
In the first series of experiments, replicate two-mm-punch biopsies were obtained from 10 different individuals. The tissue pieces were incubated in organ culture for 8 days under control conditions or treated with RA or MDI 301 (0.5–1.0 µg/ml) as described in the Materials and Methods section. At the end of the incubation period, histological features were assessed. Consistent with past reports, tissue incubated in control medium (i.e., serum-free, growth factor-free KBM supplemented with Ca2+ to a final concentration of 1.4 mM) maintained normal histological features (Fig. 1a). In contrast, skin exposed to either RA or MDI 301 demonstrated epidermal hyperplasia. There was no significant difference between the two retinoids. This can be seen from the histological images presented in Fig. 1b (RA) and Fig. 1c (MDI 301) and from the quantitative assessment shown in the lower part of Fig. 1. Hyperplasia was accompanied by features of abnormal differentiation consisting of a loss of granular cells, areas of incomplete keratinization and, in extreme cases, ancanthosis in the upper epidermis. These features were, perhaps, more prominent in cultures of RA-treated skin than in skin exposed to MDI 301 (Fig. 1b, c), but were, in fact, present in a majority of specimens treated with either agent.
Fig. 1.
Upper panel Histological features of untreated, RA-treated and MDI 301-treated human skin in organ culture. Tissue from normal (hip) skin was maintained in organ culture for 8 days under serum-free, growth factor-free conditions alone or in the presence of 0.5 µg/ml RA or 0.5 µg/ml MDI 301. At the end of the incubation period, the tissue was fixed in 10% buffered formalin and examined at the light microscopy level after sectioning and staining with hematoxylin and eosin. a Control; b RA-treated; c MDI 301-treated (X320). Lower panel Epidermal thickness measurements. Values are means and standard errors based on organ cultures from ten individuals with the average from control tissue set to 1.0. Statistical significance of the difference between untreated and retinoid-treated samples was determined by ANOVA followed by paired-group comparisons. * indicates P < 0.05 relative to control skin
In addition to examining organ-cultured human skin for changes in epidermal structure, effects of retinoid treatment on dermal features were also examined. Neither agent induced observable histological changes in the connective tissue structure over the 8-day period (Fig. 1). This is not surprising since previous studies have shown that several weeks to months are required before improvement in collagen structure is observed with topical application of RA to human skin [16, 33].
MMP-2, MMP-9 and TIMP-1 in organ cultures of retinoid-treated human skin: comparison of RA and MDI-301
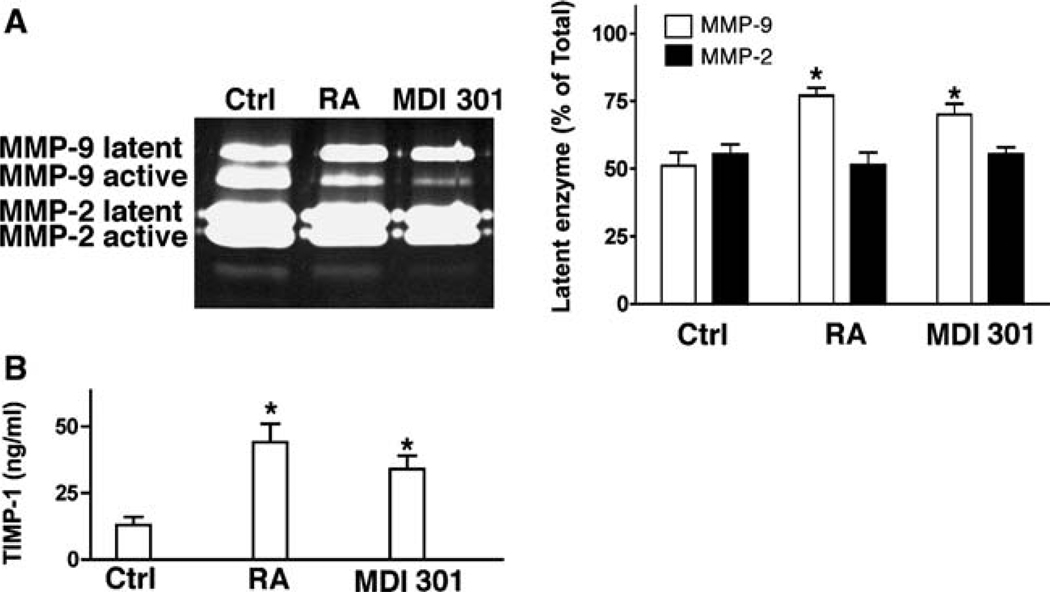
Organ culture fluids were harvested on day 4 and assayed for the presence of latent and active forms of both MMP-2 and MMP-9. Consistent with our past findings [21], approximately equal amounts of active and latent MMP-9 were present in control organ culture fluids. Also consistent, the relative amount of active MMP-9 was reduced in organ culture fluid from RA-treated skin relative to that of control cultures. As seen in Fig. 2a, treatment of skin organ cultures with MDI 301 produced the same change in overall ratio of latent to active MMP-9 as did RA. In contrast, neither retinoid significantly affected the levels of active or latent MMP-2 (Fig. 2a). The same serum-free, growth factor-free culture fluids were examined for TIMP-1 levels by ELISA. As indicated in Fig. 2b, there was a significant increase in levels of the MMP inhibitor in culture fluid from tissue treated with either retinoid in comparison with levels in culture fluid from control tissue.
Fig. 2.
MMP-2, MMP-9 and TIMP-1 production in untreated, RA-treated and MDI 301-treated human skin in organ culture. a MMPs. Left Typical gelatin zymogram demonstrating latent and active MMP-2 and MMP-9 in organ culture fluid from an untreated (ctrl), RA-treated and MDI 301-treated tissue. A higher percentage of MMP-9 in the active form can be seen in culture fluid from untreated skin as compared to RA-treated or MDI 301-treated skin. Latent and active forms of MMP-2 can also be seen but there is little difference between control and retinoid-treated samples. Right Latent enzyme expressed as a percentage of total enzyme (densitometry values from latent forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures from nine volunteers. Statistical significance of the differences among the three groups was determined by ANOVA followed by paired-group comparisons. *Indicates P < 0.05 relative to control skin. b TIMP-1. Organ culture fluid was collected on day-4 from untreated and RA-treated and MDI-301-treated skin and assayed for TIMP-1 by ELISA. Values shown are means and standard errors, based on organ cultures from nine individuals. Statistical significance of the difference between untreated and retinoid-treated samples was determined by ANOVA followed by paired-group comparisons. * indicates P < 0.05 relative to control skin
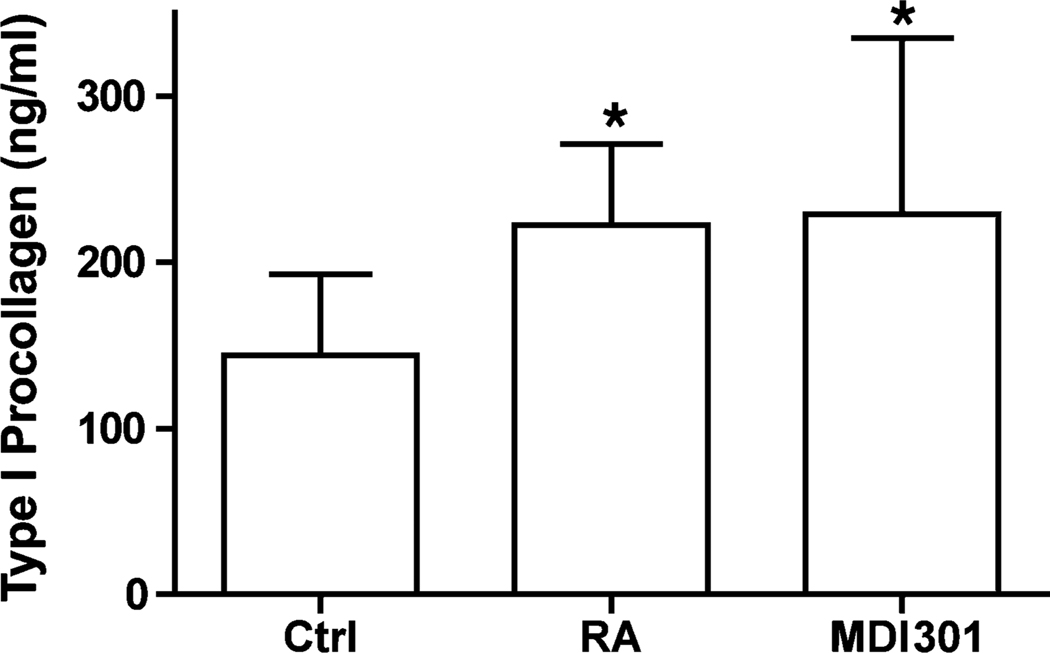
Collagen production in retinoid-treated human skin in organ culture: comparison of RA and MDI 301
Serum-free, growth factor-free culture fluids obtained from organ cultures after 2 days of incubation were examined for type-I procollagen by ELISA. As indicated in Fig. 3, there was a significant increase in levels of type-I procollagen in culture fluid from tissue treated with either retinoid in comparison with levels in control culture fluid. No difference between the two retinoids was observed.
Fig. 3.
Type-1 procollagen production in untreated, RA-treated and MDI 301-treated human skin in organ culture. Organ culture fluid was collected on day-2 and assayed for Type-1 procollagen by ELISA. Values shown are means and standard errors, based on organ cultures from nine individuals. Statistical significance of the difference between untreated and retinoid-treated samples was determined by ANOVA followed by paired-group comparisons. * indicates P < 0.05 relative to control skin
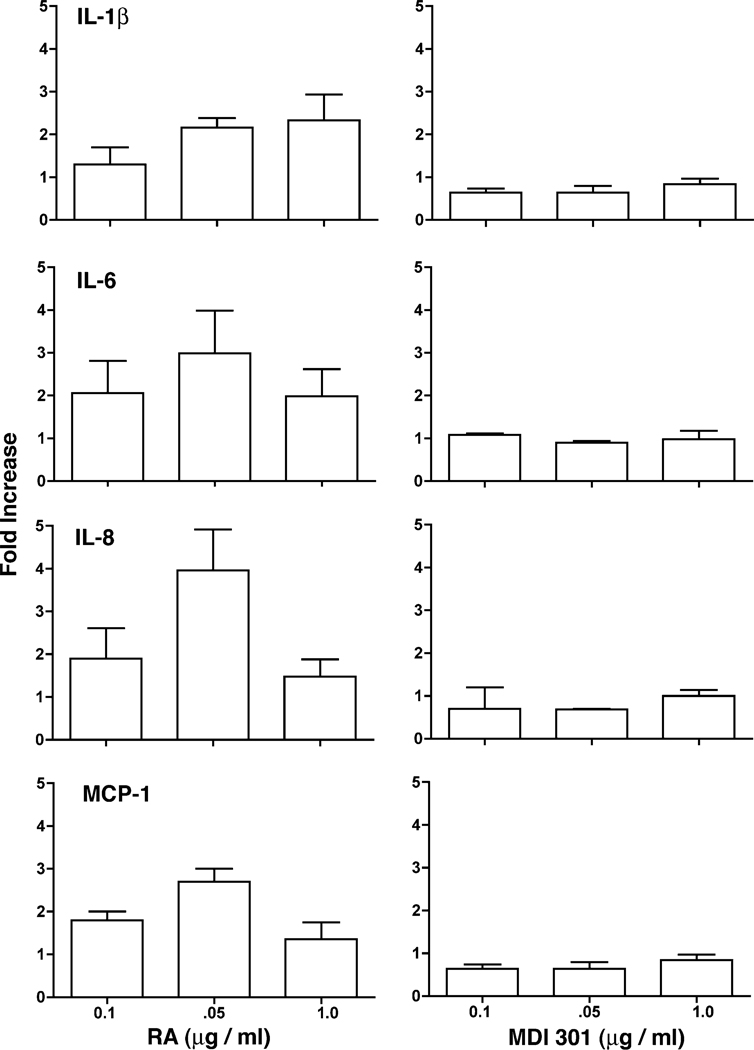
Effects of RA and MDI 301 on cytokine elaboration and leukocyte adhesion molecule expression in organ-cultured skin
Serum-free, growth factor-free culture fluids were obtained from organ cultures after 2 days of incubation and examined for pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and MCP-1) by ELISA. As indicated in Fig. 4, increased levels of all four cytokines were observed in culture fluid from tissue treated with RA in comparison with levels in control culture fluid. Tissue treated with MDI 301 did not show increased expression of these pro-inflammatory cytokines.
Fig. 4.
Cytokine production in retinoid-treated skin. Organ culture fluid was collected on day-2 and assayed for each of the cytokines by ELISA. Values obtained with control culture fluid from each individual was set to 1.0 and the values obtained in retinoid-treated skin normalized to the control value. Values shown are means and standard errors, based on organ cultures from six individuals
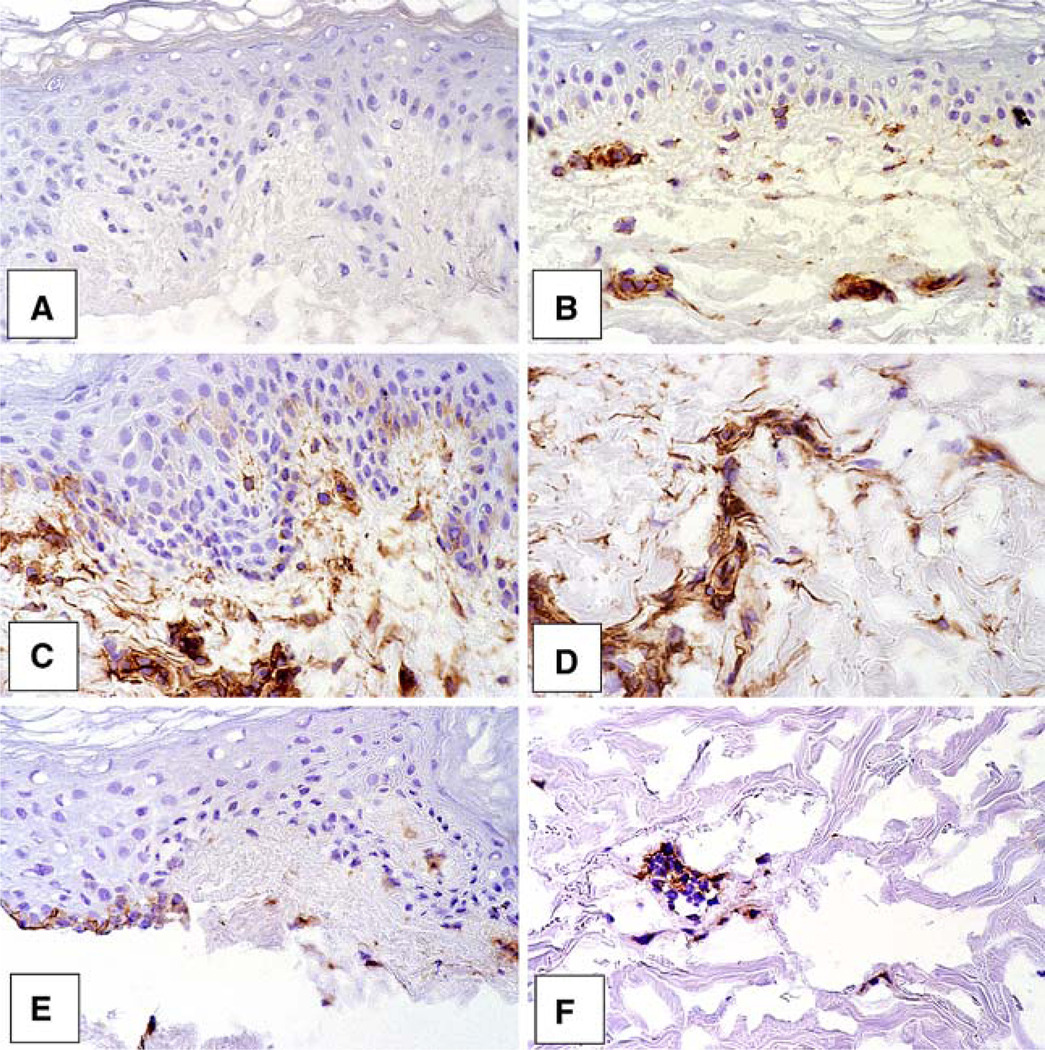
Organ cultures were incubated for 2 days under control conditions or in the presence of either RA or MDI 301 (1 µg/ml). At the end of the incubation period, the tissue was frozen in OCT and stained with antibody to ICAM-1. Staining was stronger in the RA-treated skin than in skin exposed to MDI-301 (Fig. 5). Differences were especially evident in the lower dermis (Fig. 5). When sections from the same tissues were stained with an antibody to another leukocyte adhesion molecule (E-Selectin), similar results were obtained. That is, staining was more intense in RA-treated skin than in the other groups (not shown).
Fig. 5.
Expression of ICAM-1 in control and retinoid-treated skin. Organ cultures were incubated for two days. At the end of the incubation period, tissue was frozen in OCT and stained with anti-ICAM-1 antibody by the immunoperoxidase method. a Non-specific IgG in a control tissue. b ICAM-1 expression in a control tissue. c and d ICAM-1 expression in RA-treated tissue. e and f ICAM-1 staining in MDI 301-treated tissue. Vascular staining is more intense in RA-treated sections than in MDI 301-treated skin sections
Effects of RA and MDI 301 on keratinocyte adhesive function in monolayer culture
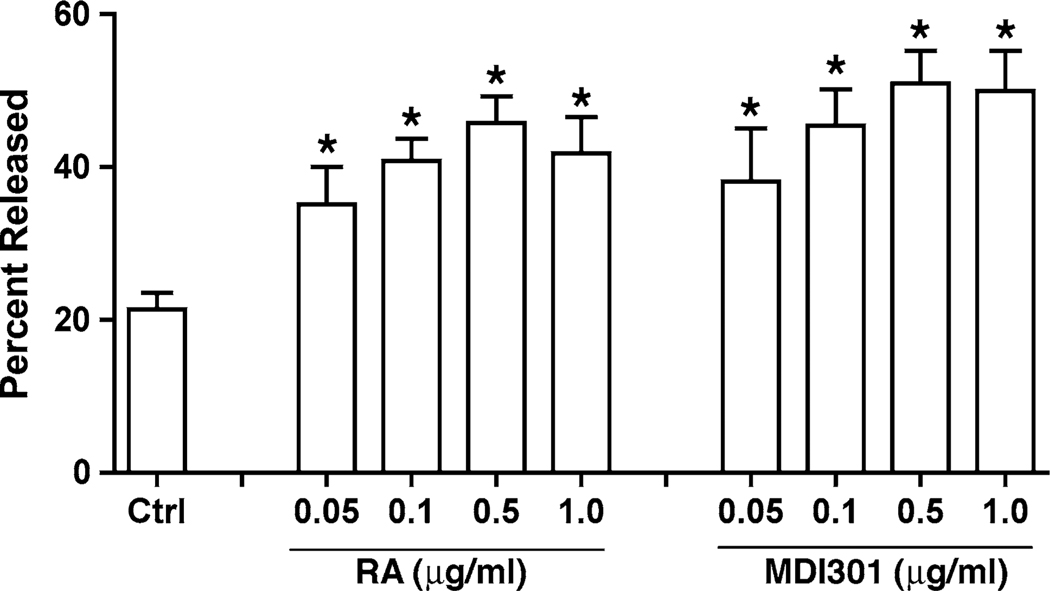
Epidermal keratinocytes were established in monolayer culture and exposed to RA or MDI 301 over a range of concentrations. After 2 days, retinoid-treated keratinocytes were examined for alterations in cell-substrate adhesive function. Both retinoids significantly increased keratinocyte sensitivity to trypsin/EDTA-mediated release from the substratum (Fig. 6). Concentrations that decreased cell–substrate adhesiveness spanned the range of concentrations that have previously been shown to stimulate keratinocyte proliferation (0.01–0.5 µg/ml) as well as concentrations that are growth-inhibitory [28]. The dose–response curves for the two agents were virtually identical.
Fig. 6.
Effects of RA and MDI 301 on keratinocyte adhesive function in monolayer culture. Keratinocyte sensitivity to trypsin/EDTA-mediated release from the substratum was assessed as described in the Materials and Methods section. Values shown represent average percentage of cells released at the 15-min time point + standard errors based on five separate experiments, each with triplicate or quadruplicate samples per data point. Statistical significance of the difference between untreated and retinoid-treated cells was determined by ANOVA followed by paired-group comparisons. *indicates P < 0.05 relative to untreated control keratinocytes
Effects of RA and MDI 301 on fibroblast survival
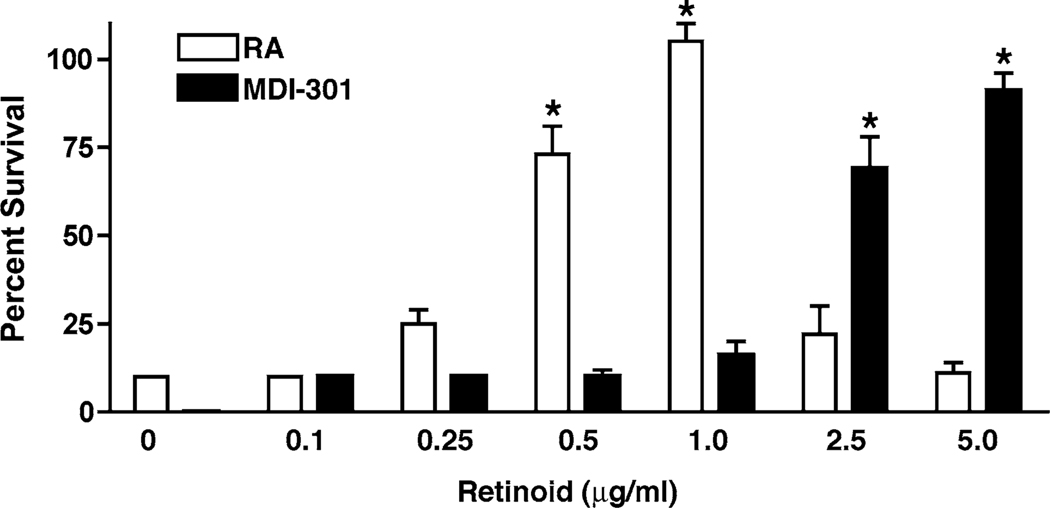
Next, RA and MDI 301 were examined for effects on fibroblast survival under low-ca2+ conditions in monolayer culture. Consistent with past results, human dermal fibroblasts lysed when cultured in low-Ca2+ (0.15 mM), serum-free, growth factor-free culture medium (31). Also consistent with our past findings, RA prevented cell death in a dose-dependent fashion in the low-Ca2+ medium (Fig. 7). MDI-301, likewise, prevented fibroblast death under low-Ca2+ conditions. However, in comparison with RA, effective concentrations were higher (Fig. 7). With RA, the ED50 for prevention of fibroblast death in the serum-free, growth factor-free, low-Ca2+ environment was 0.3 µg/ml while with MDI-301, the corresponding ED50 was 2.0 µg/ml.
Fig. 7.
Effects of RA and MDI 301 on survival of dermal fibroblasts under low-Ca2+ conditions. Fibroblast survival was assessed in KBM (serum-free, growth factor-free, 0.15 mM Ca2+) as described in the Materials and Methods section. Values shown represent average percentage of cells released at each time point + standard errors based on five separate experiments, each with duplicate samples per data point. Statistical significance of the difference between untreated and retinoid-treated cells was determined by anova followed by paired-group comparisons. *indicates P < 0.05 relative to untreated control fibroblasts
Discussion
RA and the other biologically active derivatives of vitamin A (all-trans retinol) have hormone-like activities in the skin. Where the skin has become hyperplastic (for example, in psoriasis and in certain malignancies), retinoids effectively suppress proliferation [6, 9]. On the other hand, where skin cell proliferation has slowed (for example, in aged skin), retinoid stimulate growth and result in epidermal thickening [32]. Retinoid stimulatory effects also extend to the dermis, where suppression of the major collagen-degrading enzymes [7, 8] and stimulation of procollagen synthesis [13, 18, 19] improve appearance [16, 17, 33] and improve the healing of wounds that subsequently occur [2, 20, 25, 34]. In spite of the beneficial effects, the capacity of RA to irritate the skin makes it less than desirable for many users. At one time it was believed that retinoid efficacy and retinoid irritation could not be separated. This may not be entirely correct. Others have shown that vitamin A mediates events similar to RA but is less irritating in human skin [15]. It has also been shown that by carefully controlling the dosage, irritation can be mitigated under conditions in which beneficial activities remain [12]. In a past study we showed that a synthetic, ester-substituted retinoid (i.e., MDI 301) was similar to RA in its ability to induce epidermal hyperplasia in hairless mice and to induce a thickening of the collagen band immediately beneath the epidermis [28]. These changes in skin structure were seen in spite of the fact that unlike RA, there was essentially no irritation in the skin of the MDI 301-treated animals. Although hairless rodents are commonly used as a model for retinoid responses in skin, rodent skin is significantly different from human skin, and it is important to establish beneficial effects in human skin.
Here we show that human skin in organ culture responds to MDI 301 with similar responses as observed with RA. The two retinoids induced a comparable degree of hyperplasia in the epidermis. At supra-optimal concentrations, both retinoids induced abnormalities in the upper epidermis (hyperkeratosis, acantholysis and partial separation of the differentiating cells from the basal cells). Along with the reduced epidermal cohesiveness observed in whole skin, treatment of keratinocytes with either RA or MDI 301 in monolayer culture resulted in decreased cell-substrate adhesion. The two retinoids were also comparable in stimulating type-I procollagen synthesis and inhibiting MMP function. MMP reduction was accompanied by increased production of TIMP-1 with both agents. Based on these data, we suggest that MDI 301 will ultimately prove to be an effective skin-repair agent.
In addition to examining events related to efficacy, we also compared the two retinoids for effects on cellular responses that may contribute to irritation. MDI-301 was clearly less active than RA in inducing production of pro-inflammatory cytokines and in up-regulating leukocyte adhesion molecules (events closely tied to skin inflammation). What accounts for these differences, however, is unknown. Retinoid irritation is a complex and not completely understood problem. It has been suggested that irritation is, in some manner, connected to epidermal hyperplasia. Rapidly proliferating keratinocytes elaborate large amounts of pro-inflammatory cytokines [22, 23]. In addition to inducing hyperplasia, retinoid treatment also alters the pattern of differentiation in the epidermis and reduces barrier function [5, 11, 14]. The loss of barrier function rather than hyper-proliferation per se may be directly responsible for promoting pro-inflammatory events [35]. Our data do not contradict these suggestions. On the other hand, our findings strongly imply that hyperplasia and irritation are not inextricably linked. Even changes in epidermal barrier function (to the extent that the observations made here—altered differentiation, separation of upper epidermal layers from the basal layer and decreased adhesiveness in vitro—reflect alterations in the epidermal barrier) appear not to be directly responsible for irritation. In all these regards, the effects of RA and MDI 301 were comparable. Yet RA stimulated the production of pro-inflammatory cytokines under conditions in which MDI-301 did not.
Although there was no observable difference in the epidermal response to the two retinoids (either in organ culture or with keratinocytes in monolayer culture) that might suggest a basis for the difference in irritancy, the two retinoids were significantly different in their capacity to prevent fibroblast lysis under low-Ca2+ conditions (ED50 = 0.3 µg/ml with RA as compared with 2.0 µg/ml with MDI 301). Whether this difference is related, in any way, to the difference in irritancy is not known. Of interest in this regard, it has been demonstrated previously that certain detergents such as sodium lauryl sulfate also preserve fibroblast viability under low-Ca2+ conditions [27]. Since retinoid has a detergent-like structure (polar head group connected to a hydrophobic, fatty acid tail) and have been shown to function in a detergent-like manner [24], perhaps irritation is a function of the detergent property. If this turns out to be the case, then it may be that MDI 301, with its ester substitution for the free acid group, is inherently less detergent-like than RA.
Another possibility is that the lower capacity of the ester-substituted retinoid to induce irritation reflects altered penetrability. In a past study comparing RA and MDI-301 for anti-cancer activity, it was noted that both retinoids induced epidermal hyperplasia in nude mice following intraperitoneal injection [1]. While there was no significant skin irritation in either group of mice, mice injected with either RA or MDI 301 developed a comparable degree of splenomegaly with enlarged germinal centers (indicative of a systemic inflammatory response).
Regardless of the underlying mechanism, it is clear from our recent study that MDI 301 is inherently less irritating that RA when used topically in hairless rodents [28]. The findings presented here demonstrate that MDI-301 is comparable to RA in its ability to induce events that underlie repair of damaged skin in human skin, but unlike RA, does not induce elaboration of pro-inflammatory cytokines or up-regulate expression of leukocyte adhesion molecules in the skin. Our studies, therefore, (1) suggest that repair efficacy and irritation are not inextricably linked; (2) provide insight into possible mechanisms of irritation; and (3) provide a rationale for further development of MDI 301 as a skin-repair agent.
Acknowledgment
This study was supported in part by grants AR49621 and GM77724 from the USPHS.
References
- 1.Appelyard VCL, O’Neill MAO, Murray KE, Bray SE, Varani J, Zhang J, Thompson AM. Activity of MDI-301, a novel, synthetic retinoid, in xenografts. Anticancer Res. 2004;15:991–996. doi: 10.1097/00001813-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft G, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerentology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 3.Basak PY, Eroglu E, Altuntas I, Agalar F, Basak K, Sutcu R. Comparison of the effects of tretinoin, adapalene and collagenase in an experimental model of wound healing. Eur J Dermatol. 2002;12(2):145–148. [PubMed] [Google Scholar]
- 4.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias PM, Fritsch P, Lampe M, Williams M, Brown B, Nemanic MK, Grayson S. Retinoid effects on epidermal structure, differentiation and permeability. Lab Invest. 1981;44:531–540. [PubMed] [Google Scholar]
- 6.Esgleyes-Ribot T, Chandraratna RA, Lew-Kaya DA, Sefton J, Duvic M. Response of psoriasis to a new topical retinoid, AGN 190168. J Am Acad Dermatol. 1987;30:581–590. doi: 10.1016/s0190-9622(94)70066-4. [DOI] [PubMed] [Google Scholar]
- 7.Fisher GJ, Datta SC, Talwar HS, Wang Z-Q, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature (London) 1996;379:335–338. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med. 1977;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald P, Teng M, Chandraratna RAS, Heyman RA, Allegretto EA. Retinoic acid receptor a expression correlates with retinoid-induced growth inhibition of human breast cancer cells regardless of extrogen receptor status. Cancer Res. 1997;57:2642–2650. [PubMed] [Google Scholar]
- 10.Fligiel SEG, Varani J, Datta SH, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photoaged skin in vivo and after exposure to MMP-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 11.Floyd EE, Jetten AM. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: Down-regulation by retinoids. Mol Cell Biol. 1989;9:4846–4851. doi: 10.1128/mcb.9.11.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths CE, Kang S, Ellis CN, Kim KJ, Finkel LJ, Ortiz-Ferrer LC, White GM, Hamilton TA, Voorhees JJ. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin cream. Arch Dermatol. 1995;131:1037–1044. [PubMed] [Google Scholar]
- 13.Griffiths CEM, Russman G, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–534. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 14.Jetten AM, George MA, Pettit GR, Herald CL, Rearick JL. Action of phorbol esters, bryostatins and retinoic acid on cholesterol sulfate synthesis: relation to the multistep process of differentiation in human epidermal keratinocytes. J Invest Dermatol. 1989;93:108–115. doi: 10.1111/1523-1747.ep12277374. [DOI] [PubMed] [Google Scholar]
- 15.Kang S, Duell EA, Fisher GJ, Datta SC, Wang Z-Q, Reddy AP, Tavakkol A, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid-binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:549–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- 16.Kligman AM, Dogadkina D, Lavker RM. Effects of topical tretinoin on non-sun-exposed protected skin of the elderly. J Am Acad Dermatol. 1993;29:25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- 17.Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15:836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- 18.Kligman LH. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986;15:779–785. doi: 10.1016/s0190-9622(86)70234-x. 884–777. [DOI] [PubMed] [Google Scholar]
- 19.Kligman LH, Duo CH, Kligman AM. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12:139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- 20.Lateef H, Abatan OI, Aslam MN, Stevens MJ, Varani J. Pretreatment of diabetic rats with all-trans retinoic acid increases healing of subsequently-induced abrasion wounds. Diabetes. 2005;54:855–861. doi: 10.2337/diabetes.54.3.855. [DOI] [PubMed] [Google Scholar]
- 21.Lateef H, Stevens M, Varani J. All-trans retinoic acid suppresses matrix metalloproteinase production/activation and increases collagen synthesis in diabetic skin in organ culture. Am J Pathol. 2004;165:167. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maas-Szabowski N, Shimotooyodome A, Fusenig NE. Keratintocyte growth regulation in fibroblast cocultures via double paracrine mechanism. J Cell Sci. 1999;112:1843–1853. doi: 10.1242/jcs.112.12.1843. [DOI] [PubMed] [Google Scholar]
- 23.Maas-Szabowski N, Stark H-J, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in fibroblasts. J Invest Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 24.Meeks RG, Zaharevitz D, Chen RF. Membrane effects of retinoids: possible correlation with toxicity. Arch Biochem Biophys. 1981;207:141–147. doi: 10.1016/0003-9861(81)90019-9. [DOI] [PubMed] [Google Scholar]
- 25.Popp C, Kligman AM, Stoudemayer TJ. Pretreatment of photoaged forearm skin with topical tretinoin accelerates healing of full-thickness wounds. Brit J Dermatol. 1995;132:46–53. doi: 10.1111/j.1365-2133.1995.tb08623.x. [DOI] [PubMed] [Google Scholar]
- 26.Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 27.Varani J, Astrom A, Griffiths CEM, Voorhees JJ. Stimulation of fibroblast and keratinocyte proliferation by sodium lauryl sulfate. J Invest Dermatol. 1991;97:917–921. doi: 10.1111/1523-1747.ep12491682. [DOI] [PubMed] [Google Scholar]
- 28.Varani J, Fligiel H, Zhang J, Aslam MN, Lu Y, Dehne LA, Keller ET. Separation of retinoid-induced epidermal and dermal thickening from skin irritation. Arch Dermatol Res. 2003;295:255–262. doi: 10.1007/s00403-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varani J, Gibbs DF, Inman DR, Shah B, Fligiel SEG, Voorhees JJ. Inhibition of epithelial cell adhesion by retinoic acid: relationship to reduced extracellular matrix production and alterations in Ca2+ levels. Am J Pathol. 1991;138:887–895. [PMC free article] [PubMed] [Google Scholar]
- 30.Varani J, Perone P, Griffiths C, Inman D, Fligiel S, Voorhees J. All-trans retinoic acid stimulates events in organ cultured human skin that underlie repair. J Clin Invest. 1994;94:1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varani J, Shayevitz J, Perry D, Mitra RS, Nickoloff BJ, Voorhees JJ. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990;136:1275–1281. [PMC free article] [PubMed] [Google Scholar]
- 32.Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung J, Wang ZQ, Datta SH, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JS, Ellis CN, Voorhees JJ. Topical tretinoin improves photoaged skin: a double blind, vehicle-controlled study. JAMA. 1988;259:527–532. [PubMed] [Google Scholar]
- 34.Wicke C, Halliday B, Allen D, Roche NS, Scheuenstuhl H, Spencer MM, Roberts AB, Hunt TK. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135:1265–1270. doi: 10.1001/archsurg.135.11.1265. [DOI] [PubMed] [Google Scholar]
- 35.Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1a expression and release froma pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- 36.Yuen DE, Stratford AF. Vitamin A activation of transforming growth factor-beta1 enhances porcine ileum wound healing in vitro. Ped Res. 2004;55:935–939. doi: 10.1203/01.pdr.0000127023.22960.85. [DOI] [PubMed] [Google Scholar]