Abstract
Rationale
Increased signal-detection accuracy on the 5-choice serial reaction time (5-CSRT) task has been shown with drugs that are useful clinically in treating attention deficit hyperactivity disorder (ADHD), but these increases are often small and/or unreliable. By reducing the reinforcer frequency, it may be possible to increase the sensitivity of this task to pharmacologically-induced improvements in accuracy.
Methods
Rats were trained to respond on the 5-CSRT task on a fixed ratio (FR) 1, FR 3, or FR 10 schedule of reinforcement. Drugs that were and were not expected to enhance performance were then administered before experimental sessions.
Results
Significant increases in accuracy of signal detection were not typically obtained under the FR 1 schedule with any drug. However, d-amphetamine, methylphenidate, and nicotine typically increased accuracy under the FR 3 and FR 10 schedules.
Conclusions
Increasing the FR requirement in the 5-CSRT task increases the likelihood of a positive result with clinically effective drugs, and may more closely resemble conditions in children with attention deficits.
Keywords: 5-choice serial reaction time task, sustained attention, attention deficit hyperactivity disorder, fixed ratio, psychostimulants, rats
A number of animal models of attention deficit/hyperactivity disorder (ADHD) have been proposed with varying degrees of success in predicting clinical outcome (for recent reviews, see Russell 2007; Sagvolden et al. 2005; van der Kooij & Glennon 2007). One animal model of attention which has been used extensively is the 5-CSRT task (see Robbins 2002). In this task the subject (typically a rodent) is faced with an array of holes which can be briefly illuminated from behind. The response of poking its nose in the hole most recently illuminated is reinforced. Drugs that are used clinically to treat ADHD have been shown to increase the percentage of correct responses (nose poking in the hole that was most recently illuminated), and often to decrease response latency and the number of illuminations that were not followed by a response (omissions). As with many cognitive tasks in animals, however, pharmacologically-induced increases in performance are typically small in magnitude and often not reliably obtained at a particular dose. For example, d-amphetamine has been shown to significantly increase signal-detection accuracy on this task (Bizarro et al. 2004; Grottick & Higgins, 2002), but other reports show small increases that are not statistically significant (Bizarro & Stolerman 2003; Cole & Robbins 1987). Methylphenidate has also been reported to increase accuracy on this task (Bizarro et al. 2004; Navarra et al. 2007; Paine et al. 2006), with one report only showing an effect in low-performing rats (Puumala et al. 1996). Atomoxetine has been shown to both increase the percentage of correct responses (Navarra et al. 2007), or have no effect (Robinson et al. 2007). Nicotine, which is not used clinically to treat ADHD, also increases accuracy on this task, although these increases are sometimes only seen under specific experimental conditions (Bizarro et al. 2004; Bizarro & Stolerman 2003; Grottick & Higgins, 2002; Hahn & Stolerman 1998; Mizra & Stolerman 1998; Young et al. 2004).
Previous attempts to increase the sensitivity of the 5-CSRT task to pharmacologically-induced increases in accuracy usually involved some manipulation to decrease baseline levels of performance accuracy, thereby making larger increases possible. Prefeeding rats before experimental sessions did not significantly affect accuracy, and changed the effective dose of nicotine without changing the magnitude of that effect (Bizarro & Stolerman 2003; Grottick & Higgins, 2002). Increasing the level of food deprivation also did not significantly affect accuracy or the effects of nicotine or amphetamine on accuracy (Bizarro & Stolerman 2003). Alterations to the duration of the inter-trial interval have reliably resulted in decreased baseline accuracy (Bizarro et al. 2004; Mizra & Stolerman 1998; Navarra et al. 2007). However, these decreases in baseline accuracy were not associated with further increases in accuracy produced by d-amphetamine, nicotine, methylphenidate, or atomoxetine (Bizzaro et al. 2004; Navarra et al. 2007). Mizra and Stolerman (1998) also tested the effects of nicotine with very short brief stimulus presentations (0.25 s), but found no increases in accuracy after nicotine administration.
All of these previously studied manipulations arrange a reinforcer to be delivered contingent upon each correct response recorded during the response period in the illuminated nose-poke hole, a fixed-ratio one (FR 1) response schedule. These conditions typically support high levels of baseline accuracy. However, it has been argued that children with ADHD primarily exhibit sustained attention deficits in environments in which infrequent or intermittent reinforcement is arranged (Aase & Sagvolden 2006; Douglas 1985; Douglas and Parry 1994; van der Meere 1996). In the present study, the sensitivity of the 5-CSRT task to behaviorally active drugs was examined under conditions in which the response requirement was altered. In three separate groups of rats, performance was assessed with the schedule of required accurate responses set to an FR 1, FR 3, or FR 10. It was hypothesized that increases in response requirement would be associated with decreases in baseline performance and would more closely resemble conditions where ADHD symptoms are observed clinically. The effects of drugs used clinically to treat ADHD (d-amphetamine, methylphenidate, and atomoxetine) were compared to nicotine and several other compounds that are not used clinically.
Methods
Subjects
Twenty-three male Sprague Dawley rats (Taconic Farms, Hudson, NY) served as subjects. Diet was controlled to maintain the subjects at approximately 85% of their adult free-feeding weights, which resulted in weights ranging from 325 to 360 g. When not in session, subjects were individually housed with fresh water continuously available. Husbandry and other care were in accordance with NIDA institutional animal care and use guidelines and the Guide for the Care and Use of Laboratory Animals (1996).
Apparatus
Sessions were conducted in rodent operant-conditioning chambers designed for the 5-choice serial reaction time task (MED-NP5L package; Med-Associates Inc., St. Albans, VT, USA). On one wall of the chamber were five holes capable of being illuminated from behind. The opposite wall held a food tray into which 45-mg food pellets could be delivered. The chambers were contained within light-proof, ventilated enclosures that provided sound attenuation. White noise was delivered to the chamber at all times to mask extraneous noise.
Procedure
Sessions were conducted during the light cycle (7:00 AM to 7:00 PM) with sessions for each subject conducted at approximately the same time each day. All subjects were initially trained to poke their noses into a hole under an FR 1 schedule of reinforcement. An 8-s stimulus light was randomly illuminated behind one of the five holes of the operant conditioning chamber, and responses to the lit hole (correct responses) produced a 45-mg food pellet to a hopper on the opposite wall, followed by a 5-s intertrial interval (ITI). Responses to any other hole (incorrect responses) were followed by the ITI only, and responses during the ITI had no scheduled consequence. After the ITI a randomly selected hole was illuminated, which marked the beginning of a new trial. This 8-s stimulus duration was gradually decreased to 1 s over successive sessions, but the response period remained 5.5 s from the onset of the stimulus (i.e., responses were still correct for a period after the stimulus was dimmed). Any response during the response period immediately ended the trial, so only one response per trial was possible. Sessions ended after 50 food presentations or 40 minutes (FR 3 and FR 10 groups) or 15 minutes (FR 1 group), whichever occurred first. This training procedure was in place for a median duration of 30 session (interquartile range = 24 to 38) before testing (FR 1 group) or FR escalation (FR 3 and FR 10 groups; see below).
After responding stabilized with a stimulus duration of 1 s, subjects were split into groups. Seven subjects continued on the schedule described above and comprised the FR 1 group. For the remaining 16 subjects, the FR (number of correct responses required to produce a food pellet) was gradually increased with a target of FR 10. When the FR was greater than one, correct responses that did not fulfill the response requirement and incorrect responses both led to a 5-s ITI. Only correct responses counted toward the FR requirement and incorrect responses did not reset the response requirement. Correct responses that fulfilled the response requirement were reinforced as described above. The FR was gradually increased until either an FR 10 was reached or responding was not maintained at or above approximately 50% correct responses. Ten subjects reached an FR 10, and these subjects comprised the FR 10 group. Of the remaining six subjects, 5 reached an FR ≥ 3 before performance deteriorated, and these rats were placed on an FR 3 schedule and comprised the FR 3 group. One subject did not respond at any response requirement greater than FR 1, and was subsequently studied under a variety of conditions that were not successful in developing responding under intermittent reinforcement. Data for this subject are not presented in this report.
Once responding for any individual subject was deemed stable (at least 50% accuracy with no apparent increasing or decreasing trend for at least five sessions), drug testing began. Injections were administered intraperitoneally immediately before subjects were placed in the operant chamber, and a five-minute blackout period elapsed before the session start. Sessions were conducted five days per week, with drug tests occurring the second and fifth days. The first and fourth days of each week were control sessions, with vehicle administrations on the fourth day and no injections administered on the first and third days. Not all subjects received all drug treatments. The order that drugs were allocated to individual subjects is detailed in Online Resource 1 and resulted in five subjects per drug condition per FR group. The median duration of the drug testing period was 237.5 days (interquartile range = 175 to 311 days).
Drugs
Methylphenidate HCl, d-amphetamine SO4, atomoxetine (R-tomoxetine) HCl, morphine SO4, nicotine hydrogen tartate, pentobarbital Na, desipramine HCl, nortriptyline, HCl, clonidine HCl, and selegiline (R(−) deprenyl) HCl were obtained from Sigma Aldrich (St. Louis, MO, USA). All drugs were dissolved in sterile water and doses in mg refer to the salt forms.
Data Analysis
Four dependent variables were of interest in the current study. First, percent correct was defined as the percent of trials in which a response was emitted to the correct hole within the response period (correct responses divided by the sum of correct and incorrect responses). Latency was defined as the time to any response during the response period after a brief stimulus presentation was initiated. Trials in which responses were not emitted were not included in latency calculations. Omissions were defined as the proportion of trials in which no response was emitted during the response period, expressed as a percent of total trials. ITI responses were defined as those responses that were emitted in any hole during the ITI immediately preceding a stimulus presentation.
The dependent measures from control sessions were compared among groups using a two-way ANOVA with Bonferroni-corrected post-hoc tests (FR value and the drug for which the sessions served as control were the two factors), while FR 1 performance was compared with a one-way ANOVA. Dependent measures were compared after drug pretreatments using a one-way repeated-measures ANOVA with Bonferroni-corrected post-hoc tests comparing each drug dose to the control value. Percent correct and response latency data were calculated for any session if the number of trials omitted by the subject was less than 90% (Online Resource 2 shows the number of trials with suppressed responding for each drug dose). At least 25 responses were emitted in all sessions that remained after this exclusion criterion was applied. Data for any dose of any drug were included in statistical analyses and graphical presentations if at least two subjects omitted less than 90% of trials. ITI response data and percent omissions were included for all subjects at all doses. Statistical tests were conducted using Systat SigmaStat 3.5 (San Jose, CA, USA). In the case of missing data with repeated measures ANOVAs, SigmaStat conducts a mixed-models ANOVA which allows for the inclusion of all available data while still accounting for between-subjects variance in the model. In four instances, percent correct data were not normally distributed and a repeated-measures ANOVA on ranks (Friedman test) was conducted. Both the parametric and nonparametric statistics in each of these cases produced the same result (not statistically significant).
Results
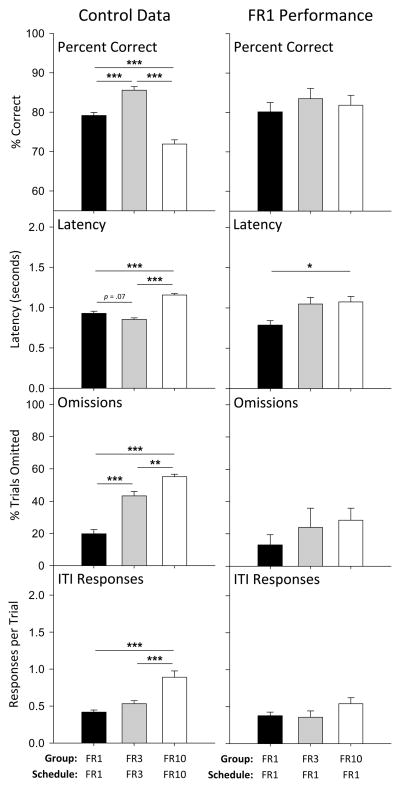
Control performances differed among groups maintained on the FR 1, FR 3, or FR 10 schedules (Figure 1, left column). In particular, accuracy (the percentage of correct responses) significantly differed among groups when maintained at the final FR schedule value [F(2, 120) = 48.4, p < 0.001]. Compared to the FR 1 group (mean [M] = 79.2%), accuracy was higher in the FR 3 group (M = 85.6%) and lower in the FR 10 group (M = 71.9%), and each group differed significantly from the other two (all p < 0.001). Though reduced, accuracy under the FR 10 schedule was much higher than the 20% chance level. Latency to respond also differed among the groups after FR escalation [F(2, 120) = 48.9, p < 0.001], with latency marginally lower in the FR 3 group (M = 0.85 s, p = 0.073) compared to the FR 1 group (M = 0.93 s), and highest in the FR 10 group (M = 1.2 s, p < 0.001 vs. FR 1 and FR 3). Percent trials omitted increased with response requirement [F(2, 120) = 47.0, p < 0.001]; the FR 10 group (M = 55.1%) omitted more trials than the FR 3 (M = 43.3%, p = 0.006) and FR 1 (M = 20.0%, p < 0.001) groups, and the FR 3 group omitted more trials than the FR 1 group (p < 0.001). The frequency of ITI responses increased with FR value [F(2, 120) = 17.3, p < 0.001], with the FR 10 group (M = 0.89) emitting significantly more responses per trial than the FR 3 (M = 0.53, p < 0.001) or FR 1 (M = 0.42, p < 0.001) groups. For each of these measures, there was no main effect of drug for which the control session preceded, indicating that no baseline shift was apparent.
Figure 1.
Performance in the absence of any drug treatments in the FR 1 (black bars), FR 3 (grey bars), and FR 10 (white bars) groups. Control data in the left panels are the mean of the sessions immediately prior to drug test sessions, the same data that comprise the control data for statistical analyses of drug effects. Data in the right panels represent the performance of each group responding on an FR 1 schedule of reinforcement, taken from the mean of the last five sessions of responding on an FR 1 schedule either before drug testing was initiated (FR 1 group) or before FR escalation (FR 3 and FR 10 groups). Baseline percent correct, latency, omissions, and ITI responses differed among the three FR groups, and asterisks depict significant Bonferroni-corrected post-hoc tests between specific group pairings (* p < 0.05, ** p < .01, *** p < 0.001).
Differences among groups were typically absent in stable performances under the FR 1 schedule before the changes in schedule requirement (Figure 1, right column). Percent correct in the three groups when maintained under the FR 1 schedule did not differ by group [F(2, 19) = 0.3, p = 0.719], however differences in latency were evident [F(2, 19) = 5.0, p = 0.017], with the FR 1 group (M = 0.79 s) exhibiting a significantly lower latency than did the group that was eventually maintained under the FR 10 schedule (M = 1.07 s, p = .021). Neither the percentage of trials omitted [F(2, 19) = 1.0, p = 0.390] nor ITI responses per trial differed among groups before FR escalation [F(2, 19) = 1.8, p = 0.194].
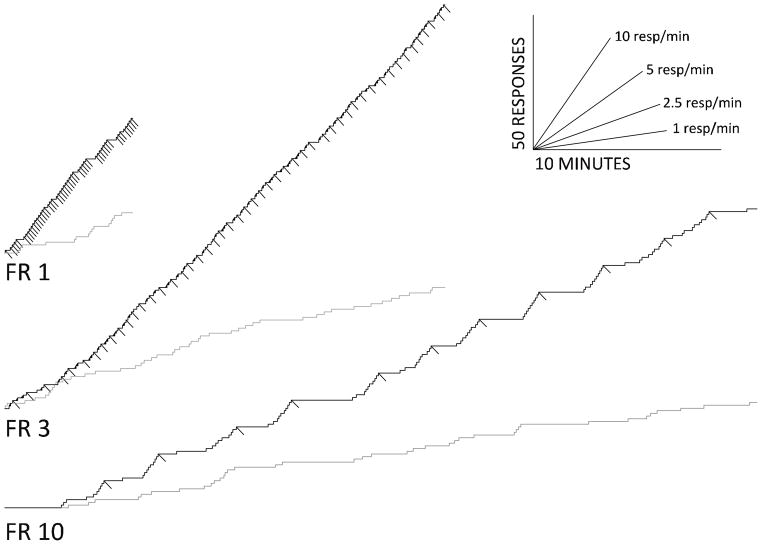
Within session response patterning differed considerably under the three FR schedules (Figure 2). Responding under the FR 1 schedule was characterized by relatively few omitted trials with responding at a consistent rate throughout the session. The infrequent pauses in correct responses were often though not universally correlated with periods of incorrect responding (gray line), that in this example increased toward the end of the session. Responding on the FR 3 schedule was characterized by more omitted trials, which were more likely to occur immediately after a pellet delivery. Therefore the pattern of correct responses often approached a typical pattern of “break and run” seen with less complex responding under FR schedules. Overall accuracy was significantly higher in this group, though there were consistent incorrect responses throughout the session (gray line). These incorrect responses often occurred after a short pause, and then decreased before the correct responses fulfilled the FR requirement. Subjects responding under the FR 10 schedule omitted the largest percentage of trials, which were more likely to occur immediately after a pellet delivery. The pattern of correct responses often approached a “break and run” pattern; however there were also instances in which the acceleration of correct responses between reinforcers was more gradual. As under the FR 3 schedule, incorrect responding under the FR 10 schedule was often characterized by a pause followed by a transition to a higher rate, which subsequently decreased before reinforcement.
Figure 2.
Cumulative records from three representative sessions on the FR 1, FR 3, FR 10 schedules with no drug pretreatment. The black lines depict responses to the illuminated nose-poke hole during the response period and downward deflections of these lines mark pellet deliveries. The gray lines mark incorrect responses during the response period. The lines in the lower-right portion of the figure indicate scaling of time and responses, respectively.
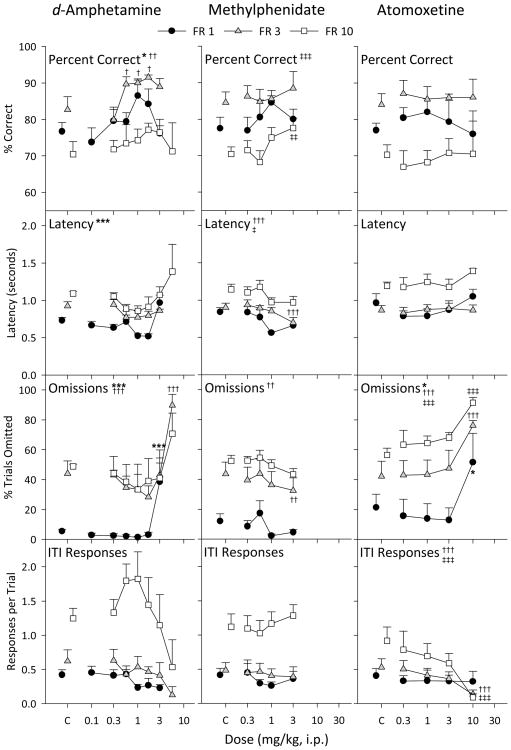
Dose of d-amphetamine significantly altered accuracy in the FR 1 [F(6, 24) = 3.10, p = 0.022] and FR 3 [F(5, 16) = 5.89, p = 0.003] groups, but not in the FR 10 group (Figure 3, left column). Accuracy was significantly increased after 0.56, 1.0, and 1.7 mg/kg d-amphetamine in the FR 3 group (all p values < 0.05), but no individual dose reached statistical significance compared to control in the FR 1 or FR 10 groups. Latency to respond was altered in the FR 1 [F(5, 16) = 6.58, p < 0.001], but not FR 3 or FR 10 groups. The percentage of trials omitted was also altered by d-amphetamine in the FR 1 [F(6, 24) = 7.94, p < 0.001] and FR 3 [F(6, 24) = 9.18, p < 0.001] groups, with the highest doses tested in each group significantly increasing omissions (3.0 mg/kg in the FR 1 group and 5.6 mg/kg in the FR 3 group, both p < 0.001). The frequency of ITI responses was not significantly altered by any of the doses tested under any of the FR values examined.
Figure 3.
Effects of drug pretreatments in the FR 1 (black circles), FR 3 (grey triangles), and FR 10 (white squares) groups on percent correct, latency, omissions, and ITI responses. The effects of d-amphetamine, methylphenidate, and atomoxetine pretreatments are displayed in the left, center, and right columns, respectively. Control points above C represent performance in sessions immediately preceding each of the drug sessions for that group, consisting of both sessions in which saline was administered or no injection was given. Significant main effects of dose for each group are represented by asterisks (FR 1), crosses (FR 3), or double crosses (FR 10) next to the panel title. Symbols next to a specific point indicate significant Bonferroni-corrected post-hoc tests for that dose as compared to the control point (one symbol, p < 0.05, two symbols, p < .01, three symbols, p < 0.001).
Methylphenidate altered accuracy in the FR 10 group [F(4, 16) = 8.13, p < 0.001], with a significant increase (p = 0.005) at 3.0 mg/kg (Figure 3, center column). Latency was generally decreased with increasing dose, and this effect was significant in the FR 3 [F(4, 16) = 13.3, p < 0.001] and FR 10 [F(4, 16) = 3.52, p = 0.030] groups, with 3.0 mg/kg significantly decreasing latency in the FR 3 group (p < 0.001). Omissions also tended to decrease with increasing dose of methylphenidate. This trend was significant in the FR 3 group [F(4, 16) = 5.86, p = 0.004], specifically at 3.0 mg/kg (p = 0.005). ITI responses per trial were not altered by methylphenidate in any group.
Neither accuracy nor latency was altered with atomoxetine up to 10.0 mg/kg in any of the groups (Figure 3, right column). At the 10.0 mg/kg dose omissions were increased in each group (all p values < 0.05) [FR 1, F(4, 16) = 4.68, p = 0.011; FR 3, F(4, 16) = 12.3, p < 0.001; FR 10, F(4, 16) = 9.22, p < 0.001], and ITI responses were decreased in the FR 3 group [F(4, 16) = 13.3, p < 0.001] and FR 10 [F(4, 16) = 13.3, p < 0.001] groups.
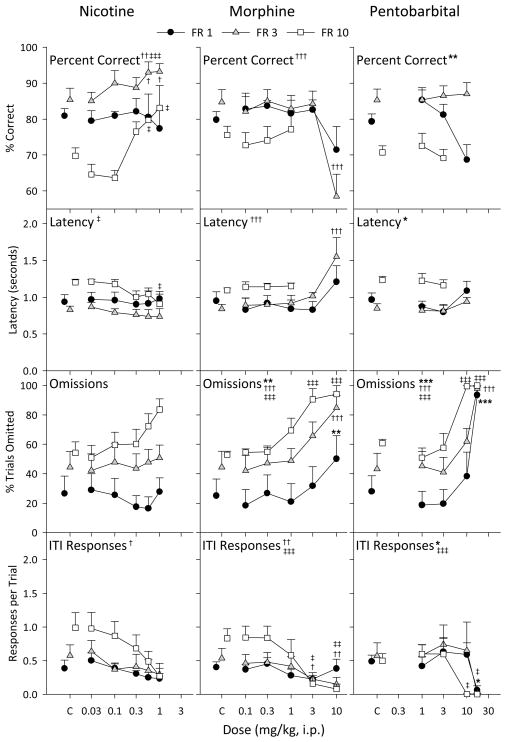
Nicotine significantly increased accuracy in the FR 3 [F(5, 20) = 4.81, p = 0.005] and FR 10 [F(5, 16) = 10.7, p < 0.001] groups at 0.56 and 1.0 mg/kg (all p values < 0.05), but had no effect in the FR 1 group (Figure 4, left column). Latency was only decreased at 1.0 mg/kg (p = 0.018) in the FR 10 group [F(5, 16) = 3.95, p = 0.016]. Omissions were not significantly altered in any FR group, and while ITI responses tended to decrease with increasing nicotine dose, this effect was only significant in the FR 3 group [F(5, 20) = 3.12, p = 0.030].
Figure 4.
Effects of nicotine, morphine, and pentobarbital pretreatments in the left, center, and right columns, respectively. All other details as in Figure 3.
Morphine only affected the measures of performance at the highest doses that disrupted behavior in general (Figure 4, center column). Accuracy [F(5, 16) = 7.59, p < 0.001] and latency [F(5, 16) = 9.65, p < 0.001] was altered in at 10.0 mg/kg (both p < .001) in the FR 3 group. Morphine dose-dependently increased omissions in each group [FR 1, F(5, 20) = 5.20, p = 0.003; FR 3, F(5, 20) = 8.63, p < 0.001; FR 10, F(5, 20) = 10.4, p < 0.001] and ITI responses were dose-dependently decreased with morphine in the FR 3 [F(5, 20) = 4.49, p = 0.007] and FR 10 groups [F(5, 20) = 6.74, p < 0.001].
Pentobarbital, like morphine, had little effect on any measure up to doses that disrupted behavior in general (Figure 4, right column). This was true for percent correct in the FR 1 group [F(3, 11) = 6.73, p = 0.008] and latency in the FR 3 group [F(3, 11) = 5.90, p = 0.012]. Omissions were dramatically and dose-dependently increased in all groups [FR 1, F(4, 15) = 12.9, p < 0.001; FR 3, F(4, 16) = 15.8, p < 0.001; FR 10, F(4, 16) = 29.6, p < 0.001] at 17.0 mg/kg and in the FR 10 group at 10 mg/kg (all p values < 0.001). ITI responses were dose-dependently decreased with pentobarbital, which was significant in the FR 1 [F(4, 15) = 4.29, p = 0.016] and FR 10 groups [F(4, 16) = 8.57, p < 0.001].
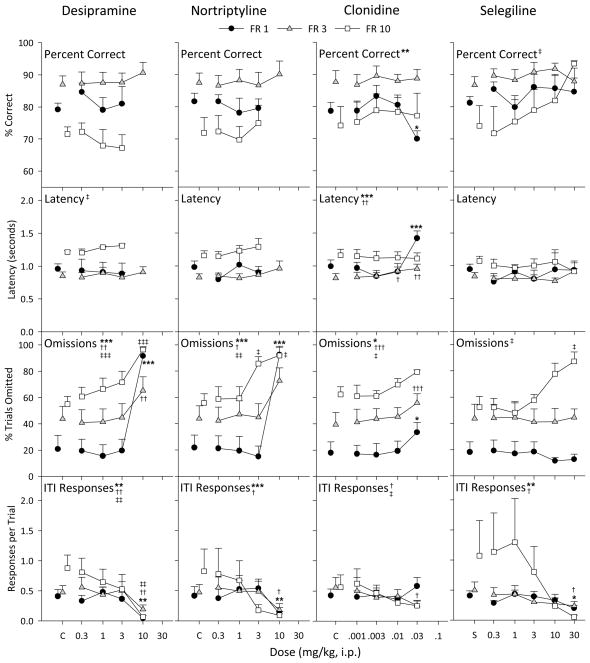
Desipramine did not have a selective effect on accuracy or latency, only disrupting behavior at high doses (Figure 5, first column). Latency was only altered in the FR 10 group [F(3, 10) = 3.89, p = 0.045] with no significance of any doses in post-hoc tests. However, percent trials omitted was dose-dependently increased in each group [FR 1, F(4, 16) = 31.5, p < 0.001; FR 3, F(4, 16) = 5.93, p = 0.004; FR 10, F(4, 16) = 9.85, p < 0.001], and ITI responses were dose-dependently decreased in all groups [FR 1, F(4, 16) = 6.43, p = 0.003; FR 3, F(4, 16) = 6.69, p = 0.002; FR 10, F(4, 16) = 6.62, p = 0.002].
Figure 5.
Effects of desipramine, norttriptyline, clonidine, and selegiline pretreatments in the first, second, third, and fourth columns, respectively. All other details as in Figure 3.
The pattern of behavior with nortriptyline was similar to that observed with desipramine (Figure 5, second column). Accuracy and latency were not affected at any desipramine dose tested in any group. Omissions were dose-dependently increased in each group [FR 1, F(4, 16) = 10.5, p < 0.001; FR 3, F(4, 16) = 3.06, p = 0.047; FR 10, F(4, 16) = 5.67, p = 0.005], and ITI responses were also dose-dependently decreased in each group, although this effect was only significant in the FR 1 [F(4, 16) = 38.5, p < 0.001] and FR 3 groups [F(4, 16) = 4.14, p = 0.017].
Clonidine had little effect on behavior except at 0.03 mg/kg, the highest dose tested (Figure 5, third column). Accuracy was only decreased in the FR 1 group [F(4, 16) = 5.76, p = 0.005] at 0.03 mg/kg (p = 0.037), which corresponded with an increase in latency [F(4, 16) = 14.0, p < 0.001] at 0.03 mg/kg (p < 0.001). Doses of 0.01 and 0.03 mg/kg (both p < 0.05) also slightly increased latency in the FR 3 group [F(4, 16) = 5.64, p = 0.005]. Omissions were dose-dependently increased in each group [FR 1, F(4, 16) = 4.10, p = 0.018; FR 3, F(4, 16) = 12.0, p < 0.001; FR 10, F(4, 16) = 3.12, p = 0.045], and ITI responses were dose-dependently decreased in the FR 3 [F(4, 16) = 3.16, p = 0.043] and FR 10 [F(4, 16) = 3.75, p = 0.024] groups.
Selegiline had a significant effect on percent correct in the FR 10 group [F(5, 15) = 3.00, p = 0.045], but not at the other FR values (Figure 5, fourth column). None of the individual doses in the FR 10 group had a significant effect in post-hoc tests, though the effects at 30 mg/kg approached significance (p = 0.054). This dose also increased trials omitted, such that only two subjects were included in the average for this dose. Latency was not altered in any group, but omissions were dose-dependently increased in the FR 10 group [F(5, 20) = 4.79, p = 0.005]. ITI responses were significantly decreased in the FR 1 [F(5, 20) = 4.32, p = 0.008] and FR 3 [F(5, 20) = 3.24, p = 0.027] groups at 30 mg/kg (both p values < 0.05).
Discussion
Increasing the FR value on the 5-CSRT task increased sensitivity to d-amphetamine, methylphenidate, and nicotine on accuracy without altering the profile of other drugs not expected to enhance response accuracy. While there were trends, neither d-amphetamine nor methylphenidate significantly increased accuracy at any of the doses tested in the FR 1 group. Further, nicotine in the FR 1 group did not affect percent correct at doses up to 1.0 mg/kg. However, in at least one of the groups studied at higher FR schedule values, these drugs did significantly increase percent correct. Nicotine significantly increased accuracy at 0.56 and 1.0 mg/kg in both the FR 3 and FR 10 groups, d-amphetamine significantly increased accuracy in the FR 3 group, and methylphenidate increased accuracy in the FR 10 group. Atomoxetine, morphine, pentobarbital, desipramine, nortriptyline, clonidine, and selegiline did not significantly increase accuracy at any dose tested at any of the FR values studied. Taken together, these results indicate that increasing the response requirement in the 5-CSRT task increases the sensitivity to accuracy-increasing effects of drug administration without altering the profile of drugs that have not been shown to enhance accuracy on this task.
In the absence of any drug administration, baseline performance differed among the three FR groups. Percent correct was lowest in the FR 10 group, and was actually highest in the FR 3 group. This effect may have been due to the non-random way in which subjects were assigned to the FR 3 and FR 10 groups. For all the subjects in these two groups, the FR value was increased until either an FR 10 was reached or response accuracy or rate was substantially lowered. Thus, the FR 3 group consisted of those subjects that did not respond reliably and at 50% accuracy or greater at FR 10. The highest baseline accuracy rates in the FR 3 group may have resulted from the history of training at the higher FR values, but this notion remains untested. Baseline latency to respond, trials omitted, and ITI responses also differed among the three FR groups. Latency was highest in the FR 10 group, but the FR 3 group actually had the lowest average latencies. Latencies in this group tended to be lower than even the FR 1 group, although this effect was not statistically significant. Response latency and percent correct on the 5-CSRT task are known to be inversely correlated (Puumala et al. 1996), and it is possible that the non-random group selection procedure also resulted in this ordering of latencies. In addition, some differences in latency were evident before the increases in FR value, suggesting that the differences in this measure were not due entirely to schedule parameter. Trials omitted and ITI responses were both positively correlated with response requirement, with the FR 10 group both omitting the most trials and emitting the most ITI responses per trial. The effect on trials omitted was not surprising, as increases in FR response requirement increases post-reinforcement pause duration (Ferster & Skinner 1957). In the current procedure, pausing after reinforcement manifests as one or more omitted trials. Further, omitted trials tended to occur immediately after reinforcement (Figure 2), creating a temporal pattern of responding typical of FR schedules.
Statistically significant increases in percent correct were more likely to be observed with nicotine and the psychostimulants commonly used to treat ADHD at the higher FR values than at FR 1. Although nicotine is not currently an approved drug for ADHD treatment, a number of clinical trials show beneficial effects on attention with transdermal nicotine in adults with or without ADHD, including alleviation of ADHD symptoms in those with an ADHD diagnosis (Conners et al. 1996; Levin & Rezvani 1998; Levin et al. 1996; Levin et al. 1998; Levin et al. 2001). These findings suggest that intermittent reinforcement of performance in the 5-CSRT task increases its predictive power for compounds that may be clinically useful.
Percent correct was not altered in any group with atomoxetine pretreatments up to doses that significantly increased trials omitted. Atomoxetine has previously been shown to either not affect accuracy on the 5-CSRT task (Robinson et al. 2007), or do so inconsistently and non-dose-dependently (Navarra et al. 2007). Thus, a limitation of the predictive validity of the 5-CSRT seems to be the lack of concordance between the clinical utility of atomoxetine and increases in performance on this task. Atomoxetine has also been shown to decrease ITI responding, a purported measure of impulsivity (Robinson et al. 2007; Navarra et al. 2007). This was also observed in the current study in the FR 3 and FR 10 groups, but only at a dose of 10 mg/kg which also increased omissions. ITI responses in the current study are probably not equivalent to the “premature response” measure often discussed as a measure of impulsivity (for recent review see Dalley et al. 2008) because the consequences of each are different. In the current study, there were no programmed consequences contingent upon ITI responses, whereas previous studies in which ITI responses are presented as a behavioral measure of impulsivity punish those responses with a timeout. Since most definitions of impulsivity require that the behavior in question persists despite being disadvantageous (Ainslie 1975; Logue 1995), it is probably not appropriate to consider ITI responses in the current experiment as indicative of impulsivity.
The other drugs administered had roughly similar profiles: no significant effect on any measure up to doses that increased trials omitted. Neither morphine, pentobarbital, desipramine, nortiptyline, clonidine, nor selegeline increased percent correct or decreased response latency, while all typically increased trials omitted and decreased ITI responses at the highest dose or doses administered. None of these drugs have demonstrated clinical utility in treating ADHD, further supporting the predictive validity of the 5-CSRT procedure, though the results with atomoxetine warn that the predictive validity of this procedure may be restricted to compounds sharing a particular mechanism.
Longer sessions under the FR 3 and FR 10 schedules compared to the FR 1 schedule may have contributed to the differences observed under the different schedules. To address this, an additional analysis was conducted (not shown) that compared only responding in the first 5 min of all sessions This analysis indicated that data from these 5-min windows were largely confirmative of data from the entire session. No increases or insignificant increases in percent correct were obtained with all drugs in the first 5 min of sessions under the FR 1 schedule, whereas increases in percent correct under the FR 3 and FR 10 schedules during the first 5 min of the sessions were similar to those described above for entire sessions. Thus, durations of sessions contributed minimally if at all to the enhancement of the effects obtained with d-amphetamine, methylphenidate and nicotine under conditions of intermittent reinforcement.
The current results not only provide further evidence that the 5-CSRT task has predictive validity, but it may point to important factors involved in ADHD that should be incorporated in studies of drugs used to treat the disorder. It has been argued that children with ADHD primarily exhibit sustained attention deficits in environments in which infrequent or intermittent reinforcement is arranged (Aase & Sagvolden 2006; Douglas 1985; Douglas and Parry 1994; van der Meere 1996). This consideration coincides with the results in the current experiments in which significant increases in accuracy of detecting an occasional but brief signal were obtained when reinforcement was intermittent. This was even true for the FR 3 group which had higher baseline accuracy than the FR 1 group, but less frequent reinforcer deliveries. Perhaps the intermittency of reinforcement on this task is a more relevant factor than level of baseline performance in contributing to its validity as a model of disorders of attention.
Acknowledgments
Funding: This research was supported by funds from the National Institute on Drug Abuse Intramural Research Program.
The authors would like to express their gratitude to Bettye Campbell and Dawn French for their expert technical assistance. This research was supported by the National Institute on Drug Abuse Intramural Research Program.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Contributor Information
Mikhail N. Koffarnus, Department of Psychiatry, Johns Hopkins University School of Medicine, 5200 Eastern Ave., Suite 142W, Baltimore, MD 21224
Jonathan L. Katz, Psychobiology Section, National Institute on Drug Abuse–Intramural Research Program, National Institutes of Health, 5500 Nathan Shock Drive, Baltimore, MD 21224
References
- Aase H, Sagvolden T. Infrequent, but not frequent, reinforcers produce more variable responding and deficient sustained attention in young children with attention-deficit/hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2006;47:457–471. doi: 10.1111/j.1469-7610.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:63–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behavioural Pharmacology. 2004;15:195–206. [PubMed] [Google Scholar]
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology. 2003;170:271–277. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacology Bulletin. 1996;32:67–73. [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacology, Biochemistry and Behavior. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Douglas VI. The response of ADD children to reinforcement: theoretical and clinical implications. In: Bloomingdale LM, editor. Attention deficit disorder: identification, course and rationale. Medical & Scientific Books; New York: 1985. pp. 49–66. [Google Scholar]
- Douglas VI, Parry PA. Effects of reward and nonreward on frustration and attention in attention deficit disorder. Journal of Abnormal Child Psychology. 1994;22:281–302. doi: 10.1007/BF02168075. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of Reinforcement. Copley Publishing Group; Acton, MA: 1957. [Google Scholar]
- Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: Effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacology. 2002;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Institute for Laboratory Animal Research, National Research Council, National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Hahn B, Stolerman IP. Nicotine-induced attentional enhancement in rats: effects of chronic exposure to nicotine. Neuropsychopharmacology. 2002;27:712–722. doi: 10.1016/S0893-133X(02)00348-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Experimental and Clinical Pschopharmacology. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. European Journal of Pharmacology. 2000;393:141–146. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- Logue AW. Self-control: Waiting until tomorrow for what you want today. Englewood Cliffs; Prentice Hall, NJ: 1995. [Google Scholar]
- Mizra NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology. 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Progress in Neuro-Psychopharmacolgy & Biological Psychiatry. 2007;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biological Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jäkälä P, Koivisto E, Riekkinen P, Jr, Sirviö J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiology of Learning and Memory. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. Journal of Neuroscience Methods. 2007;161:185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Glennon JC. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2007;31:597–618. doi: 10.1016/j.neubiorev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- van der Meere JJ. The role of attention. In: Sandberg ST, editor. Monographs in Child and Adolescent Psychiatry. Hyperactivity Disorders of Childhood. Cambridge University Press; Cambridge, United Kingdom: 1996. pp. 109–146. [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the α7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]