Abstract
Aging impairs the control of many skilled movements including speech. The purpose of this paper was to investigate whether young and older adults adapt to lower lip perturbations during speech differently. Twenty men (10 young, 26 ± 3 years of age; 10 older, 60 ± 9 years of age) were requested to repeat the word (“papa”) 300 times. In 15% of the trials, the subjects experienced a mechanical perturbation on the lower lip. Displacement and neural activation (EMG) of the upper and lower lips were evaluated. Perturbations to the lower lip caused a greater increase in the maximum displacement of the lower lip for older adults compared with young adults (34.7 ± 19% vs. 13.4 ± 17%; P = 0.017). Furthermore, young adults exhibited significantly greater 30–100 Hz normalized EMG power for the lower lip compared to the upper lip (P < 0.005). In young adults, changes from normal to perturbed trials in the 30–50 Hz frequency band of the EMG were negatively correlated to the changes from normal to perturbed trials in the lower lip maximum displacement (R2 = 0.48; P = 0.025). It is concluded that young adults adapt better to lower lip perturbations compared with older adults and that the associated neural activation strategy of the involved muscle is different for the two age groups.
Keywords: EMG, Wavelet analysis, Speech, Cortical drive
Introduction
As we grow older, our ability to adapt to perturbations may be altered. Healthy aging may affect the efficiency with which we adapt and react to perturbations for the following three reasons. First, independent of disease, the aging neuromotor system (nervous system and muscles) undergoes significant changes that result in less efficient neural processing. Such changes include the loss of cortical neurons and altered synaptic connections (Morrison and Hof 2007), reduced number of motor units (Lexell and Downham 1992) and muscle fibers (Grimbly and Saltin 1983), and altered discharge characteristics of motor units (Kamen et al. 1995). Second, motor output is significantly affected by the age-associated changes in the neuromotor system (Galganski et al. 1993). Motor output changes include slower and more variable movements (Christou and Tracy 2005; Karlsson and Carlsson 1990), decreased amplitude of movement with increased variability (Lexell and Downham 1992), as well as increased latency of response to sensory stimuli (Bugnariu and Sveistrup 2006; Chung et al. 2005; Nardone et al. 1995). In the orofacial motor system, older adults exhibit less accuracy of speech movements (Amerman and Parnell 1990), as well as increased variability of orofacial movement (Ballard et al. 2001; Wohlert and Smith 1998). Finally, recent evidence suggests that the adjustments to the motor output and muscle activity differ for young and older adults when trying to adapt to a novel goal-directed task. When learning sequential tasks, for example, older adults exhibit different sub-sequences than young adults (Shea et al. 2006). Furthermore, young and older adults organize agonist and antagonist muscle activity differently to improve motor performance with practice (Christou et al. 2007).
Although several studies have examined the ability of older adults to respond to perturbations, the majority of these studies have focused on postural and stepping perturbations (Nardone et al. 1995; Pavol and Pai 2007; Zettel et al. 2007). To our knowledge, no studies have examined the ability of the aging speech motor system to respond and adapt to perturbations. The purpose of the current study, therefore, was to determine whether the aging speech motor system adapts differently to random perturbations during speech production. Part of the findings has been reported in abstract form (Marzullo et al. 2009).
Methodology
Twenty healthy men (10 young, 26 ± 3 years of age; 10 older, 60 ± 9 years of age) participated in the study. Only male subjects were used to control for possible sex-related differences in adapting to perturbations (Sung and Park 2009). All the participants were native speakers of American English and reported no history of speech, language, reading, or other neurological impairments or substance abuse. All participants reported normal hearing and vision (either corrected/uncorrected). Participants were paid $20/h for participating. The Institutional Review Board of the University of Iowa approved the procedures, and informed consent was obtained in accordance with the Declaration of Helsinki.
Experimental arrangement
Participants were seated upright in a dental chair. The head was positioned so that the participant's Frankfurt line (i.e. inferior border of the orbit to upper margin of the auditory meatus) was aligned with the horizontal plane and the head was immobilized with a cephalostat mounted from the ceiling (Fig. 1). A custom-made bite block was used to fix each participant's jaw with a 1-cm incisor gap. Bite blocks were made from standard dental impression material placed between the participant's right molars. A standard strain-gauge cantilever system was used to track movement of the lips and jaw (Muller and Abbs 1979). Strain gauges were mounted at midline on the vermilion border of the upper lip and lower lip and on the underside of the jaw with double-sided tape and polyethylene tubing (see Fig. 1). They were calibrated prior to each set of experimental trials such that 1.0 V represented 10-mm displacement. Surface electromyography (EMG) recorded the muscle activity of the orbicularis oris superior and inferior muscle (i.e. upper lip EMG and lower lip EMG) (Biocommunication Electronics preamplifier model 301 and amplifier model 205, Biocommunication Electronics, Madison, WI). Surface electrodes (AgAgCl; Beckman Instruments, Inc., Fullerton, CA), 8 mm in diameter, were mounted to the right of midline just above and below the vermilion border with double-sided tape at a 1 cm inter-electrode distance. A Sennheiser cardioid microphone, which recorded onset of the tone used to cue responses, was positioned 1 meter from the mouth.
Fig. 1.
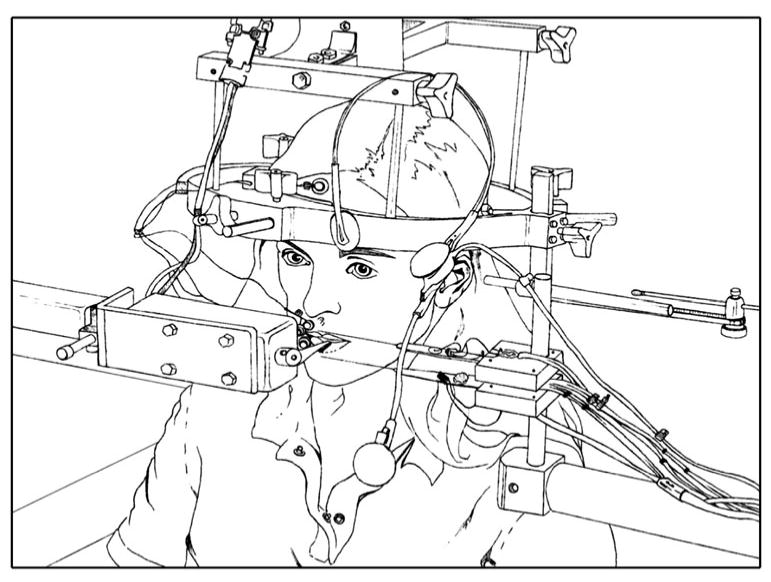
Experimental setup. Participants were seated in a dental chair, and their head was positioned in the Frankfurt horizontal plane and immobilized with a cephalostat mounted from the ceiling. A bite block was used to fix the jaw with a 1-cm incisor gap. The movement of the lips and jaw was tracked with a standard strain-gauge cantilever system. Surface electromyography (EMG) recorded the muscle activity of the upper and lower lips. Perturbations in the lower lip were delivered using a commercial lever system
Perturbation to the lower lip was delivered using a Dual-Mode Muscle Lever System 305B (Aurora Scientific, Inc.). This system was designed for studying dynamic mechanical characteristics of muscle tissue and can control and measure force of the perturbation load. The lever arm of the device was positioned at midline on the lower lip. This arm is 40-mm long and 1-mm thick, tapering from 10-mm wide at the base to 1-mm wide at the point. The system design requires that the 1-mm-thick edge of the lever arm sits on the superior surface of the lower lip. With this narrow contact area, the arm did not effectively displace the lower lip tissue, and as a consequence, the tip of the arm was custom-modified by the manufacturer so that this 1-mm-thick edge was broadened to a 4-mm-thick edge along the final 10 mm of the arm. Specifically, a 4 mm × 10 mm × 1 mm plate was affixed along the end 10 mm of the arm. This increased the contact area with the lip to achieve the desired lip depression on application of the perturbation load, without interfering with lip closure for speech production on unperturbed trials. The manufacturer checked calibration of the unit after modification. According to the manufacturer, the modification did not alter the weight of the arm sufficiently to affect initial calibration. We checked the calibration by applying known forces to the arm and comparing this value with the voltage measured. The modified end of the lever arm was positioned on the center of the lip's superior surface but not touching the teeth. It applied a constant tracking load of 3–5 g. Perturbation of the lower lip was achieved by ramping the load to 30 g for 1,000 ms duration, with a rise time of 12 ms.
All physiological signals were digitized online at 5,000 Hz using DATAQ Instruments Inc., DI-720-P Data Acquisition System and Windaq Pro+ recording software installed on a desktop PC. Signals included (a) speech; (b) inferior–superior displacement of the lower lip and upper lip; (c) surface EMG from the lower and upper parts of the orbicularis oris muscle; (d) the signal from a laptop computer to the Muscle Lever System that triggered each perturbation ; and (e) output of the Muscle Lever System.
Perturbation delivery was controlled using a customized Matlab routine on the laptop computer. This program generated a 1,000 Hz, 250-ms duration tone, cuing participants to produce the response “papa”. A 1 volt signal was sent from the computer to the Lever System electronics for controlled delivery of the 30 g load to the lower lip.
Procedure
Participants were instructed to maintain the vowel “ah”. On hearing a tone, they were to say “papa” with stress on the first syllable, take a breath, and then resume “ah” production. Sustaining “ah” ensured a relatively steady initial position of the articulators for all trials. Participants were unaware of the purpose of the study. They were forewarned that occasionally the lever arm would move on their lip but to try to disregard it and speak as usual.
Experimental trials involved producing the speech target “papa” 300 times with perturbations presented just prior to the first “p” of 15% of trials (N = 44). Trials were produced at a habitual speech rate (i.e. 220–240 syllables per minute). Rate was modeled using a metronome, and the participant was given the opportunity to match and practice the speech rate. Perturbed trials were randomized across the experiment based on the following constraints: at least 2 unperturbed trials were placed between perturbed trials, and no perturbations were delivered on the first 5 trials. Participants were instructed they could stop the experiment at any time for a break. After each block of 150 trials, all participants rested for 5 min and were offered a drink.
Prior to each set of 300 experimental trials, participants were given 10 unperturbed practice trials. During this time, they were given feedback to ensure they were speaking at a normal loudness level (70 dB at 15-cm microphone-mouth distance) and at the target speech rate. Participants' average reaction time from onset of the tone to onset of the lower lip displacement was recorded by the Matlab routine operating on the laptop computer and this was used to time delivery of perturbations in the experimental trials. Participants were given a second set of 10 trials if they missed or anticipated any alerting tone on the initial set. This meant that an accurate estimate of reaction time for each participant could be calculated. No participant required more than 2 sets of 10 practice trials. Based on this individual average reaction time, onset of the perturbation was timed to occur during the steady state “ah” production just prior to the onset of lower lip closure for the first “p” in “papa” (Gracco and Abbs 1985).
Experimental data analysis
Data were analyzed off-line using custom-written programs in Matlab® (Math Works™ Inc., Natick, MA, USA). Visual inspections of the raw displacement and processed EMG signals were performed to select the data that comprised each trial (Fig. 2). To aid the visual inspection of each trial, EMG signals were full-wave rectified and low-pass filtered (Butterworth order 4) at 40 Hz. To analyze the displacement and EMG of the lips, data were divided into non-perturbed (normal) and perturbed trials. The interference and not the rectified EMG was used for analysis.
Fig. 2.
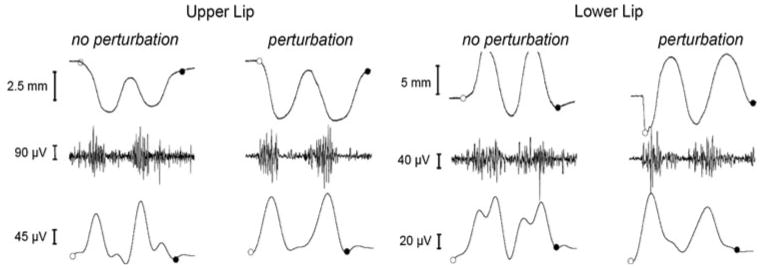
Example of a normal trial (left side) and a perturbed trial (right side) performed by a young adult. This figure shows the displacement, interference EMG, and rectified filtered EMG of the upper and lower lips. Beginning and end of each trial were selected manually by visual inspection. Open circles represent the beginning of the trial, and filled circles represent the end. Lip displacement was defined as the absolute movement amplitude (mm) of the upper or lower lips. The EMG interference (μV) was obtained at 5,000 Hz. The EMG signals were rectified and low-pass filtered at 40 Hz to facilitate the visual inspection. A direct perturbation effect can be seen as an abrupt decrease in the lower lip displacement prior to the trial
Lip displacement and EMG variables
Our behavioral variable was the maximum displacement of the lower and upper lips measured as the maximum absolute movement amplitude of each lip (mm). The neural variable was the normalized power of the EMG wavelet of the upper and lower lips for frequencies below 100 Hz. We used sub-100 Hz EMG frequencies because power in the EMG signal from 12 to 100 Hz reflects the modulation of the motor neuron pool with voluntary effort and it is not associated with the shape of the motor unit action potential (Myers et al. 2003; Neto and Christou 2010; Christou and Neto 2010). We determined the importance of four frequency bands: 5–13, 13–30 (beta drive), 30–50 (low-gamma drive), and 50–100 Hz (high-gamma drive) because they have been previously associated with specific cortical drives (Brown 2000) and associated with changes in voluntary effort (Neto et al. 2010 and task failure (Pereira et al. 2010). Interference EMG signals were used in this study because they appear to provide more accurate information about the oscillatory input to the muscle compared with rectified EMG signals (Neto and Christou 2010; Christou and Neto 2010).
Wavelet analysis
Morlet wavelet analysis was used to obtain the normalized power of different frequency bands of the EMG signals obtained from the orbicularis oris superior and inferior muscle. Morlet wavelet represents a set of functions with the form of small waves created by dilations and translations from a simple generator function (Eq. 1), which is called Morlet mother wavelet (Addison 2002).
| (1) |
where η is dimensionless time, and w0 is dimensionless frequency (in this study, we used w0 = 6, as suggested by Grinsted et al. 2004).
The wavelet transform applies the wavelet function as a band-pass filter to the time series (Eq. 2).
| (2) |
where s represents the dilation parameter (scale shifting), τ represents the location parameter (time shifting), and the basic function Ψs,τ(t) is obtained by dilating and translating the mother wavelet Ψ0(t) (Addison 2002). For the w0 = 6 Morlet wavelet, the scale is almost equal to the Fourier period (Fourier Period = 1.03 s).
The importance of wavelet transform to the analysis of EMG signals obtained during dynamic tasks is that the wavelet transform can detect if there are any transient events that take place within a chosen window of time by determining how the amplitude versus frequency characteristics of the signal changes with time (Zazula et al. 2004).
In this paper, wavelet transforms were calculated using a Matlab function developed by Torrence and Compo (1998) (available at URL: http://www.paos.colorado.edu/research/wavelets). An algorithm was developed in Matlab 7.0.1 (MathWorks Inc.) to measure the relative importance of different frequency bands of the interference EMG signals (please see next section for more details). We defined the normalized wavelet scale-averaged power spectrum (NWPS, Fig. 3) as the squared weighted modulus of the wavelet transform normalized by the sum of the squared weighted modulus over all scales for each instant to time (Eq. 3; Neto et al. 2010; Pereira et al. 2010).
Fig. 3.
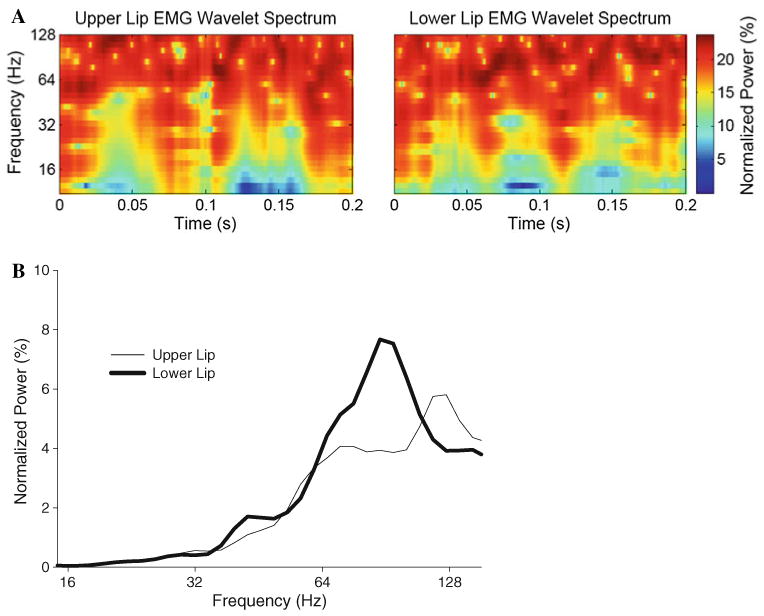
Examples of normalized wavelet spectra (a) and global wavelet power spectra (GWS, b) from the upper and lower lips EMG obtained from a young subject during a perturbed trial. The normalized wavelet spectrum shows the relative importance through time (x-axis) of the frequency content of the signal (y-axis) with intensities ranging from 0 to 100% (color coded from blue to red or white to black). The GWS shows the average intensity across time of the frequency content of the signal (thin line upper lip; thick line lower lip)
| (3) |
The normalized wavelet scale-averaged power spectrum (hereafter referred to as the normalized wavelet spectrum; Fig. 3) shows the relative importance through time of each scale (can be related to a frequency) of the signal with intensities ranging from 0 to 100%. Thus, the normalized wavelet spectrum can be used to compare the strength of different EMG oscillations within the same signal at different times, or between two different signals.
Normalized wavelet spectra were plotted for each movement window obtained from the raising and lowering of the lips. Because the bands of interest were active during the whole duration of the analyzed windows, the relative importance of each frequency band for each window was estimated as a percentage of the power within the band and the total power of the signal in each window.
Statistical analysis
A mixed three-way ANOVA (2 age groups × 2 perturbation conditions × 2 lips) with age as the between-subject factor and perturbation and lips as within-subject factors compared the maximum displacement of upper and lower lips values obtained from the young and older subjects during the normal and perturbed trial conditions. Additionally, a mixed two-way ANOVA (2 age groups × 2 lips) compared the relative change (%) in maximum displacement from normal to perturbed trials' values obtained for the upper and lower lips from the young and older subjects. Finally, a mixed four-way ANOVA (2 age groups × 2 perturbation conditions × 2 lips × 4 frequency bands) with age as the between-subject factor and perturbation, lips and bands as within-subject factors compared the normalized power of the 5–13, 13–30, 30–50, and 50–100 Hz frequency bands obtained from the EMG of the upper and lower lips EMG of the young and older subjects during the normal and perturbed trial conditions. Significant interactions from the ANOVA models were followed by appropriate post hoc analyses. For example, differences between age groups were followed with independent t tests, whereas differences between perturbation conditions were followed with dependent t tests. Multiple t test comparisons were corrected using Bonferroni corrections. Finally, a regression analysis was used to investigate whether changes in any specific band of the EMG power spectra from normal to perturbed trials were associated with changes in lip maximum displacement between normal and perturbed trials for young and older subjects. All statistical analyses were performed with the SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). The alpha level for all statistical tests, except when corrected, was 0.05. Data are reported as mean ± standard deviation (SD) within the text and as mean ± standard error of the mean (SEM) in the figures. Only the significant main effects and interactions are presented, unless otherwise noted.
Results
Lip displacement
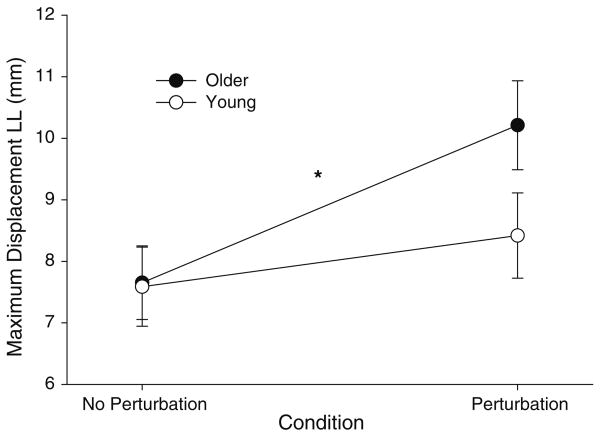
There was a significant condition main effect on lip displacement (F1,18 = 62.396, P < 0.001), which indicated that perturbation to the lower lip increased the maximum displacement of the lower lip (7.62 ±1.91 mm to 9.32 ± 2.36 mm; P < 0.001) and the maximum displacement of the upper lip (4.24 ± 1.18 mm to 4.78 ± 1.30 mm; P < 0.001). There was also a significant age x condition interaction (F1,18 = 11.9, P < 0.001), which indicated that the displacement of the lips with perturbation was greater for the older adults than the young adults. Finally, there was a significant age × condition × lips interaction (F1,18 = 8.422, P = 0.01) on the maximum displacement of the lips. The interaction indicated that although perturbation to the lower lip increased the maximum displacement of the upper and lower lips for both age groups, age differences were observed only for the lower lip displacement (Fig. 4). Specifically, only older adults exhibited significantly greater displacement of the lower lip with perturbations (P < 0.001 for older; P = 0.058 for young).
Fig. 4.
Age-associated changes in the maximum displacement of the lower lip (LL) with perturbations. The displacement of the lower lip significantly increased with perturbations only for older adults (P < 0.001 for older; P = 0.058 for young). The increase in the displacement of the lower lip relative to that of the non-perturbed trials was significantly greater (P = 0.017) for older adults (34.7 ± 19%) than young adults (13.4 ± 17%)
In addition, we examined the relative change in maximum displacement from normal to perturbed trials for the upper and lower lips of young and old adults using a two-way mixed ANOVA model. There was a significant age × lips interaction (F1,18 = 8.422, P = 0.01) for this model, which indicated that the increase in the displacement of the lower lip relative to that of the non-perturbed trials was significantly greater (P = 0.017) for older adults (34.7 ± 19%) than young adults (13.4 ± 17%). The interaction also indicated that only older adults exhibited differential increase in the maximum displacement for the upper and lower lips with perturbations. Specifically, perturbation to the lower lip of older adults induced a significantly higher percentage change for the lower lip compared with the upper lip (34.7 ± 19.1% for the lower lip and 17.8 ± 14.1% for the upper lip; P = 0.018). In contrast, perturbation to the lower lip of young adults induced a similar percent change for the upper and lower lip (13.4 ± 17.0% for the lower lip and 9.9 ± 9.1% for the upper lip; P = 0.587). The difference in the maximum displacement of the upper and lower lips for older adults suggests that lip coupling may have been weaker for older adults compared with that of young adults.
Normalized power of the EMG wavelet for the upper and lower lips
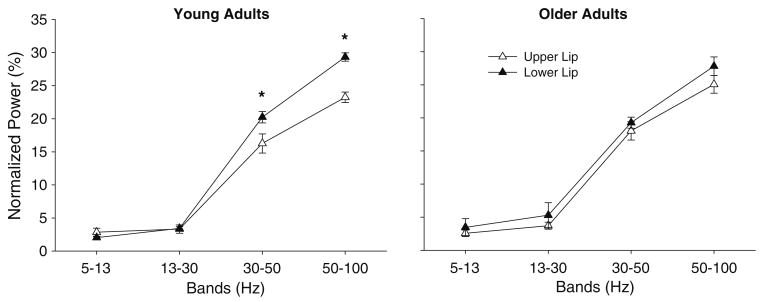
There was a significant age × lip × bands interaction (F3,54 = 3.394, P = 0.024) on the normalized power of the EMG wavelet (Fig. 5). Post hoc analyses demonstrated that young adults exhibited greater normalized power of the EMG wavelet from 30 to 50 Hz (20.2 ± 2.6% vs. 16.2 ± 4.3%; P = 0.005) and 50–100 Hz (29.3 ± 1.9% vs. 23.2 ± 2.3%; P < 0.001) for the lower lip compared with the upper lip. In contrast, older adults exhibited similar power in each frequency band for the upper and lower lips (P > 0.12).
Fig. 5.
Normalized wavelet EMG power from 5 to 100 Hz for upper and lower lips for young and older adults. Young adults exhibited significantly greater 30–100 Hz normalized wavelet EMG power for the lower lip compared with the upper lip (P < 0.005)
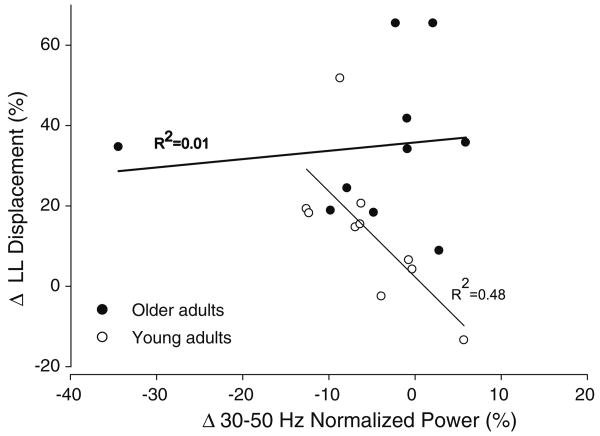
To determine whether the altered activation of the lower lip muscle contributed to the smaller displacement of the lower lip with perturbation in young adults, we performed linear regressions between the change in normalized power of the EMG wavelet and the change in lower lip displacement from normal to perturbed trials. For the young adults, there was only one significant association between the change in normalized power of the EMG wavelet from 30 to 50 Hz and the change in lower lip displacement (R2 = 0.48; P = 0.025). This association indicated that the greater the increase in power from 30 to 50 Hz, the smallest the lower lip displacement (Fig. 6). In contrast, for older adults, there was no association between changes in any frequency band and changes in lip displacement (R2 = 0.01; P > 0.3).
Fig. 6.
Associations of the relative change in the lower lip displacement and 30–50 Hz normalized wavelet EMG power (%) from normal to perturbed trials for older and young adults. There was a significant negative correlation only for young adults (R2 = 0.48; P = 0.025)
Discussion
The current study examined the ability of young and older adults to respond to random perturbations to the lower lip. The results demonstrated the following novel findings: (1) perturbations caused greater changes to the speech motor system of older adults than young adults; (2) only young adults were able to adapt to perturbations by changing the oscillatory activity in the lower part of the orbicularis oris muscle, which may have acted as an antagonist muscle to opening of the mouth.
Aging and unexpected perturbations
Perturbations to the lower lip caused a greater effect on the lower lip maximum displacement of older adults than young adults (see Fig. 4). The greater lower lip displacement in older adults with perturbations may be due to the following: (1) altered anticipatory neural control strategies to counteract perturbation of the lower lip; (2) slower perioral reflex response, which allowed greater displacement during perturbations.
Our results demonstrate that young adults have used different anticipatory strategies than older adults to counteract perturbations to the lower lip. During the speech production of the word “papa”, the upper part of the orbicularis oris muscle is responsible to lower the upper lip, whereas the lower part of the orbicularis oris muscle is responsible to raise the lower lip. We found that only young adults were able to differentially activate their lower and upper lips (Fig. 5). Specifically, we found that only young adults increased the oscillatory drive of the lower lip muscle (experimental lip—random perturbations were given) from 30 to 100 Hz relative to that of the upper lip (control lip—no perturbations). Furthermore, we found that young adults who amplified the oscillatory drive of the lower lip from 30 to 50 Hz exhibited lesser lower lip displacement with perturbations. This band of the EMG spectrum has been shown to increase with voluntary cortical input and/or higher forces (Neto et al. 2010). These results show that only young adults were able to minimize lower lip displacement during perturbation trials by manipulating the oscillatory activation of the lower lip. Increasing the oscillatory drive of the lower lip muscle, which acts as an antagonist to lowering the lower lip, may have been an anticipatory strategy used by young adults to counteract perturbations to the lower lip.
Another possible mechanism to explain the amplified effects of perturbations on the lower lip displacement in older adults may be a weaker perioral reflex. Previous findings suggest that the perioral reflex was more responsive in young women compared with older women during upper lip stretches (Wohlert 1996). The perioral reflex occurred less frequently in older women and when it did, its amplitude was lower and its latency longer (Wohlert 1996). Therefore, the greater displacement of the lower lip to perturbations in older adults may have been due to a combination of altered anticipatory strategy and weaker perioral reflex response.
Our results, using the speech motor system as a model, support the literature that compared the ability of young and older adults to respond to gait and postural perturbations. Older adults typically exhibit impaired responses to gait (Menant et al. 2009) and postural (Doumas et al. 2009) perturbations compared with young adults. For example, older adults demonstrate longer times to respond to perturbations and allow greater movement of the limbs following perturbations. The impairments during gait perturbations may also be related to altered synergistic activation of muscles (Hubley-Kozey et al. 2008) or slower long-latency reflex (Ito and Gomi 2007). Therefore, aging appears to have an impaired response to perturbations in the limb motor system and speech motor system. Because the muscles involved in the two systems are different, lip muscles do not have tendons or muscle spindles (Goodmurphy and Ovalle 1999), the findings suggest that central processes may influence the responses such as altered anticipatory strategies (e.g. changing the oscillatory input to the involved muscles) or reflexes that involve the cortex. It should be emphasized that the older adults used in this study were around 60 years old. It is possible, therefore, that these age-associated differences in the neural control of lips during perturbations may be even greater for older individuals (over the age of 70).
Speech motor system
The findings from young adults replicate previous studies (Gracco and Abbs 1985; Shaiman 2002) and show that the perturbed lower lip and the unperturbed upper lip operate as a coordinative unit, both responding similarly to a perturbation (increase of 13.4 ± 17% for the lower lip vs. increase of 9.9 ± 9% for upper lip) to ensure successful production of speech. This was not the case, however, for older adults who increased significantly more their lower lip displacement (34.7 ± 19%) compared with the upper lip (17.8 ± 14%). Hence, this study reports novel findings that perturbations in young adults can alter the movement coordination between the upper and lower lips during speech likely by changing the oscillatory input to the muscle that controls the perturbed lip. The reported effects of healthy aging on movement coordination during both unperturbed and perturbed speech production are important for interpreting the changes that occur in speech motor disorders. These disorders most commonly result from diseases of aging such as stroke and Parkinson's disease.
In summary, the data presented here are the first to report the effects of healthy aging on the response to perturbations to the speech motor system. Lower lip perturbations caused a greater displacement of the lower lip in older adults compared to young adults. Only young adults were able to adapt to perturbations and maintain coupling of upper and lower lips during perturbation trials. Specifically, young adults changed the oscillatory input to the lower lip muscle, which was acting as an antagonist to the perturbation-induced lowering of the lower lip. The perturbation paradigm is a useful approach for revealing changes in speech motor control with healthy aging that may underlie subtle perceptible changes in the quality of speech production. These findings are critical to interpreting the impact on speech motor control of neuromotor damage associated with diseases of aging, as well as predicting responsiveness to rehabilitation.
Acknowledgments
This research was supported by a NIH NIDCD grant DC005698 and a University of Iowa Biological Sciences Funding Program Grant to K. J. Ballard and R01 AG031769 to Evangelos A. Christou. We thank Arik Wald for computer programming; Jerald Moon for technical expertise; John W. Folkins for intellectual discussion; Michael Molley, Jeffrey Tyler, Valerie Flemmer, Vanessa Shaw, Rebekah Abel, Stacey Meyers, Kira Spencer, and Abbie Hammes for assistance with data acquisition.
Contributor Information
Ana Carolina de Miranda Marzullo, Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL 32611-8205, USA; Universidade Camilo Castelo Branco, São Paulo, Brazil.
Osmar Pinto Neto, Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL 32611-8205, USA; Universidade Camilo Castelo Branco, São Paulo, Brazil.
Kirrie J. Ballard, Faculty of Health Sciences, University of Sydney, Sydney, Australia
Donald A. Robin, Research Imaging Center, Departments of Neurology, and Radiology, The University of Texas Health Sciences Center, San Antonio, TX, USA
Lauren Chaitow, Faculty of Health Sciences, University of Sydney, Sydney, Australia.
Evangelos A. Christou, Email: eachristou@ufl.edu, Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL 32611-8205, USA.
References
- Addison PS. The illustrated wavelet transform handbook. Taylor and Francis Group; New York: 2002. [Google Scholar]
- Amerman JD, Parnell MM. Auditory impressions of the speech of normal elderly adults. Br J Disord Commun. 1990;25:35–43. doi: 10.3109/13682829009011961. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Robin DA, Woodworth G, Zimba LD. Age-related changes in motor control during articulator visuomotor tracking. J Speech Lang Hear Res. 2001;44:763–777. doi: 10.1044/1092-4388(2001/060). [DOI] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Bugnariu N, Sveistrup H. Age-related changes in postural responses to externally- and self-triggered continuous perturbations. Arch Gerontol Geriatr. 2006;42:73–89. doi: 10.1016/j.archger.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Christou EA, Neto OP. Identification of oscillations in muscle activity from surface EMG: reply to Halliday and Farmer. J Neurophysiol. 2010;103(6):3548. doi: 10.1152/jn.00222.2010. [DOI] [PubMed] [Google Scholar]
- Christou EA, Tracy BL. Aging and motor output variability In: Movement system variability. Human Kinetics; Champaign: 2005. pp. 199–215. [Google Scholar]
- Christou EA, Poston B, Enoka JA, Enoka RM. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J Neurophysiol. 2007;97:3340–3350. doi: 10.1152/jn.01138.2006. [DOI] [PubMed] [Google Scholar]
- Chung SG, Van Rey EM, Bai Z, Rogers MW, Roth EJ, Zhang LQ. Aging-related neuromuscular changes characterized by tendon reflex system properties. Arch Phys Med Rehabil. 2005;86:318–327. doi: 10.1016/j.apmr.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Doumas M, Rapp MA, Krampe RT. Working memory and postural control: adult age differences in potential for improvement, task priority, and dual tasking. J Gerontol B Psychol Sci Soc Sci. 2009;64:193–201. doi: 10.1093/geronb/gbp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–2115. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- Goodmurphy CW, Ovalle WK. Morphological study of two human facial muscles: orbicularis oculi and corrugator supercilii. Clin Anat. 1999;12:1–11. doi: 10.1002/(SICI)1098-2353(1999)12:1<1::AID-CA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gracco VL, Abbs JH. Dynamic control of the perioral system during speech: kinematic analyses of autogenic and nonautogenic sensorimotor processes. J Neurophysiol. 1985;54:418–432. doi: 10.1152/jn.1985.54.2.418. [DOI] [PubMed] [Google Scholar]
- Grimbly G, Saltin B. The ageing muscle. Clin Physiol. 1983;13:209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes Geophys. 2004;11:561–566. [Google Scholar]
- Hubley-Kozey C, Deluzio K, Dunbar M. Muscle co-activation patterns during walking in those with severe knee osteoarthritis. Clin Biomech (Bristol, Avon) 2008;23:71–80. doi: 10.1016/j.clinbiomech.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Ito T, Gomi H. Cutaneous mechanoreceptors contribute to the generation of a cortical reflex in speech. Neuroreport. 2007;18:907–910. doi: 10.1097/WNR.0b013e32810f2dfb. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Carlsson GE. Characteristics of mandibular masticatory movement in young and elderly dentate subjects. J Dent Res. 1990;69:473–476. doi: 10.1177/00220345900690021101. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D. What is the effect of ageing on type 2 muscle fibres? J Neurol Sci. 1992;107:250–251. doi: 10.1016/0022-510x(92)90297-x. [DOI] [PubMed] [Google Scholar]
- Marzullo AC, Neto OP, Ballard KJ, Robin DA, Christou EA. Age-associated differences in neuromotor adaptation following lower lip perturbations. 2009 Annual Neuroscience Symposium on Alzheimer's Disease; College Station, TX. 2009. [Google Scholar]
- Menant JC, Steele JR, Menz HB, Munro BJ, Lord SR. Effects of walking surfaces and footwear on temporo-spatial gait parameters in young and older people. Gait Posture. 2009;29:392–397. doi: 10.1016/j.gaitpost.2008.10.057. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- Muller EM, Abbs JH. Strain gauge transduction of lip and jaw motion in the midsagittal plane: refinement of a prototype system. J Acoust Soc Am. 1979;65:481–486. doi: 10.1121/1.382348. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O'Malley M, Vaughan CL, Heneghan C, St Clair Gibson A, Harley YX, Sreenivasan R. Rectification and non-linear pre-processing of EMG signals for corticomuscular analysis. J Neurosci Methods. 2003;124:157–165. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Nardone A, Siliotto R, Grasso M, Schieppati M. Influence of aging on leg muscle reflex responses to stance perturbation. Arch Phys Med Rehabil. 1995;76:158–165. doi: 10.1016/s0003-9993(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Neto OP, Christou EA. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol. 2010;103:1093–1103. doi: 10.1152/jn.00792.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto OP, Baweja HS, Christou EA. Increased voluntary drive is associated with changes in the common oscillations from 13–60 Hz of interference but not rectified electromyography. Muscle Nerve. 2010 doi: 10.1002/mus.21687. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavol MJ, Pai YC. Deficient limb support is a major contributor to age differences in falling. J Biomech. 2007;40:1318–1325. doi: 10.1016/j.jbiomech.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R, Schettino L, Machado M, Silva PAV, Neto OP. Task failure during standing heel raises is associated with increased power from 13 to 50 Hz in the activation of triceps surae. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaiman S. Articulatory control of vowel length for contiguous jaw cycles: the effects of speaking rate and phonetic context. J Speech Lang Hear Res. 2002;45:663–675. doi: 10.1044/1092-4388(2002/053). [DOI] [PubMed] [Google Scholar]
- Shea CH, Park JH, Braden HW. Age-related effects in sequential motor learning. Phys Ther. 2006;86:478–488. [PubMed] [Google Scholar]
- Sung PS, Park H. Gender differences in ground reaction force following perturbations in subjects with low back pain. Gait & Posture. 2009;29:290–295. doi: 10.1016/j.gaitpost.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. B Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- Wohlert AB. Reflex responses of lip muscles in young and older women. J Speech Hear Res. 1996;39:578–589. doi: 10.1044/jshr.3903.578. [DOI] [PubMed] [Google Scholar]
- Wohlert AB, Smith A. Spatiotemporal stability of lip movements in older adult speakers. J Speech Lang Hear Res. 1998;41:41–50. doi: 10.1044/jslhr.4101.41. [DOI] [PubMed] [Google Scholar]
- Zazula D, Karlsson S, Doncarli C. Advanced signal processing techniques. In: Merletti R, Parker P, editors. Electromyography: physiology, engineering, and noninvasive applications. Wiley; New Jersey: 2004. pp. 259–304. [Google Scholar]
- Zettel JL, Scovil CY, McIlroy WE, Maki BE. Gaze behavior governing balance recovery in an unfamiliar and complex environment. Neurosci Lett. 2007;422:207–212. doi: 10.1016/j.neulet.2007.06.020. [DOI] [PubMed] [Google Scholar]