Abstract
Dependence on illicit drugs during pregnancy is a major public health concern as there may be associated adverse maternal, fetal, and neonatal consequences. Sweat patches (n = 389) were collected from 39 pregnant volunteers who provided written informed consent for this Institutional Review Board-approved protocol and wore patches, replaced approximately weekly, from study entry until delivery. Patches were analyzed for opiates (heroin, 6-acetylmor-phine, 6-acetylcodeine, morphine and codeine) and cocaine (cocaine, benzoylecgonine, ecgonine methyl ester, anhydroecgonine methyl ester) by solid phase extraction and gas chromatography mass spectrometry. Seventy-one percent (276) of collected sweat patches were ≥5 ng per patch (limit of quantification) for one or more analytes. Cocaine was present in 254 (65.3%) patches in concentrations ranging from 5.2 to 11,835 ng per patch with 154 of these high enough to satisfy the proposed Substance Abuse and Mental Health Services Administration guidelines for a confirmatory drug test (25 ng per patch). Interestingly, 6-acetylmorphine was the most prominent opiate analyte documented in 134 patches (34.4%) with 11.3% exceeding the proposed opiate Substance Abuse and Mental Health Services Administration cut-off (25 ng per patch). Heroin was identified in fewer patches (77), but in a similar concentration range (5.3–345.4 ng per patch). Polydrug use was evident by the presence of both cocaine and opiate metabolites in 136 (35.0%) patches. Sweat testing is an effective method for monitoring abstinence or illicit drug use relapse in this high-risk population of pregnant opiate- and/or cocaine-dependent women.
Keywords: sweat patch, heroin, opiates, cocaine, sweat
INTRODUCTION
Dependence on illicit opioids and cocaine during pregnancy is a major public health concern. Pregnant drug-dependent women and their children are at risk for a variety of adverse outcomes, often requiring intensive and expensive medical care. Recent findings suggest children exposed to drugs in utero may be at increased risk for attentional and cognitive difficulties and a variety of other neurobehavioral problems.1,2 In a US survey, more than 6% of women reported polysubstance use (including tobacco, alcohol, and prescription medicines) and 4.7% admitted use of one or more illicit substances during pregnancy.3 Innovative strategies are needed to reduce illicit drug use by pregnant drug-dependent women.4 Buprenorphine and methadone treatment given to treat opioid dependence during pregnancy improve maternal and neonatal outcomes and reduce the severity of, but do not eliminate, the neonatal abstinence syndrome.5
Drug testing monitors patients in substance abuse treatment programs, can serve as a deterrent for illicit drug use, is a key component of contingency management programs that reward drug abstinence, and is an objective measure of the efficacy of new pharmacotherapies and behavioral treatments. Although urine is traditionally the specimen of choice, oral fluid, sweat, and hair have been proposed as alternative matrices for drug testing.6–10 Advantages of these alternate matrices include noninvasiveness of specimen collection and different drug detection windows. Oral fluid monitors recent drug use, whereas sweat and hair provide greater detection windows, throughout the sweat patch wear period (usually 1 week) and months to years for hair, depending upon the length. Sweat testing offers advantages of less embarrassment during collection than thrice weekly observed urine testing,11 and the presence of the patch also acts as a reminder that the patient is under surveillance. Adulteration is substantially reduced as compared with urine, and most importantly, sweat offers cumulative drug use data over the week.12–14 Due to the delayed process of drug incorporation into sweat, the patch can detect drug use from a few hours before the patch is applied until its removal.15–17
The mechanisms of drug incorporation into sweat are not fully understood. Passive diffusion of drugs from capillaries in the skin into perspiration seems to be the main pathway, but excretion of substances via sebum and intercellular diffusion also contribute.18 Excretion into sweat depends upon a drug’s physicochemical properties such as molecular mass, pKa, protein binding, and lipophilicity. Therefore, parent drugs that more easily cross membranes are expected to accumulate in sweat in greater concentrations than polar hydrophilic metabolites.19,20
Excretion of opiates,13,20,21 cocaine,14,15,20 amphet-amines,12,22 and cannabinoids23 in sweat were studied after controlled drug administration. Most research demonstrated that sweat testing is suitable for monitoring illicit drug use and, depending upon the drug’s characteristics, may be more sensitive than urine testing. There is no reliable on-site test for drugs in sweat. Sweat patch analysis requires extraction and sensitive chromatographic methods in combination with mass spectrometry to achieve an effective limit of quantification (LOQ).
Opioid and cocaine biomarkers in sweat were quantified by a recently developed gas chromatography mass spectrometry (GCMS) assay24 with LOQs of 5 ng per patch. In addition to the analytes recommended by the Substance Abuse Mental Health Services Administration (SAMHSA) [6-acetyl-morphine (6AM), morphine, codeine, cocaine, and benzoylecgonine (BE)], we also examined heroin, 6-acetylcodeine (6AC), ecgonine methyl ester (EME), and anhydroecgonine methyl ester (AEME), a pyrolysis metabolite of cocaine and suggested indicator of crack cocaine, in our evaluations. Data were analyzed at both the LOQ and proposed cut-offs of the SAMHSA Mandatory Guidelines for the Federal Workplace Drug Testing Programs10 for cocaine and opiate sweat testing to determine if these cut-offs were pertinent for this population of pregnant drug-dependent women. Proposed cut-offs are 25 ng per patch for cocaine and/or BE, and codeine, morphine, and/or 6AM.
METHODS
Human Participants
Opioid-dependent women between the ages 19 and 40 years, and of gestational age 9–29 weeks, qualifying for methadone maintenance at the Center for Addiction and Pregnancy (CAP) at Johns Hopkins Bayview Medical Center participated in the study. Women were required to meet the Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria for opioid dependency. Women resided for several weeks at CAP for initiation of methadone treatment and again during the postpartum period. Throughout gestation, participants received outpatient methadone maintenance drug abuse treatment, weekly individual and group counseling, and specialized prenatal care. Additionally, the week before methadone initiation, most women received morphine capsules during transition to study pharmacotherapy. The Johns Hopkins Bayview Medical Center and the National Institute on Drug Abuse Institutional Review Boards approved the research and participants provided written informed consent and received vouchers (rewards) for negative urine specimens as part of a behavioral contingency management treatment program.
Specimen Collection
Women visited CAP 7 days/wk to receive methadone treatment throughout gestation for a mean of 17.2 ± 6.2 (range 5–32) weeks. Sweat was collected with Pharmchek sweat patches provided by Pharmchem Inc (Haltom City, TX). After trained personnel thoroughly cleansed the skin with isopropyl alcohol swabs, patches were applied to the back or arm. Sweat patches were worn for approximately 1 week, although some patches were worn for shorter or longer periods based on the woman’s attendance at the clinic (range 2–24 days). Sweat patches had to remain on the skin, with no signs of tampering, to be included. After removal, patches were stored at − 20°C until analysis. The number of sweat patches for each participant was dependent on gestational age at enrollment and adherence to scheduled appointments. Serial weekly patches were collected throughout study participation from enrollment until several weeks after delivery.
Chemicals and Reagents
Cocaine, BE, EME, AEME, codeine, morphine, heroin, 6AC, 6AM (1 mg/mL), and internal standards, cocaine-d3 and heroin-d9 (100 µg/mL) were purchased from Cerilliant Corporation (Round Rock, TX). AEME-d3 and 6AC-d3 (100 µg/mL) were obtained from Lipomed Inc (Cambridge, MA). N,O-Bis (trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane was from Pierce Chemical Co. (Rockford, IL) and Clean Screen ZSDAU020 (10 mL/200 mg) extraction columns from United Chemical Technologies (Bristol, PA). Acetic acid, sodium acetate, ammonium hydroxide, sodium chloride, sodium hydroxide, urea, methylene chloride, 2-propanol, methanol, ethyl acetate, and acetonitrile were supplied by JT Baker (Phillipsburg, NJ), and ammonium chloride, triethylamine, and lactic acid by Sigma-Aldrich (St. Louis, MO). Organic solvents were high-performance liquid chromatographic grade. PharmCheck sweat patches were generously donated by PharmChem Inc. (Fort Worth, TX). Artificial sweat solution was prepared in house to contain 327 mmol/L ammonium chloride, 166 mmol/L lactic acid, 83 mmol/L urea, 42 mmol/L acetic acid, 34 mmol/L sodium chloride in deionized water; pH was adjusted to 4.7 with 2 mol/L sodium hydroxide.
Analytical Procedures
Sweat patch specimens were analyzed for cocaine, BE, EME, AEME, heroin, 6AC, 6AM, codeine, and morphine by GCMS according to a new multianalyte assay by Brunet et al.24 Briefly, deuterated internal standard solution containing 100 ng of AEME-d3, cocaine-d3, 6AC-d3, and heroin-d9 was added to sweat patches before extraction. Sweat patches were placed into screw-top vials, 6 mL of sodium acetate buffer (pH 4.0) was added, and vials were mixed for 10 minutes on a horizontal reciprocating shaker. Extracts were collected and extraction repeated with 3 mL of buffer, and contents combined. Five milliliters of the combined buffered extract was applied to preconditioned solid phase extraction (SPE) columns, columns were washed with water, 0.1 M acetic acid, and acetonitrile and eluted with methylene chloride: 2-propanol: ammonium hydroxide (78:20:2). After evaporation, extracts were derivatized with N,O-bis (trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane and analyzed by GCMS. A 3-mL portion of the original buffered patch extract was reserved for heroin analysis if 6AM concentrations were greater than the limit of detection (2.5 ng per patch). A separate SPE extraction for heroin was performed with the same columns and ethyl acetate: triethylamine (98/2) as the elution solvent. This separate extraction was necessary to prevent hydrolysis of heroin with the elution solvent used in the first SPE extraction.
Quantitative analyses were performed on an Agilent 6890 gas chromatograph interfaced with an Agilent 5973 mass-selective detector operated in electron impact mode (Agilent Technologies, Wilmington, DE). Chromatographic separation was achieved with a HP-5MS capillary column (30 m × 0.32 mm internal diameter × 0.25-µm film thickness). Two calibration curves were required due to large variability in analyte concentrations and availability of only a single patch per participant per week for sweat testing. Drugs were quantified by linear regression with a 1/x weighting factor. Low curves ranged from 5 to 1000 ng per patch for all analytes, including heroin. Additional high calibration curves (1000–10,000 ng per patch) were constructed for cocaine, BE, and 6AM by modifying GCMS injection parameters. Estimates of imprecision were calculated using 4 replicate controls from 5 analytical runs (n = 20) according to Krouwer and Rabinowitz.25 For all runs, the pooled within-run component of imprecision, expressed as % coefficients of variation (%CV), was less than 4.9% for all control concentrations (15, 150, 750, 1500, 3000, and 8000 ng per patch). Between-run imprecision (%CV) for all analytes, at all concentrations, was less than 7.0%. Total imprecision of the method (%CV) was reported as less than 7.1%. Extraction efficiencies at the same concentrations were ±11.0% of target concentrations.
RESULTS
Human Participants and Clinical Specimens
Thirty-nine pregnant opioid-dependent women (mean age 30.1 ± 5.3 years; range 19–40) between 9 and 29 weeks of gestation participated in the study. Four hundred and twenty sweat patches were collected. Thirty-one patches (7.4%) were excluded due to patches returned unattached to the skin and for unclear application or removal dates. Mean ± SD duration of patch wear was 7.0 ± 2.5 days (median 7.0 days; range 2–24 days). Median sweat patches collected for each participant were 10.0 ± 6.3 patches (range 1–32 patches).
Cocaine and Metabolites in Sweat
A total of 389 sweat patches, from 39 participants, were tested for cocaine biomarkers. Among the participating women, all patches were positive for cocaine and/or metabolites in 15 subjects; only 1 woman had no cocaine positive sweat patches. Of the remaining 23 subjects, more than half of the sweat patches collected from 14 women were positive for cocaine. Cocaine was the primary analyte detected in positive sweat patches with a total of 65.3% (n = 254) positive at the GCMS LOQ of 5 ng per patch. Cocaine metabolites were never present without concurrent parent drug; cocaine concentrations ranged from 5.2 to 11835.0 ng per patch. Cocaine was present in the highest concentration in all but 6 specimens containing higher BE concentrations. BE was the second most frequently identified analyte in 152 sweat patches ranging in concentration from 5.0 to 5338.5 ng per patch. EME (104) and AEME (56) were present in fewer patches above the method’s LOQ. Table 1 presents the number of positive specimens, median, mean, and range of all cocaine analytes detected in sweat, with comparisons at the LOQ and proposed SAMHSA confirmatory test cut-off concentrations.
TABLE 1.
Total Positive Sweat Patches and Detection Rates for Cocaine and Metabolites; BE, EME, and AEME, at the Method’s LOQs of 5 ng per Patch and Proposed SAMHSA Cut-offs of 25 ng per Patch for Cocaine and BE Only
| ≥LOQ (5 ng per patch) |
≥25 ng per patch |
||||||
|---|---|---|---|---|---|---|---|
| Total Patches (N = 389) | N | % | Median (ng per patch) |
Mean ± SD (ng per patch) |
Range (ng per patch) |
N | % |
| Cocaine | 254 | 65.3 | 35.4 | 457.7 ± 1387.0 | 5.2–11835.0 | 154 | 39.6 |
| BE | 152 | 39.1 | 12.5 | 94.2 ± 452.1 | 5.0–5338.5 | 52 | 13.4 |
| EME | 104 | 26.7 | 20.8 | 96.7 ± 224.6 | 5.0–1486.2 | 47 | 12.1 |
| AEME | 56 | 14.4 | 11.9 | 19.2 ± 16.5 | 5.2–88.5 | 15 | 3.9 |
| Combinations | |||||||
| Cocaine only | 97 | 24.9 | — | — | — | 94 | 24.2 |
| BE only | 0 | 0 | — | — | — | 1 | 0.3 |
| Cocaine and BE | 46 | 11.8 | — | — | — | 11 | 2.8 |
| Cocaine and AEME | 3 | 0.8 | — | — | — | 1 | 0.3 |
| Cocaine and EME | 2 | 0.5 | — | — | — | 8 | 2.1 |
| Cocaine, BE, and AEME | 4 | 1.0 | — | — | — | 1 | 0.3 |
| Cocaine, BE, and EME | 53 | 13.6 | — | — | — | 26 | 6.7 |
| Cocaine, BE, AEME, and EME | 49 | 12.6 | — | — | — | 13 | 3.3 |
Sweat patches were collected from pregnant women enrolled in methadone maintenance treatment.
Figure 1 monitors cocaine use during pregnancy and the early post-partum period for 2 representative subjects. Data from subject A exhibit high concentrations of all 4 analytes at the beginning of the study and evidence of a relapse to cocaine use after delivery. Between patch numbers 2 and 13, only low concentrations of cocaine (<35.9 ng per patch) were detected. In contrast, subject B had elevated concentrations of cocaine throughout the monitoring period, yet remained cocaine-free for 6 weeks after delivery.
FIGURE 1.
Excretion profile of cocaine and metabolites in sweat patches collected over time from pregnant women enrolled in meth-adone maintenance treatment.
Heroin and Metabolites in Sweat
Three hundred and eighty-nine sweat patches, collected from 39 participants, were analyzed for heroin and metabolites, with 158 (40.6%) testing positive (≥5 ng per patch) for one or more opiates. Five pregnant opioid-dependent women remained opiate free for the duration of the study. In contrast, all sweat patches from 2 subjects were positive for at least one opiate; the majority of other subjects (18 of 32) had fewer than 50% opiate-positive patches. 6AM was the most frequent opiate detected in 134 of 158 opiate-positive patches (84.8%) followed by morphine, present in 127 sweat patches (80.4%), and both 6AM and morphine in 102 positive patches (64.6%). Heroin, codeine, and 6AC were detected in up to 19.8% of all patches at the LOQ. Morphine was independently identified in an additional 19 opiate positive specimens (12.0%). 6AM had the highest sweat concentrations with a median of 13.9 ng per patch (range 5.0–385.4) followed by heroin with relatively similar median concentrations of 12.6 ng per patch (range 5.3–345.4). Although 6AC was present in 19 patches, concentrations ranged from 5.1 to 24.0 ng per patch, and it was never the only opiate analyte detected. An additional 23 patches contained codeine in concentrations ranging from 5.0 to 195.6 ng per patch. A detailed overview of mean, median, and range of all opiate analyte concentrations is given in Table 2.
TABLE 2.
Total Positive Sweat Patches and Detection Rates for Opiates; Heroin, 6AM, Morphine, Codeine, and 6AC, at the Method’s LOQs (5 ng per Patch) and Proposed SAMHSA Cut-offs (25 ng per Patch for Morphine, Codeine, and/or 6AM) in Sweat Patches Collected From Pregnant Women in Methadone Maintenance Treatment
| ≥LOQ (5 ng per patch) |
≥25 (ng per patch) |
||||||
|---|---|---|---|---|---|---|---|
| Total Patches (N = 389) | N | % | Median (ng per patch) |
Mean ± SD (ng per patch) |
Range (ng/patch) |
N | % |
| 6AM | 134 | 34.4 | 13.9 | 40.0 ± 64.6 | 5.0–385.4 | 44 | 11.3 |
| Morphine | 127 | 32.6 | 10.5 | 23.1 ± 37.5 | 5.1–312.5 | 29 | 7.5 |
| Codeine | 39 | 10.0 | 11.4 | 18.6 ± 31.6 | 5.0–195.6 | 4 | 1.0 |
| Heroin | 77 | 19.8 | 12.6 | 34.3 ± 57.4 | 5.3–345.4 | 24 | 6.2 |
| 6AC | 19 | 4.9 | 8.6 | 10.7 ± 5.4 | 5.1–24.0 | 0 | 0.0 |
| Combinations | |||||||
| 6AM only | 16 | 4.1 | — | — | — | 11 | 2.8 |
| Morphine only | 19 | 4.9 | — | — | — | 2 | 0.5 |
| Heroin only (limit of detection ≤ 6AM ≤ LOQ) | 4 | 1.0 | — | — | — | 3 | 0.8 |
| Heroin and 6AM | 11 | 2.8 | — | — | — | 6 | 1.5 |
| 6AM and morphine | 34 | 8.7 | — | — | — | 10 | 2.5 |
| 6AM, morphine, and codeine | 10 | 2.6 | — | — | — | 2 | 0.5 |
| Heroin, 6AM, and morphine | 31 | 8.0 | — | — | — | 14 | 3.6 |
| Heroin, 6AM, morphine, and codeine | 13 | 3.3 | — | — | — | 1 | 0.3 |
| Heroin, 6AM, morphine, codeine, and 6AC | 14 | 3.6 | — | — | — | 0 | 0 |
| Other combinations | 6 | 1.5 | — | — | — | 1 | 0.3 |
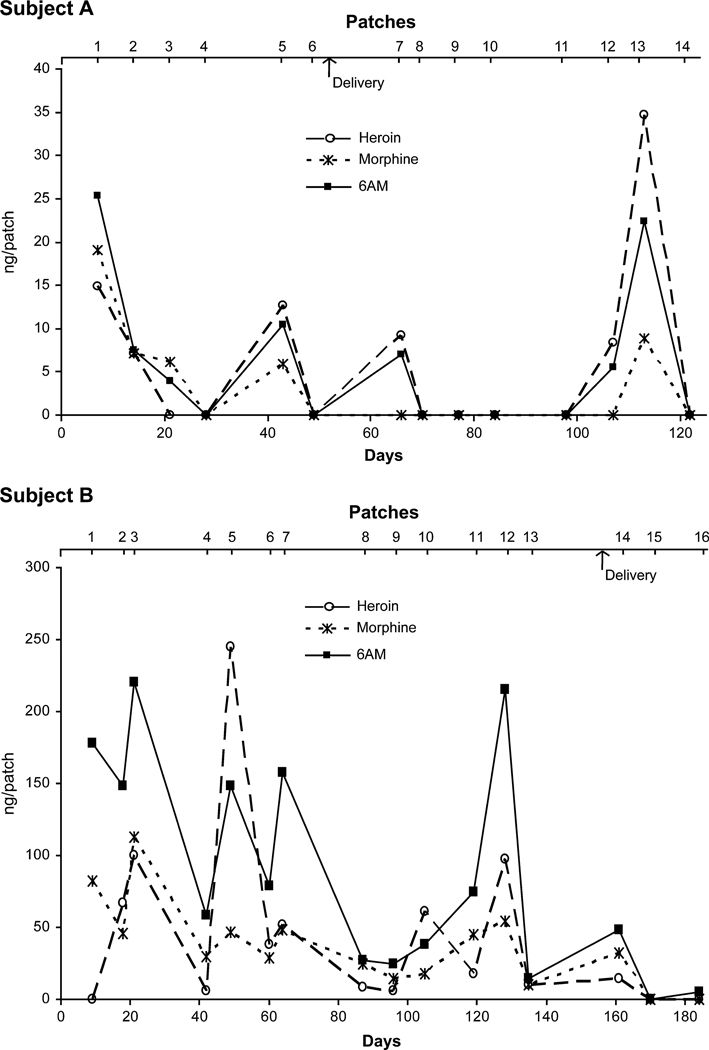
Figure 2 monitors opiate concentrations, for the same 2 subjects presented for cocaine, over the course of the study. Subject A demonstrated occasional heroin use over time. The pattern was similar to that observed for cocaine, indicating simultaneous cocaine and heroin relapse throughout pregnancy. Subject B had elevated concentrations of 6AM, heroin, and morphine throughout the study. Lower concentrations of 6AC and codeine (between 5 and 20 ng per patch) also were detected in this subject’s sweat. Only the first and the last 2 patches were negative for heroin.
FIGURE 2.
Excretion profile of opiates and metabolites in sweat patches collected over time from pregnant women enrolled in methadone maintenance treatment.
Comparison of Sweat Testing Results With Method LOQs and Proposed SAMHSA Cut-off Concentrations
SAMHSA recommended cut-off concentrations for cocaine and/or BE in sweat are 25 ng per patch. At these cut-offs, a total of 154 patches (39.6%) were positive for cocaine. Ninety-four of these were positive for cocaine alone; 51 contained both cocaine and BE, whereas only 1 patch was positive for BE without cocaine. We also evaluated results for AEME or EME at 25 ng per patch, however, there were no additional positive results. Fifteen and 47 patches were positive for AEME and EME at concentrations greater than 25 ng per patch, respectively, but contained concentrations lower than cocaine in each patch. The higher SAMHSA cut-off concentration detected 99 fewer sweat patches compared with the method’s LOQ of 5 ng per patch.
For opiates, SAMHSA also recommends a positive sweat patch cut-off of 25 ng per patch for 6AM, morphine, and/or codeine. Utilizing these thresholds, 47 patches (12.1%) would have been SAMHSA positive for at least 1 of the 3 opiates. Including a positive threshold of 25 ng per patch for 6AC did not detect any additional opiate use. However, 3 sweat patches contained heroin above 25 ng per patch with lower levels of morphine (9–13 ng per patch) and 6AM (17–22 ng per patch). The addition of heroin to the confirmatory list of opiates would increase the percentage of positive patches to 12.9% at the SAMHSA cut-off.
In Figure 3, sweat patch results for 2 subjects are illustrated with dashed lines representing the LOQ of the method (5 ng per patch) and the proposed SAMHSA cut-off (25 ng per patch). The results for subject C, document cocaine use throughout pregnancy with concentrations for 8 patches above both cut-offs, but less than 168 ng per patch. However, BE concentrations were always between the LOQ and SAMHSA cut-off, and would have been missed with a higher detection threshold. Two patches collected within the first 3 weeks were also positive for 6AM, with 3 patches positive for morphine during the same monitoring period. One of these patches contained >25 ng per patch morphine, but results indicate abstinence of heroin during most of the study.
FIGURE 3.
Time course of cocaine and opiate excretion in sweat patches with evaluation at SAMHSA and LOQ cut-offs.
Data from subject D showed heavy cocaine use with elevated concentrations of both cocaine (<11,835 ng per patch) and BE (<5389 ng per patch) in all patches throughout the study. Simultaneous heroin abuse also was demonstrated in the first month of the study with high concentrations of 6AM, morphine, and codeine.
Evaluation of Drug Concentrations and Metabolite Ratios
Substantial intersubject variability in excretion of cocaine, opiates, and metabolites in sweat was observed. Thirty-four of 39 subjects used cocaine and opiates at some point during their pregnancy. Concentrations and frequency of use varied greatly. Evaluating paired positive concentrations of cocaine and BE (152 patches), cocaine and EME (104 patches), and heroin and 6AM (77 patches), we found correlations (r) of 0.29, 0.77, and 0.77, respectively. A visual inspection of correlation graphs showed 3 obvious outliers. Removing these outliers, 2 for BE and 1 for EME, improved correlations for both cocaine and BE (r = 0.89) and cocaine and EME (r = 0.91). However, despite rather high correlations between paired analyte concentrations, there was too much variability to correctly predict one analyte concentration based on the other analyte concentration.
In patches with paired cocaine and BE concentrations, the mean metabolite to parent concentration ratio was 0.24 ± 0.33, with a range of 0.02–2.05. These results, large ranges and standard deviations, were typical for all metabolite to parent ratios evaluated (Table 3). In addition, median values also were different from calculated means, indicating an asymmetrical distribution of data. Expectedly, 1-way analysis of variance (participant as grouping variable) revealed significant differences (α = 0.05) between metabolite to parent concentration ratios among all participants (up to 26) evaluated with paired analyte concentrations. In addition, no correlation was observed for the metabolite to parent concentration ratios and duration of patch wear suggesting that hydrolysis of heroin or 6AM was not extensive during monitoring.
TABLE 3.
Metabolite to Parent Drug Concentration (ng per Patch) Ratios for Paired Sweat Patches Collected from Pregnant Women Enrolled in Methadone Maintenance
| Metabolite: Parent | N | Median | Range |
|---|---|---|---|
| BE: cocaine | 152 | 0.12 | 0.02–2.05 |
| EME: cocaine | 104 | 0.09 | 0.004–0.89 |
| AEME: cocaine | 56 | 0.02 | 0.001–0.69 |
| 6AM: heroin | 77 | 1.44 | 0.34–28.66 |
| 6AC: heroin | 17 | 0.16 | 0.04–1.08 |
DISCUSSION
In substance abuse treatment, criminal justice and workplace drug testing programs, monitoring illicit drug use is a critical component. Numerous biologic matrices are available including traditional matrices blood and urine, and alternative matrices such as oral fluid, sweat, and hair. Sweat patches generally monitor drug exposure history over 1 week. Sweat patches are readily accepted by patients, but there was some loss (1.2%) of patches during the 7-day wear period, as previously reported in other studies.12,26 Specimen processing of sweat patches is more complex than for traditional analyses; however, our new method allows simultaneous quantification of opiates, cocaine and metabolites from a single patch with appropriate LOQs. This method includes parent compounds (heroin and cocaine) usually found in sweat and important metabolites such as BE, EME, AEME, 6AM, and 6AC. Monitoring these metabolites can differentiate licit opiate medication and heroin use and also might indicate the smoked route of cocaine administration.
Our study population was composed of pregnant opioid-dependent women, most of whom also abused cocaine. These women were enrolled in methadone maintenance treatment and received specialized prenatal care and individual and group counseling, child care for additional children, and multiple other services. Similar to a study by Taylor et al,11 there is no evidence that the subjects were influenced by wearing the patch. The pattern of consumption most frequently observed in that study was a continuous use of cocaine, with occasional use of heroin. A few subjects almost completely reduced consumption of both drugs during the study, as delivery approached; however, relapse after birth also was noted. The high number of patches positive for cocaine or opiates demonstrates the difficulty in treating this high risk population of drug users.
A total of 254 patches (65.3%) tested positive for cocaine at the LOQ of the method (5 ng per patch). In previously published studies, cocaine was always the analyte most frequently detected and the analyte found in highest concentrations in sweat after cocaine use.15,27 Our data support these findings, with cocaine concentrations as high as 11,835 ng per patch. Preston et al15 detected cocaine concentrations of up to 66,225 ng per patch in weekly sweat patches after illicit cocaine use, whereas Kacinko et al14 reported cocaine concentrations of up to 197 ng per patch in weekly patches after controlled administration. However, BE and EME have been quantified at varying rates. Cone et al20 reported finding EME more frequently than BE in sweat after smoked, intravenous, or intranasal administration of cocaine. In a controlled subcutaneous administration study of cocaine hydrochloride, Kacinko et al also detected EME (36.5%) more often than BE (21.3%) in positive patches. Although mean concentrations of EME and BE were similar, EME was generally present in higher concentrations than BE.14 Fucci and De Giovanni28 detected cocaine in sweat from 2 of 10 patients enrolled in a substance abuse program, but unfortunately, an elevated LOQ of 20 ng per patch prevented drawing conclusions about BE and EME. In the present study, BE was present in 48 more patches (12.3%) than EME. Although mean BE (94.2 ng per patch) and EME (96.7 ng per patch) concentrations were similar, maximum concentrations were almost 4 times higher for BE (5338.5 ng per patch) than EME (1486.2 ng per patch) in specimens collected after illicit cocaine use. Preston et al found cocaine in 494 of 499 cocaine positive sweat patches after illicit cocaine use, followed by BE (388) and EME (362). Mean and median concentrations for BE and EME were similar.15
In the present study, AEME was detected in 56 patches, with a mean concentration of 19.2 ng per patch, or approximately 5% of cocaine concentrations. We did not have information on route of administration in our population although crack cocaine was generally reported. Currently, research is underway to determine concentrations of AEME and other minor cocaine analytes in street and US Pharmacopea cocaine that may negate their use as biomarkers of smoked cocaine.
A total of 158 patches (40.6%) also were positive for opiates at the method’s LOQ (5 ng per patch). The primary analytes detected were 6AM, morphine, and heroin, with codeine and 6AC detected in fewer patches at lower concentrations. Heroin use was evident as 6AM and/or heroin was detected in almost all of the positive opiate patches (139 of 158). There were 19 sweat patches in which morphine was detected independently. Five of these patches, obtained from 2 subjects, were collected within the first 24 days, and could have been positive from morphine administered before methadone initiation, morphine ingestion, or residual morphine after heroin abuse. Interestingly, most of the other 14 patches contained heroin and/or 6AM in previous patches and were collected weeks after methadone administration, indicating that residual morphine could be excreted from heroin use or potentially another source of morphine. The presence of 6AC, a contaminant of illicit street heroin, also was detected in 19 sweat patches. This metabolite may be a useful marker for patients undergoing pharmaceutical heroin treatment, but concentrations are quite low in sweat and LOQ must be adequate to detect this analyte. Sweat testing was shown to be an effective means of identifying illicit heroin exposure.
Several studies evaluated the use of sweat patches and opiate consumption in substance abuse treatment programs, but most of those studies involved only a small number of subjects. Kintz et al29 identified heroin use in 8 and codeine in 4 sweat patches from 20 known heroin abusers receiving buprenorphine treatment. Patches were worn for 5 days during this volunteer outpatient study. Expectedly, 6AM concentrations were highest when heroin was taken, and codeine was the primary analyte after codeine usage. Morphine was present after heroin and codeine administration, with the exception of 1 subject with low codeine concentrations. Following heroin, morphine concentrations were similar to those of heroin and codeine in sweat, whereas after codeine, morphine concentrations were approximately 10% of codeine levels.
After codeine administration, codeine was the only analyte detected in sweat. No metabolites were present, even after a high dose of 120 mg/70 kg.13 After heroin administration, Kintz et al21 demonstrated heroin as the primary analyte in sweat patches worn for 24 hours. A longer duration of patch wear could promote hydrolysis of heroin to 6AM in the patch. Cone et al reported the same finding in 2 subjects who wore concurrent patches for 1, 2, 3, or 5 days after receiving 20 mg of heroin. Heroin was the primary analyte in patches worn the first 2 days, and 6AM in patches worn 3 or 5 days.20 In the same study, Cone tested sweat patches from heroin users (n = 17) and reported similar concentrations of heroin and 6AM (up to 400 ng per patch) with lower concentrations of morphine (up to 156 ng per patch); codeine was not quantified. Patches were worn for 3–7 days, but no distinction between duration of patch wear was provided with results.
In another study of substance abuse treatment patients, 274 of 389 sweat patches tested positive for opiates with a 7.5 ng per patch cut-off for 6AM, morphine, and codeine and 12.5 ng per patch cut-off for heroin.16 Median concentrations for heroin and 6AM were 26.3 and 34.0 ng per patch, respectively. These concentrations were substantially higher than those found in our study, however, it may be due in part to the higher LOQ. The number of positive patches for heroin was fairly equivalent in the 2 studies, but we detected fewer positive patches containing 6AM, morphine, and codeine. These discrepancies may be due to the different populations studied, 44 adults (29 men and 15 women)16 compared with 39 pregnant women in the present study, different amounts of heroin used, or differences in metabolism between pregnant women and the general population.
Another objective of this study was to evaluate if the recommended SAMHSA cut-offs for detection of drugs of abuse in sweat also were pertinent in this population of pregnant drug-dependent women. SAMHSA guidelines include monitoring of 3 analytes for opiates (6AM, morphine, and codeine) and 2 for cocaine (cocaine and BE), each at a confirmatory cut-off concentration of 25 ng per patch. These cut-offs, established for federal workplace drug testing, are also used in criminal justice,30 and substance abuse treatment programs.15,16,31 Spiehler et al32 also confirmed that 25 ng per patch was the optimal cut-off for cocaine in a study of known drug users receiving controlled drug administration. Using these cut-offs in our study population identified 61% of the cocaine positive patches and only 29.7% of the opiate positive patches. The inclusion of AEME as a recommended SAMH-SA analyte may be of interest as it offers differentiation between cocaine and crack use, and EME could also be added, as it is present in sweat with concentrations and frequencies similar to BE. However, no additional positive specimens were identified in the current study with AEME and EME. In a high-risk population of pregnant heroin and cocaine users, high sensitivity (low LOQ) could certainly be justified to identify any drug exposure. Appropriate interventions could be performed to help the woman and her current children and to protect the unborn fetus. In addition, resources could be made available to the exposed neonate throughout development. However, based on our previous cocaine research, self-administered illicit cocaine could be detected up to 3 weeks after last use, during residence on a secure research unit during abstinence.14 Thus, many of the cocaine positive patches at the method’s LOQ could have been due to residual excretion of previously self-administered drug, and not to new cocaine exposure. In contrast, Schwilke et al13 reported that 44 weekly washout patches from 13 participants, applied up to 3 weeks before controlled codeine administration were all negative for 6AM, morphine, and codeine at the method LOQ (2.5 ng per patch).
It is noteworthy that sweat is one of the few matrices in which heroin is readily detected. The presence of heroin, 6AM, and 6AC allows unequivocal differentiation between licit opiate use and heroin abuse. Interestingly, 3 sweat patches contained heroin above 25 ng per patch, with lower concentrations of other opiate metabolites. 6AC could identify illicit heroin use in subjects receiving pharmaceutical heroin treatment. The SAMHSA cut-off of 25 ng per patch seems to be appropriate for detecting cocaine use, as cocaine and BE are usually present in sweat in high concentrations, however, using the same cut-off concentrations for opiates could possibly miss episodes of heroin consumption. For monitoring heroin use in high-risk populations, the lower LOQ offers much greater sensitivity. It is not clear, especially among heavy chronic users of cocaine or heroin, if the patch is detecting a new episode of drug use or that analytes represent residual excretion of recent drug intake before patch application. Kacinko et al found that during a 3-week washout period before controlled administration of cocaine, 5 participants (n = 20) had sweat patch concentrations >5 ng per patch cocaine in the third week of continuously monitored abstinence.14 After 3 codeine doses within 1 week, Schwilke et al13 demonstrated that codeine was only detectable at SAMHSA cut-off concentrations in the week of administration, but was detectable for 3 weeks at an LOQ of 2.5 ng per patch. Levisky et al questioned the presence of drug in adipose tissue and skin as a possible factor in positive sweat patch results. Although their experiments were not specifically designed to evaluate residual drug excretion in sweat, the question is raised.33 More controlled administration studies are needed in chronic heavy users to differentiate new drug use from residual drug excretion.
Illicit drug use by opiate-dependent pregnant women in methadone maintenance was monitored by drug excretion in sweat. Women received methadone pharmacotherapy and behavioral therapy in this outpatient study. Type, time, and amount of drug consumption were unknown, negating determination of windows of drug detection. However, the value of naturalistic studies such as these demonstrates realistic cocaine and opiate biomarker concentrations. Controlled drug administration studies on drug excretion in sweat have generally failed to demonstrate dose-concentration relationships.20,34,35 Duration of patch wear, variability in sweat production, and stability of drugs on the patch are some of the complex factors involved in interpretation of drug concentrations in sweat. In general, sweat monitoring is considered qualitative in nature. Sweat patches allow the detection of illicit drug use while simultaneously monitoring compliance with buprenorphine or methadone treatment.
In conclusion, sweat testing was an effective means of monitoring illicit drug use in a population of opiate-dependent pregnant women in methadone maintenance treatment. Sweat patches are readily accepted by subjects and allow monitoring over an entire week, limiting the number of required monitoring visits. These data will inform clinicians and treatment personnel of the advantages and disadvantages of monitoring heroin and cocaine use, and provide guidance for interpretation of sweat test results. The data also document a need for further controlled drug administration studies to evaluate drug excretion and optimal cut-offs for drug detection in sweat. In populations requiring continuous drug monitoring, sweat patches provide an important alternative to routine urine testing.
ACKNOWLEDGMENTS
Supported by the National Institutes of Health, National Institute on Drug Abuse Intramural Research Program, and National Institute on Drug Abuse extramural research grant DA12403 (PI: HE Jones). We thank all those who helped in the course of this study, including CAP research staff for collection of specimens.
REFERENCES
- 1.Freier MC, Griffith DR, Chasnoff IJ. In utero drug exposure: developmental follow-up and maternal-infant interaction. Semin Perinatol. 1991;15:310–316. [PubMed] [Google Scholar]
- 2.Wheeler SF. Substance abuse during pregnancy. Primary Care. 1993;20:191–207. [PubMed] [Google Scholar]
- 3.Havens JR, Simmons LA, Shannon LM, et al. Factors associated with substance use during pregnancy: results from a national sample. Drug Alcohol Depend. 2009;99:89–95. doi: 10.1016/j.drugalcdep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Jansson LM, Svikis D, Lee J, et al. Pregnancy and addiction. A comprehensive care model. J Subst Abuse Treatm. 1996;13:321–329. doi: 10.1016/s0740-5472(96)00070-0. [DOI] [PubMed] [Google Scholar]
- 5.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Cone EJ, Huestis MA. Interpretation of oral fluid tests for drugs of abuse. Ann N Y Acad Sci. 2007;1098:51–103. doi: 10.1196/annals.1384.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone EJ, Sampson-Cone A, Huestis MA. Interpreting alternative matrix test results. In: Karch SB, editor. Drug Abuse Handbook. Boca Raton: CRC Press; 2006. pp. 814–828. [Google Scholar]
- 8.Dams R, Choo RE, Lambert WE, et al. Oral fluid as an alternative matrix to monitor opiate and cocaine use in substance-abuse treatment patients. Drug Alcohol Depend. 2007;87:258–267. doi: 10.1016/j.drugalcdep.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kintz P, Bernhard W, Villain M, et al. Detection of cannabis use in drivers with the drugwipe device and by GC-MS after intercept device collection. J Anal Toxicol. 2005;29:724–727. doi: 10.1093/jat/29.7.724. [DOI] [PubMed] [Google Scholar]
- 10.SAMHSA Administration. 2004:1. Available at: http://workplace.samhsa.gov/
- 11.Taylor JR, Watson ID, Tames FJ, et al. Detection of drug use in a methadone maintenance clinic: sweat patches versus urine testing. Addiction. 1998;93:847–853. doi: 10.1046/j.1360-0443.1998.9368476.x. [DOI] [PubMed] [Google Scholar]
- 12.Barnes AJ, Smith ML, Kacinko SL, et al. Excretion of methamphetamine and amphetamine in human sweat following controlled oral metham-phetamine administration. Clin Chem. 2008;54:172–180. doi: 10.1373/clinchem.2007.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwilke EW, Barnes AJ, Kacinko SL, et al. Opioid disposition in human sweat after controlled oral codeine administration. Clin Chem. 2006;52:1539–1545. doi: 10.1373/clinchem.2006.067983. [DOI] [PubMed] [Google Scholar]
- 14.Kacinko SL, Barnes AJ, Schwilke EW, et al. Disposition of Cocaine and its metabolites in human sweat after controlled cocaine administration. Clin Chem. 2005;51:2085–2094. doi: 10.1373/clinchem.2005.054338. [DOI] [PubMed] [Google Scholar]
- 15.Preston KL, Huestis MA, Wong CJ, et al. Monitoring cocaine use in substance abuse treatment patients by sweat and urine testing. J Anal Toxicol. 1999;23:313–322. doi: 10.1093/jat/23.5.313. [DOI] [PubMed] [Google Scholar]
- 16.Huestis MA, Cone EJ, Wong CJ, et al. Monitoring opiate use in substance abuse treatment patients with sweat and urine drug testing. J Anal Toxicol. 2000;24:509–521. doi: 10.1093/jat/24.7.509. [DOI] [PubMed] [Google Scholar]
- 17.Bush DM. The US Mandatory Guidelines for Federal Workplace Drug Testing Programs: Current status and future considerations. Forensic Sci Int. 2008;174:111–119. doi: 10.1016/j.forsciint.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Skopp G, Pötsch L. Perspiration versus saliva—basic aspects concerning their use in roadside drug testing. Int J Legal Med. 1999;112:213–221. doi: 10.1007/s004140050239. [DOI] [PubMed] [Google Scholar]
- 19.Huestis MA, Oyler JM, Cone EJ, et al. Sweat testing for cocaine, codeine and metabolites by gas chromatography-mass spectrometry. J Chromatogr. 1999;733:247–264. doi: 10.1016/s0378-4347(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 20.Cone EJ, Hillsgrove MJ, Jenkins AJ, et al. Sweat testing for heroin, cocaine, and metabolites. J Anal Toxicol. 1994;18:298–305. doi: 10.1093/jat/18.6.298. [DOI] [PubMed] [Google Scholar]
- 21.Kintz P, Brenniesen R, Bundeli P, et al. Sweat testing for heroin and metabolites in a heroin maintenance program. Clin Chem. 1997;43:736–739. [PubMed] [Google Scholar]
- 22.Pichini S, Navarro M, Pacifici R, et al. Usefulness of sweat testing for the detection of MDMA after a single-dose administration. J Anal Toxicol. 2003;27:294–303. doi: 10.1093/jat/27.5.294. [DOI] [PubMed] [Google Scholar]
- 23.Huestis MA, Scheidweiler KB, Saito T, et al. Excretion of Delta9-tetrahydrocannabinol in sweat. Forensic Sci Int. 2008;174:173–177. doi: 10.1016/j.forsciint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet BR, Barnes AJ, Scheidweiler KB, et al. Development and validation of a solid-phase extraction gas chromatography-mass spec-trometry method for the simultaneous quantification of methadone, heroin, cocaine and metabolites in sweat. Anal Bioanal Chem. 2008;392:115–127. doi: 10.1007/s00216-008-2228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 26.Barnes AJ, De Martinis BS, Gorelick DA, et al. Disposition of MDMA and metabolites in human sweat following controlled MDMA administration. Clin Chem. 2009;55:454–462. doi: 10.1373/clinchem.2008.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberty HJ, Johnson BD, Fortner N, et al. Detecting crack and other cocaine use with fastpatches. Addict Biol. 2003;8:191–200. doi: 10.1080/1355621031000117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fucci N, De Giovanni N. Methadone in hair and sweat from patients in long-term maintenance therapy. Ther Drug Monit. 2007;29:452–454. doi: 10.1097/FTD.0b013e31811f1bbe. [DOI] [PubMed] [Google Scholar]
- 29.Kintz P, Tracqui A, Mangin P, et al. Sweat testing in opioid users with a sweat patch. J Anal Toxicol. 1996;20:393–397. doi: 10.1093/jat/20.6.393. [DOI] [PubMed] [Google Scholar]
- 30.Sunshine I, Sutliff JP. Sweat it out. In: Wong SHY, Sunshine I, editors. Handbook of Analytical Therapeutic Drug Monitoring and Toxicology. New York: CRC Press; 1997. pp. 253–264. [Google Scholar]
- 31.Chawarski MC, Fiellin DA, O’Connor PG, et al. Utility of sweat patch testing for drug use monitoring in outpatient treatment for opiate dependence. J Subst Abuse Treatm. 2007;33:411–415. doi: 10.1016/j.jsat.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiehler V, Fay J, Fogerson R, et al. Enzyme immunoassay validation for qualitative detection of cocaine in sweat. Clin Chem. 1996;42:34–38. [PubMed] [Google Scholar]
- 33.Levisky JA, Bowerman DL, Jenkins WW, et al. Drug deposition in adipose tissue and skin: evidence for an alternative source of positive sweat patch tests. Forensic Sci Int. 2000;110:35–46. doi: 10.1016/s0379-0738(00)00146-8. [DOI] [PubMed] [Google Scholar]
- 34.Kintz P. Drug testing in addicts: a comparison between urine, sweat, and hair. Ther Drug Monit. 1996;18:450–455. doi: 10.1097/00007691-199608000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Kintz P, Bundeli P, Brenneisen R, et al. Dose-concentration relationships in hair from subjects in a controlled heroin-maintenance program. J Anal Toxicol. 1998;22:231–236. doi: 10.1093/jat/22.3.231. [DOI] [PubMed] [Google Scholar]